Introduction
Primary leiomyosarcomas (LMS) are rare and account for between 1 and 4% of all soft tissue sarcomas with an annual incidence of 0.04% in the USA [1–5]. LMSs are categorized as either subcutaneous or deep, with subcutaneous lesions further dichotomized as either superficial or cutaneous [6]. Superficial and cutaneous LMSs are respectively believed to be derived from erector pili muscles of hair follicles and vascular smooth muscle of the arterial tunica media layer [6, 7]. Deep LMSs arise from smooth muscle tissue of various organs such as the uterus. The clear distinction between subcutaneous and deep lesions is important due to differing rates of recurrence and metastasis as well as prognosis [8].
Leiomyosarcomas are believed to arise de novo and not from predisposing or precancerous lesions. Unlike other sarcomas such as angiosarcomas in which risk factors such as radiation exposure are well-described [9], predispositions for LMSs are not well understood although associations with radiation exposure [10, 11] and chemotherapy [12] have been found. Li-Fraumeni syndrome and retinoblastoma are associated with LMS occurrence [13, 14]. Currently, the optimal method of treatment is surgery with recommended resection margins ranging from 1 to 5 cm, dependent on the use of adjuvant radiation [15, 16]. If inoperable, radiation with or without chemotherapy is recommended although success rates vary [17].
Although LMSs most commonly arise in the uterus and gastrointestinal tract [2], they can also primarily arise in the head and neck, which are found to be more aggressive and associated with worse prognoses [18]. LMSs in the head and neck can arise in the larynx, auricular area, and intracranial space [7, 19, 20]. There are several reported cases of intracranial LMSs, particularly in AIDS patients [21–27]. Primary scalp LMSs are very rare and only one previous case has been reported [28]. We present the case of a patient with a primary skull-derived LMS with intracranial extension and dural, but not brain, involvement.
Case Presentation
A 65-year-old woman noticed a swelling over her right eyebrow in January 2018. Her past medical history was significant for smoking, tonsillectomy and adenoidectomy, dilation and curettage, total thyroidectomy with subsequent hypothyroidism, hypertension, and deep vein thrombosis. There was no prior history of exposure to radiation or toxins. The lesion was painless and non-ulcerated but was rapidly enlarging. The patient had it surgically removed by an outside surgeon in June of 2018. The mass was reported to consist of a gelatinous material and to have caused erosion of the outer table of the skull. The initial pathology was interpreted to be a malignant spindle cell neoplasm most consistent with malignant schwannoma. The patient presented to Roswell Park Comprehensive Cancer Center after re-growth of the mass. A review of the pathology provided a diagnosis of leiomyosarcoma (intermediate grade 2) with weak SMA positivity and patchy p63 positivity. S100 HMW CK, CKAE1/3, CD 68, CD 31, and Factor XIIIa were all negative. The patient underwent brain MRI which showed a soft tissue mass involving the right frontal bone with superficial extension into the scalp as well as an intracranial extension (Fig. 1). No T2 flair signal change was noted in the brain parenchyma pre- or postoperatively (image not shown). As the periosteum and bone usually provide excellent barriers to sarcoma penetration, especially in the case of leiomyosarcoma, which is not one of the most aggressive sarcomas, we think that this patient’s leiomyosarcoma originated within the diploë of the skull and is not of cutaneous origin. The patient also underwent imaging for restaging purposes, which did not show evidence of tumor elsewhere in the body. The patient then underwent volumetric-modulated arc radiotherapy to a dose of 5000 cGy in 25 fractions to the lesion completed in August 2018. Six weeks following completion of radiation therapy, the patient underwent a combined approach consisting of right frontal trough craniotomy with circumferential dural incision for en bloc resection of the leiomyosarcoma. In detail, the incision was outlined with a surgical marking pen by the sarcoma team to avoid transgressing the tumor and to define the craniotomy. After circumferential incision around the lesion, four burr holes were created with a Stryker drill. A through craniectomy was created between the burr holes using the cutting bit of the Stryker drill. The inner table of the skull was then removed with Kerrison punch rongeurs and a complete circumferential removal of bone was performed with about 2 cm of margin around the outer edge of the tumor. At no point was the tumor violated. The dura was then incised with a 15 blade within the trough craniectomy and the durotomy was extended with Metzenbaum scissors circumferentially along the trough. The entire specimen was then lifted out with the scalp bone and dura attached. The inner surface of the specimen was inspected and it did not appear the tumor violated the dura. The surface of the brain was inspected and while there was an indentation in the frontal pole where the tumor had compressed, the surface of the arachnoid and cerebral cortex looked completely intact. No CSF analysis was therefore performed and also no lumbar drain had been placed pre- or postoperatively. The frontal sinus was not violated. Following the craniotomy, the fascia lata was sewn into the dural defect and this was covered by a layer of DuraGen and EvoSeal. A cranioplasty was performed with titanium mesh, scalp rotational flap reconstruction for coverage of the cranial defect and split-thickness skin grafting (Figs. 2 and 3). The patient tolerated the procedure well and was discharged home on postoperative day three in good condition. The patient has done well in follow-up (Fig. 4).
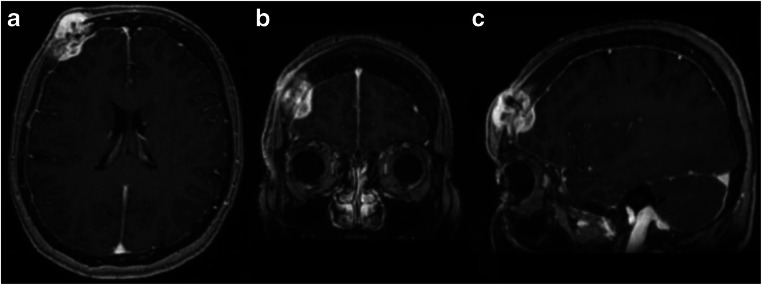
Fig. 1.
a Axial, b coronal, and c sagittal T1-weighted post-contrast MRI with right frontal bone mass measuring 3.2 × 2.2 cm in diameter with extension to the subcutaneous scalp, as well as intracranial extension into the epidural space. No evidence of brain parenchymal involvement or vasogenic edema was noted
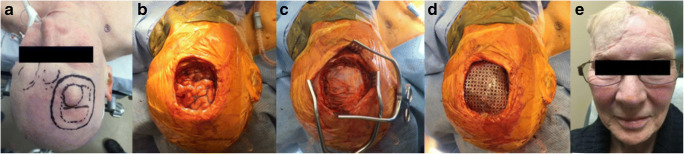
Fig. 2.
Intraoperative images showing a pre-surgical appearance of mass with overlying intact skin, b en bloc resection of the scalp, calvarium, and dura mater with fascia lata dural graft covering cerebral cortex (c), d titanium mesh cranioplasty prior to rotational scalp flap coverage, and postoperative cosmetic result with rotational scalp flap and skin graft (e)
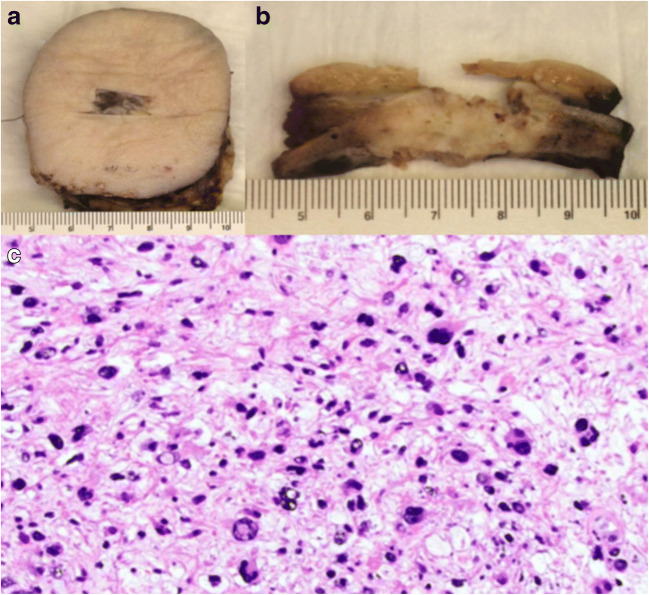
Fig. 3.
a Operative specimen with a tag for orientation, b full-thickness section of mass, and c microscopic images of leiomyosarcoma (grade 2) with radiation treatment effect (× 200). Mitotic index 3/10 HPF; necrosis 5%
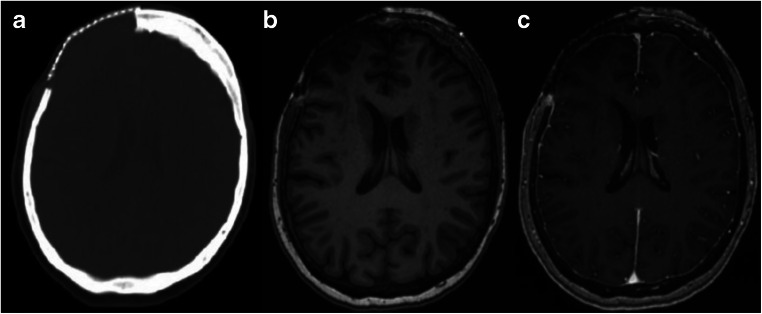
Fig. 4.
Postoperative imaging. a Axial CT of the head without contrast status post craniectomy with right frontal cranioplasty mesh in place and b axial MRI imaging without and c with contrast showing postoperative changes in the right frontal region; no enhancement is noted in the postsurgical area
Discussion
Very few cases of primary leiomyosarcomas of the calvarium have been documented with most having been diagnosed in patients with HIV [21–27]. LMS tumors arise from mesenchymal tissue, which may differentiate into a variety of structures including hair follicles, blood vessels, tendons, and bone. The pathogenesis of primary cutaneous LMS is still unknown, and a few reports link them to prior radiation [29], EBV-virus infection [30], or malignant transformation of a primary leiomyoma [5]. Although there are currently no standardized treatment regimens for leiomyosarcomas of the head and neck, some efficacy of combined radiation and surgical resection has been observed [27, 31].
This patient’s lesion was discussed by a multidisciplinary team comprised of members from head and neck surgery, neurosurgery, pathology, plastic surgery, and radiation medicine and the optimal treatment was discussed and planned. By consensus, the patient underwent preoperative radiation prior to performing a complete en bloc resection of the tumor, encompassing the overlying scalp, periosteum, full-thickness calvarium, and dura mater with wide negative margins.
Preoperative radiation, as opposed to postoperative adjunctive radiation, was decided because of the need for scalp and skull reconstruction in addition to the resection. By having different specialties involved and openly communicating each specialty’s considerations and plan for treatment, the patient was able to receive optimal streamlined care. This multidisciplinary planning, as shown in this case, becomes crucial especially in patients with rare diagnoses in difficult anatomical locations where standardized treatment regimens are not well established.
After preoperative radiation, the patient underwent an en bloc resection rather than staged resection to prevent the local recurrence of the LMS. This was feasible because no major vascular or neural structures traversed the tumor or its margins. There are no published articles comparing the outcomes of en bloc resections vs. staged resections of head and neck LMS masses. However, several articles report success with en bloc and complete resections for osteogenic sarcomas and inferior vena cava LMS with no recurrence at up to 5-year follow-up [32, 33], which further highlights the association of complete resection of sarcomas with negative margins and increased survival [34].
Conclusion
We present a case of a woman with recurrent primary leiomyosarcoma of the calvarium extending intracranially, which was treated with preoperative radiation and en bloc resection. We believe that treating such lesions in a multidisciplinary team approach is helpful to optimize the outcome.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stout AP, Hill WT. Leiomyosarcoma of the superficial soft tissues. Cancer. 1958;11(4):844–854. doi: 10.1002/1097-0142(195807/08)11:4<844::AID-CNCR2820110425>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.McLeod AJ, Zornoza J, Shirkhoda A. Leiomyosarcoma: computed tomographic findings. Radiology. 1984;152(1):133–136. doi: 10.1148/radiology.152.1.6729102. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135(3):451–457. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Workman AD, Farquhar DR, Brody RM, Parasher AK, Carey RM, Purkey MT, Nagda DA, Brooks JS, Hartner LP, Brant JA, Newman JG. Leiomyosarcoma of the head and neck: a 17-year single institution experience and review of the National Cancer Data Base. Head Neck. 2018;40(4):756–762. doi: 10.1002/hed.25054. [DOI] [PubMed] [Google Scholar]
- 5.Zacher M, Heppt MV, Brinker TJ, Hayani KM, Flaig MJ, Berking C. Primary leiomyosarcoma of the skin: a comprehensive review on diagnosis and treatment. Med Oncol. 2018;35(10):135. doi: 10.1007/s12032-018-1196-2. [DOI] [PubMed] [Google Scholar]
- 6.Aneiros-Fernandez J, Antonio Retamero J, Husein-Elahmed H, Ovalle F, Aneiros-Cachaza J. Primary cutaneous and subcutaneous leiomyosarcomas: evolution and prognostic factors. Eur J Dermatol. 2016;26(1):9–12. doi: 10.1684/ejd.2015.2681. [DOI] [PubMed] [Google Scholar]
- 7.Ozturk K, Keles B, Arbag H, Yondeml IF, Avunduk MC. Postauricular subcutaneous leiomyosarcoma. Auris Nasus Larynx. 2004;31(3):323–328. doi: 10.1016/j.anl.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Bogart SF, Sacks HG, DeMarco LC. Metastatic leiomyosarcoma of the palate. J Oral Maxillofac Surg. 1990;48(12):1338–1340. doi: 10.1016/0278-2391(90)90495-N. [DOI] [PubMed] [Google Scholar]
- 9.Farran Y, Padilla O, Chambers K, Philipovskiy A, Nahleh Z. Atypical presentation of radiation-associated breast angiosarcoma: a case report and review of literature. Am J Case Rep. 2017;18:1347–1350. doi: 10.12659/AJCR.905157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SG, See AC, Williamson PA, Archer DJ, Evans PH. Radiation induced sarcoma of the head and neck. Head Neck. 1999;21(4):346–354. doi: 10.1002/(SICI)1097-0347(199907)21:4<346::AID-HED9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Brockstein B, Mundt A, Haraf DJ, Ferguson M, Montag A. Radiation-induced leiomyosarcoma: does antimetabolite chemotherapy contribute? A report of three cases. Sarcoma. 2003;7(3–4):167–172. doi: 10.1080/13577140310001650330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez Sevilla C, Admella-Salvador C, Romero-Martin JA, Llopis-Manzanera J, Barranco-Sanz MA. Bladder leiomyosarcoma 25 years after treatment with cyclophosphamide in patient with history of retinoblastoma. Urol Int. 2018;100(1):119–121. doi: 10.1159/000437442. [DOI] [PubMed] [Google Scholar]
- 13.Sabater-Marco V, Ferrando-Roca F, Morera-Faet A, Garcia-Garcia JA, Bosch SB, Lopez-Guerrero JA. Primary cutaneous Leiomyosarcoma arising in a patient with Li-Fraumeni syndrome: a neoplasm with unusual histopathologic features and loss of Heterozygosity at TP53 gene. Am J Dermatopathol. 2018;40(3):225–227. doi: 10.1097/DAD.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 14.Gao P, Seebacher NA, Hornicek F, Guo Z, Duan Z. Advances in sarcoma gene mutations and therapeutic targets. Cancer Treat Rev. 2018;62:98–109. doi: 10.1016/j.ctrv.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Porter CJ, Januszkiewicz JS. Cutaneous leiomyosarcoma. Plast Reconstr Surg. 2002;109(3):964–967. doi: 10.1097/00006534-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Ko YI, Lim JS, Han KT, Kim MC. Leiomyosarcoma of the face. Arch Craniofac Surg. 2014;15(1):36–39. doi: 10.7181/acfs.2014.15.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich CT, Feiz-Erfan I, Spetzler RF, Isaacs JD, Hott JS, Nakaji P, Coons SW, Joganic EJ, Kresl JJ, Milligan JM, Lettieri SC. Sinonasal leiomyosarcoma: review of literature and case report. Laryngoscope. 2005;115(12):2242–2248. doi: 10.1097/01.mlg.0000183767.97518.09. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery E, Goldblum JR, Fisher C. Leiomyosarcoma of the head and neck: a clinicopathological study. Histopathology. 2002;40(6):518–525. doi: 10.1046/j.1365-2559.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 19.Tewary AK, Pahor AL. Leiomyosarcoma of the larynx: emergency laryngectomy. J Laryngol Otol. 1991;105(2):134–136. doi: 10.1017/S0022215100115166. [DOI] [PubMed] [Google Scholar]
- 20.Selcuk OT, Renda L, Erol B, Osma U, Eyigor H, Ozturk H. A case of laryngeal leiomyosarcoma and review of the literature. Ann Maxillofac Surg. 2015;5(2):274–276. doi: 10.4103/2231-0746.175772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aeddula NR, Pathireddy S, Samaha T, Ukena T, Hosseinnezhad A. Primary intracranial leiomyosarcoma in an immunocompetent adult. J Clin Oncol. 2011;29(14):e407–e410. doi: 10.1200/JCO.2010.33.4805. [DOI] [PubMed] [Google Scholar]
- 22.Almubaslat M, Stone JC, Liu L, Xiong Z. Primary intracranial leiomyosarcoma in an immunocompetent patient. Clin Neuropathol. 2011;30(3):154–157. doi: 10.5414/NPP30154. [DOI] [PubMed] [Google Scholar]
- 23.Francisco CN, Alejandria M, Salvana EM, Andal VMV (2018) Primary intracranial leiomyosarcoma among patients with AIDS in the era of new chemotherapeutic and biological agents. BMJ Case Rep 2018 [DOI] [PMC free article] [PubMed]
- 24.Fujimoto Y, Hirato J, Wakayama A, Yoshimine T. Primary intracranial leiomyosarcoma in an immunocompetent patient: case report. J Neuro-Oncol. 2011;103(3):785–790. doi: 10.1007/s11060-010-0450-z. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Havens PL, Southern JF, Firat SY, Jogal SS. Epstein-Barr virus-associated intracranial leiomyosarcoma in an HIV-positive adolescent. J Pediatr Hematol Oncol. 2010;32(4):e144–e147. doi: 10.1097/MPH.0b013e3181c80bf3. [DOI] [PubMed] [Google Scholar]
- 26.Torihashi K, Chin M, Yoshida K, Narumi O, Yamagata S. Primary intracranial leiomyosarcoma with intratumoral hemorrhage: case report and review of literature. World Neurosurg. 2018;116:169–173. doi: 10.1016/j.wneu.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Dong L, Huang Y, Zhang B, Ma H, Zhou Y, Shu C, Cheng G. Primary intracranial leiomyosarcoma: review of the literature and presentation of a case. Onkologie. 2012;35(10):609–616. doi: 10.1159/000342676. [DOI] [PubMed] [Google Scholar]
- 28.Pop M, Botar Jid C, Hotoleanu C, Vasilescu D, Sfrangeu S. Superficial leiomyosarcoma of the scalp: a case report. Med Ultrason. 2011;13(3):237–240. [PubMed] [Google Scholar]
- 29.Niwa J, Hashi K, Minase T. Radiation induced intracranial leiomyosarcoma: its histopathological features. Acta Neurochir. 1996;138(12):1470–1471. doi: 10.1007/BF01411129. [DOI] [PubMed] [Google Scholar]
- 30.Suankratay C, Shuangshoti S, Mutirangura A, Prasanthai V, Lerdlum S, Shuangshoti S, Pintong J, Wilde H. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis. 2005;40(10):1521–1528. doi: 10.1086/429830. [DOI] [PubMed] [Google Scholar]
- 31.Hussain S, Nanda A, Fowler M, Ampil FL, Burton GV. Primary intracranial leiomyosarcoma: report of a case and review of the literature. Sarcoma. 2006;2006:52140. doi: 10.1155/SRCM/2006/52140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda H, Minamimura K, Endo Y, et al. Complete surgical resection of a leiomyosarcoma arising from the inferior vena cava. Case Rep Med. 2015;2015:342148. doi: 10.1155/2015/342148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcove RC. En bloc resection for osteogenic sarcoma. Can J Surg. 1977;20(6):521–528. [PubMed] [Google Scholar]
- 34.McGrath PC, Neifeld JP, Lawrence W, Jr, et al. Improved survival following complete excision of retroperitoneal sarcomas. Ann Surg. 1984;200(2):200–204. doi: 10.1097/00000658-198408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]