Abstract
Plague is a virulent zoonosis, vectored by fleas, posing danger to black-tailed prairie dogs (BTPDs) (Cynomys ludovicianus), black-footed ferrets (Mustela nigripes), and humans in North America. During prior research, a fipronil grain bait (0.005%) applied at rates of 1-½ cup/burrow, reduced flea abundance by > 95–100% when applied three times February–March in northern Colorado. The objective of the current study was to determine the efficacy of fipronil bait against fleas in northern Colorado at reduced application rates (½ cup/burrow) and frequencies (1–2 applications). The field study was conducted in Larimer county, Colorado USA between June-November 2018. Three test plots were selected: two treatment plots (1 vs. 2 fipronil bait applications) and one untreated control. Fipronil was applied at a rate of ½ cup (~95 g)/burrow. Fleas were collected from captured BTPDs and swabs of active burrows prior to bait application and up to 134-days post-treatment. A total of 203 BTPDs and 210 active burrows were sampled. Within the treatment plots, no fleas were collected from BTPDs up to 134-days post-treatment (100% efficacy). Five fleas were recovered from burrows within the one-application plot (<40-days post-application) with efficacy ranging from 97.1 to 100%. No fleas were recovered from burrows within the two-application plot. We caution that while fleas were present within the control plot throughout the study, abundances were low. The efficacy results are supported by those of prior field research conducted in South Dakota and suggest that fipronil bait may be applied at lower rates and frequencies than initially proposed, with potential to sustain flea removal >4-months.
Keywords: Black-tailed prairie dog, Cynomys ludovicianus, Fipronil, Oropsylla hirsuta, Fleas, Plague, Vector control, Yersinia pestis
Graphical abstract
Highlights
-
•
Fipronil grain bait fed to black-tailed prairie dogs was effective against fleas.
-
•
Controlling prairie dog fleas can reduce plague transmission to humans and wildlife.
-
•
Low application rate could reduce environmental risk and insecticide resistance.
-
•
One application of bait controlled 100% of prairie dog fleas for more than 4-months.
1. Introduction
Plague, caused by the bacterium Yersinia pestis, is a virulent vector borne zoonosis, transmissible to humans, and maintained in nature by fleas (pathogen vector) and rodents (pathogen host) (Gratz, 1999). Although human plague incidence and mortality are minimized relative to the pandemics of prior centuries (United States Centers for Disease Control and Prevention, 2020), human instances of plague still occur in the United States (US) with 16 cases and 4 deaths reported in 2015 (United States Centers for Disease Control and Prevention, 2019). In the US, plague epizootics have severe negative impacts on black-tailed prairie dog (BTPD) (Cynomys ludovicianus) populations and mortality rates in colonies can reach 100% (Stapp et al., 2004; Pauli et al., 2006). The prairie dog is considered a keystone species that significantly impacts the structure, function, and composition of ecosystems (Miller et al., 2000), and plague epizootics within BTPD populations negatively impact grassland ecosystems and wildlife species dependent upon them (Antolin et al., 2004; Eads and Biggins 2015). This is evidenced particularly by the fact that black-footed ferret (Mustela nigripes) restoration efforts are often hindered by plague (Roelle et al., 2006; Matchett et al., 2010). Thus, plague management should continue to focus on reducing the occurrence and severity of plague within BTPD colonies with the aim of preserving wild populations, protecting the wildlife species that depend on them, and reducing risk of Y. pestis transmission to humans.
Vector control through use of insecticides provides a promising solution for reducing flea abundance. Oral baits formulated with low concentrations of insecticide, can systemically control ectoparasites such as ticks and fleas feeding on rodents (Borchert et al., 2009; Poché et al., 2020, Poché et al., 2017; Pelletier et al., 2020, Poché et al., 2017, Poché et al., 2020), meaning that the insecticide is absorbed by the ectoparasite during blood feeding. Several insecticidal compounds have demonstrated promise in controlling on-host fleas, systemically, when administered orally to host rodents (Borchert et al., 2009; Davis et al., 1999; Slowik et al., 2001; Jachowski et al., 2011). Fipronil, an insecticide (phenylpyrozol) which blocks the GABA and glutamate-gated chloride channels of arthropods (Raymond-Delpech et al., 2005), is particularly promising, and has controlled a variety of arthropod pest species including fleas under field and laboratory conditions. Studies utilizing phlebotomine sand flies (Ingenloff et al., 2013) reported fipronil to be more efficacious than eprinomectin, imidacloprid, and diflubenzuron.
Recently, a fipronil grain bait (0.005%) was developed for flea control on rodents (United States Environmental Protection Agency Reg. No. 72500–28) with the aim of disrupting zoonotic plague transmission (Poché et al., 2017). The field study, which led to federal registration of the fipronil bait, was conducted in 2015 in northern Colorado (Larimer County), where the bait was applied 3x over 3-weeks from February–March. The fipronil application rates were 0.1345 mg/m2 (Day-0: 1-cup/burrow), 0.069 mg/m2 (Day-7: ½ cup/burrow), and 0.084 mg/m2 (Day-21: ½ cup/burrow), respectively. The fipronil bait successfully reduced the number of fleas parasitizing BTPDs by >95% for a minimum of 52-days post-initial bait application.
Although the results of the above study were satisfactory based upon US Environmental Protection Agency (EPA) regulatory guidelines (United States Environmental Protection Agency, 1998), a lesser application rate and application frequency would be economically and environmentally advantageous. A more recent study conducted in the northern subzone desert in southeastern Kazakhstan in 2016 (Poché et al., 2018) indicated the EPA-registered fipronil bait formulation could reduce Xenopsylla spp. fleas, parasitizing great gerbils (Rhombomys opimus), by 100% for at least 80 days post-treatment, when performing two baiting applications (½ cup/active burrow), each at a rate of 0.006 mg/m2 (Poché et al., 2018). These researchers indicated that the rate of decline in bait efficacy was not calculable because 100% efficacy was obtained for the study duration, and hence efficacy would have likely been sustained for a longer duration. This hypothesis is supported by a recent field study conducted in Buffalo Gap National Grassland, South Dakota (Eads et al., 2019). The results suggested that a single application (½ cup/active burrow) of this fipronil bait formulation, performed in July, could reduce fleas parasitizing BTPDs up to 3-months by 97–100% and could significantly suppress fleas up to 12-months (Eads et al., 2019). These studies suggest that the number of applications (1–2x) and application rates in northern Colorado can be reduced markedly and that the efficacy of fipronil bait in reducing fleas should be investigated several months post-exposure.
It would additionally be useful to monitor the off-host flea abundance within colonies (Krasnov et al., 2004). The field studies conducted by Poché et al., 2017, Poché et al., 2018 and Eads et al. (2019) did not explicitly estimate the impact of fipronil treatment upon off-host flea abundance. However, Poché et al. (2018) noted a complete absence of Xenopsylla gerbili minax within examined burrow entrances post-treatment, suggesting that control of on-host fleas with fipronil bait would subsequently reduce off-host fleas. Off-host abundance was not investigated by Eads et al. (2019) because the primary BTPD flea species, (Oropsylla hirsuta) is regarded as a “fur flea” spending most of its time on-host (Krasnov, 2008) and has demonstrated high mortality in the absence of available blood meals (Salkeld and Stapp, 2008; Wilder et al., 2008). However, prairie dog fleas have been observed within burrows 3-months to 1-year after active colonies have been removed by plague (Lechleitner et al., 1968; Fitzgerald 1970; Cully et al., 1997), suggesting that they can survive off-host for a considerable period. Fipronil bait acts systemically, and thus most directly impacts on-host blood feeding fleas. Managers might better confirm the complete removal of fleas from BTPD colonies by monitoring post-treatment off-host flea abundance within fipronil-treated localities.
The objective of this study was to determine the potential for 1–2 applications of fipronil grain bait (0.005%) to reduce fleas (Oropsylla spp.) parasitizing BTPDs and active burrows >4-months post-application in northern Colorado. Significant reductions >4-months post-exposure will further support the usefulness of fipronil bait in reducing infected flea bite risk to BTPDs, dependent wildlife species, and humans. In addition to providing protection from infected flea bites, reducing the fipronil application rates and frequencies would prove more feasible and cost effective for managers, as well as presenting reduced risk of access by non-targets, potential for bioaccumulation, and potential for insecticide resistance. This work serves as 1) a follow-up to the work conducted by Poché et al. (2017) in northern Colorado; and 2) a complimentary replication to the work conducted by Eads et al. (2019) performed in a shortgrass ecosystem within a different North American state. The results should provide insights into the usefulness of this reduced fipronil application rate in controlling BTPD fleas in northern Colorado.
2. Materials and methods
2.1. Study area
The study was performed in 2018 (June 25-November 16) in a Larimer County Colorado locality (~N 40° 42′ 28.8, W 105° 04′ 16.4) on private land. Three test plots) were selected and randomly assigned to one of three test groups: Treatment 1 (T1), Treatment 2 (T2), and untreated control (Control). Each plot was an individual BTPD colony. The perimeter of each plot was mapped using a handheld GPS (Garmin Etrex 30, Garmin, Olathe, KS). The area of each interior plot was ~4.04 ha (T1), ~4.04 ha (T2) and ~4.03 ha (Control). Treated buffer zones (≥100 m) encircled both treatment plots, resulting in total areas of ~13.68 ha (T1) and 13.07 ha (T2). The perimeters of each test plot were separated by > 500 m and were each separated by paved roads and hills, which served as natural boundaries to separate the BTPD colonies. Permission was received from the property owners prior to study initiation.
2.2. Collection of rodents and fleas
Over the course of the experiment, fleas were collected from BTPDs and active burrows at ~2-4-week intervals. BTPDs were captured using Tomahawk live traps (Tomahawk® Live Trap Company, Hazelhurst WI) (7.6 × 7.6 × 25 cm). To maximize trapping success, traps were wired open and baited with rolled oats and peanut butter for 2–3 days. After BTPDs were properly conditioned to feed from traps, the wires were removed from the traps, which continued to be baited. During each trapping period, 6–10 BTPDs were captured within each study plot.
BTPDs were captured during 7 trapping periods, 1 Pre-treatment and 6 Post-treatment (Post-): Pre-treatment (June 25–27), 1st Post- (July 16–19), 2nd Post- (August 6–9), 3rd Post- (August 27–31), 4th Post- (September 24–27), 5th Post- (October 22–24), and 6th Post- (November 13–15). Approximately 20–40 traps were activated within each plot with the location of each trap identified using a handheld GPS (Garmin Etrex 30, Garmin, Olathe, KS). Traps were opened at ~0700 h and were checked at least hourly. Captured BTPDs were marked by numbered ear tag. BTPDs recaptured within the same trapping period were immediately released at the point of capture.
Captured BTPDs were anesthetized using an isoflurane vaporizer connected to an induction chamber (17″ L x 8.5″ W x 10.5” H) for ~6–10 min. After reaching an anesthetic plane, BTPDs were removed from the chamber and fleas were collected using methods similar to those described by Davis (1999). All fleas were removed from the fur of BTPDs using a metallic flea comb and were collected using a handheld electronic aspirator. Fleas were then transferred to 50 mL conical tubes.
Fleas were collected from active burrows, utilizing a burrow swabbing technique similar to that described by Ecke and Johnson (1952) and Griffin et al. (2010), during 7 swabbing periods: Pre-treatment (June 29), 1st Post- (July 19–20), 2nd Post- (August 8–9), 3rd Post- (August 29–31), 4th Post- (September 24–27), 5th Post- (October 23–24), and 6th Post- (November 15–16). During each swabbing period, fleas were obtained from 10 active burrows within each plot. Active burrows are defined as those with fresh BTPD scat (greenish, black, dark brown) in the opening or ~0.5 m of it (Biggins et al., 1993) and with obvious fresh digging and tracks. Burrows utilized for swabbing were 1) burrows actively used by BTPDs, 2) burrows in which sunlight was able to enter the tunnel, and 3) burrows in which swabbing could be performed at sufficient burrow depths (Eads, 2017). Burrows were swabbed using an ~3.5 m metal plumbing snake. A white flannel cloth (swab) (15 cm × 15 cm) was connected to the plumbing snake using an alligator clip and was inserted into an active burrow and into the burrow chamber (≥2 m depth). Once removed from the burrow, swabs were immediately placed individually into sealable plastic bags.
All fleas collected during sampling were taken to the laboratory and stored in a −20 °C freezer. Collected fleas were separated from dirt and debris using fine-tipped forceps and the total number of fleas per BTPD and per burrow were recorded. A subsample of fleas was retained (n = 50) which were identified to the species level using published taxonomic keys (Hubbard, 1947).
2.3. Rodent grain bait (0.005% fipronil)
The fipronil grain bait evaluated during this study was manufactured with a nominal fipronil concentration of 0.005% (50 ppm). The nominal concentration in the bait was confirmed using a High-Performance Liquid Chromatography (HPLC) reverse-phase method as was described by Poché et al. (2017). The mean concentration of fipronil in the bait was 50.9 ± 2.0 ppm (n = 4, recovery = 102%, CV = 3.89%). During fipronil bait application, similar to the methodology described by Poché et al., 2017, Poché et al., 2018 and Eads et al. (2019), ½ cup (~95 g) fipronil bait was scattered within 1 m of each active burrow within each treatment plot (+buffer zone). Similar to Eads et al. (2019), a single fipronil bait application was conducted within T1 on July 2 (Day-0). Fipronil bait application was conducted within T2 on July 2 and on September 4 (Day-64). The ~60-day follow up application was performed within T2 under the assumption that a vector population could recover from initial treatment (Poché et al., 2016). The whole blood half-life of a single oral dose of fipronil in laboratory rats (4 mg/kg, 150 mg/kg) can range from 8.3 to 2.1 days (United States Environmental Protection Agency, 1996), suggesting that a follow up application could be needed to eliminate any surviving fleas. The Control plot received no fipronil bait application.
All activities involving animals during this study adhered to Animal Welfare Act (AWA) regulations and were approved by the Genesis Laboratories, Inc. Institutional Animal Care and Use Committee (IACUC) (USDA AWA, 9 CFR Parts 1–3) (May 4, 2018 edition). Colorado Parks and Wildlife Scientific Collection License (18 TR206a) was obtained prior to trapping BTPDs.
2.4. Data analyses
Parasitized BTPDs and parasitized burrows were defined as those having a minimum of 1 flea. Flea prevalence was defined as the proportion BTPDs or active burrows which were parasitized by fleas. Flea abundance was defined as the mean number of fleas per BTPD/burrow per plot per sampling period. Pre-treatment, 1st Post-, 2nd Post-, 3rd Post-, 4th Post-, 5th Post-, and 6th Post-treatment flea abundances were used to estimate the efficacy of fipronil bait. Efficacy was estimated using an equation described by Henderson and Tilton (1955). The equation is a modified form of Abbott's formula (Abbott, 1925), which adjusts for differences in flea abundance within the treatment and control during pre-treatment. The equation is presented below:
where:
T = Treatment Group, C= Control Group, b = Mean number of fleas parasitizing BTPDs or burrows during pre-treatment timepoint, a = Mean number of fleas parasitizing BTPDs or burrows during post-treatment timepoint.
Differences in the number of fleas collected from BTPDs and active burrows between each plot (T1, T2, Control) and within each plot during Pre-treatment, 1st Post-, 2nd Post-, 3rd Post-, 4th Post-, 5th Post-, and 6th Post-treatment were analyzed using a non-parametric Kruskal-Wallis test and Wilcoxon Rank-Sum test. Differences in the number of fleas collected on BTPDs and within active burrows were analyzed using a Wilcoxon Rank-Sum test. Differences in flea prevalence were analyzed using a Pearson's x2 test for independence. Statistical analyses (p < 0.005) were performed using JMP statistical software (Version 15) (SAS, Cary, NC, US).
3. Results
3.1. Fipronil bait application
A total of 18.7 kg of fipronil bait (0.005%) was used to treat ~197 active burrows within T1 (July 2). Within T2, a total of 29.6 kg (July 2) and 29.4 kg (September 4) bait were used to treat ~312 and 309 active burrows, respectively (total = 59.0 kg). The fipronil application rate within T1 and T2 were 0.007 mg/m2 (1x) and 0.011 mg/m2 (2x), respectively. Fipronil bait and fipronil application rates are presented in Table 1.
Table 1.
Application rate of bait and fipronil by within T1 plot and buffer area 13.68 ha (136,8 m2) and T2 plot and buffer area 13.07 ha (130,7 m2).
| Plot Identification | Application Date | Bait Applied (kg) | Bait Application Rate (g/m2) | Fipronil Application Rate (mg/m2) |
|---|---|---|---|---|
| T1 | July 2 | 18.7 | 0.14 | 0.007 |
| T2 | July 2 | 29.6 | 0.23 | 0.011 |
| September 4 | 29.4 | 0.22 | 0.011 |
3.2. Rodent and flea collection
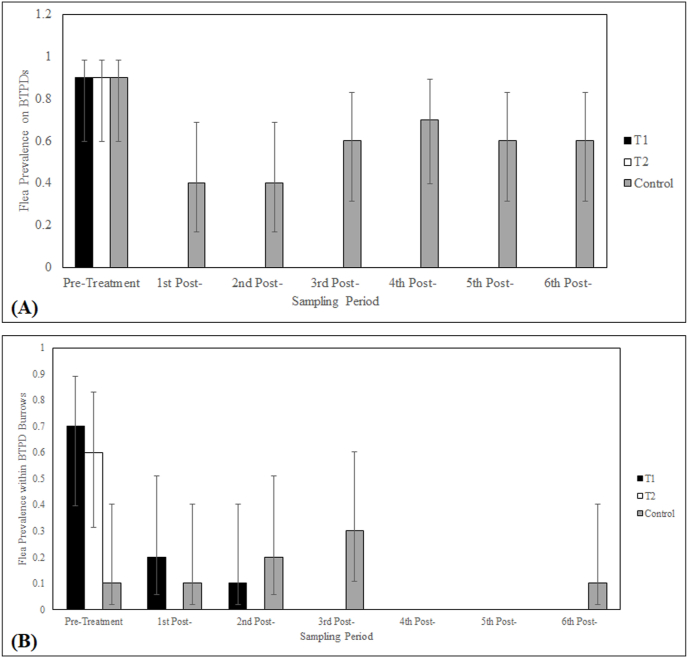
A total of 203 BTPD captures occurred over the course of the study. One hundred eleven (111) individual BTPDs were marked with ear tags and 92 were recaptured at successive timepoints. BTPD captures are summarized in Table 2. A total of 417 fleas were collected from 30 BTPDs captured pre-treatment. All plots exhibited flea prevalence (95% CI) on BTPDs of 0.9 (0.60, 0.98) during pre-treatment (Fig. 1a). The flea abundances (±SD) on captured BTPDs during Pre-treatment were 32.7 ± 44.3 (T1), 5.9 ± 6.8 (T2) and 3.1 ± 2.2 (Control). No fleas were collected on 113 captured BTPDs within T1 or T2 during the 6 post-treatment sampling periods. Seventy-eight (78) fleas were collected from 60 captured BTPDs processed within the Control plot during the 6 post-treatment sampling periods with flea prevalence being: 1st Post-: 0.4 (0.17, 0.69), 2nd Post-: 0.4 (0.17, 0.69), 3rd Post-: 0.6 (0.31, 0.83), 4th Post-: 0.7 (0.40, 0.89), 5th Post-: 0.6 (0.31, 0.83), 6th Post-: 0.6 (0.31, 0.83) (Fig. 1a). The flea abundances within the Control (1st Post-: 0.8 ± 1.5, 2nd Post-: 0.9 ± 1.3, 3rd Post-: 1.2 ± 1.3, 4th Post-: 2.0 ± 1.8, 5th Post-: 1.1 ± 1.2, 6th Post-: 1.8 ± 2.0) were low throughout the study. Efficacy of fipronil bait in reducing fleas on wild-caught BTPDs within T1 and T2 was estimated to be 100% during all 6 post-treatment collection periods. All flea abundances data and efficacy estimates are presented in Table 3, Table 4, respectively.
Table 2.
Number of black-tailed prairie dogs (Cynomys ludovicianus) captured within the three test plots.
| Sampling Period | Pre-treatment (6/25/18–6/27/18) | 1st Post-treatment (7/16/18–7/19/18) | 2nd Post-treatment (8/6/18–8/9/18) | 3rd Post-treatment (8/27/18–8/31/18) | 4th Post-treatment (9/24/18–9/27/18) | 5th Post-treatment (10/22/18–10/24/18) | 6th Post-treatment (11/13/18–11/15/18) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot ID | T1 | T2 | aC | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | |||||
| Total Captured | 10 | 10 | 10 | 10 | 7 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 6 | 10 | |||||
| Newly Marked | 10 | 10 | 10 | 9 | 6 | 9 | 2 | 5 | 8 | 3 | 5 | 7 | 2 | 2 | 3 | 6 | 3 | 4 | 3 | 0 | 4 | |||||
| Recaptured | aN | N | N | 1 | 1 | 1 | 8 | 5 | 2 | 7 | 5 | 3 | 8 | 8 | 7 | 4 | 7 | 4 | 7 | 6 | 6 | |||||
C = control plot, N = not applicable.
Fig. 1.
Flea prevalence (0.0–1.0) during pre-treatment and post-treatment for (A) captured black-tailed prairie dogs (Cynomys ludovicianus); and (B) active burrows. Vertical bars indicate the 95% confidence intervals (95% CI).
Table 3.
Black-tailed prairie dog (Cynomys ludovicianus) and burrow mean flea abundances (±SD) within each test plot, and estimated fipronil bait efficacy.
| Sampling Period | Pre-treatment (6/25/18–6/27/18) | 1st Post-treatment (7/16/18–7/19/18) | 2nd Post-treatment (8/6/18–8/9/18) | 3rd Post-treatment (8/27/18–8/31/18) | 4th Post-treatment (9/24/18–9/27/18) | 5th Post-treatment (10/22/18–10/24/18) | 6th Post-treatment (11/13/18–11/15/18) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot ID | T1 | T2 | aC | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | |||||
| BTPDs | 32.7 (±44.3) | 5.9 (±6.8) | 3.1 (±2.2) | 0 | 0 | 0.8 (±1.5) | 0 | 0 | 0.9 (+1.3) | 0 | 0 | 1.2 (+1.3) | 0 | 0 | 2.0 (+1.8) | 0 | 0 | 1.1 (+1.2) | 0 | 0 | 1.8 (±2.0) | |||||
| Burrowsa | 10.5 (±8.6) | 1.1 (±1.1) | 0.1 (±0.3) | 0.2 (±0.4) | 0 | 0.1 (±0.3) | 0.3 (+0.9) | 0 | 0.3 (+0.6) | 0 | 0 | 0.6 (+1.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 (±0.3) | |||||
C = control plot, Fleas were collected from 10 active burrows within each plot during each sampling period.
Table 4.
Efficacy (%) reduction in flea abundance on BTPDs and within BTPD burrows.
| Sampling Method | Plot ID |
aEfficiancy |
|||||
|---|---|---|---|---|---|---|---|
| 1st Post-treatment | 2nd Post-treatment | 3rd Post-treatment | 4th Post-treatment | 5th Post-treatment | 6th Post-treatment | ||
| BTPDs | T1 | 100% | 100% | 100% | 100% | 100% | 100% |
| T2 | 100% | 100% | 100% | 100% | 100% | 100% | |
| Burrows | T1 | 98.1% | 97.1% | 100% | aNC | NC | 100% |
| T2 | 100% | 100% | 100% | NC | NC | 100% | |
NC = not calculable due to no fleas being collected within any of the three plots.
The flea abundances reported in Table 3 were used to calculate efficacy. Calculations were performed using the equation described by Henderson and Tilton (1955).
A total of 210 active burrows were swabbed for fleas over the course of the study. During Pre-treatment, 117 fleas were collected from within 30 active burrows. Flea prevalence in burrows during Pre-treatment was 0.7 (0.4, 0.89) (T1), 0.6 (0.31, 0.83) (T2) and 0.1 (0.02, 0.40) (Control) (Fig. 1B) and flea abundances were 10.5 ± 8.6 (T1); 1.1 ± 1.1 (T2); and 0.1 ± 0.3 (Control). Flea prevalence within burrows within T1 during post-treatment was 0.2 (0.06, 0.51) (1st Post-), 0.1 (0.02, 0.40) (2nd Post-), and 0 for the remaining sampling periods (Fig. 1B), and flea abundances were 0.2 ± 0.4 (1st Post-), 0.3 ± 0.9 (2nd Post-), and 0 for the remaining sampling periods. No fleas were collected within burrows during any of the post-treatment sampling periods within T2. Flea abundances for active burrows within the Control during post-treatment were low throughout post-treatment: 0.1 ± 0.3 (1st Post-), 0.3 ± 0.9 (2nd Post), 0.6 ± 1.2 (3rd Post-), 0 (4th Post-), 0 (5th Post), and 0.1 ± 0.3 (6th Post-). Efficacy of fipronil bait in reducing fleas collected from active burrows within T1 was: 98.1% (1st Post-), 97.1% (2nd Post-), 100% (3rd Post-), and 100% (6th Post-). Efficacy of fipronil bait within T2 was 100% at 1st Post-, 2nd Post-, 3rd Post-, and 6th Post. No fleas were collected within the three test plots (Control included) at 4th Post- and 5th Post-, and therefore efficacy could not be estimated. All flea abundances and are presented in Table 3.
Pre-treatment flea abundance in T1 was significantly greater relative to T2 (Wilcoxon; p = 0.0062) and Control (Wilcoxon; p = 0.0003). During all post-treatment periods, flea abundance was significantly greater within Control relative to T1 (Kruskal-Wallis/Wilcoxon; p < 0.0001) and T2 (Kruskal-Wallis/Wilcoxon; p < 0.0001). In T1 and T2, flea abundance at all post-treatment sampling periods was significantly reduced relative to pre-treatment (Kruskal-Wallis/Wilcoxon; p < 0.0001). Flea abundance within pre-treatment Control was not significantly different relative to any post-treatment sampling period but was nearly significant when compared with 1st Post- (Wilcoxon; p = 0.0532). During pre-treatment, flea abundance was significantly higher on BTPDs relative to active burrows within Control (Wilcoxon; p = 0.0004) and T2 (Wilcoxon; p = 0.0257) but not T1 (Wilcoxon; p = 0.7043). The probability of flea prevalence was greater on BTPDs than in active burrows (Pearson's x2; p < 0.0001) and was significantly reduced during post-treatment relative to pre-treatment (Pearson's x2; p < 0.0001).
Fifty (50) fleas were morphologically identified by species: 49 were identified as O. hirsuta (18 males, 31 females), and 1 was identified as female Pulex spp.
4. Discussion
These results support those of Eads et al. (2019), suggesting that fipronil bait, applied once in July at a rate of 0.007 mg fipronil/m2 (1/2 cup per active burrow) in northern Colorado, could potentially reduce fleas (O. hirsuta) up to 100% for 134-days (>4-months) post-exposure. Fleas parasitizing BTPDs were reduced by 100% in areas treated with fipronil bait. When calculable, fleas were reduced by 97.1–100% within the area treated with a single fipronil application (T1). The results obtained from the T1 plot are considerably reinforced by the results of Eads et al. (2019) who recorded 97–100% flea reductions up to 3-months after a single application, suggesting that these two studies may serve as complimentary investigations demonstrating the usefulness of fipronil bait in controlling fleas of BTPDs in at least two locations in North America. The current study suggested application rates ~13.7x and ~8.7x lower than the average application rate used over three applications performed by Poché et al. (2017) could affectively control fleas parasitizing BTPDs. High frequency application of a single insecticide can lead to resistant vector populations through the process of selection pressure (Nauen, 2007). Therefore, reducing the fipronil application rate (1/2 cup/active burrow) to ensure the bait is taken up quickly by the BTPD population, and reducing the number and frequency of applications should reduce risk of potential insecticidal resistance. Although research suggests that the potential for direct resistance of fleas to fipronil is uncertain or improbable (Tingle et al., 2003; Rust et al., 2015), treated flea populations should continue to be monitored for potential resistance (Rust, 2016). Reduced application rates and treatment frequency should minimize the probability of resistance occurring and the results of the current study, in addition to those presented by Poché et al. (2018) and Eads et al. (2019), suggest low rates and frequencies are achievable. The results of the current study, in conjunction with the those of Poché et al. (2017) and Eads et al. (2019) should provide wildlife managers and human health practitioners with important insights concerning the usefulness and challenges associated with utilizing fipronil bait to control fleas parasitizing BTPDs in shortgrass ecosystems within multiple North American States.
The EPA conducted a thorough risk assessment of this fipronil bait prior to registering it (Reg. No. 72500–28) as a product for use against BTPD fleas in parks, golf courses, non-crop rights-of-way, and other non-crop areas. As previously reported by Poché et al., 2017, Poché et al., 2018 small mammals would be required to eat more than their own body weight of 0.005% fipronil bait in one sitting to reach the oral LD50 reported for mammals (97 mg/kg) (United States Environmental Protection Agency, 1996), an improbable feat. Additionally, the reduced amount of bait applied (1/2 cup per active burrow) and the infrequent bait application schedule (1 application per ~3–4 months) during the current study and that of Eads et al. (2019), relative to the research of Poché et al. (2017), should further prevent chronic exposure and fipronil bioaccumulation in BTPDs. During the current study, no evidence of non-target animal death or distress was observed. This finding is consistent with prior field studies evaluating low dose fipronil and imidacloprid flea baits (Borchert et al., 2009; Jachowski et al., 2011; Poché et al., 2017, Poché et al., 2018. However, the above researchers acknowledge that an absence of non-target collateral cannot be completely confirmed because observations were performed above ground during the day. The potential for non-target collateral should continue to be investigated and proper precautions should be taken. Additional fipronil bait research might consider further evaluating any impact of application on non-target species and strive to provide additional recommendations to further minimize potential for non-target risk.
This study's greatest limitations are likely low flea abundance and a small BTPD sample size. Low flea abundance within the Control plot (particularly within active burrows) may have weakened the baseline, suggesting that additional testing may be needed in areas with greater flea abundance. Throughout the study, we note that landowners would periodically irrigate a considerable proportion of the Control plot. On multiple occasions, the flannel swabs utilized during swabbing were observed to be soaked with water upon retrieval from burrows, indicating partial flooding of burrow systems. It is likely that recurring irrigation had a negative impact on flea abundance within this locality. Flea survivorship can decline in response to high temperature (Krasnov et al., 2001), and given this study was initiated during the summer months, it is possible that low numbers within the control plot were a partial byproduct of temperature-related mortality. However, the microclimates within burrow systems should be more conducive to flea survivorship relative to above ground, and the effects of temperature on flea survivorship should be further studied (Tripp et al., 2009). The low sample size of 6–10 BTPDs/burrows per plot was selected based on EPA recommendation (United States Environmental Protection Agency, 1998). However, because on-host flea abundance typically follows a negative binomial distribution within prairie dog colonies (Tripp et al., 2009; Eads et al., 2016), as was the case during the current study, a greater number of samples would be ideal to better insure that all parasitized BTPDs would be included in the sample. The distribution of the data led to the use of non-parametric statistical methods. Additionally, the continuous trapping conducted over the course of the study resulted in the majority of BTPDs being recaptures during the latter post-exposure timepoints. However, considering the only fleas we found on any BTPDs following pre-treatment were collected within the control plot, it is improbable that recaptures would have impacted the results collected from the treatment plots. While a sizable number of control group fleas and larger BTPD sample would have made these results more robust, we still contend that the complete removal of fleas within the treatment areas suggests fipronil bait significantly impacted flea abundance within these areas. An average of 32.7 fleas per BTPD was collected within T1 during pre-treatment, ~10.5x greater than the average recorded within the Control plot during the same period. After a single fipronil application, no fleas were collected from BTPDs for the remainder of the study, including the months of August–October, during which flea abundance would be expected to be high (Tripp et al., 2009). Two applications also led to the complete removal of fleas within T2. Although flea abundance was low within the Control plot, fleas were collected from multiple captured BTPDs during each post-treatment timepoint. Use of the equation described by Henderson and Tilton (1955) allowed efficacy to be adjusted based upon changes in flea abundance within the Control plot. Although only 6–10 BTPDs were captured per plot at each sampling period, if we consider the entire post-exposure period (zero fleas collected from 60 to 53 BTPDs collected within T1 and T2, respectively), the study suggests that fipronil significantly impacted flea abundance. While future studies of greater sample size (plots + BTPDs) and flea abundances would add confidence to the reproducibility of these data, we argue that the results of this study are still important and resulted in 97.1–100% reduction in flea abundance. The current efficacy results replicate those of Eads et al. (2019) (97–100% efficacy), suggesting that fipronil bait application has potential to efficaciously control fleas within another shortgrass ecosystem in northern Colorado.
Overall, on-host flea abundance was significantly greater than off-host abundance. Low flea abundance within burrows was particularly obvious within the Control plot, where flea abundance was low in general. In contrast, flea numbers within active burrows in T1, while lower than BTPD numbers, did not differ significantly. This suggested that burrow swabbing would be a more reliable measure in areas of heavy on-host flea abundance, and hence, areas at risk of epizootic plague outbreaks. Although numerous fleas were collected off-host within the treatment plots (particularly T1) during pre-treatment, 100% (T2) and 98.1% (T1) efficacy were obtained at 1st Post-after a single application. The fast-acting nature of fipronil on fleas actively blood feeding on BTPDs is intuitive, however it is also encouraging that >98.1–100% efficacy was achieved for off-host fleas within 2-weeks of fipronil bait application. The potential for immediate efficacy against off-host fleas may be a byproduct of the majority of fipronil being excreted in feces (United States Environmental Protection Agency, 1996). Immature fleas feed on organic material such as feces within the burrows (Krasnov, 2008). This is supported by previous findings indicating that fipronil bait is highly efficacious against phlebotomine sand fly larvae feeding on rodent and cattle feces (Ingenloff et al., 2013: Mascari et al., 2013: Poché et al., 2013). Given the low flea abundance within the Control plot burrows, we report these results with caution. Additional research is needed before the impact of fipronil bait on flea abundance within burrows can be fully understood.
In considering future studies, researchers should aim to evaluate how seasonality of fipronil bait application might impact its efficacy against fleas. During the study conducted by Poché et al. (2017), treatment was performed 3x from February to March and resulted in 95–100% mortality. Additional research conducted by Tripp et al. (2016) suggested that deltamethrin treatment performed in autumn provided longer flea control than did treatment performed in spring. The results of a single fipronil bait application may vary dependent on timing and thus additional studies evaluating the impact of timing on fipronil bait efficacy would be useful. Additionally, should flea abundance gradually recover, studies should be designed in which spring or summer treatment is followed by a subsequent autumn treatment (Poché et al., 2018), perhaps ~4–6 months post-treatment. The long-term efficacy of fipronil bait in reducing flea abundance should also be evaluated up to 12-months post-treatment in northern Colorado as was done previously in South Dakota (Eads et al., 2019). Finally, while this study serves as complimentary to the work conducted by Eads et al. (2019), researchers should consider the potential for a study in the same area in Colorado with an expanded sample size to include several control and treatment plots.
The results suggest the potential for fipronil bait (0.005%), applied one to two times at a rate of ½ cup (95 g)/burrow, to efficaciously control fleas (O. hirsuta) parasitizing BTPDs and occupying active burrows up to 134-days (>4-months) post-treatment. These results are supported by the results of Eads et al. (2019) and suggest that fipronil bait may provide a means of controlling fleas of BTPDs in shortgrass ecosystems within at least two US states. Additional studies conducted in areas known to have high flea density, in addition to studies evaluating treatment efficacy up to 12-months post-exposure as was done by Eads et al. (2019), would be useful to further estimate the impact of fipronil baits on on-host and off-host flea abundance in northern Colorado. Additionally, future studies might expand upon the current results and those of Eads et al. (2019) by evaluating the use of a single fipronil bait application implemented at different times throughout various seasons. Through reducing on-host and off-host flea abundance, fipronil bait application has potential to protect BTPDs from epizootic plague for several months and subsequently reduce negative impacts on dependent wildlife species and risk of plague infection in humans.
Declaration of competing interest
Authors have no conflict of interest.
Acknowledgements
We would like to thank Daniel W. Tripp of Colorado Parks and Wildlife for assisting with all flea identification performed during this study. His generosity and time devoted were gratefully appreciated. We thank Gregory Franckowiak and Joseph Conner for assisting with field collection. We additionally thank Jane Andales for assisting with HPLC bait analyses. Finally, we thank Scimetrics Limited Corp. (Wellington, Colorado, USA) for providing all fipronil bait utilized during this research. This research did not receive any specific grants from funding agencies in the public, commercial or no-for-profit sectors.
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;17 [PubMed] [Google Scholar]
- Antolin M.F., Gober P., Luce B., Biggins D.E., Van Pelt W.E., Seery D.B., Lockhart M., Ball M. The influence of sylvatic plague on North American wildlife at the landscape level, with special emphasis on black-footed ferret and prairie dog conservation. Trans. N. Am. Wildl. Nat. Resour. Conf. 2004;67:104–127. [Google Scholar]
- Biggins D.E., Miller B.J., Hanebury L.R., Oakleaf B., Farmer A.H., Crete R., Dood A.A. Technique for evaluating black-footed ferret habitat. Management of prairie dog complexes for the reintroduction of the black-footed ferret. U S Fish Wildl. Serv. Biol. Rep. 1993;13:73–88. [Google Scholar]
- Borchert J.N., Davis R.M., Poché R.M. Field efficacy of rodent bait containing the systemic insecticide imidacloprid against the fleas of California ground squirrels. J. Vector Ecol. 2009;34(1):92–98. doi: 10.1111/j.1948-7134.2009.00011.x. [DOI] [PubMed] [Google Scholar]
- Cully J.F., Barnes A.M., Quan T.J., Maupln G. Dynamics of plague in a Gunnison's prairie dog colony complex from New Mexico. J. Wildl. Dis. 1997;33(4):706–719. doi: 10.7589/0090-3558-33.4.706. [DOI] [PubMed] [Google Scholar]
- Davis R.M. Use of orally administered chitin inhibitor (lufenuron) to control flea vectors of plague on ground squirrels in California. J. Med. Entomol. 1999;36:562–567. doi: 10.1093/jmedent/36.5.562. [DOI] [PubMed] [Google Scholar]
- Eads D.A., Biggins D.E. Plague bacterium as a transformer species in prairie dogs and the grasslands of western North America. Conserv. Biol. 2015;29(4):1086–1093. doi: 10.1111/cobi.12498. [DOI] [PubMed] [Google Scholar]
- Eads D.A., Biggins D.E., Long D.H., Gage K.L., Antolin M.F. Droughts may increase susceptibility of prairie dogs to fleas: incongruity with hypothesized mechanisms of plague cycles in rodents. J. Mammal. 2016;97(4):1044–1053. [Google Scholar]
- Eads D.A. Swabbing prairie dog burrows for fleas that transmit Yersinia pestis: influences on efficiency. J. Med. Entomol. 2017;54(5):1273–1277. doi: 10.1093/jme/tjx090. [DOI] [PubMed] [Google Scholar]
- Eads D.A., Biggins D.E., Bowser J., Broerman K., Livieri T.M., Childers E., Dobesh P., Griebel R.L. Evaluation of five pulicides to suppress fleas on black-tailed prairie dogs: encouraging long-term results with systemic 0.005% fipronil. Vector Borne Zoonotic Dis. 2019;19(6):400–406. doi: 10.1089/vbz.2018.2339. [DOI] [PubMed] [Google Scholar]
- Ecke D.H., Johnson C.W. Plague in Colorado and Texas. Publ. Health Monogr. 1952;6:1–37. [PubMed] [Google Scholar]
- Fitzgerald J.P. Colorado State University; Fort Collins, Colorado, US: 1970. Ecology of Plague in Prairie Dogs and Associated Small Mammals in South Park, Colorado. PhD. Thesis; p. 90. [Google Scholar]
- Gratz N. Rodent reservoirs and flea vectors of natural foci of plague. In: Dennis D.T., Gage K.L., Gratz N.G., Poland J.D., Thikhomirov E., editors. Plague Manual: Epidemiology, Distribution, Surveillance and Control. World Health Organization; Geneva: 1999. pp. 63–96. (Chapter 4), Pub No WHO/CDS/CSR/EDC/992. [Google Scholar]
- Griffin K.A., Martin D.J., Rosen L.E., Sirochman M.A., Walsh D.P., Wolfe L.L., Miller M.W. Detection of Yersinia pestis DNA in prairie dog-associated fleas by polymerase chain reaction assay of purified DNA. J. Wildl. Dis. 2010;46:636–643. doi: 10.7589/0090-3558-46.2.636. [DOI] [PubMed] [Google Scholar]
- Henderson C.F., Tilton E.W. Tests with acaricides against the brow wheat mite. J. Econ. Entomol. 1955;48:157–161. [Google Scholar]
- Hubbard C.A. The Iowa State College Press; Ames, IA, US: 1947. Fleas of Western North America: Their Relation to Public Health; p. 533. [Google Scholar]
- Ingenloff K., Garlapati R., Poché D., Singh M.I., Remmers J.L., Poché R.M. Feed‐through insecticides for the control of the sand fly Phlebotomus argentipes. Med. Vet. Entomol. 2013;27(1):10–18. doi: 10.1111/j.1365-2915.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- Jachowski D.S., Skipper S., Gompper M.E. Field evaluation of imidacloprid as a systemic approach to flea control in black‐tailed prairie dogs, Cynomys ludovicianus. J. Vector Ecol. 2011;36(1):100–107. doi: 10.1111/j.1948-7134.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- Krasnov B.R., Khokhlova I.S., Fielden L.J., Burdelova N.I. Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: pulicidae) J. Med. Entomol. 2001;38:629–637. doi: 10.1603/0022-2585-38.5.629. [DOI] [PubMed] [Google Scholar]
- Krasnov B.R., Shenbrot G.I., Khokhlova I.S., Degen A.A. Flea species richness and parameters of host body, host geography and host ‘milieu’. J. Anim. Ecol. 2004;73(6):1121–1128. [Google Scholar]
- Krasnov B.R. Cambridge University Press; Cambridge, UK: 2008. Functional and Evolutionary Ecology of Fleas: a Model for Ecological Parasitology. [Google Scholar]
- Lechleitner R.R., Kartman L., Goldenberg M.I., Hudson B.W. An epizootic of plague in Gunnison's prairie dogs (Cynomys gunnisoni) in south‐central Colorado. Ecol. 1968;49(4):734–743. [Google Scholar]
- Mascari T.M., Stout R.W., Foil L.D. Oral treatment of rodents with fipronil for feed-through and systemic control of sand flies (Diptera: psychodidae) J. Med. Entomol. 2013;50(1):122–125. doi: 10.1603/me12157. [DOI] [PubMed] [Google Scholar]
- Matchett M.R., Biggins D.E., Carlson V., Powell B., Rocke T. Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana. Vector Borne Zoonotic Dis. 2010;10(1):27–35. doi: 10.1089/vbz.2009.0053. [DOI] [PubMed] [Google Scholar]
- Miller B., Reading R., Hoogland J., Clark T., Ceballos G., List R., Forrest S., Hanebury L., Manzano P., Pacheco J., Uresk D. The role of prairie dogs as a keystone species: response to Stapp. Conserv. Biol. 2000;14(1):318–321. [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007;63(7):628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Pauli J.N., Buskirk S.W., Williams E.S., Edwards W.H. A plague epizootic in the black-tailed prairie dog (Cynomys ludovicianus) J. Wildl. Dis. 2006;42(1):74–80. doi: 10.7589/0090-3558-42.1.74. [DOI] [PubMed] [Google Scholar]
- Poché R.M., Garlapati R., Singh M.I., Poché D.M. Evaluation of fipronil oral dosing to cattle for control of adult and larval sand flies under controlled conditions. J. Med. Entomol. 2013;50(4):833–837. [PubMed] [Google Scholar]
- Poché D.M., Grant W.E., Wang H.H. Visceral leishmaniasis on the Indian subcontinent: modelling the dynamic relationship between vector control schemes and vector life cycles. PLoS Neglected Trop. Dis. 2016;10(8) doi: 10.1371/journal.pntd.0004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché D.M., Hartman D., Polyakova L., Poché R.M. Efficacy of a fipronil bait in reducing the number of fleas (Oropsylla spp.) infesting wild black‐tailed prairie dogs. J. Vector Ecol. 2017;42(1):171–177. doi: 10.1111/jvec.12252. [DOI] [PubMed] [Google Scholar]
- Poché D.M., Torres-Poché Z., Yeszhanov A., Poché R.M., Belyaev A., Dvořák V., Sayakova Z., Polyakova L., Aimakhanov B. Field evaluation of a 0.005% fipronil bait, orally administered to Rhombomys opimus, for control of fleas (Siphonaptera: pulicidae) and phlebotomine sand flies (Diptera: psychodidae) in the Central Asian Republic of Kazakhstan. PLoS Neglected Trop. Dis. 2018;12(7) doi: 10.1371/journal.pntd.0006630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Rocheleau J., Aenishaenslin C., Beaudry J., Masson G.D., Lindsay L.R., Ogden N.H., Bouchard C., Leighton P.A. Evaluation of fluralaner as an oral acaricide to reduce tick infestation in a wild rodent reservoir of Lyme disease. Parasites Vectors. 2020;13:73. doi: 10.1186/s13071-020-3932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché D.M., Franckowiak G., Clarke T., Tseveenjav B., Polyakova L., Poché R.M. Efficacy of a low dose fipronil bait against blacklegged tick (Ixodes scapularis) larvae feeding on white-footed mice (Peromyscus leucopus) under laboratory conditions. Parasites Vectors. 2020;13:391. doi: 10.1186/s13071-020-04258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond-Delpech V., Matsuda K., Sattelle B.M., Rauh J.J., Sattelle D.B. Ion channels: molecular targets of neuroactive insecticides. Invertebr. Neurosci. 2005;5:119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Roelle J.E., Miller B.J., Godbey J.L., Biggins D.E. Proceedings of the Symposium on the Status of the Black-Footed Ferret and its Habitat. vols. 28–29. US Department of the Interior, US Geological Survey; Fort Collins, Colorado: 2006. Recovery of the black-footed ferret: progress and continuing challenges. January, 2004. [Google Scholar]
- Rust M.K., Vetter R., Denholm I., Blagburn B., Williamson M.S., Kopp S., Rees R. Susceptibility of adult cat fleas (Siphonaptera: pulicidae) to insecticides and status of insecticide resistance mutations at the Rdl and knockdown resistance loci. Parasitol. Res. 2015;114:7–18. doi: 10.1007/s00436-015-4512-1. [DOI] [PubMed] [Google Scholar]
- Rust M.K. Insecticide resistance in fleas. Insects. 2016;7(1):10. doi: 10.3390/insects7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld D.J., Stapp P. Prevalence and abundance of fleas in black-tailed prairie dog burrows: implications for the transmission of plague (Yersinia pestis) J. Parasitol. 2008;94(3):616–621. doi: 10.1645/GE-1368.1. [DOI] [PubMed] [Google Scholar]
- Slowik T.J., Lane R.S., Davis R.M. Field trial of systemically delivered arthropod development-inhibitor (fluazuron) used to control woodrat fleas (Siphonaptera: ceratophyllidae) and ticks (Acari: ixodidae) J. Med. Entomol. 2001;38(1):75–84. doi: 10.1603/0022-2585-38.1.75. [DOI] [PubMed] [Google Scholar]
- Stapp P., Antolin M.F., Ball M. Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Niño events. Front. Ecol. Environ. 2004;2:235–240. [Google Scholar]
- Tingle C.C., Rother J.A., Dewhurst C.F., Lauer S., King W.J. Reviews of Environmental Contamination and Toxicology. Springer; New York, NY: 2003. Fipronil: environmental fate, ecotoxicology, and human health concerns; pp. 1–66. [DOI] [PubMed] [Google Scholar]
- Tripp D.W., Gage K.L., Montenieri J.A., Antolin M.F. Flea abundance on black-tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics. Vector Borne Zoonotic Dis. 2009;9(3):313–321. doi: 10.1089/vbz.2008.0194. [DOI] [PubMed] [Google Scholar]
- Tripp D.W., Streich S.P., Sack D., Martin D.J., Griffin K.A., Miller M.W. Season of deltamethrin application affects flea and plague control in white-tailed prairie dog (Cynomys leucurus) colonies, Colorado, USA. J. Wildl. Dis. 2016;52(3):553–561. doi: 10.7589/2015-10-290. [DOI] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention . 2020. Plague: History.https://www.cdc.gov/plague/history/index.html Accessed. [Google Scholar]
- United States Centers for Disease Control and Prevention . 2019. Plague: Maps and Statistics.https://www.cdc.gov/plague/maps/index.html Accessed. [Google Scholar]
- United Stated Environmental Protection Agency . 1996. New Pesticide Fact Sheet. Fipronil.https://nepis.epa.gov/Exe/ZyNET.exe/P1001KCY.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C95thru99%5CTxt%5C00000021%5CP1001KCY.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL EPA 737-F-96-005. Accessed. [Google Scholar]
- United States Environmental Protection Agency . 1998. Office of Prevention, Pesticides and Toxic Substances.https://nepis.epa.gov/Exe/ZyNET.exe/P100IJCA.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C95thru99%5CTxt%5C00000034%5CP100IJCA.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL Product Performance Test Guidelines, OPPTS 810.3300: Treatments to Control Pests of Humans and Pets. Accessed. [Google Scholar]
- Wilder A.P., Eisen R.J., Bearden S.W., Montenieri J.A., Gage K.L., Antolin M.F. Oropsylla hirsuta (Siphonaptera: ceratophyllidae) can support plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus) by early-phase transmission of Yersinia pestis. Vector Borne Zoonotic Dis. 2008;8(3):359–368. doi: 10.1089/vbz.2007.0181. [DOI] [PubMed] [Google Scholar]