Abstract
Background
Hepatocyte growth factor (HGF) is a multifunctional growth factor that promotes various biological processes. However, the effect of HGF on bone metabolism in rheumatoid arthritis (RA) remains unknown. Here, we investigated the role of HGF in regulating osteoclastogenesis and bone resorption in RA.
Methods
The expression of HGF in RA patients and collagen-induced arthritis (CIA) mice was examined. The role of HGF on osteoclastogenesis was analysed by osteoclastogenesis and bone resorption assays. The effect of HGF inhibition was evaluated in a CIA mice model. The mechanism of HGF in regulating osteoclastogenesis and bone resorption was explored by a series of in vitro studies.
Results
HGF was overexpressed in CIA and RA. HGF stimulated osteoclastogenesis in vitro. SU11274, a selective small molecule blocker of c-Met, impeded the effect of HGF on osteoclastogenesis and bone resorption. HGF regulated osteoclastogenesis by JNK and AKT-GSK-3β-NFATc1 signallings. SU11274 protected CIA mice from pathological bone loss.
Conclusions
These data strongly suggest that the highly expressed HGF in the joint tissues contributes to bone loss in RA. Inhibition of HGF/c-Met could effectively alleviate pathological bone loss and inflammatory symptoms in CIA mice. HGF/c-Met may be used as a new target for the treatment of bone loss in RA.
Keywords: HGF, Osteoclast, Rheumatoid arthritis
Introduction
Bone homeostasis depends on the balance between bone formation by osteoblasts and bone absorption by osteoclasts [1]. Osteoclast overdifferentiation and hyperfunction can lead to osteoporosis, Paget’s disease and rheumatoid arthritis (RA) [[2], [3], [4]]. Rheumatoid arthritis is a systemic immune disease characterized by inflammatory arthritis [5]. Bone loss in patients with advanced rheumatoid arthritis is irretrievable, and this is closely related to the excessive osteoclast activity.
Hepatocyte growth factor (HGF) is a kind of cytokine found in 1984 that can promote the proliferation of hepatocytes [6]. Studies have shown that HGF is a multifunctional growth factor secreted by various cells [7]. When combined with its unique receptor c-Met [8], HGF efficiently activates multiple signal pathways, such as PI3K-Akt, MEK-MAPK, and STAT3 [[9], [10], [11]]. In addition, HGF is interrelated with multiple myeloma, osteosarcoma, and bone metastasis [[12], [13], [14]]. Recently, researchers depicted that HGF receptor c-Met also existed on the membrane of osteoclast and its precursor cell, and HGF could substitute for M-CSF to promote the differentiation of human peripheral blood mononuclear cells (PBMCs) differentiate into osteoclasts [15,16].
Furthermore, the amount of HGF in serum, synovium, synovial fluid and articular adipose tissue of RA was significantly higher than that of normal people or patients with osteoarthritis [17]. HGF definitely contributed to intra-articular angiogenesis and pannus formation in RA patients, and the inhibition of HGF/c-Met signaling pathway could notably alleviate RA inflammation and articular bone loss in mice [18,19]. However, it is not clear whether HGF has effects on osteoclastogenesis in RA. Herein, through experiments in vitro and in vivo, we found that HGF could enhance the differentiation of osteoclasts, and boost the bone absorption ability of osteoclasts. Impeding the HGF/c-met signaling could effectively prevent RA mice from articular bone loss.
Materials and methods
Reagents
The cell-culture reagents were provided by Gibco BRL. Recombinant murine sRANKL (#315–11) and Recombinant murine HGF (#315–23) were purchased from PeproTech. Recombinant mouse MCSF protein (#ab129146), and the following antibodies: p65 (#ab16502), TRAF6 (#ab33915), JNK (#ab124956), HGF (#ab83760), were obtained from Abcam. The following antibodies were purchased from Cell Signaling Technology: p-p65 (#3033), p-JNK (#9255), Met (#8198), p-Met (#3077), Akt (#4685), p-Akt (#4060), GSK-3β (#9315S), p-GSK-3β (#9323). NFATc1 (#sc-17834) and GAPDH (#sc-32233) antibodies were provided by Santa Cruz Biotechnology. β-actin (#60008-1-Ig) and Histone-3 (#17168-1-AP) antibodies were purchased from Proteintech Group. SU11274 was obtained from Selleck. Immunization Grade Chick type II collagen (#20012), Complete Freund’s Adjuvant (#7001), and Incomplete Freund’s Adjuvant (#7002) were purchased from Chondrex.
Collagen-induced arthritis (CIA)
Male C57BL/6 mice (7–8 weeks old) were injected subcutaneously with 400 μg Immunization Grade Chick type II collagen (emulsified in 200 μL of Complete Freund’s Adjuvant) at the base of tail. In order to boost immunization, mouse were injected with 400 μg Immunization Grade Chick type II collagen (emulsified in 200 μL of Incomplete Freund’s Adjuvant) 21 days later. Afterwards, clinical arthritis scores were performed every 2 days. Paws were scored individually as follows: 0 = normal, 1 = mild swelling and erythema confined to the midfoot and ankle joint, 2 = mild swelling and erythema extending to the midfoot and ankle joint, 3 = moderate swelling and erythema extending from the metatarsal joints to the ankle, and 4 = severe swelling and erythema encompassing the foot, ankle and digits [20]. The total clinical scores of each mouse (0–16) were recorded. 21 days after the first injection, C57BL/6 mice were intraperitoneally injected with PBS (0.5 mL) or SU11274 (5 μM in 0.5 mL PBS) every 2 days for a month. Then, the femur and tibia of the left hind limb were obtained, and TRAP staining of distal femur and micro-CT (SkyScan1176 In-Vivo Micro-CT, BRUKER, Kontich, Belgium) scanning of tibia were conducted. All animal experiments were performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The protocols were approved by the Ethics Review Board of Soochow University (Suzhou, Jiangsu, China).
Immunofluorescence staining
The synovium tissues of knee joints from patients undergoing total knee arthroplasty (5 cases in RA group and 5 cases in OA group) were obtained and fixed with 4% buffered formalin at 4°C for 24 hours. The tissues were then transferred to a 30% sucrose solution for 48 hours. After that, the samples were embedded with Optimal Cutting Temperature compound (SAKURA, #4583), and cut into 20 μm thick sections. The synovium slices were rinsed by PBS for 3 times, and permeabilized with 0.3% Triton X-100 (Amresco, #0694) for 30 minutes at room temperature. The slices were then washed with PBS for 3 times again and blocked with bovine serum albumin (Sangon Biotech, #9048-46-8) for 1 hours. Afterwards, the samples were incubated overnight at 4°C with rabbit anti-HGF (1:300, Abcam, #ab83760). Following three washes in PBS, the slices were incubated with the corresponding secondary antibodies (1: 400, Abcam, #ab150077) at room temperature for 1 hours. Finally, the slices were mounted with fluorescent mounting medium (Dako, #S3023) and examined by a fluorescence microscope (Zeiss Axio Scope A1, Germany).
Cell cultures and osteoclast differentiation
RAW264.7 cells were purchased from Cell Resource Center of Shanghai Institute of Life Sciences, Chinese Academy of Sciences. Bone marrow monocytes (BMMs) were obtained from male C57BL/6 mice (5–6 weeks old) by flushing the medullary cavity of femur and tibia [21]. For osteoclast differentiation, cells were cultured with 50 ng/mL of mouse recombinant receptor activator of nuclear factor-κB ligand (RANKL) in the absence (RAW264.7) or presence (BMMs) of 30 ng/mL macrophage colony stimulating factor (M-CSF) for 3 days. For comparison, cells were treated with HGF (50 ng/mL) or HGF (50 ng/mL) plus SU11274 (0.5 μM).
Tartrate-resistant acid phosphatase (TRAP) staining assay
Cells were seeded at 10,000 (RAW264.7) or 20,000 (BMMs) per well in 24-well plate and stimulated with the indicated concentration of cytokines for 3 days. Mice femurs were immediately fixed in 4% paraformaldehyde solution for 24 hours at 4°C. After that, the specimens were decalcified with EDTA (Solarbio, #E1171) for 2 weeks at 4°C. Decalcified samples were embedded into Optimal Cutting Temperature compound (SAKURA, #4583) and sectioned (10 μm thick) with cryostat. To perform the TRAP staining, Acid Phosphatase, Leukocyte (TRAP) Kits (Sigma–Aldrich, #387A) were used according to the procedure provided by the manufacturer. Pictures were taken using a Carl-Zeiss Axio Observer A1 inverted microscope. Image analysis was performed using Image J v1.8.0 software.
Quantitative PCR
The synovium of the mouse was obtained from the ankle joint of its left hind limb. Cells were seeded at 1 × 105 (RAW264.7) or 2 × 105 (BMMs) per well in 6-well plate and stimulated with the indicated concentration of cytokines for 5 days. A RNA-Quick Purification Kit (ES Science, #RN001) was used to isolate the total RNA. cDNA was reverse-transcribed from 0.5 μg total RNA with Fast All-in-One RT Kit (ES Science, #RT001). qPCR was performed through the ChamQ Universal SYBR qPCR Master Mix (Vazyme, #Q711-02). Primer (Sangon Biotech) sequences are as followings: nfatc1 (5′-GGAGAGTCCGAGAATCGAGAT-3′, 5′-TTGCAGCTAGGAAGTACGTCT-3′), acp5 (5′-CACTCCCACCCTGAGATTTGT-3′, 5′-CCCCAGAGACATGATGAAGTC-3′), ctsk (5′-CTCGGCGTTTAATTTGGGAGA-3′, 5′-TCGAGAGGGAGGTATTCTGAG-3′), hgf (5′-ACTTCTGCCGGTCCTGTTG-3′, 5′-CCCCTGTTCCTGATACACCT-3′), β-actin (5′-CTAGTACGTTGCTATCCAGGC-3′, 5′-CTCCTTAATGTCACGCACGAT-3′).
Bone resorption assay
Cells were seeded at 10,000 (RAW264.7) or 20,000 (BMMs) per well in 24-well Corning Osteo Assay Surface Plate (Corning, #3987), and stimulated with the indicated concentration of cytokines for 7 days. Then, Cells were removed by the bleach solution. Pictures were taken using a Carl-Zeiss Axio Observer A1 inverted microscope. Image analysis was performed using Image J v1.8.0 software.
Western blot analysis
RAW264.7 cells were seeded at 50,000 or 5 × 105 per well in 6-well plate and stimulated with the indicated concentration of cytokines for 5 days or the indicated times. For the phosphorylation assay, cells were starved for 8 hours and pretreated with PBS for 40 minutes. At 30 days after the last injection of collagen, the right ankle tissues of CIA mice were dissected. The synovium tissues of knee joints from patients undergoing total knee arthroplasty (5 cases in RA group and 5 cases in OA group) were obtained according to the Declaration of Helsinki. Then, the total proteins were isolated. Assays were performed as described previously [22] using following antibodies: NFATc1 (1:500), TRAF6 (1:2000), p65 (1:1000), p-p65 (1:2000), JNK (1:2000), p-JNK (1:1000), HGF (1:1000), Met (1:500), p-Met (1:500), Akt (1:1000), p-Akt (1:1000), GSK-3β (1:5000), p-GSK-3β (1:5000), GAPDH (1:5000), β-actin (1:5000) and Histone-3 (1:1000).
Nuclear and cytoplasmic extraction
RAW264.7 cells were seeded at 2 × 105 per well in 6-well plate and stimulated with the indicated concentration of cytokines for 3 days. For Western Blot analysis, the cytoplasmic and nuclear proteins were extracted with Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, #78833) according to the protocol provided by the manufacturer.
Statistical analysis
Data are provided as means ± standard deviation. Statistical differences were analyzed by paired-samples T-Test, One-way ANOVA, followed by post hoc Bonferroni test (SPSS version 20.0). Values of p < 0.05 were considered statistically significant. All experiments were conducted for at least three times.
Results
HGF was overexpressed in CIA and RA
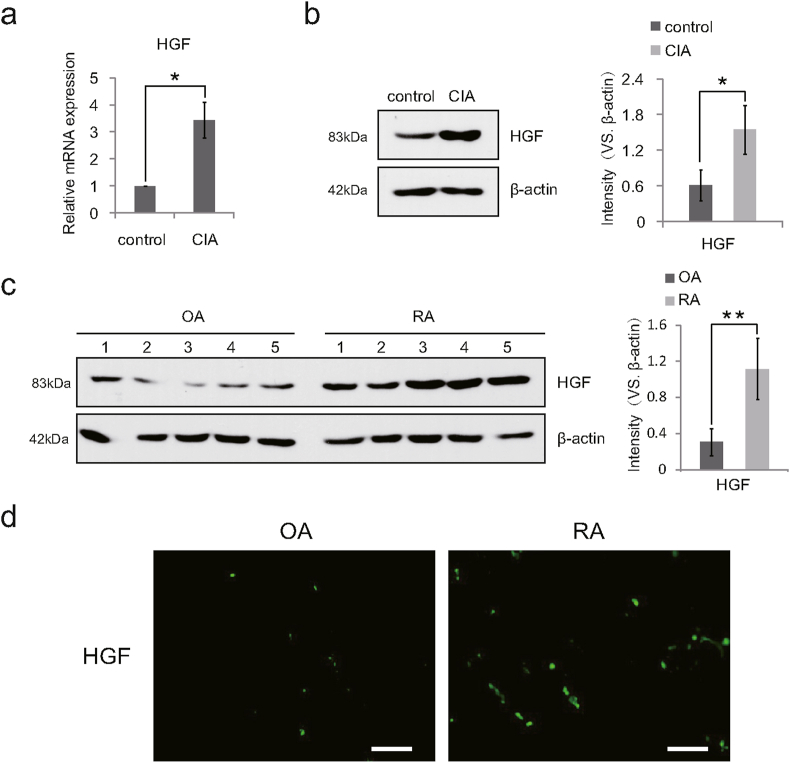
Studies have shown that HGF mRNA was significantly overexpressed in synovium, synovial fluid and adipose tissue of RA patients and CIA mice [17]. We wondered whether the expression of HGF in the joint tissues of RA patients and CIA mice also increased remarkably at the protein level. Male C57BL/6 mice (7–8 weeks old) were selected to induce CIA model and establish blank control group (6 in each group). At 30 days after the last injection of Immunization Grade Chick type II collagen, the synovium of left ankles from CIA mice were extracted for the detection of HGF mRNA expression by qPCR. The right ankles of mice were harvested and immediately soaked in liquid nitrogen. After that, the total proteins were extracted and analyzed by Western Blotting to detect the expression of HGF protein. Consistent with previous studies, the results showed that the expression of HGF mRNA and protein in the ankle tissues of CIA mice was significantly higher than the control group (Fig. 1a and b). The synovium tissues of knee joints from patients undergoing total knee arthroplasty (5 cases in RA group and 5 cases in OA group) were resected and assessed by Western Blotting and immunofluorescence staining. Compared with the OA group, the expression of HGF in RA patients increased at the protein level (Fig. 1c and d). These findings indicated that HGF mRNA and protein were both over expressed in CIA mice and RA patients.
Figure 1.
HGF was overexpressed in CIA and RA. (a) Total mRNA was isolated from the ankle synovium of mice, and the relative expression of HGF mRNA was tested. β-actin mRNA was detected as internal control (n = 6). (b) Western Blot results confirming the overexpression of HGF in the ankle synovium tissue of CIA mice (n = 6) and (c) in the knee synovium of RA patients (n = 5) with quantification. (d) Comparison of HGF expression (green) in the synovium tissues of OA and RA patients by immunofluorescence staining. Representative fluorescent micrographs were shown (n = 5). Scale bars, 100 μm. Data are expressed as means ± SD; ∗p < 0.05; ∗∗p < 0.01. Experiments were repeated 3 times, and similar results were obtained.
HGF stimulated osteoclastogenesis in vitro
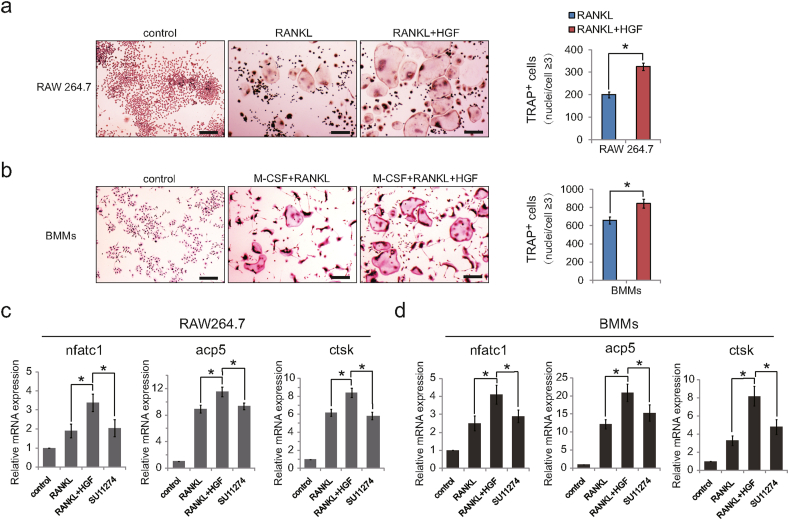
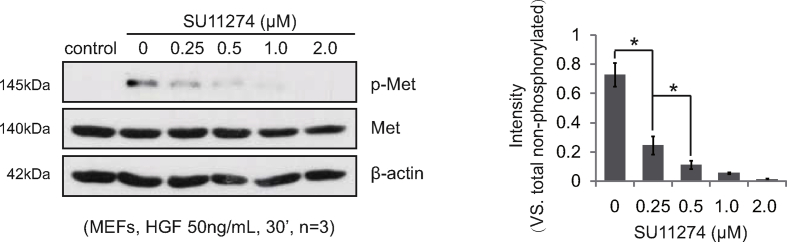
Although HGF could substitute for M-CSF to promote PBMCs differentiation into osteoclasts [15,16], its specific role in osteoclast differentiation remains unclear. Under the stimulation of HGF, the number of osteoclasts increased dramatically in widely used osteoclast precursor cells murine RAW264.7 and BMMs (Fig. 2a and b). Meanwhile, gene expression analysis showed that HGF boosted the expression of osteoclast encoding genes nfatc1, acp5, and ctsk (Fig. 2c and d). To further verify the effect of HGF on osteoclast differentiation, SU11274, a selective small molecule blocker of c-Met which was the unique receptor of HGF [23], was adopted. Previously, related preliminary experiments were carried out. To find the appropriate dosage of SU11274, mouse embryonic fibroblasts (MEFs), a type of tool cells, were pretreated with SU11274 at different concentrations for 6 hours. In the presence of HGF (50 ng/mL), the phosphorylation level of c-Met in MEFs cells decreased gradually with the increase of the concentration of SU11274. Furthermore, after treatment with SU11274 (0.5 μM), the level of p-c-Met decreased by more than 85% (Supplementary Fig. 1). Therefore, SU11274 (0.5 μM) was added into the culture medium of osteoclast precursor cells. Interestingly, HGF lost the ability to promote the expression of nfatc1, acp5, and ctsk (Fig. 2c and d). These results showed that HGF indeed promoted osteoclastogenesis and the expression of osteoclast related genes in vitro.
Figure 2.
HGF promoted osteoclastogenesis in vitro. Cells were cultured with RANKL alone (50 ng/mL) or M-CSF (30 ng/mL) plus RANKL (50 ng/mL) for 3d or 5d. Representative TRAP staining images of (a) RAW264.7 cells, (b) BMMs with osteoclast quantitation (n = 6). RANKL + HGF: stimulated with HGF (50 ng/mL) for 3 d; Scale bars, 100 μm. Total mRNA was respectively isolated from (c) RAW264.7 cells and (d) BMMs. SU11274: stimulated with HGF (50 ng/mL) and SU11274 (0.5 μM) for 5d (n = 3). Relative expression of nfatc1, acp5, and ctsk mRNA were tested. β-actin mRNA was detected as internal control (n = 3). Data are expressed as means ± SD; ∗p < 0.05. Experiments were repeated 3 times, and similar results were obtained.
SU11274 impeded the effect of HGF on osteoclastogenesis and bone resorption
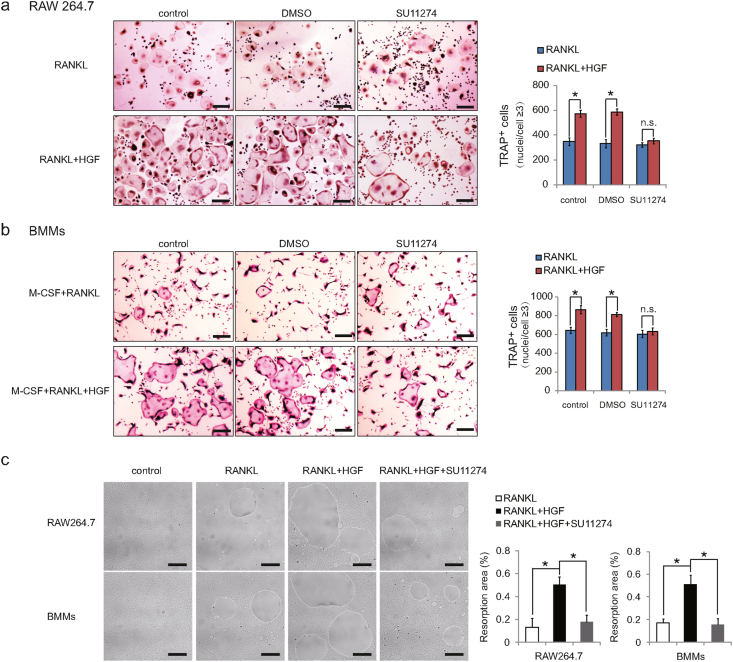
To further confirm the role of HGF in osteoclast differentiation, RAW264.7 cells and BMMs were incubated with SU11274. Since the solvent of SU11274 is DMSO, DMSO group was also set up to exclude the interference of DMSO. Three days after induction of differentiation, TRAP staining assay showed that the number of osteoclasts in SU11274 group was almost the same as that in the control group, while DMSO had no significant effect on osteoclast differentiation (Fig. 3a and b). These results revealed that SU11274 blocked the catalytic effect of HGF on osteoclast differentiation. To explore the effect of HGF on bone resorption ability of osteoclast, bone resorption assay was put into practice in vitro. Cells were seeded on 24-well Corning Osteo Assay Surface Plate and incubated with the indicated concentration of cytokines for 7 days. The data suggested that under the stimulation of HGF, the bone absorption area of osteoclasts was much larger than that of RANKL group (Fig. 3c), and HGF prominently enhanced the bone absorption ability of osteoclasts. However, SU11274 distinctly reduced the range of pit resorption caused by HGF (Fig. 3c), implying that the ability of HGF to promote bone resorption was restrained in SU11274 group. These results demonstrated that HGF could promote osteoclast differentiation and bone resorption, while SU11274 was able to neutralize the promotion.
Figure 3.
HGF-enhanced osteoclastogenesis and bone resorption was counteracted by SU11274. Representative TRAP staining images and analysis of osteoclasts cultured on day 3 derived from (a) RAW264.7 cells and (b) BMMs. RANKL (50 ng/mL); HGF (50 ng/mL); SU11274 (0.5 μM) (n = 6). Scale bars, 100 μm. (c) Resorption pit assay of RAW264.7 cells and BMMs. Cells were incubated with or without HGF (50 ng/mL) in the absence or presence of SU11274 (0.5 μM) for 7d (n = 6). Scale bars was 100 μm. At least 3 independent experiments were analyzed, and results are shown as means ± SD; ∗p < 0.05; n. s., not significant.
HGF regulated osteoclastogenesis by JNK and AKT-GSK3β-NFATc1 signallings
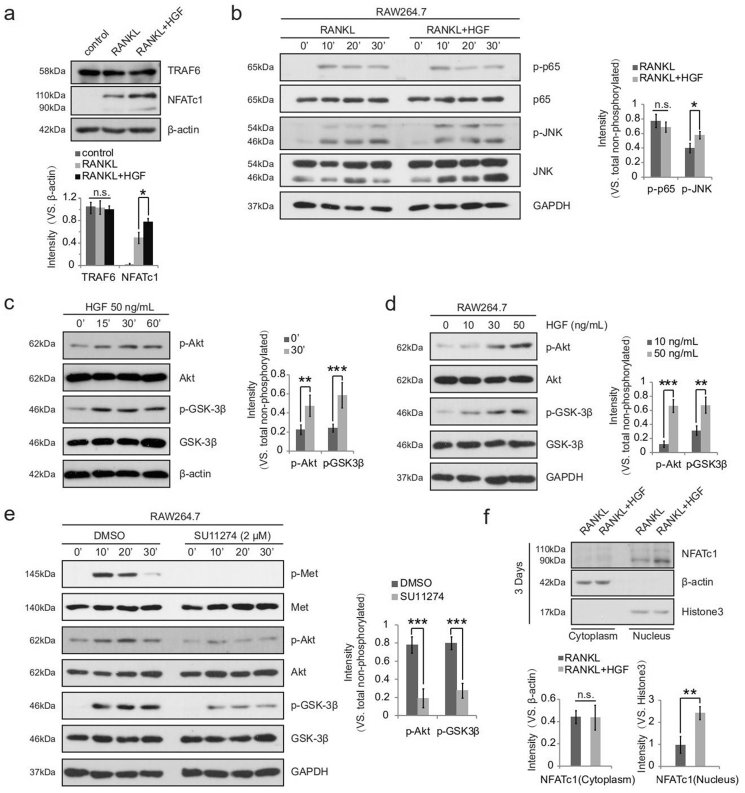
Studies confirmed that NF-κB and MEK-MAPK-JNK signaling pathways activated by RANKL/RANK, both play important positive roles in osteoclast formation [24,25]. To clarify the mechanism of HGF in promoting osteoclast differentiation, RAW264.7 cells were first used to detect the effect of HGF on the expression of related cytokines involving in NF-κB and MEK-MAPK-JNK pathways via Western Blotting. The results demonstrated that HGF enhanced the expression of NFATc1, but had no significant effect on TRAF6 (Fig. 4a). Further experiments showed that HGF increased the phosphorylation level of JNK, but did not affect the activation of p65 (Fig. 4b). Because the increase of p-JNK could promote osteoclast formation by up-regulating AP-1 and then the expression of NFATc1 [26]. HGF/c-Met might stimulate osteoclast formation by activating the MEK-MAPK-JNK signaling pathway in osteoclast precursor cells.
Figure 4.
HGF activated multiple signaling pathways to regulate Osteoclastogenesis. RAW264.7 cells were stimulated with RANKL (50 ng/mL) in the absence or presence of HGF (50 ng/mL) for 5 d (a) or the indicated times (b), and Western Blot was used to detect the listed proteins (n = 3) (c, d) Western blot analysis to detect Akt and GSK-3β phosphorylation induced by HGF (applied at the indicated concentrations for the indicated times) in RAW264.7 cells (n = 3). RAW264.7 cells were pretreated with DMSO or SU11274 (2.5 μM) for 6 h, followed by HGF (50 ng/mL) stimulation. Cells were further cultured for applied time, and expression of listed proteins is shown (e). Phosphorylated (p) kinases (vs. total kinases) were quantified (n = 3). (f) Western blot analysis of NFATc1, β-actin (cytoplasm loading control) and Histone3 (nuclei loading control) in cytoplasm and nucleus lysates of pre-osteoclasts (n = 3). Results are expressed as means ± SD; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n. s., not significant. Experiments were repeated 3 times, and similar results were obtained.
In addition, It has been proven that the Akt-GSK-3β-NFATc1 signaling cascade is critical for osteoclastogenesis [27,28]. GSK-3β could inhibit the translocation of NFATc1 from cytoplasm to nucleus, while GSK-3β inactivated by Akt-mediated phosphorylation on Serine 9 (Ser9) which indirectly promoted osteoclast formation [29,30]. Consequently, we wondered whether HGF/c-Met could also activate Akt-GSK-3β-NFATc1 signaling pathway in osteoclast precursor cells. We found that Akt was fast activated by HGF, leading to the phosphorylation of GSK-3β in a dose-dependent manner (Fig. 4c and d). Given the drastic increase in Akt activation, we then applied the c-Met inhibitor SU11274 for further validation. Intriguingly, in the presence of SU11274, the phosphorylation level of Akt and GSK-3β was extremely reduced (Fig. 4e). Furthermore, we also investigated the cytoplasmic and nuclear distribution of NFATc1 in cells stimulated by HGF for 3days. While similar NFATc1 content was observed in the cytoplasm, more NFATc1 was noticed in the nucleus of cells stimulated by HGF (Fig. 4f). These results indicated that HGF might also activate Akt-GSK-3β-NFATc1 signaling pathway, and ultimately promotes osteoclast differentiation.
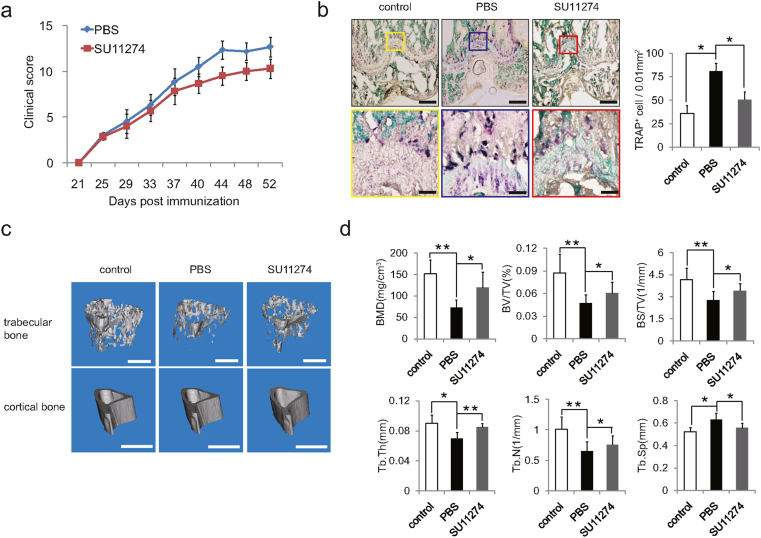
SU11274 protected CIA mice from pathological bone loss
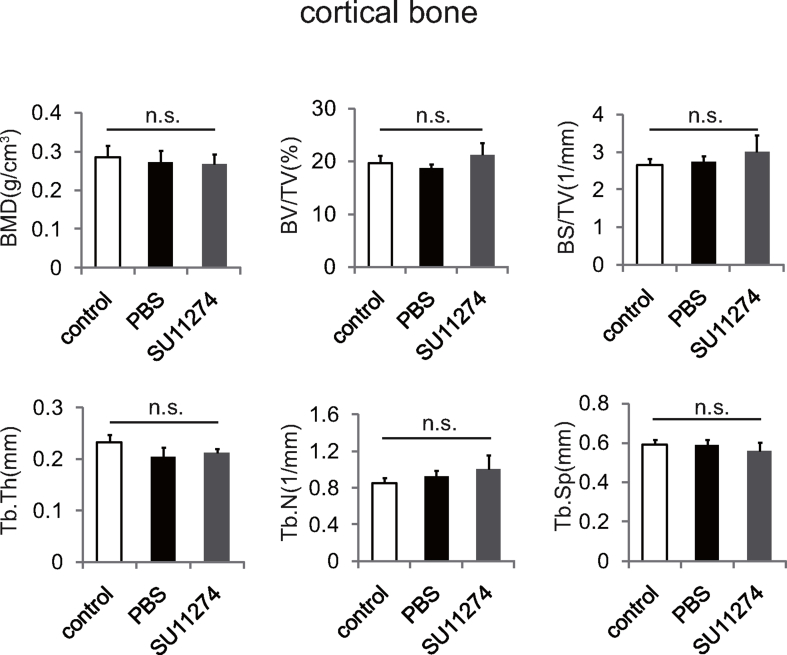
To clarify the clinical significance of HGF in the pathogenesis of rheumatoid arthritis, CIA mice were intraperitoneally injected with PBS or SU11274 (once every other day for 30 days) respectively. The results showed that the arthritis clinical score of CIA mice was markedly declined from 40 days after SU11274 injection (Fig. 5a). Afterwards, we wondered whether inhibition of HGF/c-Met could affect osteoclast formation in CIA mice. The distal femurs of CIA mice were used for morphological analysis. TRAP staining assay depicted that the number of osteoclasts in femur of CIA mice increased dramatically, while decreased noticeably after SU11274 injection (Fig. 5b). To determine the influence of HGF/c-Met on bone degradation in RA, the mice tibias of three groups were collected and used for micro-CT scanning. Compared with PBS injection, SU11274 significantly increased the bone mass of trabecular bone in CIA mice (Fig. 5c and d), but had no effect on cortical bone (Supplementary Fig. 2). These results suggested that SU11274, which could inhibit HGF/c-Met, alleviated the bone destruction of trabecular bone in the joints of CIA mice.
Figure 5.
SU11274 ameliorated pathological bone loss in CIA mice. (a) Time course changes in arthritis clinical score from CIA mice treated with PBS, and SU11274 respectively (n = 6). (b) Representative TRAP staining images and parameters of distal femoral osteoclasts from CIA mice (n = 12). Scale bars were 100 μm and 25 μm. (c) Representative micro-CT images (trabecular bones, Scale bars, 0.5 mm; cortical bones, Scale bars, 1 mm) from the hind paws of mice (n = 6). (d) The values of micro-CT parameters (BMD, BV/TV, BS/TV, Tb.Th, Tb.N and Tb. Sp) of the proximal tibial bones from the CIA mice after treatment in the respective group (n = 6). All data are shown as means ± SD; ∗p < 0.05, ∗∗p < 0.01.
Discussion
This study uncovered a new role of HGF in regulating osteoclast formation in RA pathogenesis. We further elaborated that the inhibition of HGF could alleviate the pathological bone destruction and chronic inflammation in RA.
Previous studies have shown that the content of HGF in serum, synovium, synovium fluid and joint adipose tissue of RA patients was significantly increased [31,32], which was consistent with our results (Fig. 1). However, the specific role of HGF in the pathological process of RA is still unclear, and even controversial. Several studies demonstrated that HGF could promote various biological processes, e.g. intra-articular angiogenesis, synovial cells proliferation, and chronic inflammation in RA. Meanwhile, inhibition of HGF receptor c-Met could relieve the inflammatory response and bone loss in RA [18,19,33]. Similarly, we found that after the injection of SU11274, a selective small molecule blocker of c-Met, the arthritis clinical score declined in CIA mice (Fig. 5a). On the contrary, it has been shown that at the beginning of collagen induction, the mice injected with high dose of HGF subcutaneously displayed a weaker immune response and a milder joint inflammation [34]. This divergence might be caused by different timing of HGF/c-Met intervention on CIA mice. In our study, mice were intervened on the 21st day after the first injection of collagen. We speculated that HGF may have immunosuppressive effect in the early stage of RA, while in the later stage it would promote the development of RA.
Currently, more studies are required in finding the direct effect of HGF on bone metabolism in RA. Studies showed that HGF could substitute for M-CSF to support osteoclastogenesis [15,16]. We further confirmed that HGF could directly promote the osteoclast precursor cells to differentiate into osteoclasts in vitro (Fig. 2a and b), and also to enhance the bone absorption ability of osteoclasts (Fig. 3c). In addition, the underlying mechanism were also explored. Studies depicted that HGF could stimulate a variety of cells and activate several signaling pathways including PI3K-Akt and MEK-MAPK [[9], [10], [11]]. Meanwhile, the critical roles of Akt-GSK-3β-NFATc1 signaling and JNK in osteoclast differentiation are well-documented [25,28]. Western Blot assay revealed that HGF distinctly activated JNK and Akt-GSK-3β-NFATc1 signaling pathways and facilitated the nuclear translocation of NFATc1 (Fig. 4). Also, the number of osteoclasts in the distal femur of CIA mice decreased dramatically after blocking HGF/c-Met in vivo (Fig. 5b). These results indicated that HGF could promote osteoclast formation in the joint of CIA mice, and HGF/c-Met might be used as a new therapeutic target to alleviate pathological bone destruction in RA.
However, the effect of HGF on osteoblasts in RA remains largely unknown. Also, previous results about effect of HGF on osteoblasts were inconsistent. Some investigators suggested that HGF promoted osteoblast formation by activating p38 and 1,25-dihydroxyvitamin D [35,36]. Nevertheless, several literatures demonstrated that the inhibition of HGF/c-Met would promote osteoblast differentiation [19]. Here, we observed that the bone mass of trabecular bone in the adjacent area of CIA mice joints increased significantly after SU11274 injection, while the bone mass of cortical bone was not affected (Fig. 5d, Supplementary Fig. 2). Further investigation of the influence of HGF on osteoblasts in RA is needed.
Conclusion
To our knowledge, this is the first study to clarify that HGF directly promotes osteoclast differentiation and enhances bone absorption by activating JNK and Akt-GSK-3β-NFATc1 signaling. Inhibition of HGF/c-Met could effectively alleviate pathological bone loss and inflammatory symptoms in CIA mice. HGF/c-Met may be used as a new target for the treatment of bone loss in RA.
Author contributions
C.-m.H. and X.-z.Z. designed research; C.-m.H., Y.-f.Z., J.-y.B., C.S., X.S. and H.-j.S. performed research; C.-m.H., Y.-f.Z., J.-y.B. and X.-z.Z. analyzed data; and C.-m.H. and X.-z.Z. wrote the paper. All authors approved the final version of the manuscript for submission.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81873995, 81171712), the Key laboratory of spinal cord injury repair of Suzhou (SZS201807), the Natural Science Foundation of Jiangsu province (BK20151213), the Social development program of Jiangsu province (BE2019662), and the Health commission of Jiangsu province (H2017066).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2020.10.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
MEFs were seeded at 5 × 105 per well in 6-well plate and pretreated by SU11274 with different concentrations for 6 h. Then, MEFs were stimulated with HGF (50 ng/mL) for 30 min. Expression of listed proteins is shown, and p-Met (vs. total Met) were quantified (n = 3). Results are shown as means ± SD; ∗p < 0.05. Experiments were repeated 3 times, and similar results were obtained.
Supplementary Figure 2.
The values of micro-CT parameters (BMD, BV/TV, BS/TV, Tb.Th, Tb.N and Tb. Sp) of the cortical bone of tibial bones from the CIA mice after treatment in the respective group (n = 6). All data are represented means ± SD; n. s., not significant.
References
- 1.Ikebuchi Y., Aoki S., Honma M., Hayashi M., Sugamori Y., Khan M. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 2.Saito H., Gasser A., Bolamperti S., Maeda M., Matthies L., Jahn K. TG-interacting factor 1 (Tgif1)-deficiency attenuates bone remodeling and blunts the anabolic response to parathyroid hormone. Nat Commun. 2019;10:1354. doi: 10.1038/s41467-019-08778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divisato G., Formicola D., Esposito T., Merlotti D., Pazzaglia L., Del Fattore A. ZNF687 mutations in severe Paget disease of bone associated with giant cell tumor. Am J Hum Genet. 2016;98:275–286. doi: 10.1016/j.ajhg.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laha D., Deb M., Das H. KLF2 (kruppel-like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy. 2019;15:2063–2075. doi: 10.1080/15548627.2019.1596491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X., Liu J., Liang C., Badshah S.A., Zheng K., Dang L. Osteoblastic PLEKHO1 contributes to joint inflammation in rheumatoid arthritis. EBioMedicine. 2019;41:538–555. doi: 10.1016/j.ebiom.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kara F., Yildirim A., Gumusdere M., Karatay S., Yildirim K., Bakan E. Association between hepatocyte growth factor (HGF) gene polymorphisms and serum HGF levels in patients with rheumatoid arthritis. Eurasian J Med. 2014;46:176–181. doi: 10.5152/eajm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikebuchi F., Oka K., Mizuno S., Fukuta K., Hayata D., Ohnishi H. Dissociation of c-Met phosphotyrosine sites in human cells in response to mouse hepatocyte growth factor but not human hepatocyte growth factor: the possible roles of different amino acids in different species. Cell Biochem Funct. 2013;31:298–304. doi: 10.1002/cbf.2898. [DOI] [PubMed] [Google Scholar]
- 8.Li B., Leung J.C.K., Chan L.Y.Y., Yiu W.H., Li Y., Lok S.W.Y. Amelioration of endoplasmic reticulum stress by mesenchymal stem cells via hepatocyte growth factor/c-met signaling in obesity-associated kidney injury. STEM CELLS Translational Medicine. 2019;8:898–910. doi: 10.1002/sctm.18-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J.J., Li Y., Chen W.S., Liang Y., Wang G., Zong M. Shp2 deletion in hepatocytes suppresses hepatocarcinogenesis driven by oncogenic β-Catenin, PIK3CA and MET. J Hepatol. 2018;69:79–88. doi: 10.1016/j.jhep.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y., Ding H., Kim D., Bach D.H., Hong J.Y., Xu Y. Antitumor activity of DFX117 by dual inhibition of c-met and PI3Kalpha in non-small cell lung cancer. Cancers. 2019;11:627. doi: 10.3390/cancers11050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y., Kang K., Lee S.B., Seo D., Yoon S., Kim S.J. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019;70:97–107. doi: 10.1016/j.jhep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Sun M., Jiang Y., Zhang T., Sun W., Wang H. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Canc. 2019;145:979–993. doi: 10.1002/ijc.32180. [DOI] [PubMed] [Google Scholar]
- 13.Saltarella I., Morabito F., Giuliani N., Terragna C., Omede P., Palumbo A. Prognostic or predictive value of circulating cytokines and angiogenic factors for initial treatment of multiple myeloma in the GIMEMA MM0305 randomized controlled trial. J Hematol Oncol. 2019;12:4. doi: 10.1186/s13045-018-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamasaki K., Mukai S., Sugie S., Nagai T., Nakahara K., Kamibeppu T. Dysregulated HAI-2 plays an important role in renal cell carcinoma bone metastasis through ligand-dependent MET phosphorylation. Cancers. 2018;10:190. doi: 10.3390/cancers10060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamopoulos I.E., Xia Z., Lau Y.S., Athanasou N.A. Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochem Biophys Res Commun. 2006;350:478–483. doi: 10.1016/j.bbrc.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 16.Taylor R.M., Kashima T.G., Knowles H.J., Athanasou N.A. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92:1398–1406. doi: 10.1038/labinvest.2012.108. [DOI] [PubMed] [Google Scholar]
- 17.Feuerherm A.J., Borset M., Seidel C., Sundan A., Leistad L., Ostensen M. Elevated levels of osteoprotegerin (OPG) and hepatocyte growth factor (HGF) in rheumatoid arthritis. Scand J Rheumatol. 2001;30:229–234. doi: 10.1080/030097401316909585. [DOI] [PubMed] [Google Scholar]
- 18.Maruotti N., Cantatore F.P., Crivellato E., Vacca A., Ribatti D. Angiogenesis in rheumatoid arthritis. Histol Histopathol. 2006;21:557–566. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 19.Shibasaki S., Kitano S., Karasaki M., Tsunemi S., Sano H., Iwasaki T. Blocking c-Met signaling enhances bone morphogenetic protein-2-induced osteoblast differentiation. FEBS Open Bio. 2015:341–347. doi: 10.1016/j.fob.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi B.Y., Choi Y., Park J.S., Kang L.J., Baek S.H., Park J.S. Inhibition of Notch1 induces population and suppressive activity of regulatory T cell in inflammatory arthritis. Theranostics. 2018;8:4795–4804. doi: 10.7150/thno.26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K.-J., Yeon J.-T., Choi S.-W., Moon S.-H., Ryu B.J., Yu R. Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre-osteoclasts. Bone. 2015;81:208–216. doi: 10.1016/j.bone.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Marshall J., Zhou X.Z., Chen G., Yang S.Q., Li Y., Wang Y. Antidepression action of BDNF requires and is mimicked by Galphai1/3 expression in the hippocampus. Proc Natl Acad Sci U S A. 2018;115:E3549–E3558. doi: 10.1073/pnas.1722493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H.J., Yoon A., Ryu J.Y., Cho Y.J., Choi J.J., Song S.Y. c-MET as a potential therapeutic target in ovarian clear cell carcinoma. Sci Rep. 2016;6:38502. doi: 10.1038/srep38502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Ayoub A., Xiu Y., Yin X., Sanders J.O., Mesfin A. TGFβ-induced degradation of TRAF3 in mesenchymal progenitor cells causes age-related osteoporosis. Nat Commun. 2019;10:2795. doi: 10.1038/s41467-019-10677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K., Qiu P., Yuan Y., Zheng L., He J., Wang C. Pseurotin A inhibits osteoclastogenesis and prevents ovariectomized-induced bone loss by suppressing reactive oxygen species. Theranostics. 2019;9:1634–1650. doi: 10.7150/thno.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei L., Sang W., Chen Z., Zheng L., Jin K., Lou C. Small molecule inhibitor RepSox prevented ovariectomy-induced osteoporosis by suppressing osteoclast differentiation and bone resorption. J Cell Physiol. 2018;233:9724–9738. doi: 10.1002/jcp.26914. [DOI] [PubMed] [Google Scholar]
- 27.Luo J., Yang Z., Ma Y., Yue Z., Lin H., Qu G. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22:539–546. doi: 10.1038/nm.4076. [DOI] [PubMed] [Google Scholar]
- 28.Wu M., Chen W., Lu Y., Zhu G., Hao L., Li Y.P. Galpha13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3beta-NFATc1 signalling pathway. Nat Commun. 2017;8:13700. doi: 10.1038/ncomms13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Wang Y., Xiao F., Wang S., Xing G., Li Y. CKIP-1 regulates macrophage proliferation by inhibiting TRAF6-mediated Akt activation. Cell Res. 2014;24:742–761. doi: 10.1038/cr.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choo Y.-Y., Tran P.T., Min B.-S., Kim O., Nguyen H.D., Kwon S.-H. Sappanone A inhibits RANKL-induced osteoclastogenesis in BMMs and prevents inflammation-mediated bone loss. Int Immunopharm. 2017;52:230–237. doi: 10.1016/j.intimp.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Kontny E., Prochorec-Sobieszek M. Articular adipose tissue resident macrophages in rheumatoid arthritis patients: potential contribution to local abnormalities. Rheumatology. 2013;52:2158–2167. doi: 10.1093/rheumatology/ket287. [DOI] [PubMed] [Google Scholar]
- 32.Rabquer B.J., Koch A.E. NK4 therapy: a new approach to target angiogenesis and inflammation in rheumatoid arthritis. Arthritis Res Ther. 2013;15:119. doi: 10.1186/ar4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kontny E., Zielinska A., Skalska U., Ksiezopolska-Orlowska K., Gluszko P., Maslinski W. Distinct secretory activity and clinical impact of subcutaneous abdominal adipose tissue in women with rheumatoid arthritis and osteoarthritis. Inflammation. 2017;40:106–116. doi: 10.1007/s10753-016-0459-3. [DOI] [PubMed] [Google Scholar]
- 34.Okunishi K., Dohi M., Fujio K., Nakagome K., Tabata Y., Okasora T. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol. 2007;179:5504–5513. doi: 10.4049/jimmunol.179.8.5504. [DOI] [PubMed] [Google Scholar]
- 35.Aenlle K.K., Curtis K.M., Roos B.A., Howard G.A. Hepatocyte growth factor and p38 promote osteogenic differentiation of human mesenchymal stem cells. Mol Endocrinol. 2014;28:722–730. doi: 10.1210/me.2013-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K., Perez-Stable C., D’Ippolito G., Schiller P.C., Roos B.A., Howard G.A. Human bone marrow-derived stem cell proliferation is inhibited by hepatocyte growth factor via increasing the cell cycle inhibitors p53, p21 and p27. Bone. 2011;49:1194–1204. doi: 10.1016/j.bone.2011.08.023. [DOI] [PubMed] [Google Scholar]