Abstract
Oxidative stress drives the pathogenesis of atrial fibrillation (AF), the most common arrhythmia. In the cardiovascular system, cystathionine γ-lyase (CSE) serves as the primary enzyme producing hydrogen sulfide (H2S), a mammalian gasotransmitter that reduces oxidative stress. Using a case control study design in patients with and without AF and a mouse model of CSE knockout (CSE-KO), we evaluated the role of H2S in the etiology of AF. Patients with AF (n = 51) had significantly reduced plasma acid labile sulfide levels compared to patients without AF (n = 65). In addition, patients with persistent AF (n = 25) showed lower plasma free sulfide levels compared to patients with paroxysmal AF (n = 26). Consistent with an important role for H2S in AF, CSE-KO mice had decreased atrial sulfide levels, increased atrial superoxide levels, and enhanced propensity for induced persistent AF compared to wild type (WT) mice. Rescuing H2S signaling in CSE-KO mice by Diallyl trisulfide (DATS) supplementation or reconstitution with endothelial cell specific CSE over-expression significantly reduced atrial superoxide, increased sulfide levels, and lowered AF inducibility. Lastly, low H2S levels in CSE KO mice was associated with atrial electrical remodeling including longer effective refractory periods, slower conduction velocity, increased myocyte calcium sparks, and increased myocyte action potential duration that were reversed by DATS supplementation or endothelial CSE overexpression. Our findings demonstrate an important role of CSE and H2S bioavailability in regulating electrical remodeling and susceptibility to AF.
Keywords: Hydrogen sulfide, Oxidative stress, Atrial fibrillation, Electrical remodeling, Endothelial function
Graphical abstract
Central Illustration Figure: 116 patients with (n = 51) and without atrial fibrillation (AF) (n = 65) had plasma sulfide pools measured in the base control human study (left). Patients with AF had significantly reduced acid labile sulfide. Paroxysmal AF patients (n = 26) had significantly lower free sulfide compared to patients with persistent AF (n = 25). Patients who had rhythm conversion had increase in free sulfide but not acid labile sulfide at 30 days follow up. Cystathionine ϒ-lyase knock out mice (CSE-KO) has reduced atrial sulfide levels, increased reactive oxygen species (ROS), longer atrial effective refractory period (AERP), longer action potential duration (APD) of atrial myocytes, slower conduction velocity, increased calcium sparks and increased AF inducibility and duration. Sulfide supplementation by retro-orbital diallyl tri-sulfide (DATS) or by endothelial overexpression of CSE reversed these changes in CSE-KO mice.
Abbreviations:
- ROS
Reactive Oxygen Species
- CSE
Cystathionine γ-lyase
- AF
Atrial Fibrillation
- H2S
Hydrogen Sulfide
- DATS
Diallyl Trisulfide
- AERP
Atrial Effective Refractory Period
- MEA
Mult-electrode Array
- ACEI
Angiotensin Converting Enzyme Inhibitor
- ARB
Angiotensin Receptor Blocker
- BB
Beta Blocker
1. Introduction
Among conditions that affect the heart's natural rhythm, atrial fibrillation (AF) is the most common arrhythmia. By the year 2030, it has been predicted that an estimated 12.1 million subjects in the US will have AF. Approximately 1 in 3 Caucasians and 1 in 5 African Americans will develop AF in their lifetimes. Subjects with AF have a 50% higher risk of mortality than those without the disease. The cost to the United States health care system attributable to AF in 2005 was $26 billion [1]. Current treatment options for AF are limited in their efficacy and/or carry a significant risk of prohibitive side effects. Reactive oxygen species (ROS) are commonly elevated in conditions that lead to AF and are thought to drive multiple aspects of AF pathogenesis, suggesting that preventing ROS may represent a therapeutic target to treat AF [[2], [3], [4]].
The mammalian gasotransmitter hydrogen sulfide (H2S) reduces oxidative stress either by directly scavenging ROS or by amplifying anti-oxidant defense mechanisms [5]. Cystathionine γ-lyase (CSE) and cystathionine beta synthase (CBS) are the two major enzymes involved in the endogenous H2S production in non-neuronal tissue [6]; specifically, CSE is thought to be the primary source of H2S in the cardiovascular system. Patients with heart failure have reduced circulating sulfide levels compared to those of controls [7]. In addition to its anti-oxidant effects, H2S can induce ion channel modifications and anti-apoptotic and anti-inflammatory signaling, all of which are mechanisms implicated in the pathogenesis of AF [8]. However, the link between H2S and AF remains unknown.
Therefore, we investigated whether H2S could be a serum biomarker and a molecular signal connecting risk factors, atrial ROS, and downstream pro-arrhythmic substrates. We used a case control design to evaluate differences in plasma pools of H2S in human patients with AF and a validated CSE knockout (CSE-KO) mouse model to study the effects of low H2S on structural and electrical changes in the atria leading to AF. Finally, we used an endothelial CSE transgenic system in a global CSE-KO mouse model to study the coupling between the vascular endothelium affected by all of the risk factors for AF and atrial remodeling and resultant atrial arrhythmia.
2. Methods
2.1. Experimental animal studies
C57BL/6J mice were purchased from Jackson Labs. The CSE-KO mice in C57BL/6J background bred in house, which have been previously described [9], have a global deletion of CSE. Twenty-week-old CSE-KO and C57BL6 (WT) mice of both sexes were used in this study. Mice were housed at the Louisiana State University Health Sciences Center-Shreveport animal resources facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments using animals were approved by the LSU Institutional Animal Care and Use Committee (LSU IACUC Protocol# P18-016) and were housed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and studies performed according to ARRIVE guidelines.
2.2. ecCSEKO transgenic mice
Global CSE-KO and endothelial-specific CSE over-expressing mice (CSE-KO/ecCSETg), created in the Kevil laboratory by breeding global CSE-KO mice with VE-Cadh-Cre/tTa (ecCSETg) mice, were maintained in-house at our animal facility. The litters obtained from these breeders were genotyped with primers using PCR for CSE-KO and TRE/tTA to confirm the CSE-KO/ecCSETg genotype.
2.3. Diallyl trisulfide
Diallyl trisulfide (DATS) was purchased from Cayman Chemical. DATS (200 μg/kg) was administered via the retro-orbital capillary plexus twice a day for 3 consecutive days prior to the electrophysiology (EP) studies.
2.4. Intracardiac electrophysiology studies (EP studies)
In vivo electrophysiology pacing studies were performed using age- and sex-matched 20-week-old mice of both sexes. Mice were anesthetized with isoflurane (2% induction and 1–1.5% for maintenance of anesthesia; Apollo Tech 3 Vaporizer; NorVap), and placed in a supine position with limbs taped onto surface EKG electrodes using a temperature-controlled surgical platform (Rodent Surgical Monitor, Indus Instruments) that maintained core body temperatures at 37.0 ± 0.5 °C.
Atrial intracardiac electrograms were recorded using a 1.1F octapolar EP catheter (EPR-800; Millar Instruments) inserted via the right internal jugular vein. A 2-lead body surface EKG and up to 4 intracardiac bipolar electrograms (Labchart Pro software, version 8; AD Instruments) were recorded using a computer based acquisition system (Powerlab 16/30; AD Instruments). Surface and intracardiac recordings were taken simultaneously to measure baseline parameters.
Right atrial pacing was performed using 2 ms current pulses delivered by an external stimulator (STG-3008; Multi Channel Systems). The atrioventricular effective refractory period (AVNERP) and atrial effective refractory period (AERP) were determined by applying a series of atrial pacing trains at a fixed cycle length of 100 ms (S1) along with a premature S2 stimulus. The S1–S2 was gradually reduced in each pacing train from 70 ms to 20 ms. The AVNERP is defined as the longest S1–S2 coupling interval at which a premature stimulus delivered to the atrium is not followed by a QRS complex. The AERP is defined as the longest S1–S2 coupling interval in which the atrium failed to produce a propagated beat with S2.
AF was defined as the occurrence of rapid and fragmented atrial electrograms with irregular AV nodal conduction and ventricular rhythm for a least 1 s. Persistent AF was defined as any AF lasting 30 s or more [10]. Inducibility of AF was determined by decremental burst pacing using an automated stimulator. Burst pacing started at a cycle length of 40 ms, decreasing by 2 ms every 2 s to a cycle length of 20 ms in one protocol and 8 ms in the next protocol. AF inducibility was also determined by twenty 20 ms cycle length train episodes. Each AF inducibility protocol was repeated three times 1 min after the previous burst concluded or after AF terminated. The mouse was considered inducible if at least three of the nine protocols produced AF. Positive AF was also recorded if any protocol produced AF that lasted at least 5 min.
After completion of all pacing protocols, 1.5 mg/kg isoproterenol, a β adrenoreceptor agonist, was injected intraperitoneally (i.p.) to attain a 20–30% increase in basal heart rate. Five minutes following isoproterenol injection, atrial burst pacing protocols were repeated to further study the inducibility of AF [11,12].
2.5. Multi-electrode array studies
Measurement of atrial electrophysiology using multi-electrode array was performed as described previously [13]. Mouse hearts were isolated after euthanasia and the left atria were dissected while being perfused with Krebs solution supplemented with 30 mM 2,3-butanedione monoxime. The atrial tissue was then moved to the array perfused with Krebs solution (37 °C; 95% O2, 5% CO2). Electrical stimulation using a multielectrode array system (MEA), which allows noninvasive synchronous multifocal recording of extracellular field potentials [14], was used to assess atrial tissue electrophysiology. The MEA (MED64™; Alpha Med Scientific Inc, Osaka, Japan) consists of 64 microelectrodes arranged in an 8x8 matrix, with a 50-μm electrode diameter and an interelectrode distance of 150 μm. After placing atrial tissue samples in the center of the MEA dish, a holder was used to maintain contact with the electrodes on the array, which was then perfused continuously with oxygenated Krebs solution at 37 °C. The atrial tissue was stimulated using bipolar pulses (2x threshold, 2-ms duration, 4-Hz frequency) through one of the MEA microelectrodes. Field potential waveforms and timing were acquired simultaneously from all 64 microelectrodes. To measure conduction properties, isolated atria were stimulated (4 Hz) from one electrode and field potential recordings were obtained and processed using MED64-Möbius™ software to delineate local activation times using the minimum derivative of the field potential. To measure conduction velocity, the distance between the sites was divided by the local activation time at the destination electrode as described previously [15].
2.6. Measurement of biological pools of H2S
Plasma and heart atrial samples were analyzed for free sulfide, acid labile sulfide (ALS), and total sulfide levels as reported previously [16]. Blood samples were collected from the mice via retro-orbital bleed into lithium heparin tubes and centrifuged at 1500×g for 4 min at 4 °C. The plasma were collected in vials containing 100 mM Tris-HCl buffer (pH 9.5, 0.1 mM DTPA) in a ratio of 1:5 (v/v) and snap-frozen until further analysis. Hearts were harvested from WT, CSE-KO, and CSE-KO/ecCSETg mice. The atria were separated and homogenized in 100 mM Tris-HCl buffer (pH 9.5, 0.1 mM DTPA) in a ratio of 1:10 (w/v). Plasma and atria were derivatized with monobromobimane (MBB) and analyzed using a reversed-phase high-performance liquid chromatography (RP-HPLC) system with an Agilent Eclipse XDB-C18 column (5 μm, 80 Å, 4.6 mmx250 mm) for sulfide-dibimane fluorescence detection (excitation: 390 nm; emission: 475 nm) as reported previously [16].
2.7. Superoxide measurements
Mouse heart superoxide production was measured using the hydroethidine HPLC method as previously reported [17,18]. Briefly, all mice were injected intraperitoneally with 0.3 mg of HE/30 mg mouse. After 1 h the animals were anesthetized with 1% isoflurane, blood was collected via retro-orbital bleed into lithium heparin tubes, centrifuged at 1500×g for 4 min at 4 °C, and the plasma snap-frozen for superoxide measurements. Extra blood was removed via volume exchange with 10 mL 1xPBS via the heart and excision of the portal vein. Hearts were collected in cold 50 mM phosphate buffer (pH 7.4), homogenized, centrifuged for 5 min 10,000×g at 4 °C, and analyzed on an Shimadzu HPLC system (Shimadzu Corporation) equipped with fluorescence detector (excitation 490 nm; emission 567 nm). 2-OH-ethidium (2-OH-E+) concentration was normalized to total protein and reported as picomoles per milligram of protein for tissue and nanomole concentrations for plasma. 2-hydroxyethidium(2-OH-E+) is a specific product of the reaction between superoxide and hydroethidine (HE), and the theoretical molar stoichiometric ratio of superoxide over 2-OH-E+ is 2:1. In oxidizing environments, HE-derived radical reactions may also produce other products, such as ethidium (E+), or dimerization products (HE-HE, E-E, E-HE etc). Among them, only 2-OH-E+ and E+ contribute significantly to the fluorescence intensity. By using HPLC with fluorescent detection, the peak area of the 2-OH-E+ can be specifically measured allowing for accurate determination of concentration. In this manner, quantification of 2-OH-E+ provides an accurate assessment of superoxide levels in a biological sample.
2.9. Patch clamp electrophysiology
Atrial myocytes were enzymatically isolated from hearts of male and female mice (20 weeks of age). Briefly, mice were intraperitoneally injected with 5000 U/kg heparin (Sigma-Aldrich, St. Louis, MO) and euthanized by cervical dislocation. The heart was quickly removed and mounted on a Langendorff apparatus followed by a 3-min retrograde perfusion with oxygenated (100% O2) Ca2-free Tyrode's solution containing (in mmol/l): 140 NaCl, 5.4 KCl, 0.5 MgCl2, 10 glucose, and 10 HEPES (pH 7.4; 37 °C). Hearts were then perfused with the same Tyrode's solution but containing Liberase TH enzymes (0.025 mg/mL; Sigma-Aldrich) and bovine serum albumin (BSA; 1 mg/mL; Sigma-Aldrich). Left and right atrial tissue was then removed and minced, and atrial myocytes were dispersed in KB solution containing (in mmol/l): 80 KOH, 40 KCl, 25 KH2PO4, 3 MgSO4, 50 glutamic acid, 20 taurine, 1 EGTA, 10 glucose, and 10 HEPES (pH 7.2 with KOH; 20–22 °C). Cells were stored at room temperature (20–22 °C) for at least 1 h before use. All chemicals used to make the solutions for cell isolations were obtained from Sigma-Aldrich.
Whole cell patch-clamp recordings were performed at 37 °C. Borosilicate glass pipette microelectrodes (Warner Instruments, Hamden, CT) with tip resistances of 2–3 MΩ when filled with pipette solution were used. Electrodes were connected to a MultiClamp 700B microelectrode amplifier equipped with a CV-7B head stage (Axon Instruments, Molecular Devices, San Jose, CA). Electrical signals were sampled at 4 kHz and digitized with an Axon analog/digital converter (Digidata 1440A). Data acquisition and analysis were performed using Clampfit software (version 10.3, Axon Instruments, Molecular Devices). For current-clamp recordings, action potentials were evoked by electrical stimulation with 1-ms, 2-nA current pulses at a frequency of 1 Hz. The bath solution contained (in mmol/L): 126 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, 20 HEPES, and 11 glucose (pH = 7.4 with NaOH). The pipette solution contained (in mmol/L): 90 K-aspartate, 30 KCl, 10 NaCl, 5.5. glucose, 1.0 MgCl2, 10 EGTA, 4.0 Na-GTP, and 10 HEPES (pH = 7.2 with KOH). All chemicals used to make the bath and pipette solutions for recordings were obtained from Sigma-Aldrich.
2.8. Trichrome staining
Hearts from control and CSE-KO mice were excised and fixed in 10% formalin. Samples were processed, embedded in paraffin, and cut into 10 μm sections. Slides were de-paraffinized with xylene and rehydrated in gradations of ethanol through distilled water. Masson's Trichrome staining was performed using established protocols.
2.10. Ca2+ sparks imaging
Atrial myocytes were isolated as described previously [19]. In brief, mice were euthanized using isoflurane followed by cervical dislocation after anesthesia was confirmed. The hearts were quickly removed and placed in Complete Tyrode's solution containing 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES-NaOH, and 5.5 mM d-glucose, pH 7.4. The left and right atrial tissues were dissected away and placed in 2.5 mL 35 ± 1 °C Low Ca2+/Mg2+ Tyrode's solution. The atrial tissue was washed gently three times before being digested in Low Tyrode's solution with elastase and a collagenase/protease blend (containing 140 mM NaCl, 5.4 mM KCl, 0.5 mM MgCl2, 0.2 mM CaCl2, 1.2 mM KH2PO4, 50 mM taurine, 5.5 mM d-glucose, 5 mM HEPES-NaOH, pH 6.9 and ollagenase type II, elastase, protease, and BSA). Following enzyme digestion of the tissue strips for 20 min at 37 °C using gentle mechanical agitation, the tissue was gently washed three times in 2.5 mL Modified Kraft-Brühe (KB) solution containing 70 mM l-glutamic acid, 20 mM KCl, 80 mM KOH, 10 mM d-β–OH–butyric acid, 10 mM KH2PO4, 10 mM taurine, 1 mg/mL BSA, and 10 mM HEPES-KOH, pH 7.4. Single cells were then dissociated using constant trituration at approximately 0.5–1 Hz for 10–15 min. After dissociation, calcium (up to 1.3 mM) was reintroduced using multiple concentrations of a NaCl/CaCl2 adaptation solution. Following calcium re-adaptation, the cardiomyocytes were collected by centrifugation at a rate of 2000×g for 3 min. The cardiomyocytes were then plated onto a laminin-coated glass-bottom 4-well slide. Cells were allowed to adhere for 1–2 h. Fluo-4 AM was loaded to the cardiomyocytes and allowed to incubate for 30 min. After de-esterifcation, the cells were perfused with Complete Tyrode's solution. Confocal imaging was performed using a Nikon Eclipse Ti-E inverted confocal microscope equipped with a 40×0.75 NA objective. The cells were excited using a 488-nm laser line. Calcium sparks were measured during resting conditions by performing line scan recording at a sampling rate of 2 FPS along the longitudinal axis of the cardiomyocyte. Calcium sparks were quantified in the following areas: amplitude (F/F0), full-width at half-maximum amplitude (μm), duration at half-maximum amplitude (ms), time to peak (ms), Tau (ms), and frequency of sparks.
2.11. Human subjects
Human subjects for this study were recruited from the Cardiology and Cardiac Electrophysiology clinics and in-patient medicine wards at Ochsner-LSU Hospital in Shreveport between July 2018 and June 2019. Healthy controls were recruited by institution-wide study advertisements. Patients between 18 and 89 years of age who were able to provide informed consent were recruited at initial or follow up visits if they had AF or other atrial arrhythmias, or other cardiovascular disease. Patients with congenital heart disease, end-stage renal disease on hemodialysis, or self-reported pregnancy were excluded. Institutional review board approval was obtained from the Louisiana State University Health Sciences Center-Shreveport IRB for this prospective study (STUDY00000962). After qualification, informed consent, and enrollment, blood was drawn by venipuncture, collected in EDTA, heparin, and serum vacutainer tubes and immediately taken to the laboratory on ice for further processing and analysis. Patients who underwent an ablation or direct current cardioversion procedure for AF had a baseline blood draw and then a subsequent blood draw immediately post procedure, within 24 h, and then at their 3-month follow up visit. All data regarding patient demographics, co-morbidities, and labs were collected and maintained in accordance with HIPAA guidelines.
2.12. Statistical analysis
The means and standard deviations of the different pools of plasma free H2S levels were calculated for the groups and compared using a two-sample Student's t-test. The baseline characteristics of the human AF patients and controls were compared using Student's t-test for continuous variables and Fisher's exact test for categorical variables. All animal studies were analyzed using Fisher's exact test if they involved categorical variables and Student's t-test if they involved continuous variables. When more than two groups were involved, a one-way ANOVA was performed with a post-hoc Tukey analysis.
3. Results
3.1. Acid labile sulfide levels are decreased in patients with AF
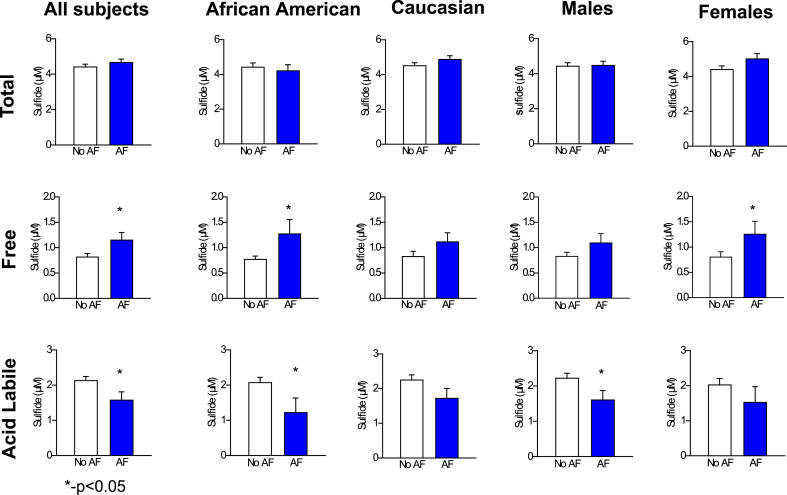
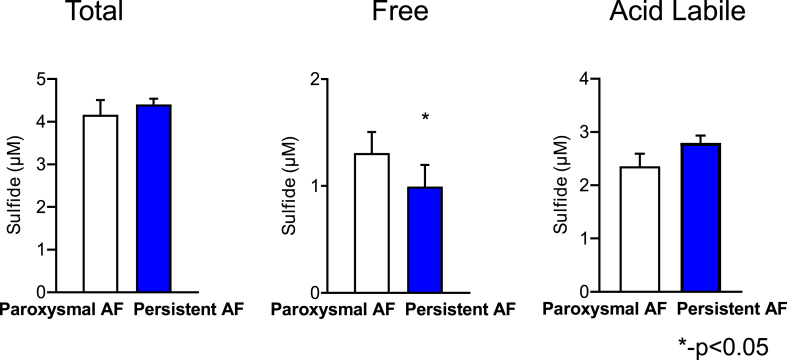
One hundred and sixteen patients with and without AF were enrolled in the study. Among these, 26 had paroxysmal AF, 25 had persistent AF, and 65 patients had no evidence or history of AF (control group). The baseline characteristics of patients with and without AF are compared in Table 1. The patients without AF were younger but comparable to the patients with AF with regard to traditional risk factors including race, sex, diabetes, sleep apnea, and smoking history. Patients with AF were more likely to have hypertension (82% versus 54% in patients without AF, p = 0.002) and heart failure (47% versus 25% in patients without AF, p = 0.02) and therefore were more likely to take a beta blocker (BB) (84% versus 55% in patients without AF, p = 0.001) and angiotensin converting enzyme inhibitor (ACEI) and/or an angiotensin receptor blocker (ARB) (73% versus 46% in patients without AF, p = 0.005). On the other hand, patients without AF in this study were more likely to have coronary artery disease and/or peripheral arterial disease (43% versus 17% in patients with AF, p = 0.005), factors that are also considered to increase the risk of AF. While the total sulfide pool (p = 0.25, Fig. 1) was not significantly different in patients with and without AF, patients with AF had significantly lower acid labile sulfide levels compared to patients without AF (1.59 μM in AF patients versus 2.14 μM in non-AF patients, p = 0.02, Fig. 1). As shown in Fig. 1, this trend was true in African Americans (AF = 1.24 μM, no AF = 2.09 μM, p = 0.02), Caucasians (AF = 1.73 μM, no AF = 2.26 μM, p = 0.08), males (AF = 1.62 μM, no AF = 2.34 μM, p = 0.03), and females (AF = 1.54 μM, no AF = 2.04 μM, p = 0.21). Previous work from our lab has shown that despite reduction in acid labile sulfide in patients with cardiovascular disease [16], plasma free H2S levels were elevated in patients with cardiovascular disease [20]. In agreement with those findings, free sulfide levels (AF = 1.16 μM, no AF = 0.82 μM, p = 0.02, Fig. 1) were increased in patients with AF compared to those without AF. Interestingly, while there was no difference in acid labile sulfide pool levels in patients with persistent AF compared to those with paroxysmal AF (p = 0.23, Fig. 2), the free sulfide levels in patients with persistent AF were significantly lower compared to those with paroxysmal AF (0.99 μM vs 1.31 μM, p = 0.02, Fig. 2). There were no significant differences in total sulfides between paroxysmal and persistent AF patients. Fifteen patients in the study underwent DC cardioversion or AF ablation, of which 11 patients had post rhythm conversion H2S levels available. While levels of free (pre = 1.8 μM sulfide, immediately post conversion = 1.47 μM sulfide and 30 days post conversion = 3.1 μM sulfide, p = 0.01) and total sulfide (pre = 5.05 μM sulfide, immediately post conversion = 4.35 μM sulfide and 30 days post conversion = 6.96 μM sulfide, p = 0.005, Supplementary Fig. 1) increased post rhythm conversion in these patients, those of acid labile sulfide remained unchanged (pre = 0.78 μM sulfide, post = 0.93 μM sulfide, p = 0.85), suggesting that the lower acid labile sulfide levels in patients with AF is not just a result of AF, as might be the case with free sulfide levels (see Table 2).
Table 1.
Demographics and co-morbidities in subjects with and without atrial fibrillation (AF). .
| No AF (n = 65) | AF (n = 51) | p-value | |
|---|---|---|---|
| Age | 52.94 ± 15.62 | 65.2 ± 12.21 | <0.0001 |
| Caucasian | 39 (60%) | 34 (67%) | 0.5619 |
| African American | 23 (36%) | 14 (27%) | 0.4247 |
| Hispanic | 1 (2%) | 0 (0%) | >0.9999 |
| Other | 3 (2%) | 3 (6%) | >0.9999 |
| Male | 35 (54%) | 34 (67%) | 0.1857 |
| Female | 30 (46%) | 17 (33%) | 0.1857 |
| CAD/PAD | 28 (43%) | 9 (17%) | 0.0047 |
| HTN | 35 (54%) | 42 (82%) | 0.0015 |
| DM | 12 (18%) | 9 (18%) | >0.9999 |
| HLD | 20 (31%) | 16 (31%) | >0.9999 |
| OSA | 6 (9%) | 11 (22%) | 0.0704 |
| CHF | 16 (25%) | 24 (47%) | 0.0177 |
| Smoking | 17 (26%) | 16 (31%) | 0.5425 |
| Alcohol Use | 8 (12%) | 5 (10%) | 0.7721 |
| CAD- coronary artery disease, PAD- peripheral arterial disease, HTN- Hypertension, DM- diabetes mellitus, HLD- hyperlipidemia, OSA- obstructive sleep apnea, CHF- congestive heart failure | |||
Fig. 1.
Total, free, and acid labile sulfide pools in All, African American, Caucasian, Male, and Female subjects based on the presence or absence of AF. Patients with AF (n = 51) had increased free sulfide, decreased acid labile sulfide, and comparable total sulfide levels when compared to patients without AF (n = 65). These findings are similar in each of the subgroups shown, with either a trend towards or a significant increase in free sulfide or reduction in acid labile sulfide levels. A Student's t-test was used for each of these comparisons.
Fig. 2.
Total, free, and acid labile sulfide pools in patients with paroxysmal (n = 26) and persistent AF (n = 25). Patients with persistent AF have lower free sulfide levels but comparable acid labile and total sulfide levels. A Student's t-test was used for each of these comparisons.
Table 2.
Comparison of sulfide pools in all patients (top half) and patients with AF (bottom half) who were taking and not taking common cardiac medications. ACEI- Angiotensin converting enzyme inhibitor, ARB- Angiotensin receptor blockers.
| Medication | Free Sulfide- All Patients |
Acid Labile Sulfide- All Patients |
Total Sulfide- All Patients |
||||||
|---|---|---|---|---|---|---|---|---|---|
| On Med | Not on Med | p-value | On Med | Not on Med | P-Value | On Med | Not on Med | P-value | |
| Beta Blocker | n = 79 | n = 37 | n = 79 | n = 37 | n = 79 | n = 37 | |||
| 0.98 ± 0.78 | 0.94 ± 0.73 | 0.8 | 1.86 ± 1.26 | 1.99 ± 1.17 | 0.61 | 4.57 ± 1.09 | 4.48 ± 1.06 | 0.7 | |
| ACEI/ARB | n = 67 | n = 49 | n = 67 | n = 49 | n = 67 | n = 49 | |||
| 1.00 ± 0.82 | 0.92 ± 0.67 | 1.87 ± 1.24 | 1.96 ± 1.23 | 0.7 | 4.63 ± 1.02 | 4.43 ± 1.05 | 0.32 | ||
| Aldosterone Inhibitors | n = 18 | n = 98 | n = 18 | n = 98 | n = 18 | n = 98 | |||
| 0.83 ± 0.62 | 1.0 ± 0.79 | 0.39 | 1.99 ± 1.23 | 1.89 ± 1.23 | 0.74 | 4.47 ± 1.04 | 4.56 ± 1.09 | 0.73 | |
| Statin | n = 63 | n = 53 | n = 63 | n = 53 | n = 63 | n = 53 | |||
| 0.97 ± 0.73 | 0.97 ± 0.82 | 0.99 | 1.87 ± 1.25 | 1.95 ± 1.21 | 0.73 | 4.68 ± 1.07 | 4.38 ± 1.09 | 0.14 | |
| Medication | Free Sulfide- AF Patients | Acid Labile Sulfide- AF Patients | Total Sulfide- AF Patients | ||||||
| On Med | Not on Med | p-value | On Med | Not on Med | P-Value | On Med | Not on Med | P-value | |
| Beta Blocker | n = 43 | n = 8 | n = 43 | n = 8 | n = 43 | n = 8 | |||
| 1.05 ± 0.93 | 1.84 ± 1.21 | 0.049 | 1.73 ± 1.46 | 0.73 ± 1.92 | 0.11 | 4.8 ± 1.08 | 3.99 ± 1.52 | 0.09 | |
| ACEI/ARB | n = 37 | n = 14 | n = 37 | n = 14 | n = 37 | n = 14 | |||
| 1.13 ± 1.02 | 1.25 ± 0.99 | 0.7 | 1.64 ± 1.5 | 1.44 ± 1.86 | 0.7 | 4.92 ± 1.01 | 4.02 ± 1.4 | 0.02 | |
| Aldosterone Inhibitors | n = 11 | n = 40 | n = 11 | n = 40 | n = 11 | n = 40 | |||
| 0.93 ± 0.78 | 1.22 ± 1.06 | 0.4 | 1.97 ± 1.52 | 1.48 ± 1.57 | 0.36 | 4.94 ± 0.83 | 4.62 ± 1.24 | 0.43 | |
| Statin | n = 31 | n = 20 | n = 31 | n = 20 | n = 31 | n = 20 | |||
| 1.13 ± 0.97 | 1.2 ± 1.08 | 0.83 | 1.64 ± 1.56 | 1.51 ± 1.58 | 0.76 | 4.99 ± 0.98 | 4.19 ± 1.29 | 0.02 | |
In addition, to evaluate the effect of common cardiac medications on sulfide levels in patients with AF, we analyzed the sulfide pools based on whether patients were on common cardiac medications or not. When all patients were considered, there were no significant differences in free, acid labile or total sulfide levels in patients on BB, ACEI and/or ARB, aldosterone inhibitors and statins compared to patients who were not on these medications. When considering patients with AF alone, those not on a BB (n = 8) had a significantly higher free sulfide level compared to those who were on them (n = 43) (1.84 μM vs 1.05, p = 0.049). AF patients not on an ACEI or ARB (n = 14) had significantly lower total sulfide level compared to patients on the medication (n = 37) (4.02 μM vs 4.92 μM, p = 0.02). Similarly, AF patients not on a statin drug (n = 20) had significantly reduced total sulfide level compared to AF patients on statin (n = 31) (4.19 μM vs 4.99 μM, p = 0.02). Interestingly, none of the cardiac medications were associated with any differences in acid labile sulfide levels in AF patients.
Taken together, these findings showed a positive association between AF and decreased plasma acid labile sulfide levels. H2S levels also correlated with the severity of AF and there was a rebound in total and free sulfide pools, but not the acid labile sulfide pool, when normal sinus rhythm was achieved by DC cardioversion or AF ablation, suggesting that low acid labile sulfide levels in these patients may not be an effect of AF.
CSE-KO mice have decreased H2S levels, increased oxidative stress and enhanced AF susceptibility.
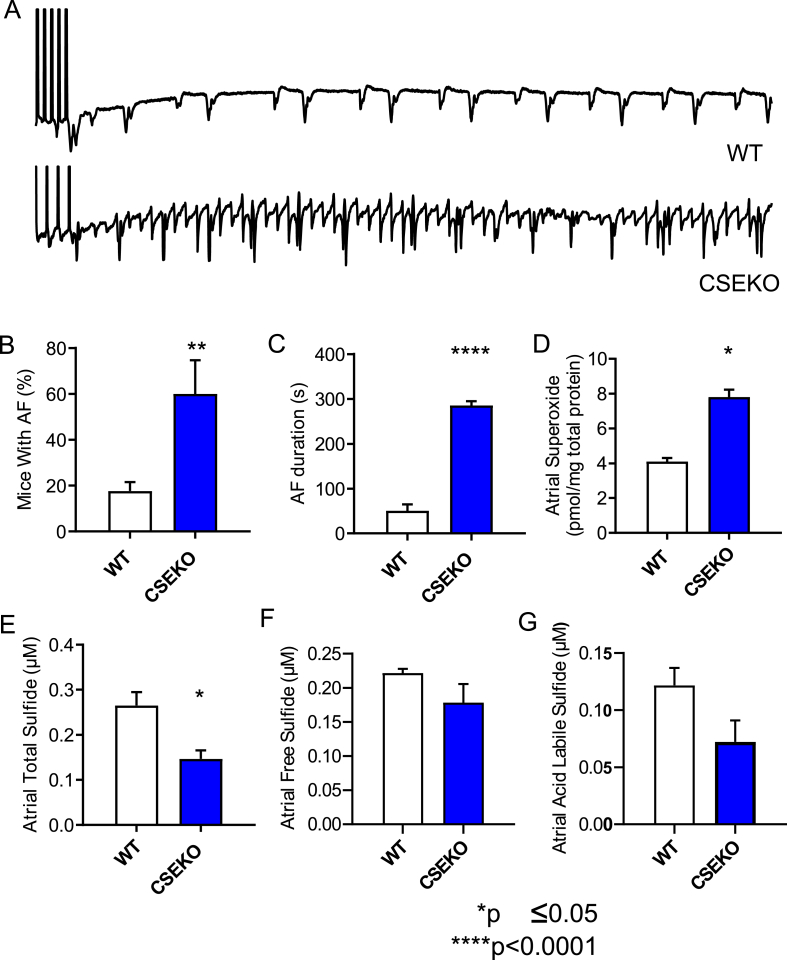
Based on our findings in human patients, we hypothesized that low H2S contributes to AF. To test the hypothesis, we evaluated the inducibility of AF in a mouse model with low levels of H2S caused by deficiency of the enzyme CSE, which biosynthesizes endogenous H2S using l-cysteine as the substrate. We performed burst pacing in the right atrium of anesthetized WT and CSE-KO mice, with or without isoproterenol, using previously published pacing protocols to test AF inducibility. CSE-KO mice had significantly higher inducibility of persistent AF, which was described as AF lasting for 30 s or more compared to WT mice [60% in CSE-KO mice (n = 15) versus 17.65% in WT mice (n = 18); p = 0.0052, Fig. 3A and B]. When all inducible AF was considered, CSE-KO mice had longer duration of AF compared to WT mice (285.5 s in CSE-KO mice versus 50.71 s in WT mice; p=<0.0001, Fig. 3C).
Fig. 3.
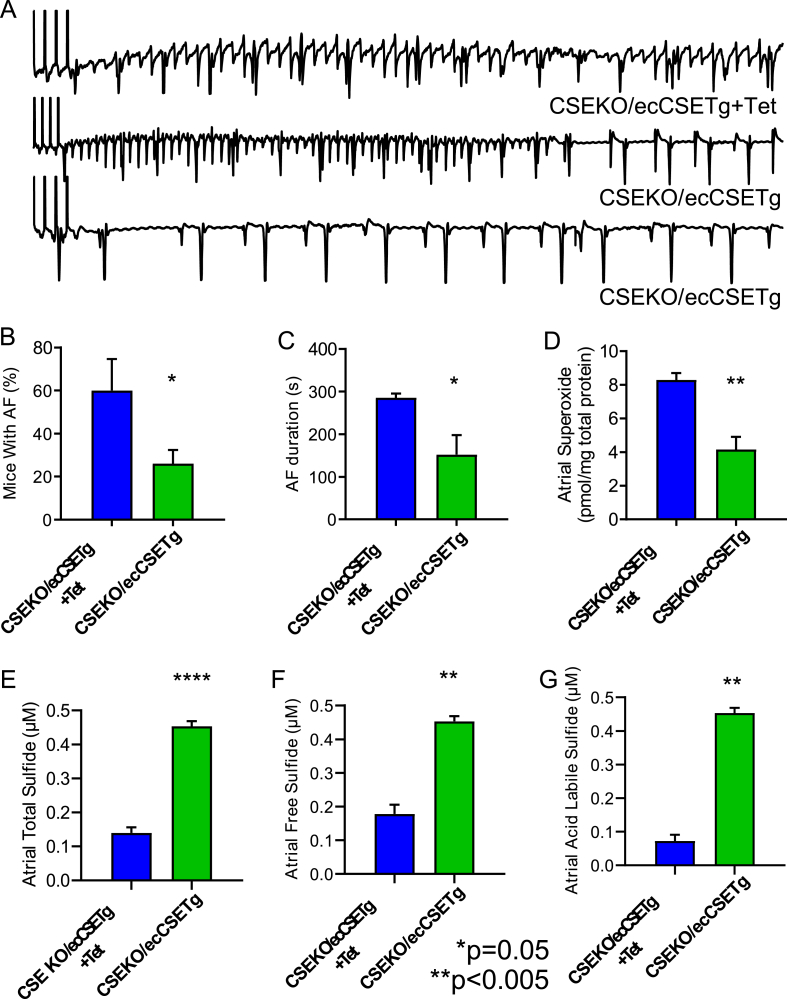
Decreased H2S bioavailability leads to oxidative stress and AF. Representative intracardiac electrograms from WT and CSE-KO mice are shown in A. Following the end of the pacing protocol depicted by vertical lines at the beginning of the EGM, the WT mouse resumed normal rhythm while the CSE-KO mouse had induced AF. CSE-KO mice (n = 15) had a higher incidence of inducible persistent AF (defined as 30 s or more) than WT mice (n = 18) (B) and had a longer duration of any inducible AF than WT mice (C). Atrial superoxide levels as measured by HPLC were higher in CSE-KO mice (n = 4) compared to WT controls (n = 3) (D). CSE-KO mice (n = 4) had significantly lower total sulfide levels and a trend (p = 0.07) towards lower levels of acid labile sulfide than WT mice (n = 4) (E–G). Fisher's exact test was used to compare groups in B, and Student's t-test was used to compare groups in C-G.
Previously published data from our group have provided evidence of lower plasma free H2S levels in CSE-KO compared to WT mice [21]. To assess whether this decrease in plasma free H2S levels translated to lower H2S levels in the atria, we measured atrial H2S levels in the CSE-KO mice and compared it to those in WT mice. As expected, we found that CSE-KO mice atria (n = 4) had significantly lower total H2S levels compared to atria of WT mice (n = 4) (0.14 μM sulfide versus 0.28 μM sulfide; p = 0.0062, Fig. 3E). CSE-KO mice tended to have lower acid labile sulfide levels than WT mice [CSE-KO, 0.72 μM sulfide (n = 4); WT, 0.12 μM sulfide (n = 4); p = 0.09, Fig. 3G], but comparable free sulfide levels, similar to observations in human subjects. In addition, we compared atrial oxidative stress, a known biological determinant of AF, between CSE-KO and WT mice. We found that oxidative stress as measured by atrial superoxide levels was significantly elevated in CSE-KO mice (n = 4) compared to WT mice (n = 3) (4.10 pmol/mg total protein versus 8.29 pmol/mg total protein; p = 0.0003, Fig. 3D). Together, these findings are consistent with the clinical data, showing that low bioavailability of H2S in CSE-KO mice leads to higher oxidative stress and enhanced AF susceptibility.
H2S supplementation reduces oxidative stress and decreases AF susceptibility in CSE-KO mice.
Given the low H2S levels in human patients with AF, and the high susceptibility of CSE-KO mice with low H2S and high superoxide levels to AF, we then tested whether administration of sulfide donors can reverse this susceptibility in CSE-KO mice. To test this hypothesis, mice were injected retro-orbitally with diallyl trisulfide (DATS) (200 μg/kg body weight) twice daily for 3 days prior to the electrophysiology studies to induce AF. Di-ally trisulfide (DATS) is a polysulfide that reacts with biological thiols including cysteine and glutathione resulting in the formation of allyl perthiol anion, which in the presence of these thiols is released as H2S. Diallyl trisulfide (DATS) is a long-lasting and slow-releasing sulfide and polysulfide donor compared to Sodium sulfide (Na2S), sodium hydrosulfide (NaHS), and other allyl sulfide derivatives such as diallyl disulfide. It's structure, chemistry and release rates have been well discussed and reviewed in the literature [22,23]. Previous work from our lab and others have demonstrated the biological functions and vasoprotective effects of DATS in comparison to the other sulfide donor Na2S [24]. [21,25].
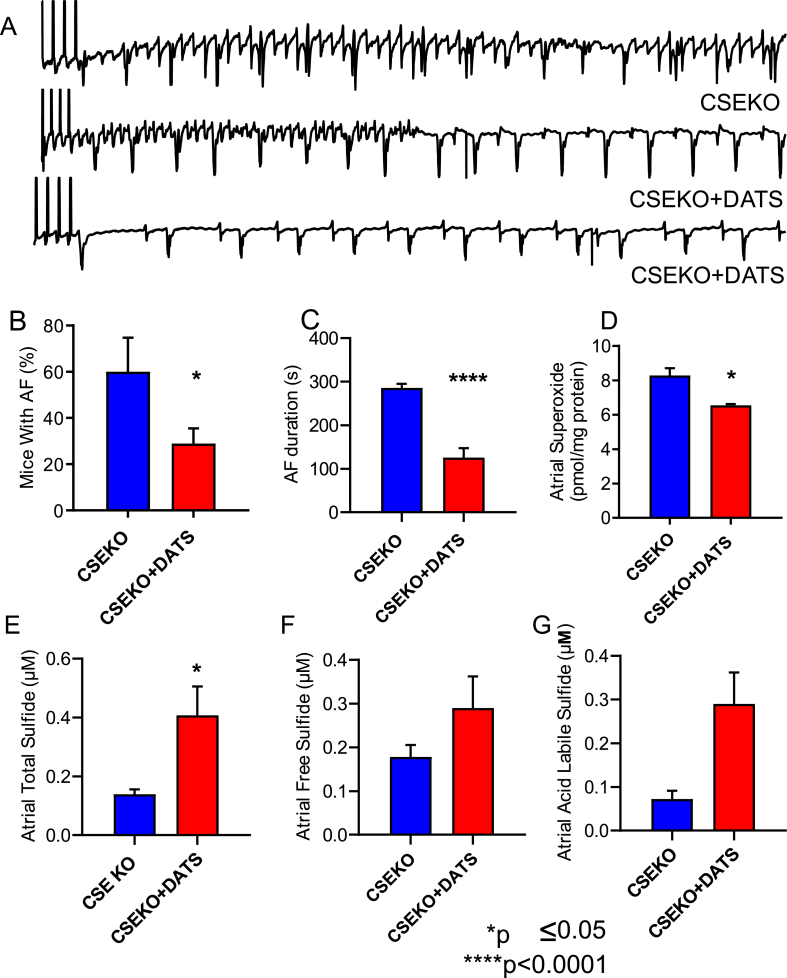
DATS-treated CSE-KO mice had a statistically significant reduction in the incidence of inducible AF [29% of DATS-treated CSE-KO mice (n = 18) versus 60% CSE-KO mice without any treatment (n = 15); p = 0.05, Fig. 4A and B]. The duration of AF induced in DATS-treated CSE-KO mice was double that of the WT mice (125.6 s versus 50.71 s) but was halved compared to untreated CSE-KO mice (285.5 s versus 125.6 s; p=<0.0001, Fig. 4C).
Fig. 4.
Supplementation of sulfide via retro-orbital injection of diallyl trisulfide (DATS) increases sulfide bioavailability, decreases oxidative stress, and decreases AF in CSE-KO mice. Representative intracardiac electrograms from CSE-KO mice with or without DATS supplementation are shown in A. Following the end of the pacing protocol depicted by vertical lines at the beginning of the EGM, the CSE-KO mouse without DATS supplementation had induced AF, while the CSE-KO mice that received DATS supplementation resumed normal rhythm. CSE-KO mice that received DATS supplementation (n = 18) had a lower incidence of inducible persistent AF (defined as 30 s or more) than CSE-KO mice that did not receive supplementation (B) and had a longer duration of any inducible AF (C). Atrial superoxide levels as measured using HPLC were lower in CSE-KO mice that received DATS supplementation (n = 3) compared to CSE-KO mice that did not (n = 4) (D). CSE-KO mice that received DATS supplementation (n = 3) had significantly higher total sulfide levels and a trend towards higher acid labile sulfide levels than CSE-KO mice that did not receive supplementation (n = 4) (E–G). Fisher's exact test was used to compare groups in B, and Student's t-test was used to compare groups in C-G.
We tested to see whether increased H2S, decreased superoxide, or both mediated this reduction in the incidence of persistent AF and the duration of AF in the CSE-KO mice treated with DATS. We found that the atrial superoxide levels were significantly reduced in the CSE-KO mice treated with DATS (n = 3) compared to the CSE-KO mice without DATS treatment (n = 4) (8.29 pmol/mg total protein versus 6.55 pmol/mg total protein; p = 0.02, Fig. 4D). Total sulfide levels in the atria of CSE-KO mice treated with DATS were significantly increased compared to those in CSE-KO mice without DATS treatment [CSE-KO + DATS (n = 3), 0.41 μM sulfide; CSE-KO (n = 4), 0.14; p = 0.01, Fig. 4E]. In addition, there was a trend towards higher acid labile sulfide levels in CSE-KO mice that received DATS treatment compared to those that did not (CSE-KO + DATS, 0.18 μM sulfide; CSE-KO, 0.07 μM sulfide; p = 0.051, Fig. 4G). These results indicate that treatment with a sulfide donor can reduce oxidative stress in the atria of mice deficient in H2S by increasing H2S levels, thereby decreasing their susceptibility to AF and subsequent complications.
Transgenic overexpression of CSE in the endothelium is sufficient to rescue global CSE-KO mice from oxidative stress and AF susceptibility.
Many of the well known risk factors for AF, including hypertension, diabetes, and obesity, can alter the substrate of the atria and make them pro-arrhythmic even before causing obvious structural changes [26]. While mechano-electrical feedback may be the foundation of AF in a large number of patients, it does not explain the pathogenesis of AF in all AF patients, especially those with lone AF [27]. Co-morbidities that increase the risk of AF have also been implicated in altering endothelial function. While endothelial dysfunction is associated with AF [28], it remains unclear whether it is a cause or an effect. Endothelial dysfunction causes oxidative stress and is pro-inflammatory [29], thus setting up the atrial tissue for electrical remodeling. Endothelial dysfunction is associated with low bioavailability of H2S [30], thus coupling vascular endothelium with atrial remodeling and atrial arrhythmias. We therefore asked whether overexpressing CSE and increasing the H2S production in the endothelial cells of global CSE-KO mice is sufficient to reduce the inducibility of AF. To determine this, we performed electrophysiology and inducibility studies in CSE-KO mice with an endothelial cell CSE transgene turned off by tetracycline. CSE-KO mice with the endothelial CSE transgene turned on demonstrated a significant reduction in AF inducibility [26% of CSE-KO mice with endothelial cell CSE transgene (n = 15) versus 60% CSE-KO mice with eCSETg turned off; p = 0.04, Fig. 5A and B] and duration (152.2 s versus 285.5 s; p = 0.02, Fig. 5C) compared to the CSE-KO mice with the endothelial cell transgene for CSE turned off with tetracycline (n = 15). This reduction in the AF inducibility and AF duration in the CSE-KO mice with the endothelial cell transgenic overexpression of CSE (n = 3) was accompanied by reduction in the atrial superoxide level (8.29 pmol/mg total protein versus 4.15 pmol/mg total protein; p = 0.003, Fig. 5D) compared to the CSE-KO mice where the endothelial transgene was turned off by administering tetracycline (n = 4). Total atrial sulfide levels [CSE-KO/ecCSETg (n = 3), 0.52 μM sulfide; CSE-KO (n = 4), 0.14 μM sulfide; p=<0.0001, Fig. 5E], free sulfide levels [CSE-KO/ecCSETg (n = 3), 0.45 μM sulfide; CSE-KO (n = 4), 0.18 μM sulfide; p = 0.003, Fig. 5F], and acid labile sulfide levels [CSE-KO/ecCSETg (n = 3), 0.21 μM sulfide; CSE-KO (n = 4), 0.07 μM sulfide; p = 0.01, Fig. 5G] in the CSE-KO mice with endothelial transgenic overexpression of CSE were significantly elevated compared to those of global CSE-KO mice. Results from these experiments show that increased endothelial H2S production is sufficient to rescue the oxidative stress and AF phenotype created by global H2S deficiency in the CSE-KO mice, suggesting that H2S may mediate the coupling between endothelial dysfunction and atrial remodeling and AF.
Fig. 5.
CSE transgenic overexpression in the endothelial cells (ecCSETg) increases sulfide bioavailability, decreases oxidative stress, and decreases AF in global CSE-KO mice. Representative intracardiac electrograms from CSE-KO mice with or without endothelial CSE transgenic overexpression are shown in A. Following the end of the pacing protocol depicted by vertical lines at the beginning of the EGM, the CSE-KO mouse with endothelial transgenic overexpression of CSE turned off by administering tetracycline (CSE-KO/ecCSETg + Tet) had induced AF while the CSE-KO mice with endothelial CSE transgenic overexpression (CSE-KO/ecCSETg) resumed normal rhythm. CSE-KO mice with endothelial CSE transgenic overexpression (n = 15) had a lower incidence of inducible persistent AF (defined as 30 s or more) than global CSE-KO mice without endothelial CSE transgenic overexpression (n = 15) (B) and had a longer duration of any inducible AF (C). Atrial superoxide levels as measured by HPLC were lower in CSE-KO mice with endothelial CSE transgenic overexpression (n = 3) compared to CSE-KO mice without endothelial CSE transgenic overexpression (n = 4) (D). CSE-KO mice with endothelial CSE transgenic overexpression (n = 3) had significantly higher total, free, and acid labile sulfide levels than CSE-KO mice without endothelial CSE transgenic overexpression (n = 4) (E–G). Fisher's exact test was used to compare groups in B, and Student's t-test was used to compare groups in C-G.
Low H2S level causes electrical remodeling of the atrial myocardium.
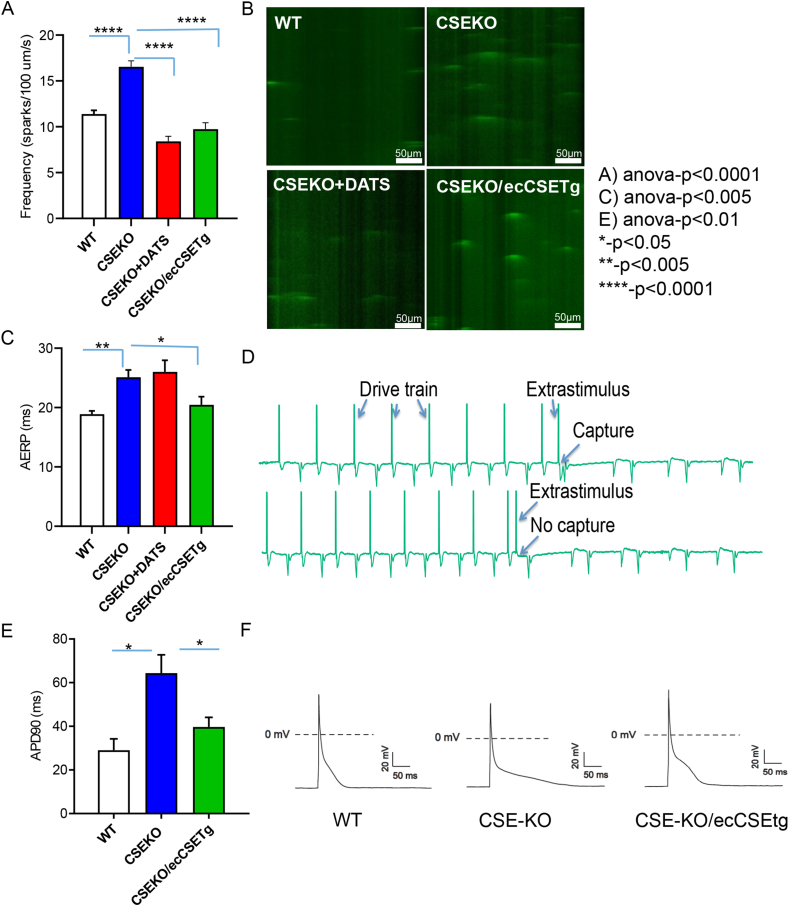
Oxidative stress leads to pro-arrhythmic electrical remodeling by direct oxidative modification of ion channels and ion channel handling proteins in addition to affecting intercellular electrical coupling [31]. In addition, oxidative stress causes a pro-inflammatory, pro-apoptotic, and pro-fibrotic milieu that promotes structural remodeling leading to rhythm disorders. We therefore examined whether the pro-arrhythmic state induced by low H2S in CSE-KO mice and the rescue of the AF phenotype by intrinsic or extrinsic H2S was related to electrical or structural remodeling or both. We first measured the atrial effective refractory period during EP studies. The effective refractory period is the longest time period following an initial stimulation during which an additional electrical stimulus will fail to excite the myocardium. A prolonged effective refractory period can result from electrical remodeling or structural remodeling of the heart. CSE-KO mice (n = 15) had a significantly prolonged AERP compared to WT mice (n = 15) (25.11 ms vs 18.9 ms; p = 0.001, Fig. 6C and D). While sulfide supplementation with DATS (n = 18) did not change AERP levels in CSE-KO mice (26.00 ms in CSE-KO mice treated with DATS vs 25.11 ms in CSE-KO mice without DATS; p = 0.69, Fig. 6C), CSE overexpression in the endothelial cells of the CSE-KO mice with a transgene for endothelial CSE (n = 15) did reduce AERP levels back to WT levels (20.45 ms in CSE with endothelial transgene for H2S compared to 25.11 ms in CSE-KO mice; p = 0.02, Fig. 6C).
Fig. 6.
Low bioavailability of H2S in CSE-KO mice caused electrical remodeling, which was reversed by sulfide supplementation by DATS administration or by transgenic overexpression of CSE in the endothelial cells (eCSETg). CSE-KO mice (n = 3) had increased calcium sparks compared to WT mice (n = 3), which was reversed by DATS administration (n = 3) and by endothelial transgenic overexpression of CSE (CSE-KO/ecCSETg, n = 3); summary data (A) and representative line scans (B) shown. The atrial effective refractory period in CSE-KO mice (n = 15) was prolonged compared to WT mice (n = 15) (C), which was reversed by transgenic overexpression of CSE in the endothelial cells (n = 15) but not by DATS administration (n = 18). Representative electrograms showing AERP are shown in D. Action potential duration (APD-90) of atrial myocytes was prolonged in CSE-KO mice (n = 3) compared to WT controls (n = 3) and was reversed by transgenic overexpression of CSE in the endothelial cells (n = 3) (E). Representative action potentials from CSE-KO, WT, and CSE-KO/ecCSETg mice are shown in F. Groups were compared using one-way ANOVA and post-hoc Tukey's test in A, C, and E.
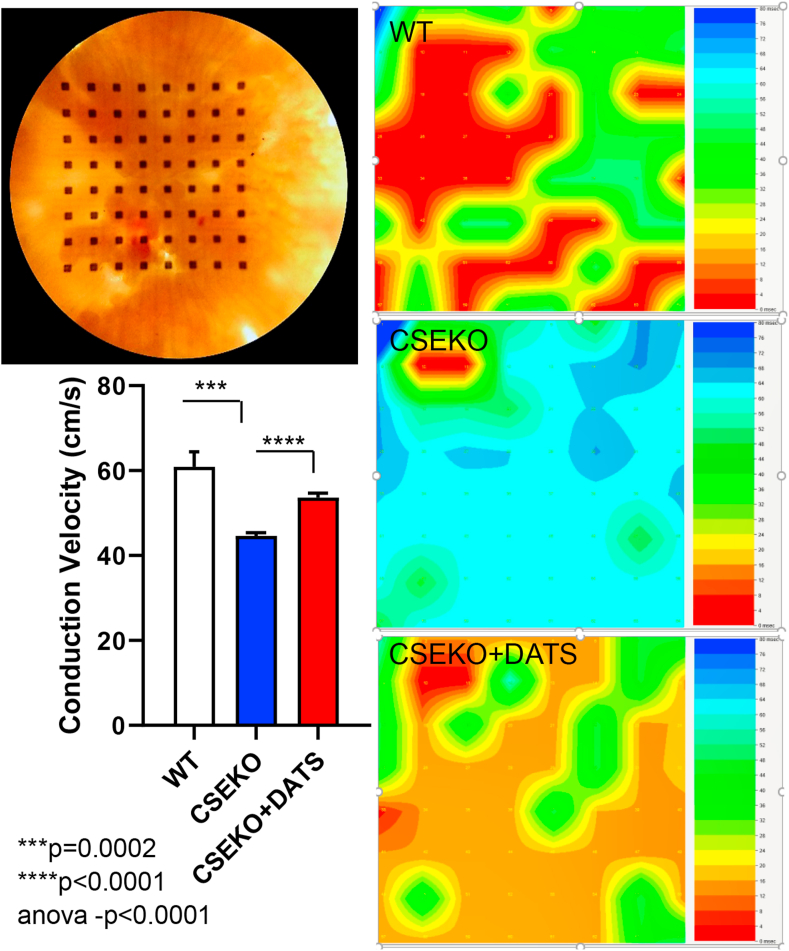
We then looked at conduction velocity at the tissue level in the atrial tissue of WT and CSE-KO mice using a multi-electrode array. The left atrial tissue was excised quickly after sacrifice of the mice, laid flat on the MEA, and stimulated from channel 1, from which we calculated the conduction velocity across the array. We found significant slowing of the conduction velocity across the left atrial tissue of CSE-KO mice (n = 3) compared to that of WT mice (n = 3) (60.88 cm/s vs 44.65 cm/s; p = 0.05, Fig. 7). Supplementation of sulfide in CSE-KO mice with DATS (n = 3), by retro-orbital injections for 3 days prior to the conduction velocity experiments, significantly increased conduction velocity across the left atrial tissue of CSE-KO mice compared to CSE-KO mice without DATS supplementation (53.63 cm/s vs 44.65 cm/s, p < 0.001, Fig. 7). We then examined whether there were cellular level electrical changes that led to tissue level changes in the effective refractory period and conduction velocity. First, we measured action potential duration (APD) in CSE-KO mice and compared to that in WT mice. We found that CSE-KO mice (n = 3) had prolonged action potential duration compared to that of WT mice (n = 3) (64.35 ms vs 29.01 ms; p = 0.02, Fig. 6E and F). This prolongation in APD was reversed by CSE overexpression in the endothelial cells of CSE-KO mice (n = 3) (39.67 ms in CSE-KO mice with endothelial CSE transgenic overexpression compared to 64.35 ms in CSE-KO mice; p = 0.02, Fig. 6F). As oxidative stress causes oxidation of calcium handling proteins and is known to cause calcium leak [32], we then assessed whether an increase in calcium sparks in CSE-KO mice was linked to oxidative stress and APD prolongation and changes in atrial ERP. Isolated atrial myocytes were loaded with calcium sensing dye and imaged using confocal microscopy. We found that the frequency of calcium sparks was increased in CSE-KO mice (n = 3) compared to WT mice (n = 3) (16.54 sparks/100 μm/s vs 11.38 sparks/100 μm/s; p=<0.0001, Fig. 6A and B). H2S supplementation in the form of either DATS (n = 3) or transgenic overexpression of endothelial CSE (n = 3) normalized calcium sparks (8.41 sparks/100 μm/s in CSE-KO mice treated with DATS compared to 16.54 sparks/100 μm/s in CSE-KO without treatment; p=<0.0001 and 9.75 in CSE KO mice with transgenic overexpression of endothelial cell CSE compared to 16.64 in CSE KO mice, p=<0.0001, Fig. 6A and B).
Fig. 7.
Low bioavailability of H2S in CSE-KO mice caused decreased conduction velocity in the atrial tissue. Representative mouse atrial tissue placed over the MEA is shown at the top left and summary data are shown at the bottom left. Groups were compared using one-way ANOVA and post-hoc Tukey's test. Representative isochronal activation maps from WT (n = 3), CSE-KO (n = 3), and CSE-KO mice supplemented with DATS (n = 3) injections are shown on the right.
Finally, we also looked at whether structural remodeling in the atrium in the form of fibrosis could explain some of the electrical changes observed in the CSE-KO mice. Masson's trichrome staining of the atria from CSE-KO mice and WT mice showed no difference in atrial fibrous tissue between these two groups (p = 0.14, Supplementary Fig. 2).
Taken together, our data show that low H2S levels cause electrical remodeling at the atrial level, as illustrated by prolonged atrial effective refractory period, at the tissue level as demonstrated by decreased conduction velocity, and at the cellular level as established by prolonged APD and increased frequency of calcium sparks. These changes are reversed partially or completely by H2S supplementation in the form of DATS; notably, increasing H2S in the endothelium alone is sufficient to reverse the changes of a global reduction in H2S.
4. Discussion
The most significant risk factors for AF have two notable pathophysiological changes in common: 1) direct or indirect hemodynamic changes, causing increased right or left atrial pressure [33], and 2) vascular endothelial dysfunction [34]. Extensive research has explored the pathways by which left or right atrial hypertension caused by the different risk factors for AF leads to the molecular mechanisms resulting in electrical and structural remodeling in the atria. However, there is a paucity of research looking at the link between endothelium and pro-arrhythmic myocardial remodeling triggering AF. ROS-induced activation of redox sensitive proteins with resultant atrial remodeling and AF has been extensively described [31,32]. However, examination of upstream sources of ROS has been limited to NADPH oxidase and the relationship between AF risk factors and the production of ROS is underexplored. In this study, we present data that suggest that low levels of H2S, an ubiquitous gasotransmitter promotes elevated superoxide likely through reduced scavenging and anti-oxidant mechanisms. CSE deficiency leads to increased atrial superoxide, decreased atrial conduction velocity, altered atrial effective refractory period, increased calcium sparks, and increased atrial myocyte action potential duration, a combination that eventually leads to AF in mice. We also show that replenishing H2S by DATS administration prevents atrial remodeling and AF. More importantly, we found that overexpression of CSE in the endothelium alone in a mouse model with global deficiency of CSE is sufficient to prevent AF, thus linking endothelial function to atrial remodeling and AF. Finally, for the very first time, we show that human subjects with AF have decreased acid labile sulfide levels compared to non-AF controls and that, while conversion to normal sinus rhythm in these individuals restores all other forms of sulfide, it does not change the acid labile sulfide levels.
H2S is a unique and ubiquitous gaseous signaling molecule that has been implicated in numerous cardiovascular diseases. Low H2S levels are associated with heart failure, coronary artery disease, peripheral arterial disease, and chronic limb ischemia. H2S is noted to play a significant role in transcriptional regulation [35] and post-translational modification of ion channels and ion channel handling proteins [36]. In addition, H2S is cardioprotective in several ways, including its anti-apoptotic, anti-inflammatory, and anti-fibrotic properties [6]. These qualities merit a deeper look at the role of H2S in the pathogenesis of AF, an atrial arrhythmia in which oxidative stress and inflammation promote cell death, fibrosis, and ion channel remodeling. This is the first study to measure and compare sulfide levels in patients with AF.
The acid labile sulfide pool within the cell is considered a stable store of sulfide from which H2S can be readily released [37,38]. These long-term stores of sulfide may be compartmentalized, with most acid labile sulfide being stored in the mitochondria [39]. Prior human studies have reported total sulfide levels in the blood of patients with different medical conditions [40,41], but the measurement techniques may have aggregated all sulfide pools together. We previously reported elevated plasma free sulfide levels in patients with vascular disease [20]. In this study, despite the fact that more controls had CAD and PAD, patients with AF had elevated plasma free H2S levels compared to controls, suggesting that AF may lead to cellular processes that mobilize free sulfide at levels even higher than other cardiovascular diseases, perhaps to compensate for oxidative stress. In addition, our findings from earlier studies show that acid labile sulfide levels are lower in patients with any cardiovascular disease compared to controls but patients with arrhythmias were not specifically included in those studies [16]. Interestingly, the acid labile sulfide levels in AF patients were significantly lower than those of non-AF patients, many of whom had cardiovascular disease, possibly signifying that their long-term stable stores of sulfide could have been depleted by compensatory release as plasma free sulfide to deal with the demands of increased oxidative stress in these patients. This hypothesis becomes even more significant when comparing patients with persistent and permanent AF. In addition to already having lower levels of acid labile sulfide stores than non-AF subjects, patients with persistent or permanent AF appear to be losing their ability to compensate, as evidenced by their lower plasma free sulfide levels. While lower sulfide levels could be the cause or effect of AF, the finding that free and total sulfide pools increased post rhythm conversion, while acid labile sulfide levels remained unchanged, signifies an intrinsic deficiency of this sulfide pool in patients with AF compared to non-AF patients. Similarly, while free and total sulfide levels were affected by common cardiac medications like BB, ACEI/ARB and statins in AF patients, acid labile sulfide levels did not differ based on whether patients were taking these medications, confirming that low acid labile sulfide levels in AF patients may be due to an inherent deficit rather than effects of AF that can be modulated by cardiac medications.
Our study shows that a lower bioavailability of H2S is associated with increased action potential duration and prolonged atrial effective refractory periods, both findings reversed by supplementation of sulfide. H2S has been shown to decrease transient outward potassium currents [42] and increase ATP-dependent potassium channel currents [43]. Consistent with these findings, it has been documented that administration of sodium hydrosulfide shortened rat atrial myocyte action potentials. Therefore, our finding, that a lower bioavailability of H2S in CSE-KO mice increased APD, is in agreement with this. Although the effects of H2S could very well be one possible explanation, H2S has other direct and indirect effects on calcium channels and calcium handling proteins which could also lead to these findings. We found increased calcium sparks in isolated atrial cardiomyocytes from mice with decreased atrial H2S levels and lower bioavailability of H2S. These results were rescued by overexpression of CSE in the endothelial cells. Oxidation of multiple calcium handling proteins has been shown to increase the leak of calcium from the sarcoplasmic reticulum and to lead to AF. Increased CaMKII activity by oxidation of CaMKII has been linked to increased calcium sparks, triggered activity, and increased susceptibility to AF [32]. In addition, oxidative stress induced ryanodine receptor oxidation has recently been shown to promote SR calcium leak and AF. Increased atrial superoxide in our mouse model of decreased bioavailability of H2S could have produced conditions suitable for this oxidation of calcium handling proteins. Interestingly, sulfhydration of CaMKII by H2S was recently shown to reduce CaMKII phosphorylation and activity in H9c2 cells [36], adding another pathway by which decreased H2S levels could directly contribute to increased CaMKII activity and increased SR calcium leak. Finally, H2S acts as a regulator of L-type calcium channels by inhibiting them [44], causing acceleration of the depolarization phase of the action potential in human atrial cells [45]. Therefore, the lack of H2S, as seen in the atria of CSE-KO mice, is not surprisingly related to longer action potentials, effective refractory periods, and increased risk of AF.
Evolving research has made it increasingly clear that oxidative stress and AF are closely associated [46,47]. Low bioavailability of H2S in CSE-KO mice is associated with enhanced oxidative stress in the ventricles [48]. We observed increased superoxide levels in the atria of CSE-KO mice, which was reversed by administration of DATS and by endothelial overexpression of CSE. These changes in superoxide levels inversely relate to the changes in sulfide levels in these animals and directly relate to the AF inducibility. In addition to the oxidation of calcium handling proteins such as CaMKII and RyR, leading to SR calcium leak and AF, there may be other pathways by which ROS induced by low H2S leads to AF. Kv1.5 channels are primarily responsible for ultra-rapid outwardly rectifying potassium currents (Ikur). Loss of function or gain of function of these channels can lead to AF [49,50]. H2S supplementation has been shown to decrease angiotensin II mediated NADPH oxidase 4 (Nox4) signaling, thereby decreasing atrial ROS in a beagle model of rapid atrial pacing [35]. In this model, Kv1.5 protein expression was also reduced by H2S supplementation, but the effects of H2S supplementation on the potassium currents or action potential duration was not measured. In our model, decreased H2S levels were associated with increased atrial superoxide and increased atrial myocyte action potential duration and AF susceptibility, suggesting that low H2S induced NADPH mediated ROS could cause electrical remodeling by changes in Ikur.
H2S is produced predominantly by CSE in the endothelium, vascular smooth muscle cells, and cardiomyocytes [51]. Endothelial dysfunction, which is commonly found in hypertension, aging, obesity, and diabetes, is indicated by a low bioavailability of H2S [30]. Whereas almost all of the strong risk factors for AF cause endothelial dysfunction [52] and oxidative stress, prior research has focused on the pathways that lead from ROS to AF and has been unsuccessful at connecting the dots between the risk factors, endothelial function, and AF, with or without mediation by ROS. While prior research has looked at endothelial function as a result of AF [28,53], our findings show H2S to be a viable link between endothelial dysfunction and AF. In our model of low bioavailability of H2S caused by CSE-KO, endothelial overexpression of CSE was sufficient to effectively prevent oxidative stress and electrical remodeling in the atrial myocardium, similar to exogenous sulfide donor administered systemically. As a gasotransmitter, H2S is able to exert its effects by simple diffusion and direct transport across cell membranes, reaching molecular targets far from the site of its biosynthesis [54]. We found that endothelial overexpression of CSE in a global CSE-KO model reversed atrial oxidative stress, evidence that supports the importance of functional cross-talk between the cell types found in several other cardiac pathologies [55], a relationship underexplored in arrhythmias in general and AF in particular.
Our study has limitations. Although most of the demographics of the human subjects in this study were matched, the AF group was significantly older than the non-AF subjects. They also had some different co-morbidities due to the conditions commonly associated with AF. Although H2S levels in human patients may be affected by AF, this is the first investigation of this molecule in AF. The fact that acid labile sulfide levels did not change post conversion while other sulfide pools increased is notable. This study delved deeper into the upstream connection between ROS and the common conditions leading to AF and did not focus on the downstream signaling changes leading to electrical remodeling, which will be assessed in future studies.
5. Conclusion
In conclusion, our findings reveal H2S to be a unique link between the risk factors for AF and downstream oxidative stress, electrical remodeling, and AF. They also convincingly demonstrate the communication between vascular endothelium and atrial cells mediated by endothelial H2S.
Funding
This publication was supported by an Institutional Development Award from the National Institutes of General Medical Sciences of the National Institutes of Health (NIH) under grant number P20GM121307 and HL149264 to CK, by R01NS100954and R01NS099188 to EG, and by HL098435, HL133497, and HL141155 to AWO.
Author contribution
P. Dominic, C.G Kevil, M.Watts, G. Kolluru, P. Dherange and AW.Orr designed research. M.Watts, G. Kolluru, P.Dherange, S.Pardue, M. Si, X.Shen, K. Trosclair, J.Glawe, Z.Al-Yafei, M.Iqbal, B.Person and K.Hamilton performed research. P.Dominic, M.Watts, G.Kolluru, S.Pardue analyzed data. M.Watts, P.Dominic, G.Kolluru, E.Glasscock, C. Kevil wrote and revised the paper. AW Orr, C Hamilton provided critical revision of the manuscript for intellectual content.
Declaration of competing interest
P.Dominic and C.Kevil have a provisional patent for the use of hydrogen sulfide compounds in the treatment of atrial fibrillation.
Acknowledgements
We thank Georgia Morgan for her assistance editing this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101817.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics—2019 update: a report from the American heart association. Circulation. 2019;139:1–473. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Yang K.-C., Dudley S.C., Jr. Oxidative stress and atrial fibrillation. Circulation. 2013;128:1724–1726. doi: 10.1161/CIRCULATIONAHA.113.005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang X., Zhang Q., Wang X., Yuan M., Zhang Y., Xu Z. Reactive oxygen species mediated oxidative stress links diabetes and atrial fibrillation. Mol. Med. Rep. 2018;17:1–8. doi: 10.3892/mmr.2018.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn J.-Y., Zhang J., Zhang Y., Chen H., Liu D., Ping P. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J. Mol. Cell. Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Q., Ying J., Xiang L., Zhang C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine. 2018;97 doi: 10.1097/MD.0000000000013065. e13065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial Infarction and Heart Failure. 2014. p. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y., Shen Z., Luo S., Guo W., Zhu Y.Z. The cardioprotective effects of hydrogen sulfide in heart diseases: from molecular mechanisms to therapeutic potential. Oxidative Medicine and Cellular Longevity. 2015:1–13. doi: 10.1155/2015/925167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii I., Akahoshi N., Yamada H., Nakano S., Izumi T., Suematsu M. Cystathionine γ-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010;285:26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westphal C., Spallek B., Konkel A., Marko L., Qadri F., DeGraff L.M. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PloS One. 2013;8 doi: 10.1371/journal.pone.0073490. e73490–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temple J., Frias P., Rottman J., Yang T., Wu Y., Verheijck E.E. Atrial fibrillation in KCNE1-null mice. Circ. Res. 2005;97:62–69. doi: 10.1161/01.RES.0000173047.42236.88. [DOI] [PubMed] [Google Scholar]
- 12.Bober S.L., Ciriello J., Jones D.L. Atrial arrhythmias and autonomic dysfunction in rats exposed to chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H1160–H1168. doi: 10.1152/ajpheart.00173.2017. [DOI] [PubMed] [Google Scholar]
- 13.Opel A., Nobles M., Montaigne D., Finlay M., Anderson N., Breckenridge R. Absence of the regulator of G-protein signaling, RGS4, predisposes to atrial fibrillation and is associated with abnormal calcium handling. J. Biol. Chem. 2015;290:19233–19244. doi: 10.1074/jbc.M115.666719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussek A., Schmidt M., Bauriedl J., Ravens U., Wettwer E., Lohmann H. Cardiac tissue slices with prolonged survival for in vitro drug safety screening. J. Pharmacol. Toxicol. Methods. 2012;66:145–151. doi: 10.1016/j.vascn.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Meiry G., Reisner Y., Feld Y., Goldberg S., Rosen M., Ziv N. Evolution of action potential propagation and repolarization in cultured neonatal rat ventricular myocytes. J. Cardiovasc. Electrophysiol. 2001;12:1269–1277. doi: 10.1046/j.1540-8167.2001.01269.x. [DOI] [PubMed] [Google Scholar]
- 16.Rajpal S., Katikaneni P., Deshotels M., Pardue S., Glawe J., Shen X. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biology. 2018;15:480–489. doi: 10.1016/j.redox.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bir S.C., Pattillo C.B., Pardue S., Kolluru G.K., Shen X., Giordano T. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-Dependent manner. Diabetes. 2014;63:270–281. doi: 10.2337/db13-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgiou C.D., Papapostolou I., Grintzalis K. Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils. Nat. Protoc. 2008;3:1679–1692. doi: 10.1038/nprot.2008.155. [DOI] [PubMed] [Google Scholar]
- 19.Voigt N., Zhou X.-B., Dobrev D. Isolation of human atrial myocytes for simultaneous measurements of Ca2+ transients and membrane currents. JoVE. 2013:1–9. doi: 10.3791/50235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D., Zhang W.W. Plasma free H2S levels are elevated in patients with cardiovascular disease. Journal of the American Heart Association. 2013;2 doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolluru G.K., Bir S.C., Yuan S., Shen X., Pardue S., Wang R. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc. Res. 2015;107:590–600. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Biggs T.D., Xian M. Hydrogen sulfide (H 2S) releasing agents: chemistry and biological applications. Chem. Commun. 2014;50:11788–11805. doi: 10.1039/C4CC00968A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolluru G.K., Shen X., Kevil C.G. Reactive sulfur species: a new redox player in cardiovascular pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020;40:874–884. doi: 10.1161/ATVBAHA.120.314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardue S., Kolluru G.K., Shen X., Lewis S.E., Saffle C.B., Kelley E.E. Hydrogen sulfide stimulates xanthine oxidoreductase conversion to nitrite reductase and formation of NO. Redox Biology. 2020;34:101447. doi: 10.1016/j.redox.2020.101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conen D., Glynn R.J., Sandhu R.K., Tedrow U.B., Albert C.M. Risk factors for incident atrial fibrillation with and without left atrial enlargement in women. Int. J. Cardiol. 2013;168:1894–1899. doi: 10.1016/j.ijcard.2012.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacà V., Galderisi M., Mondillo S., Focardi M., Ballo P., Guerrini F. Left atrial enlargement as a predictor of recurrences in lone paroxysmal atrial fibrillation. Can. J. Cardiol. 2007;23:869–872. doi: 10.1016/s0828-282x(07)70841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guazzi M., Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2008;95:102–106. doi: 10.1136/hrt.2007.135277. [DOI] [PubMed] [Google Scholar]
- 29.Vane J.R., Anggård E.E., Botting R.M. Regulatory functions of the vascular endothelium. N. Engl. J. Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 30.Sun H.-J., Wu Z.-Y., Nie X.-W., Bian J.-S. 2020. Role of Endothelial Dysfunction in Cardiovascular Diseases: the Link between Inflammation and Hydrogen Sulfide; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolke C., Bukowska A., Goette A., Lendeckel U. Redox control of cardiac remodeling in atrial fibrillation. Biochim. Biophys. Acta Gen. Subj. 2015;1850:1555–1565. doi: 10.1016/j.bbagen.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Purohit A., Rokita A.G., Guan X., Chen B., Koval O.M., Voigt N. Oxidized CaMKII triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau D.H., Nattel S., Kalman J.M., Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 34.Corban M.T., Godo S., Burczak D.R., Noseworthy P.A., Toya T., Lewis B.R. Coronary endothelial dysfunction is associated with increased risk of incident atrial fibrillation. Journal of the American Heart Association. 2020;9:1–8. doi: 10.1161/JAHA.119.014850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu G., Xu C., Tang K., Zhang J., Li Q., Peng L. H2S inhibits angiotensin II-induced atrial Kv1.5 upregulation by attenuating Nox4-mediated ROS generation during atrial fibrillation. Biochem. Biophys. Res. Commun. 2017;483:534–540. doi: 10.1016/j.bbrc.2016.12.110. [DOI] [PubMed] [Google Scholar]
- 36.Wu Dan, Hu Q., Tan B., P, Rose D., Zhu, Zhu Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca2+/calmodulin-dependent protein kinase II. Redox Biology. 2018;19:250–262. doi: 10.1016/j.redox.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson K.R. A practical look at the chemistry and biology of hydrogen sulfide. Antioxidants Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen X., Pattillo C.B., Pardue S., Bir S.C., Wang R., Kevil C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? BBA - Bioenergetics. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K., Sagara M., Aoki C., Tanaka S., Aso Y. Clinical implication of plasma hydrogen sulfide levels in Japanese patients with type 2 diabetes. Intern. Med. 2017;56:17–21. doi: 10.2169/internalmedicine.56.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhuri S, Banerjee S, Kumar A, Biswas UK. Association between serum levels of nitric oxide and hydrogen sulfide in pre-eclampsia. Biochem. Anal. Biochem. 2019;8:1–6. doi: 10.35248/2161-1009.19.8.384. [DOI] [Google Scholar]
- 42.Sheng J., Shim W., Wei H., Lim S.Y., Liew R., Lim T.S. Hydrogen sulphide suppresses human atrial fibroblast proliferation and transformation to myofibroblasts. J. Cell Mol. Med. 2013;17:1345–1354. doi: 10.1111/jcmm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong G.-Z. Hydrogen Sulfide—a potent multichannel anti-arrhythmic drug. J. Cardiovasc. Dis. Res. 2010;1:37–39. doi: 10.4103/0975-3583.59984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai L., Qian Y., Zhou J., Zhu C., Jin L., Li S. Hydrogen sulfide inhibited L-type calcium channels (CaV1.2) via up-regulation of the channel sulfhydration in vascular smooth muscle cells. Eur. J. Pharmacol. 2019;858:172455. doi: 10.1016/j.ejphar.2019.172455. [DOI] [PubMed] [Google Scholar]
- 45.Munaron L., Avanzato D., Moccia F., Mancardi D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 2013;53:77–84. doi: 10.1016/j.ceca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Samman Tahhan A., Sandesara P.B., Hayek S.S., Alkhoder A., Chivukula K., Hammadah M. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849–1855. doi: 10.1016/j.hrthm.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korantzopoulos P., Kolettis T.M., Galaris D., Goudevenos J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int. J. Cardiol. 2007;115:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 48.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. U.S.A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christophersen I.E., Olesen M.S., Liang B., Andersen M.N., Larsen A.P., Nielsen J.B. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur. Heart J. 2012;34:1517–1525. doi: 10.1093/eurheartj/ehs442. [DOI] [PubMed] [Google Scholar]
- 50.Olson T.M., Alekseev A.E., Liu X.K., Park S., Zingman L.V., Bienengraeber M. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 51.Wallace J.L., Wang R. Vol. 14. Nature Publishing Group; 2015. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter; pp. 1–17. [DOI] [PubMed] [Google Scholar]
- 52.Khan A.A., Thomas G.N., Lip G.Y.H., Shantsila A. Endothelial function in patients with atrial fibrillation. Ann. Med. 2020;52:1–11. doi: 10.1080/07853890.2019.1711158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshino S., Yoshikawa A., Hamasaki S., Ishida S., Oketani N., Saihara K. Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int. J. Cardiol. 2013;168:1280–1285. doi: 10.1016/j.ijcard.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Cirino G., Vellecco V., Bucci M. Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017;174:4021–4031. doi: 10.1111/bph.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M., Shah A.M. ROS signalling between endothelial cells and cardiac cells. Cardiovasc. Res. 2014;102:249–257. doi: 10.1093/cvr/cvu050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.