Abstract
Blood is a non-Newtonian, shear-thinning fluid owing to the physical properties and behaviors of red blood cells (RBCs). Under increased shear flow, pre-existing clusters of cells disaggregate, orientate with flow, and deform. These essential processes enhance fluidity of blood, although accumulating evidence suggests that sublethal blood trauma—induced by supraphysiological shear exposure—paradoxically increases the deformability of RBCs when examined under low-shear conditions, despite obvious decrement of cellular deformation at moderate-to-higher shear stresses. Some propose that rather than actual enhancement of cell mechanics, these observations are “pseudoimprovements” and possibly reflect altered flow and/or cell orientation, leading to methodological artifacts, although direct evidence is lacking. This study thus sought to explore RBC mechanical responses in shear flow using purpose-built laser diffractometry in tandem with direct optical visualization to address this problem. Freshly collected RBCs were exposed to a mechanical stimulus known to drastically alter cell deformability (i.e., prior shear exposure (PSE) to 100 Pa × 300 s). Samples were subsequently transferred to a custom-built slit-flow chamber that combined laser diffractometry with direct cell visualization. Cell suspensions were sheared in a stepwise manner (between 0.3 and 5.0 Pa), with each step being maintained for 15 s. Deformability and cell orientation indices were recorded for small-scatter Fraunhofer diffraction patterns and also visualized RBCs. PSE RBCs had significantly decreased visualized and laser-derived deformability at any given shear stress ≥1 Pa. Novel, to our knowledge, observations demonstrated that PSE RBCs had increased heterogeneity of direct visualized orientation with flow vector at any shear, which may be due to greater vorticity and thus instability in 5-Pa flow compared with unsheared control. These findings indicate that shear exposure and stress-strain history can alter subsequent RBC behavior in physiologically relevant low-shear flows. These findings may yield insight into microvascular disorders in recipients of mechanical circulatory support and individuals with hematological diseases that alter physical properties of blood.
Significance
The shear stresses that exist within mechanical circulatory support systems, although low enough to avoid complete cell rupture, have been recently thought to negatively impact red blood cell function. This study provides direct evidence that sublethal shear stresses alter red blood cell biophysics in a manner that affects cell dynamics when subsequently in physiologically relevant flows. This finding may illuminate some of the microvascular associated complications in individuals with hematological diseases that alter the physical properties of blood or in individuals receiving mechanical circulatory support. This study thus also lends support for further evaluation of current medical pumps that exert supraphysiological stresses on blood and the potential clinical complications that may exist independent of the primary pathology itself.
Introduction
As blood flows through the varied geometries of the cardiovascular system, its viscous properties dynamically respond to fluctuating shear rates imposed by fluid-wall interactions. Given red blood cells (RBCs) constitute 36–50% of blood and ∼99% of the formed elements, the movement and biophysical properties of single RBCs largely dictate the fluid stability and viscous behavior of blood (1). At the cellular scale, rheology is primarily dependent on RBC deformability and orientation relative to tangential shear stresses; further, these properties are vital for alignment and entry of RBCs (∼8 μm) into the smallest apertures of the vasculature (∼3-μm inner diameter) (2). The ability of RBCs to orientate and deform is predominantly governed by the cell’s physical properties: including elasticity and stability of the surface membrane and connected cytoskeleton, the viscosity of the hemoglobin-dominant cytosol relative to that of the surrounding media, and a biconcave morphology that yields a favorable surface area relative to cell volume. Thus, RBCs exhibit a unique elastic range without exceeding plastic limits and/or surface rupture (for review, see Chien (2)).
In higher shear flow, single cells adopt an elastic drop-like behavior in which the surface membrane freely rotates around the cytosol in a “tank-treading” motion (3,4). This tank-treading movement has been identified to be an important attribute that facilitates energy dissipation of external work across the membrane, transferring tangential stresses to the inner fluid and allowing maintenance of a steady shape and orientation with the flow vector (5). Tank-treading acts to decrease hydraulic resistance in cell transit of the microcirculation and across axial regions of larger vessels with higher concentrations of cells (i.e., increased hematocrit) (6); tank treading is thus imperative for optimal blood flow.
The rate of tank treading is highly dependent on external factors (i.e., applied shear rates and viscosity of the suspending fluid) and internal cellular factors (i.e., cell membrane viscosity and morphological characteristics that influence hydrodynamics of the cell) (7). Goldsmith and Marlow (8) observed RBC tank treading, elliptocyte morphology, and orientation with flow in shears ≥1.2 Pa. In flow in which shear was <1.2 Pa, however, tank treading was still observed, although cells did not elongate, and the resting biconcave morphology was maintained. In very low shears, cells were observed to flip and tumble in an unsteady motion. Fedosov et al. (9) highlighted that large deformations and membrane “buckling” are necessary to facilitate the transition of cells from tumbling to tank-treading motions and that rigid cells that require larger forces for buckling also need increased shears to facilitate this dynamic transition. Thus, if RBC sensitivity to mechanical stimuli (particularly membrane material properties) is altered with pathological processes, flow homeostasis may be compromised, causing interruptions in local fluid stability, possibly hindering the ability of RBCs to uniformly access and traverse the microcirculation.
The material properties of the RBC membrane are susceptible to shear rigidification before complete rupture with accumulated exposure to shear stresses that are supraphysiological yet sublethal (i.e., do not induce hemolysis). Recent investigations that manipulated RBC stress-strain history to induce sublethal mechanical damage to RBCs identified that sublethal shear exposure induces: shear rigidification (10,11); remodeling of the membrane biochemistry, altering cell electronegativity (12); and morphological alterations (13), possibly indicating instability of junctional attachments of spectrin to the lipid bilayer. Given strong membrane deformations are required to transition from tumbling to tank treading, RBCs that have been exposed to sublethal mechanical trauma are likely to have altered cell dynamics, may become trapped in tumbling motions, and require greater shear rates to transition to tank treading (9).
Prior exposure of RBCs to supraphysiological shear stresses may also produce an unexpected increase in cellular deformation when measured under lower shears despite obvious decrement of cellular deformation at moderate-to-higher shear stresses (10). Specifically, laser-diffractometry techniques typically employed in these studies are useful for assessing the mean RBC deformability for an entire sample; as RBCs increasingly deform, laser-diffraction patterns become more ellipsoidal in shape and, thus, indirectly provide cell mechanical information. Given this technique is an indirect population measurement, “pseudoimprovements” in cellular deformability in low shear flows may actually reflect physical properties other than solely cell deformability. Bull et al. (14) identified (with secondary confirmation in a rheoscope) that cell morphology and orientation data influence low-shear ektacytometry, with differently orientated elliptocytes producing “hazy” diffraction patterns. An adjunct observation also reported that cell orientation is an important determinant of low-shear ektacytometry values (15), with paradoxical low-shear increases in diffraction elongation indices likely representing increased heterogeneity of cell populations rather than “true” deformation (16). Given that a single diffraction pattern is the product of light scattering from millions of blood cells, different cell subpopulations (e.g., young and old) contribute to the entirety of the output being overlayed with varying intensity (17). A paradoxically elevated low-shear diffraction elongation index (EI) has been observed in blood cells obtained from clinical populations (i.e., sickle cell anemia (18)) with morphological alterations (14) that were glutaraldehyde hardened (19) and after exposure to sublethal shear damage (10,13,20, 21, 22, 23). Numerous authors hypothesize that low-shear increases in RBC deformability in such conditions known to decrease cell mechanics is likely due to nonalignment of RBCs in flow and excessive cell tumbling, although conclusive evidence is lacking.
This study thus examined the behavior of blood flow for RBCs that had previously been exposed to sublethal and supraphysiological shear stresses, with particular interest in cell orientation to flow. It was predicted these data might elucidate the paradoxical low-shear “increases” in cellular deformability as determined via laser diffractometry in conditions known to cause impaired cell mechanics.
Materials and Methods
Blood sampling and preparation
Blood was collected from healthy men (age: 27 ± 8 years; n = 10) via routine venepuncture of a prominent vein in the antecubital region of the upper limb using a 21-gauge needle and syringe; the syringe draw was performed slowly to minimize shearing during collection. Immediately after collection, the blood was transferred into a tube containing 1.8 mg⋅mL−1 K2-EDTA and thoroughly mixed. All experimental procedures were completed within 4 h of initial blood collection. This study protocol was reviewed and approved by the Griffith University Human Research Ethics Committee (reference number: 2016/712), which conforms with the Declaration of Helsinki.
Experimental design
To investigate the influence of RBC shear rigidification on subsequent low-shear cell orientation behavior, this study employed three experimental stages: 1) although previous literature has begun elucidating thresholds that delineate RBC sublethal damage (10), experiment one sought to confirm magnitudes of sublethal shear stress that induce cell rigidification; 2) using the identified shear magnitude from experiment one that induced the largest cell rigidification, experiment two sought to examine the relationship between shear-rigidified RBCs, and the pseudoimprovements detected with low-shear laser diffractometry; and 3) upon detection of the largest low-shear pseudoimprovement in experiment two, fresh blood samples were exposed to the same magnitude of shear and immediately transferred into a custom-built device that enabled deeper inspection with laser diffractometry and concurrent direct visualization during flow. Resultant data were analyzed for cellular deformation and orientation.
Experiments one and two: shear magnitudes that rigidify RBCs
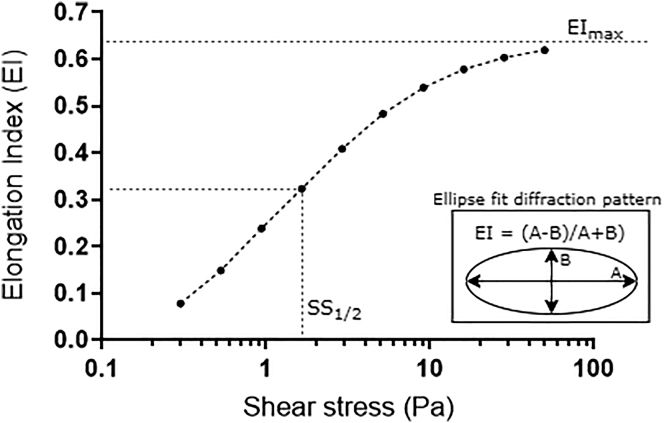
In preparation for ektacytometry investigations, a viscous solution for RBC suspension was created (5% 360 kDa polyvinylpyrrolidone in 0.1 mol⋅L−1 phosphate buffered saline (PVP-PBS) and 290 mOsmol∙kg−1). The viscosity of the PVP solution was measured using a cone-plate viscometer (0.5 DVII+ with CPE-40 spindle; Brookfield Engineering Labs, Middleboro, MA) operating at a temperature reflective of the specific experimental requirements; i.e., at room temperature (η = 39 mPa⋅s) and 37°C (η = 30 mPa⋅s). Fresh whole blood was diluted at 1:200 in the PVP solution, and 1 mL was injected into the gap of a commercial annular Couette shearing system and ektacytometer (Laser-assisted Optical Rotational Cell Analyzer; Mechatronics, Hoorn, the Netherlands) operating at 37 ± 0.2°C. As previously described (24), the rotational velocity of the outer cup was manipulated to induce discrete magnitudes and durations of shear exposure (i.e., 1–100 Pa and 1–300 s) for shear conditioning and standard RBC deformability tests. A low-powered laser (655 nm, 3 mW) was emitted through the RBC suspension to yield small-scatter diffraction patterns. Diffraction patterns are typically circular for cells at rest (due to their random orientation) and become more ellipsoidal as RBCs deform (and align with flow). An EI was determined across a range of specifically chosen shear stresses (0.3–3 Pa) by fitting an ellipse to discrete diffraction patterns using the equation EI = (A − B)/(A + B), where A is the long axis and B is the short axis of the ellipse (Fig. 1). The EI-SS curves were subsequently parameterized with a nonlinear fit of the Lineweaver-Burk equation to determine the maximal theoretical elongation that could be achieved at an infinite shear stress (i.e., EImax) and the magnitude of shear stress required to induce half of EImax (i.e., SS1/2). As previously described (25,26), the ratio of these parameters (SS1/2/EImax) provides an index of RBC deformability that facilitates effective and simple comparisons between RBC samples. Decreased RBC deformability is associated with increased SS1/2/EImax and vice versa.
Figure 1.
Parametrization of red blood cell (RBC) deformability elongation index (EI)-shear curves from laser diffractometry or ektacytometry.
For the shear stress magnitude that induced the largest increase in SS1/2/EImax in experiment one, the impact of increasing duration of exposure (5, 10, 60, and 300 s) were investigated in experiment two by inspecting EI-shear-stress relationships for pseudoimprovements in low-shear deformability. The sample that displayed the largest increase in low-shear EI was chosen as the shear condition to be used in experiment three of the study.
Development of the ektacytoscope
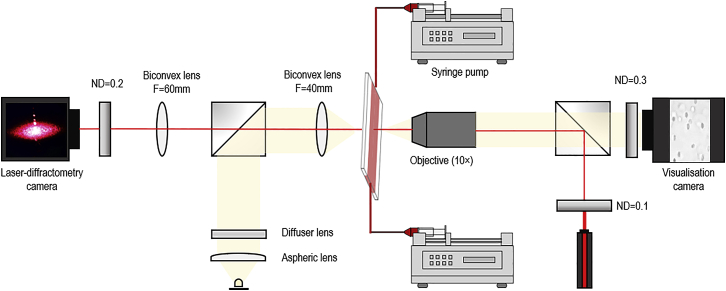
Investigating single-cell dynamics with low-shear ektacytometry required the development and deployment of a custom-built device that combined laser diffractometry and optical visualization (i.e., ektacytometer and rheometer—“ektacytoscope”) along the same axis (Fig. 2). This system enabled the concurrent visualization of a single area of a blood-shearing chamber with laser-diffractometry imaging from ektacytometry and direct cell visualization from bright-field microscopy. The shearing and visualization chamber was a slit-flow rheometer design (obtained from RheoMeditech, Seoul, South Korea), with the dimensions 40 (L) × 4 (W) × 0.18 mm (H). The pressure head in the chamber was generated by dual reciprocating syringe pumps (NE-8000X; New Era Pump Systems, Farmingdale, NY) with a flow protocol that increased wall shear stress in a stepwise manner from 0.3 to 5.0 Pa (i.e., 0.040–0.665 mL⋅min−1). The optical sectioning of the experimental setup was a depth of ∼10 μm; therefore, given the parabolic flow profile of the Poiseuille slit-flow chamber, the visualized section was determined to be within ∼10% error of each specific shear stress magnitude. The pump control system was automated and time synchronized with the data acquisition devices. The coaxial design was achieved by aligning bright-field visualization microscopy with co-localization of an epi-illuminated laser light source. The two imaging systems were overlapped coaxially using 50:50 beam splitters, and the light intensities of the laser and white-light sources were balanced with a variety of neutral density filters in each light path to optimize the captured image clarity and to compensate for backscatter, cross-illumination, and ambient light contamination. Small-scatter diffraction patterns generated from the blood sample were columnated through the condenser lens and subsequently focused onto a complementary metal-oxide-semiconductor (CMOS) sensor (ToupTek Photonics, Hangzhou, China) operating at 20 Hz. Optical visualization was recorded at the opposite end of the rail system using a charge-coupled device (CCD) camera and frame grabber (FlowSense EO 2M; Dantec Dynamics, Bristol, UK) at 16 Hz, with the interval between frames set to 200 ns. Before experimentation, care was taken to optimize the environment of the charge-coupled device camera used for direct RBC visualization, which included setting the focal distance to the chamber wall, enhancing image clarity, and correcting exposure levels. This process enhanced image analyses through provision of uniform image quality, thus minimizing the need for postprocessing manipulations (see Fig. 3 for image analysis flowchart). Bright-field microscopy and laser-diffractometry visualization were recorded in tandem and exported to image analysis software for further inspection (see Fig. 4 for typical raw images collected). Note: random subsets of data were preprocessed to extract parameters specific to the discrete experimental setup and thus facilitate image optimization.
Figure 2.
Design schematic for the custom-built, combined slit-flow ektacytometer-rheometer (i.e., the “ektacytoscope”). The designed system facilitated a coaxial assessment of blood cells in shear flow with high-speed visualization and small-scatter laser diffractometry. To see this figure in color, go online.
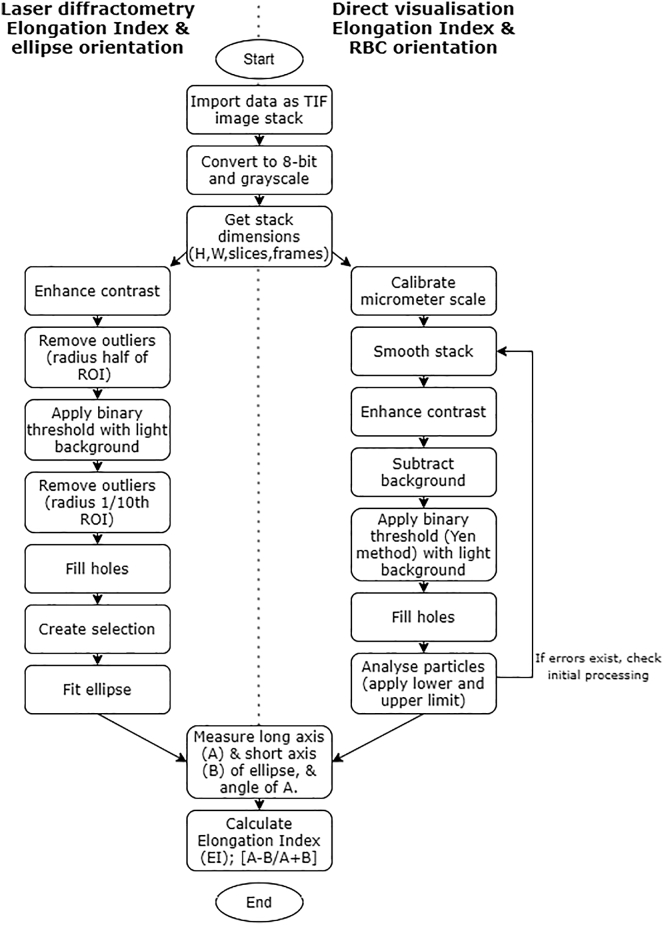
Figure 3.
ImageJ (National Institutes of Health) image analysis process for the analysis of raw laser-diffractometry data and directly recorded visualized blood samples.
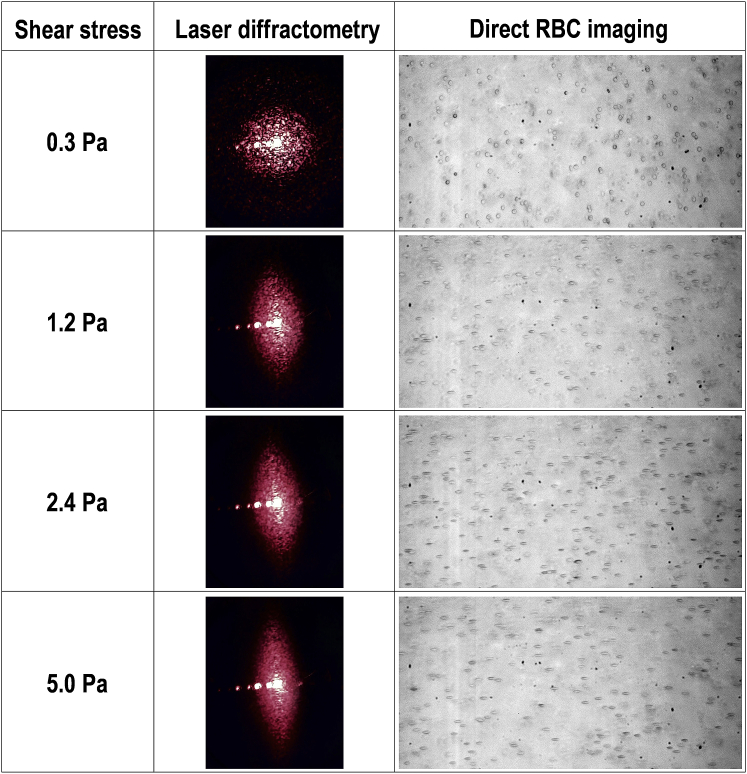
Figure 4.
Typical images obtained from the ektacytoscope displaying captured laser-diffraction patterns and simultaneously recorded visualized RBCs at four increasing shear magnitudes. To see this figure in color, go online.
Experiment three: RBC treatment phase and ektacytoscope assessment
After identification of the shear-magnitude-duration combination that yielded low-shear EI increases in experiment two, fresh RBCs were suspended in the PVP-PBS solution at 6% hematocrit, and 1 mL was then injected into the annular Couette shearing system. The suspension was sheared at 100 Pa for 300 s, and then, 600 μL was recovered and transferred into a tube containing 2.4 mL of fresh PVP-PBS (final hematocrit: 2%). This “prior shear exposure” (PSE) RBC suspension was subsequently transferred to the ektacytoscope, using 1 mL to slowly prime the tubing and slit-flow chamber. Once the system was primed and free from air bubbles, flow velocity was confirmed to be 0 via direct cell visualization and laser diffractometry. The syringe pumps were subsequently activated, and velocity was manipulated to achieve peak shear magnitudes that started at 0.3 Pa and increased to 3 Pa in 0.3-Pa increments, followed by two further steps of 4 and 5 Pa. Each shear magnitude was sustained for 15 s, with the last 10 s of each stage being extracted for subsequent analysis; this ensured transitional flow periods were avoided during subsequent data analyses. Within each experimental condition, ∼35,000 cells were directly visualized and assessed for elongation and orientation at each shear magnitude.
Data analysis
Data obtained from traditional ektacytometry in experiments one and two were analyzed with a one-way ANOVA with repeated measures, with emphasis on post hoc comparisons applying the Bonferroni correction to examine low-shear differences. Correlation analyses were performed between markers of cell rigidity (i.e., mechanical sensitivity index) and the EI-value at the lowest measured shear (i.e., 0.3 Pa) for all data from experiments one and two; the mechanical sensitivity index displays cell rigidity as percentage change in deformability relative to unsheared baseline (as previously described elsewhere (10,13,22)). Video data from experiment three were captured for visualized cells and laser-diffraction patterns, and subsequent image analysis was performed using custom software routines written in open source software (ImageJ; National Institutes of Health, Bethesda, MD). After image processing, data were analyzed for EI (deformability), angle of orientation, and ellipse fit area. Because the visualization images yielded >300 cells per frame, histograms of the EI distribution were generated to examine population differences between nonsheared (Con) and PSE RBCs. For visual orientation analyses, an ellipse was fitted to each cell in every frame, and cells that had a calculated EI above 0.2 were assessed for orientation of the long axis of the ellipse relative to the flow vector (0°). Cells below a calculated EI of 0.2 were excluded from the orientation analysis because the measured direction of the long axis of the fitted ellipse generated inconsistent results; the implemented filter thus captured cells closer to the “C = 0” orientation (27) (where the long and short axes are closer to ∼8 and ∼2 μm, respectively, i.e., the side profile) and not cells with “flat” circular morphology. Orientation data were grouped into 10° bins for frequency counts and assessed for total frequency and relative differences between PSE and Con RBCs. All statistical analyses were conducted using commercial software (Prism 7; GraphPad Software, San Diego, CA). Significance was determined at an α of 0.05. Unless otherwise stated, data are presented as mean ± standard error.
Results
Experiment one: confirmation of shear magnitudes that rigidify RBCs
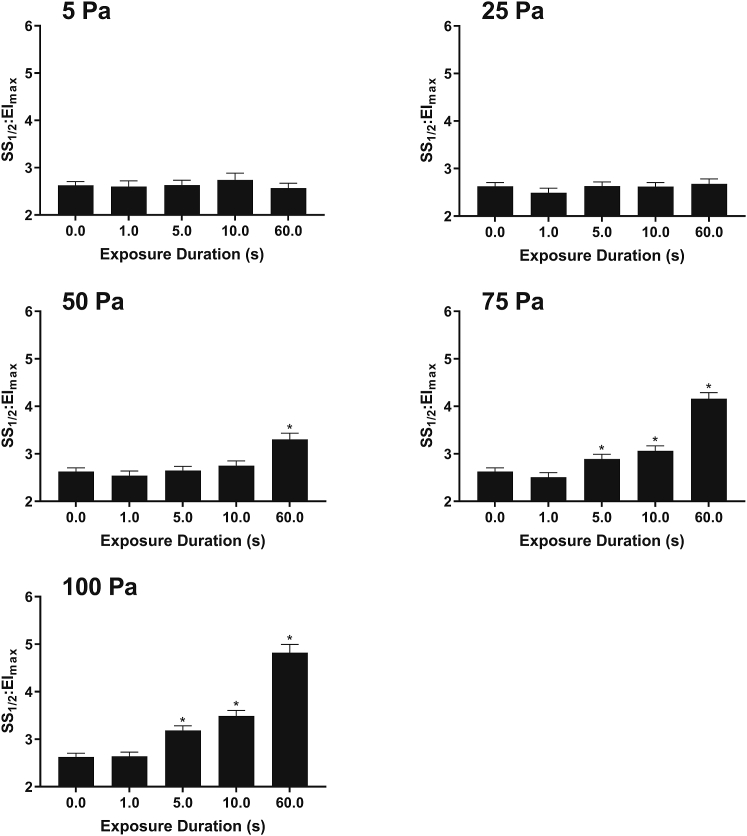
The SS1/2/EImax index (i.e., reflecting RBC deformability) obtained from ektacytometry after RBC treatment with varied magnitudes and durations of shear stress is illustrated in Fig. 5. Exposure to ≥5 s of 100 or 75 Pa or 60 s of 50 Pa significantly decreased deformability of RBCs (p < 0.05). For any matched duration, 100-Pa shear stress yielded the most pronounced increases in cell rigidity.
Figure 5.
Deformability index of fresh RBC suspensions exposed to varied magnitude and duration of nonlethal shear stress. Increases in SS1/2/EImax reflect decreases in deformability. ∗p < 0.05, significantly different to unsheared “0” sample.
Experiment two: RBC rigidity and low-shear EI
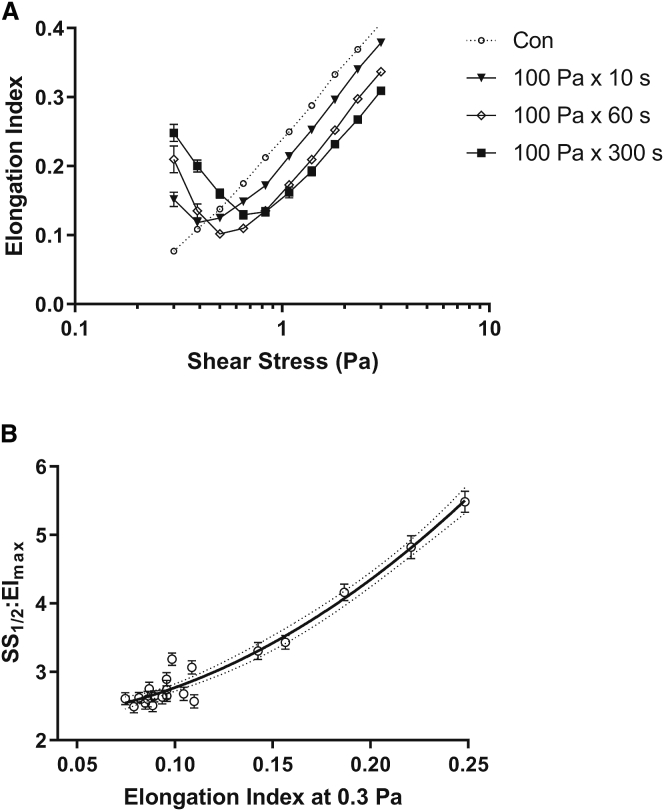
The EI-shear stress ektacytometry curves are illustrated in Fig. 6 A for unsheared Con, and RBCs previously exposed to varied durations of 100 Pa. Although Con displayed a typical deformability response to shear, at shears ≥1 Pa, all RBCs prior exposed to 100 Pa required significantly more shear to attain the same level of deformability. Below 1 Pa, however, increased exposure to prior shear augmented low-shear EI-values in a dose-response manner, with 100 Pa × 300 s of prior shear displaying the largest instantaneous EI-value at 0.3 Pa.
Figure 6.
(A) Ektacytometry deformability curves for unsheared Con and RBC suspensions previously exposed to 100 Pa for various durations. (B) Shown is the relationship of the decreased RBC deformability index and the increased EI obtained from laser-diffraction patterns at 0.3 Pa; r = 0.97.
Using the raw EI-SS curves in experiment one and the data presented in Fig. 6, A and B demonstrates a significant positive correlation between SS1/2/EImax (decreased RBC deformability) and the instantaneous EI-value at 0.3 Pa for grouped shear-magnitude combinations (r = 0.97).
Experiment three: coaxial ektacytometry and rheometry of RBC deformability
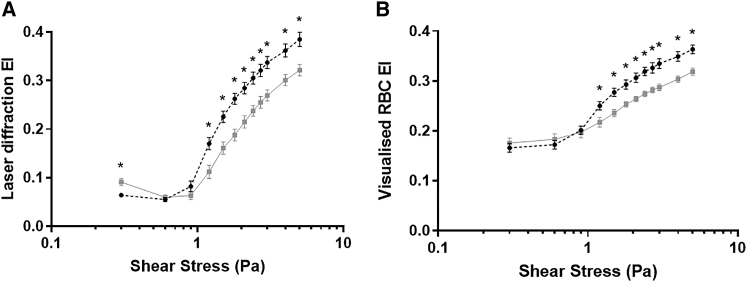
Ektacytoscope-derived deformability analyses of RBC suspensions are displayed in Fig. 7. For RBCs that had PSE to 100 Pa × 300 s, the laser-derived deformability (Fig. 7 A) was paradoxically and yet predictably (as per experiment two data) increased when measured at 0.3 Pa compared with Con. Further, PSE RBCs exhibited significantly decreased laser-derived deformability when measured at all shears >1 Pa compared with Con. Concurrent direct visualization of PSE RBC deformability (Fig. 7 B) also provided a similar general trend (i.e., less deformable than Con); however, no difference was detected at low shears for PSE RBCs. Direct visualized RBC deformability responses less than 1 Pa were significantly elevated compared with laser-diffraction measures because of several RBCs in the visualization plane being imaged when rotated away from the camera (C = 0 orientation), thus capturing the side view of an RBC, for which the long and short axes are recorded as ∼8 and ∼2 μm, respectively.
Figure 7.
EIs derived from coaxial laser diffractometry (A) and concurrent direct visualization rheometry (B) for RBCs without (Con) or after (prior shear exposure (PSE)) exposure to 100 Pa. ∗p < 0.05, significantly different to Con.
Experiment three: histograms of RBC deformation
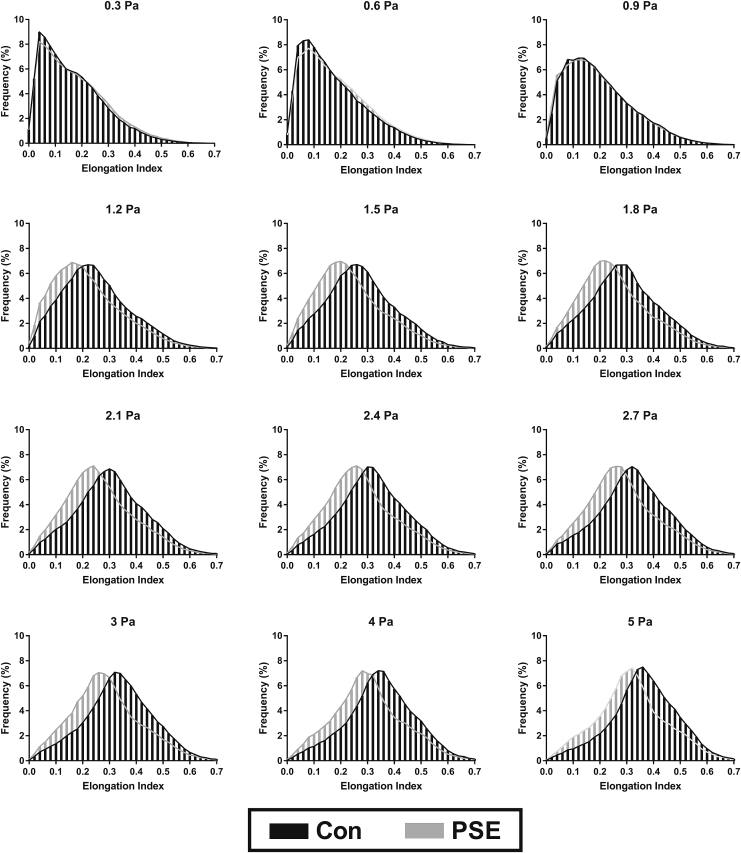
Typical approaches to estimating cellular deformability represent an averaged response for all cells within a given measurement period; however, in this study, a frame-by-frame approach was employed to facilitate assessment of all visible cells to yield a frequency histogram of cellular deformability for a given sample (Fig. 8). Data are presented for PSE cells and also unsheared Con under instantaneous exposure to shears between 0.3 and 5 Pa. At all shears above 0.9 Pa, control RBCs generated increased EI-values (i.e., increased deformability) at the modal response. At 0.9 Pa and below, no significant differences were detected between the distributions of measured EI-values for unsheared Con and PSE RBCs.
Figure 8.
Frequency distribution histograms of the EI of visualized flowing RBCs at discrete magnitudes of instantaneous shear stresses for unsheared Con and for RBCs with PSE.
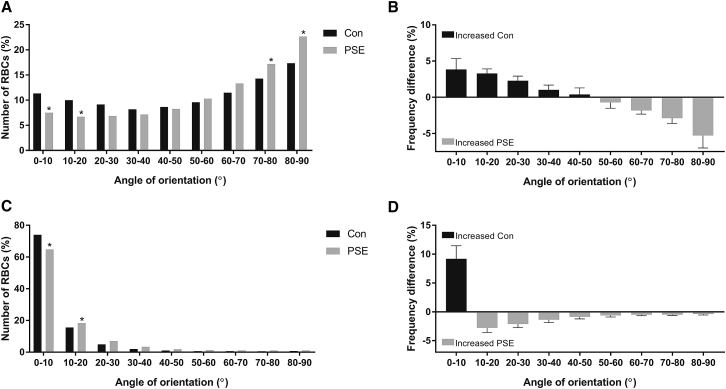
Experiment three: RBC orientation at 0.3 and 5 Pa
Given differences in laser-derived deformability at 0.3 Pa could not be explained by concurrent visualized RBC deformability, RBC orientation data were compared between PSE and Con at 0.3 (Fig. 9, A and B) and 5 Pa (Fig. 9, C and D). In 0.3-Pa shear flow, Con exhibited significantly more RBCs with greater alignment with the flow vector than PSE (i.e., the long axis closer to 0°). This trend was also maintained in 5-Pa shear flow, in which average cell orientation was instantaneously less aligned for PSE than Con (i.e., 12 and 9°, respectively).
Figure 9.
Frequency of RBCs at discrete orientations for RBCs without (Con) or after (PSE) exposure to 100 Pa in subsequent 0.3- (A and B) and 5-Pa (C and D) shear flows. ∗p < 0.05, significantly different to Con.
Discussion
Since the advent of prosthetic heart valves and the emergence of mechanical circulatory support, there has been avid interest in the effects of supraphysiological shear stresses on blood. Although prior works centered on understanding the processes involved in overt blood trauma (e.g., hemolysis; for review, see Faghih and Sharp (28)), recent attention has shifted toward more sensitive markers of functional blood trauma. The primary objective of this study was to investigate how exposure of RBCs to sublethal shear stress alters cellular dynamics (especially cell orientation) when those cells are subsequently in low-shear conditions. A secondary aim was to elucidate the observed but to date unexplained paradoxical low-shear increase in EI that is derived from laser diffractometry for RBCs that were previously exposed to high shears. The salient findings of this study were that after prior high shear exposure 1) RBC deformability was confirmed to significantly decrease via direct optical and laser-derived assessments, 2) orientation of RBCs with the mean flow vector was significantly less aligned and thus more unstable in flow, and 3) the heterogeneity of RBC populations increased, with relatively fewer cells maintaining ability to elongate appropriately. Further, it was observed that after prior exposure of RBCs to high shears, decreased RBC deformability (i.e., increased SS1/2/EImax) was correlated paradoxically with elevated low-shear ektacytometry EI-values. Collectively, these findings indicate that sublethal shear changes the physical properties of RBCs that subsequently alter the behavior of blood at flow rates that are physiologically relevant. It is postulated that when RBCs are exposed to high shear stresses, upon entry into lower shear regions, greater energy is required for cell stability and orientation with the mean flow vector. These findings may yield insight into the microvascular dysfunction reported for recipients of mechanical circulatory support and individuals with hematological conditions that alter the biophysical properties of blood.
In this study, prior exposure to sublethal shear stress significantly decreased RBC deformability and altered the ability of RBCs to orientate in flow. Cellular deformability of RBCs was recently demonstrated to be significantly decreased after prolonged exposure to >38 Pa and short-term exposure to ∼80 Pa (10). These findings are consistent with this notion; RBC deformability was impaired in a stepwise fashion with shear stress exposures >50 Pa, but no effect was observed for cells exposed to ≤25 Pa (Fig. 5). Although the discrete mechanisms of RBC rigidification after exposure to shear levels beyond elastic limits remain to be fully elucidated, physical alterations to the membrane, cytoskeleton, and cytosol appear to partly explain the observable changes, i.e., sublethal shear damage to RBCs has been reported to remodel the cell’s glycocalyx (12), alter the lipid bilayer (29), force unfolding of spectrin (30), and breakdown spectrin junctional attachments (28,31). McNamee and colleagues (32) exposed RBC to supraphysiological shears and monitored changes in membrane elastic modulus and capillary transit velocity and reported that membrane rigidification was associated with concurrent decreases in cell volume. As RBC volume decreases, the relative concentration of intracellular hemoglobin increases, thus increasing the viscosity of the cytosol (33). RBC cytosolic viscosity is a primary determinant of cell deformability and energy dissipation across the membrane (which facilitates RBC tank-treading stability in shear flow) (5,7). With increased RBC membrane rigidification and increased cytosolic viscosity after sublethal shear exposure, it is plausible that PSE RBCs exhibit altered tumbling-to-tank-treading behavior and less stability in higher shear flows.
These data support the hypothesis presented by Fedosov et al. (9) that more rigid (less deformable) cells require increased shear flows and thus energy to facilitate the dynamic transition of tumbling-to-tank-treading movements. In this study, once a majority of blood cells had achieved substantial orientation and elongated in higher shears, cells that had been previously exposed to sublethal shear were more unstable in flow, with steeper angles of orientation (Fig. 9). Given that tank-treading movements facilitate energy dissipation of tangential shear stresses to maintain steady shape elongation and orientation with the flow vector (5), it is likely that in addition to shear rigidification causing altered transitional movements of tumbling to tank treading, shear-rigidified RBCs may also have diminished the ability to tank tread and dissipate energy in all shear flows, thus being more unstable in flow than healthy RBCs. Given that the biophysical properties of RBCs have been demonstrated to significantly impact flow through microchannels of various sizes, with deformability and orientation being mediators of wall friction forces and hydraulic resistance in passage through the microcirculation (6), it is thus plausible that the findings of this study suggest that shear-induced hinderances in the ability of RBCs to orientate and deform would also likely yield consequences for microcirculatory blood flow and tissue oxygenation.
In this study, exposing RBC suspensions to sublethal shear stress decreased average cell deformability yet increased the heterogeneity of the cell populations, indicating that subpopulations of RBCs respond differently to shear. In our recent investigation (20), it was highlighted that different subpopulations have varied tolerance to sublethal damage, with older (more dense) subpopulations being more susceptible to mechanical trauma than younger cells. It is plausible that these increases in orientation and deformability distribution width is resultant of diverse subpopulations with varied mechanical susceptibility; qualitative observations identified varied cell morphologies after exposure to shear. Curiously, although the deformability of most cells decreased, a few cells remained unaltered in their ability to deform and align. Given all cells contribute to the generated laser-diffraction pattern, further inspection into the varied intensities of the captured image might facilitate more rapid discrete subpopulation detection without the need for concurrent direct visualization. Identifying and isolating cells that do not accumulate shear damage would be of value for elucidating potential cellular protective mechanisms.
This study provides direct optical evidence, for the first time, to our knowledge, indicating that low-shear, laser-diffractometry measurements of rigid RBCs misrepresent the artifact of poor cell alignment as pseudoincreases in cellular deformability. Although numerous authors have commented on the abnormal low-shear ektacytometry values with conditions that alter cell biophysics (10,13,14,18, 19, 20, 21, 22, 23), without confirmation by visual inspection, many investigations have strategically excluded these data from the analysis processes (25). In the design of this study, with the development of the ektacytoscope, the direct investigation of the parallel variables of laser diffractometry and bright-field microscopic visualization could be performed. The marked responses of sublethal low-shear EI increases observed in this study appear to be a phenomenon limited to laser-diffractometry techniques and are nonexistent with direct cell visualization. These findings identify that when low-shear diffractometry EI-values were elevated, concurrent visualized RBCs were comprised of populations with greater heterogeneity of orientation and less alignment with direction of flow (i.e., less cells with C = 0 orientation). It is thus proposed that the paradoxical increased low-shear EI reported with mechanical RBC damage in ektacytometry does not represent true cell deformability but instead is resultant of distortions because of abnormal cell behavior and chaos of orientation and alignment.
Although wall shear stresses were determined to be the most important factor when visualizing RBCs within this slit-flow test rig, it should be noted that the parabolic shear profile resulting from Poiseuille flow creates disparity between the plane of directly visualized RBCs and the generated laser-diffraction pattern (which has transmitted through the entire parabolic flow profile with regions of lower shear), thus introducing noise into the data. Initially, the heterogeneity of the cell responses due to the parabolic shear profile was theorized to influence low-shear EI diffraction responses; however, comparison of PSE RBCs in the ektacytoscope (Fig. 7 A) versus the commercial Couette system (Fig. 6 A) exhibited the opposite response. A possible explanation of the lessened low-shear EI responses in the Poiseuille system may be due to axial regions of parabolic flow initially being at shears too low to induce cell movement and overcome inertial viscous forces of the membrane, thus yielding lessened ensemble average cell movement and thus diffraction responses. Alternatively, another explanation may be due to the experimental design using the commercial Couette system for initial shear conditioning before assessment in the Poiseuille system; thus, it is possible that partial shape and damage recovery may have occurred. Wen et al. (31) partly characterized low-viscosity ektacytometry and identified that cell relaxation responses have fast and slow responses, with the slow variation being required for shape memory and complete morphological recovery. Artmann et al. (34) deployed classical micropipette techniques to induce morphological alterations to RBCs and identified that shear-induced echinocytosis could return to biconcave morphology, albeit with recovery beyond 30 min. Using recovery times similar to that in this study to transfer between the shearing systems, Kuck et al. (21) identified shear rigidification as “physiologically irreversible.” It is thus possible that in addition to the shear-induced changes in orientation and tumbling contributing to low-shear EI increases, the method of shear application and the presence of aberrant cell morphology shape modifications may also play a role.
Conclusion
The collective findings elucidate that exposure of RBCs to supraphysiological sublethal shear stresses change cell biophysics in a manner that affect cell dynamics when subsequently in low shear flow. This finding may illuminate some of the microvascular associated complications in individuals with diseases that alter RBC biophysics or in individuals receiving mechanical circulatory support. Further inspection of the small-scatter laser diffractometry with combined visualization diffractometry may provide valuable data that can yield new clinical insights for the assessment of subtle rheological disturbances.
Author Contributions
A.P.M. and M.J.S. performed the experimental concept and design; A.P.M. and T.F. developed the infrastructure; A.P.M. and T.F. conducted the experiment; A.P.M. and M.J.S. performed the data analysis and interpretation; and A.P.M., T.F., M.J.S., and G.D.T. contributed to the reagents, materials, and/or analysis tools. All authors wrote, reviewed, and approved the final manuscript.
Editor: Jay Groves.
References
- 1.Baskurt O.K., Meiselman H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 2.Chien S. Red cell deformability and its relevance to blood flow. Annu. Rev. Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 3.Schmid-Schöenbein H., Wells R. Fluid drop-like transition of erythrocytes under shear. Science. 1969;165:288–291. doi: 10.1126/science.165.3890.288. [DOI] [PubMed] [Google Scholar]
- 4.Fischer T.M., Stöhr-Lissen M., Schmid-Schönbein H. The red cell as a fluid droplet: tank tread-like motion of the human erythrocyte membrane in shear flow. Science. 1978;202:894–896. doi: 10.1126/science.715448. [DOI] [PubMed] [Google Scholar]
- 5.Fischer T.M. On the energy dissipation in a tank-treading human red blood cell. Biophys. J. 1980;32:863–868. doi: 10.1016/S0006-3495(80)85022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid-Schönbein H., Rieger H., Fischer T. Blood fluidity as a consequence of red cell fluidity: flow properties of blood and flow behavior of blood in vascular diseases. Angiology. 1980;31:301–319. [Google Scholar]
- 7.Fischer T.M. Tank-tread frequency of the red cell membrane: dependence on the viscosity of the suspending medium. Biophys. J. 2007;93:2553–2561. doi: 10.1529/biophysj.107.104505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith H., Marlow J., MacIntosh F.C. Flow behaviour of erythrocytes-i. Rotation and deformation in dilute suspensions. Proc. R. Soc. Lond. B Biol. Sci. 1972;182:351–384. [Google Scholar]
- 9.Fedosov D.A., Caswell B., Karniadakis G.E. A multiscale red blood cell model with accurate mechanics, rheology, and dynamics. Biophys. J. 2010;98:2215–2225. doi: 10.1016/j.bpj.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmonds M.J., Meiselman H.J. Prediction of the level and duration of shear stress exposure that induces subhemolytic damage to erythrocytes. Biorheology. 2016;53:237–249. doi: 10.3233/BIR-16120. [DOI] [PubMed] [Google Scholar]
- 11.Horobin J.T., Sabapathy S., Simmonds M.J. Repetitive supra-physiological shear stress impairs red blood cell deformability and induces hemolysis. Artif. Organs. 2017;41:1017–1025. doi: 10.1111/aor.12890. [DOI] [PubMed] [Google Scholar]
- 12.McNamee A.P., Tansley G.D., Simmonds M.J. Sublethal mechanical trauma alters the electrochemical properties and increases aggregation of erythrocytes. Microvasc. Res. 2018;120:1–7. doi: 10.1016/j.mvr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 13.McNamee A.P., Tansley G.D., Simmonds M.J. Biphasic impairment of erythrocyte deformability in response to repeated, short duration exposures of supraphysiological, subhaemolytic shear stress. Biorheology. 2016;53:137–149. doi: 10.3233/BIR-15108. [DOI] [PubMed] [Google Scholar]
- 14.Bull B., Feo C., Bessis M. Behavior of elliptocytes under shear stress in the rheoscope and ektacytometer. Cytometry. 1983;3:300–304. doi: 10.1002/cyto.990030413. [DOI] [PubMed] [Google Scholar]
- 15.Yao W., Wen Z., Chien S. Low viscosity Ektacytometry and its validation tested by flow chamber. J. Biomech. 2001;34:1501–1509. doi: 10.1016/s0021-9290(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 16.Parrow N.L., Violet P.-C., Levine M. Measuring deformability and red cell heterogeneity in blood by Ektacytometry. J. Vis. Exp. 2018;131:e56910. doi: 10.3791/56910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihailescu M., Costescu J. Diffraction pattern study for cell type identification. Opt. Express. 2012;20:1465–1474. doi: 10.1364/OE.20.001465. [DOI] [PubMed] [Google Scholar]
- 18.Rabai M., Detterich J.A., Wood J.C. Deformability analysis of sickle blood using ektacytometry. Biorheology. 2014;51:159–170. doi: 10.3233/BIR-140660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeth N., Kiss F., Miszti-Blasius K. Interpretation of osmotic gradient ektacytometry (osmoscan) data: a comparative study for methodological standards. Scand. J. Clin. Lab. Invest. 2015;75:213–222. doi: 10.3109/00365513.2014.993695. [DOI] [PubMed] [Google Scholar]
- 20.McNamee A.P., Richardson K., Simmonds M.J. Susceptibility of density-fractionated erythrocytes to subhaemolytic mechanical shear stress. Int. J. Artif. Organs. 2019;42:151–157. doi: 10.1177/0391398818790334. [DOI] [PubMed] [Google Scholar]
- 21.Kuck L., Grau M., Simmonds M.J. Recovery time course of erythrocyte deformability following exposure to shear is dependent upon conditioning shear stress. Biorheology. 2018;54:141–152. doi: 10.3233/BIR-17151. [DOI] [PubMed] [Google Scholar]
- 22.Simmonds M.J., Atac N., Yalcin O. Erythrocyte deformability responses to intermittent and continuous subhemolytic shear stress. Biorheology. 2014;51:171–185. doi: 10.3233/BIR-140665. [DOI] [PubMed] [Google Scholar]
- 23.Bayer R., Caglayan S., Guenther B. Biochemical Diagnostic Instrumentation. International Society for Optics and Photonics; 1994. Discrimination between orientation and elongation of RBC in laminar flow by means of laser diffraction; pp. 105–113. [Google Scholar]
- 24.Hardeman M., Goedhart P., Lettinga K. Laser-assisted optical rotational cell analyser (LORCA); I. A new instrument for measurement of various structural hemorheological parameters. Clin. Hemorheol. Microcirc. 1994;14:605–618. [Google Scholar]
- 25.Baskurt O.K., Hardeman M.R., Meiselman H.J. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Invest. 2009;69:777–788. doi: 10.3109/00365510903266069. [DOI] [PubMed] [Google Scholar]
- 26.Baskurt O.K., Meiselman H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcirc. 2013;54:99–107. doi: 10.3233/CH-2012-1616. [DOI] [PubMed] [Google Scholar]
- 27.Bitbol M. Red blood cell orientation in orbit C = 0. Biophys. J. 1986;49:1055–1068. doi: 10.1016/S0006-3495(86)83734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faghih M.M., Sharp M.K. Modeling and prediction of flow-induced hemolysis: a review. Biomech. Model. Mechanobiol. 2019;18:845–881. doi: 10.1007/s10237-019-01137-1. [DOI] [PubMed] [Google Scholar]
- 29.Dao K.M., O’Rear E.A., Peitersen S.E. Sensitivity of the erythrocyte membrane bilayer to subhemolytic mechanical trauma as detected by fluorescence anisotropy. Biorheology. 1994;31:69–76. doi: 10.3233/bir-1994-31106. [DOI] [PubMed] [Google Scholar]
- 30.Johnson C.P., Tang H.-Y., Discher D.E. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen Z., Gao T., Xiao H. Biophysical meanings of orientation and deformation of RBCs in shear flow field of low viscosity with new Ektacytometry. Sci. China C Life Sci. 1998;41:195–202. doi: 10.1007/BF02882727. [DOI] [PubMed] [Google Scholar]
- 32.McNamee A.P., Tansley G.D., Simmonds M.J. Sublethal mechanical shear stress increases the elastic shear modulus of red blood cells but does not change capillary transit velocity. Microcirculation. 2020:e12652. doi: 10.1111/micc.12652. [DOI] [PubMed] [Google Scholar]
- 33.Nash G.B., Meiselman H.J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys. J. 1983;43:63–73. doi: 10.1016/S0006-3495(83)84324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artmann G.M., Sung K.L., Chien S. Micropipette aspiration of human erythrocytes induces echinocytes via membrane phospholipid translocation. Biophys. J. 1997;72:1434–1441. doi: 10.1016/S0006-3495(97)78790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]