Abstract
HIF-1-mediated adaptation to changes in oxygen availability is a critical aspect of healthy physiology. HIF is regulated by a conserved mechanism whereby EGLN/PHD family members hydroxylate HIF in an oxygen-dependent manner, targeting it for ubiquitination by Von-Hippel-Lindau (VHL) family members, leading to its proteasomal degradation. The activity of the only C. elegans PHD family member, EGL-9, is also regulated by a hydrogen sulfide sensing cysteine-synthetase-like protein, CYSL-1, which is, in turn, regulated by RHY-1/acyltransferase. Over the last decade, multiple seminal studies have established a role for the hypoxic response in regulating longevity, with mutations in vhl-1 substantially extending C. elegans lifespan through a HIF-1-dependent mechanism. However, studies on other components of the hypoxic signaling pathway that similarly stabilize HIF-1 have shown more mixed results, suggesting that mutations in egl-9 and rhy-1 frequently fail to extend lifespan. Here, we show that egl-9 and rhy-1 mutants suppress the long-lived phenotype of vhl-1 mutants. We also show that RNAi of rhy-1 extends lifespan of wild-type worms while decreasing lifespan of vhl-1 mutant worms. We further identify VHL-1-independent gene expression changes mediated by EGL-9 and RHY-1 and find that a subset of these genes contributes to longevity regulation. The resulting data suggest that changes in HIF-1 activity derived by interactions with EGL-9 likely contribute greatly to its role in regulation of longevity.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00194-0) contains supplementary material, which is available to authorized users.
Keywords: C. elegans, Lifespan, Aging, HIF-1 signaling, Hypoxic response, RHY-1, EGL-9/PHD
Introduction
Adaptation to changes in oxygen availability is a central requirement for aerobic life. In response to hypoxia, reduced oxygen-dependent hydroxylation of hypoxia-inducible factor α (HIFα) transcription factors by members of the EGLN/proline-hydroxylase (PHD) family triggers stabilization of HIFα proteins and activation of a transcriptional stress response that promotes survival (Epstein et al. 2001). This hypoxic response plays critical roles in a variety of pathological conditions including inflammation and cancer (Balamurugan 2016; Pezzuto and Carico 2018; Ramakrishnan and Shah 2016). Constitutive stabilization of the sole C. elegans HIFα family member, HIF-1, by deletion of the Von-Hippel-Lindau ubiquitin ligase, VHL-1, which ubiquitinates HIF-1 and targets it for degradation, results in HIF-1-dependent increases in stress response and longevity (Cockman et al. 2000; Jiang et al. 2001; Leiser and Kaeberlein 2010; Mehta et al. 2009; Müller et al. 2009; Treinin et al. 2003; Zhang et al. 2009).
Genetic studies in C. elegans have identified additional players in the hypoxic signaling pathway. Activity of EGL-9, the only known C. elegans PHD family member, is inhibited by direct interaction with the H2S sensing cysteine-synthetase family member CYSL-1 (Ma et al. 2012). CYSL-1 protein levels are, in turn, reduced through an unknown mechanism by regulator of hypoxia-inducible factor-1 (RHY-1), an ER transmembrane protein with predicted acyltransferase activity (Ma et al. 2012; Shen et al. 2006). Predicted loss-of-function mutations in rhy-1 stabilize HIF-1 and produce expression patterns of HIF-1 target genes that are consistent with reduced EGL-9 activity (Shen et al. 2006).
Interestingly, while vhl-1 mutation extends C. elegans lifespan across culture conditions, the role of EGL-9/PHD is context dependent. While egl-9(RNAi) extends lifespan at 20 °C, the lifespan phenotypes of partial loss-of-function mutations in egl-9 are temperature-dependent, extending lifespan in a HIF-1-dependent manner at low temperatures (15 °C) but not at higher temperatures (20 °C and 25 °C) (Leiser et al. 2011; Leiser and Kaeberlein 2010; Miller et al. 2017; Zhang et al. 2009). The loss-of-function mutant egl-9(sa307) also reduces the lifespan of dietary restricted animals and long-lived rsks-1 mutants when animals are cultured at 25 °C, suggesting that EGL-9 activity may promote lifespan in multiple contexts (Di Chen and Kapahi 2009). Furthermore, recent work from our lab showed that rhy-1 putative knockout mutants were not long-lived at any temperature, despite their reported robust activation of hypoxic response genes (Miller et al. 2017; Shen et al. 2006).
Previous studies on the roles of EGL-9, RHY-1, and VHL-1 show that (1) HIF-1 stabilization when vhl-1 is mutated leads to robust induction of egl-9 and rhy-1, (2) EGL-9 and RHY-1 have VHL-1-independent effects on transcription of some hypoxic response genes, and (3) EGL-9 and RHY-1 play a VHL-1-independent role in pathogen and hydrogen sulfide resistance (Horsman et al. 2019; Luhachack et al. 2012; Shao et al. 2009; Shao et al. 2010; Shen et al. 2005; Shen et al. 2006). However, the possibility that EGL-9 and RHY-1 modulate longevity through a downstream, VHL-1-independent transcriptional response has not been addressed. Here, we present a genetic study demonstrating that EGL-9 and RHY-1 are necessary for lifespan extension when HIF-1 is stabilized by vhl-1 mutation. We show that, like EGL-9, RHY-1 has both longevity promoting and inhibiting activities. We further identify genes that are oppositely regulated in vhl-1 and egl-9 or rhy-1 mutants, suggesting that RHY-1 and EGL-9 promote a VHL-1-independent transcriptional response when HIF-1 is stabilized by vhl-1 mutation. Lastly, we find that RNAi knockdown of four genes downregulated in vhl-1 mutants and upregulated in egl-9 mutants, with likely functions in innate immunity, each partially rescues lifespan extension in egl-9;vhl-1 mutants. Together, our results suggest that EGL-9 modulates lifespan by regulating a VHL-1-independent transcriptional program.
Results
RHY-1 and EGL-9 promote longevity in vhl-1 mutants
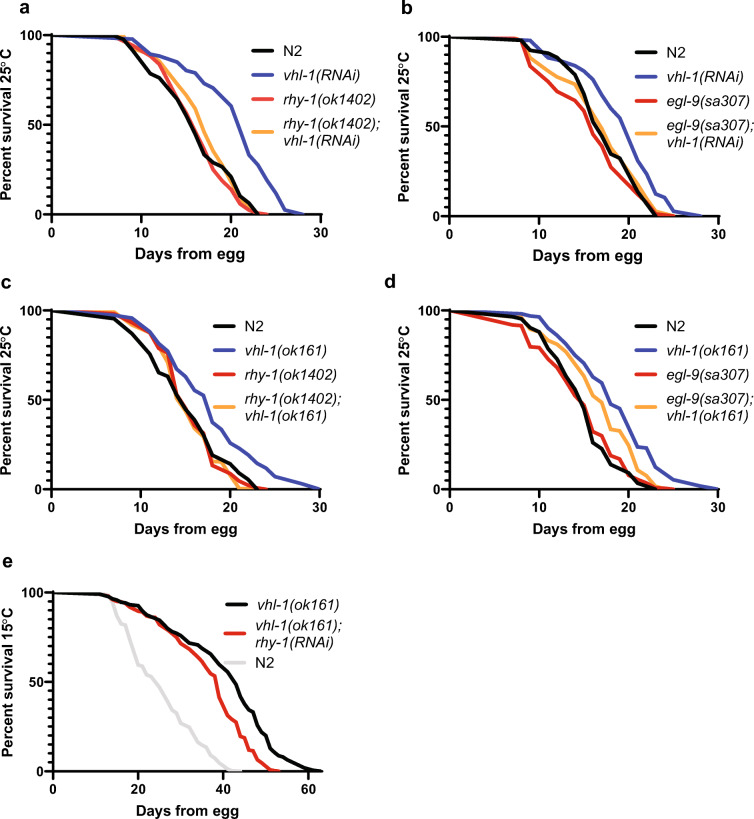
We initially hypothesized that the presence of EGL-9 and RHY-1 is necessary to promote longevity downstream of HIF-1 stabilization. This hypothesis would suggest that reduced activity of EGL-9 or RHY-1 would prevent or mitigate lifespan extension when HIF-1 is stabilized by reducing VHL-1 activity. Previous studies show that vhl-1(ok161) and egl-9(sa307) mutant strains show similar lifespan phenotypes at low temperatures (15 °C), making epistasis experiments difficult to interpret (Miller et al. 2017). However, at high temperatures, egl-9(sa307) mutants have short to wild-type lifespans, while vhl-1(ok161) mutants are long-lived (Di Chen and Kapahi 2009; Leiser et al. 2011; Miller et al. 2017). Thus, we tested whether egl-9(sa307) and/or rhy-1(ok1402) mutants suppress the extended longevity phenotype of vhl-1(RNAi)-treated or vhl-1(ok161) mutant animals at high temperature (25 °C). Our results (Fig. 1) show that rhy-1(ok1402) (Fig. 1a) and egl-9(sa307) (Fig. 1b) mutants abrogate the extended longevity phenotype caused by vhl-1(RNAi) at 25 °C. Furthermore, rhy-1(ok1402) fully abrogated the extended longevity phenotype of vhl-1(ok161) mutants (Fig. 1c), while egl-9(sa307) mutants partially suppressed the extended longevity phenotype of vhl-1(ok161) mutants at 25 °C (Fig. 1d). We also observed that rhy-1(RNAi) reduces the lifespan of vhl-1(ok161) mutants at low temperatures (15 °C (Fig. 1e)) and that the lifespan of rhy-1(ok1402) mutants are not extended by vhl-1(RNAi) at low temperatures (15 °C (Supplementary Figure 1)), suggesting that these interactions are not fully temperature-dependent. These results are consistent with a model where RHY-1 and EGL-9 act in the same pathway to promote lifespan downstream of HIF-1 stabilization.
Fig. 1.
Epistasis of hypoxic response regulators. a Lifespans of N2 (wild-type) or rhy-1(ok1402) mutants treated with empty vector or vhl-1(RNAi) at 25 °C. b Lifespans of N2 and egl-9(sa307) animals treated with empty vector and vhl-1(RNAi) at 25 °C. c Lifespans of N2, vhl-1(ok161), rhy-1(ok1402), and rhy-1(ok1402);vhl-1(ok161) animals at 25 °C. d Lifespans of N2, egl-9(sa307), vhl-1(ok161), and egl-9(sa307);vhl-1(ok161) animals at 25 °C. e Lifespans of vhl-1(ok161) animals treated with empty vector and rhy-1(RNAi) at 15 °C, along with N2 controls (p < .0001 by log-rank) Interactions of egl-9(sa307) and rhy-1(ok161) with vhl-1(ok161) and vhl-1(RNAi) were statistically different than interactions of N2 animals with vhl-1(ok161) and vhl-1(RNAi) by non-overlapping 95% confidence intervals of Mantel-Haenzel hazard ratios (Supplementary Table 1). Data are aggregated from at least three independent experiments (Supplementary Table 2)
RHY-1 has longevity promoting and inhibiting activities
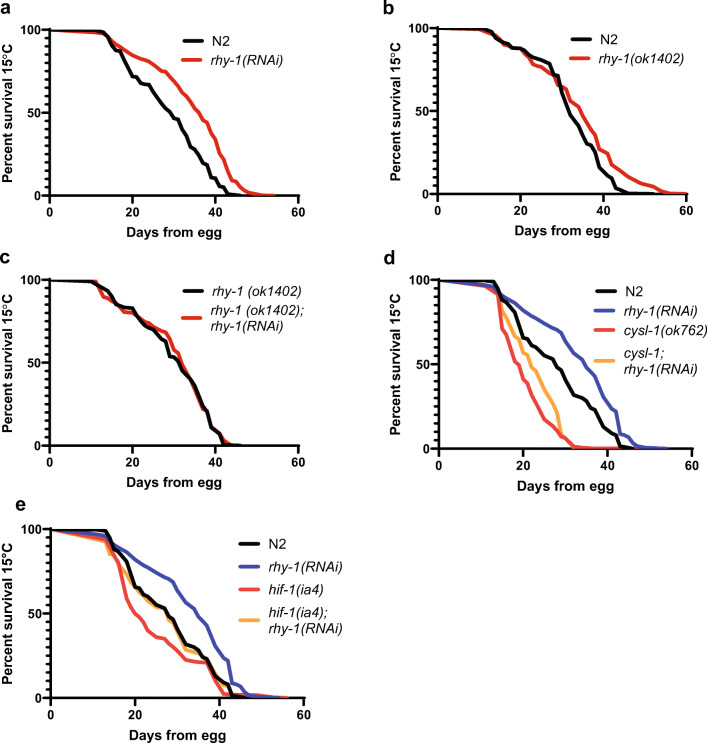
In the course of testing the effects of rhy-1(RNAi) on vhl-1(ok161) mutants at 15 °C, we observed that rhy-1(RNAi) substantially extended the lifespan of wild-type control animals (Fig. 2a). This result was surprising since we recently reported a study of the interaction between temperature and longevity and found that rhy-1(ok1402) mutants did not extend lifespan at any of the three temperatures (15 °C, 20 °C, or 25 °C) that are commonly used for longevity studies (Miller et al. 2017). We outcrossed rhy-1(ok1402) five additional times to wild-type to further eliminate any background mutations and repeated lifespan experiments. Consistent with our published data, the outcrossed rhy-1(ok1402) strain was not long-lived: observed lifespans were modestly longer than wild-type in two of four trials, identical to wild-type in one trial, and shorter than wild-type in one trial (Fig. 2b; Supplementary Figure 1). Lifespan extension by rhy-1(RNAi) was abrogated by rhy-1(ok1402), consistent with the longevity extension phenotype caused by rhy-1(RNAi) being dependent on modulation of the RHY-1 gene product (Fig. 2c). Furthermore, rhy-1(RNAi)-treated cysl-1(ok762) and hif-1(ia4) mutants are short-lived relative to rhy-1(RNAi)-treated wild-type animals, consistent with a model where HIF-1 and CYSL-1 activities are required for full lifespan extension by rhy-1(RNAi) (Fig. 2d, e). Importantly, rhy-1(RNAi) treatment does extend the lifespans of cysl-1(ok762) and hif-1(ia4) mutants relative to vector-treated controls (Fig. 2d, e), suggesting that it may modulate lifespan through a mechanism that is partially independent of the CYSL-1-HIF-1 pathway. The distinct phenotypes observed in rhy-1(ok1402) and rhy-1(RNAi) are similar to the reported differences between egl-9(RNAi), which extends lifespan at 20 °C, and the egl-9(sa307) mutant, which extends lifespan at 15 °C but not 20 °C or 25 °C (Di Chen and Kapahi 2009; Mehta et al. 2009; Miller et al. 2017; Zhang et al. 2009). These results are consistent with a model where partial reductions in activity of rhy-1 or egl-9 increase longevity by stabilizing HIF-1, while stronger reduction of rhy-1 or egl-9 causes a secondary effect that limits lifespan downstream of HIF-1 stabilization.
Fig. 2.
rhy-1-mediated regulation of longevity. a Lifespans of N2 (wild-type) animals treated with empty vector or rhy-1(RNAi) (p < 0.0001 by log-rank). b Lifespans of N2 and rhy-1(ok1402) animals (p < 0.0001 by log-rank). c Lifespans of rhy-1(ok1402) animals treated with empty vector or rhy-1(RNAi). d Lifespans of N2 and cysl-1(ok762) animals treated with vector or rhy-1(RNAi) (p < 0.001 for cysl-1(ok762);rhy-1(RNAi) vs rhy-1(RNAi) by log rank). e Lifespans of hif-1(ia4) and N2 animals treated with empty vector or rhy-1(RNAi) (p < 0.0001 for hif-1(ia4);rhy-1(RNAi) vs rhy-1(RNAi)). Interaction with rhy-1(RNAi) treatment was statistically different between N2 and hif-1(ia4) as determined by non-overlapping 95% confidence intervals of Mantel-Haenzel hazard ratios. Hazard ratios for cysl-1(ok762);rhy-1(RNAi)/cysl-1(ok762) and rhy-1(RNAi)/N2 were 0.58–0.82 and 0.44–0.64, respectively. Lifespan data are aggregated from at least three experiments (Supplementary Tables 1–2)
RHY-1 and EGL-9 control a VHL-1-independent transcriptional response
Previous studies reported that egl-9 causes vhl-1-independent changes in expression of some transcripts (Shao et al. 2009; Shen et al. 2005; Shen et al. 2006). We hypothesized that the dominance of the egl-9(sa307) and rhy-1(ok1402) lifespan phenotypes over the vhl-1(ok161) lifespan phenotype might be caused by genes whose transcription is regulated by EGL-9 or RHY-1. Concurrently with our work, Angeles et al. published an analysis of transcriptome profiles from rhy-1(ok1402), egl-9(sa307), hif-1(ia4), egl-9(sa307);hif-1(ia4), and egl-9(sa307);vhl-1(ok161) mutants. They identified a class of genes whose transcription is regulated by EGL-9 in a way that is distinct from, and dominant over, their regulation by VHL-1. We will refer to this class as EGL-9/VHL-1 antagonistic genes.
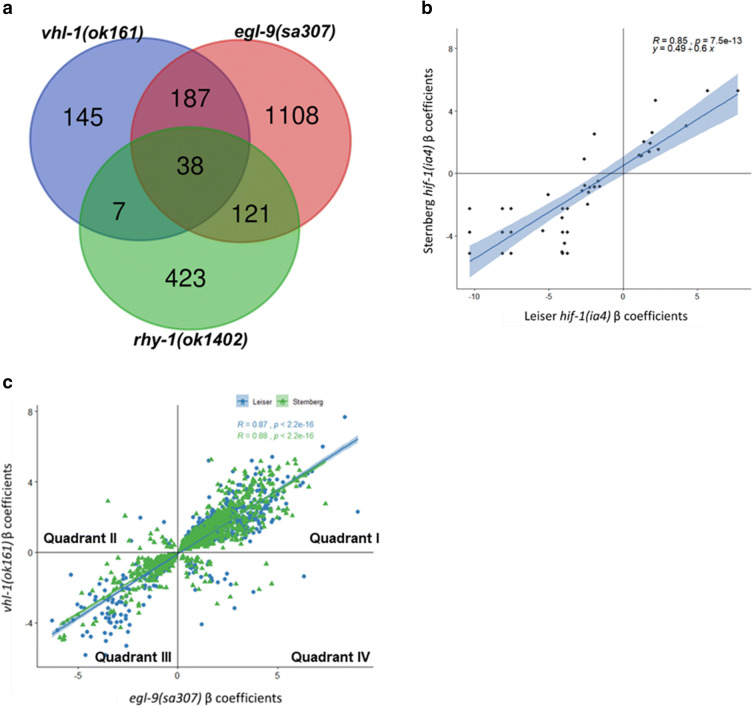
To identify genes that might modulate lifespan downstream of the hypoxic response, we had previously profiled the transcriptomes of egl-9(sa307), vhl-1(ok161), rhy-1(ok402), and hif-1(ia4) mutants. We reanalyzed our data using the methodology that Angeles et al. reported and an up-to-date bioinformatic pipeline (Angeles-Albores et al. 2018; Bray et al. 2016; Pimentel et al. 2017). Our datasets identified a subset of genes that were differentially expressed in the HIF-1-negative regulator mutants relative to wild-type, further confirming that these changes are implicated in the hypoxic response (Fig. 3a, Table 1, Supplementary Tables 3–4). Conversely, we observed low overlap between the datasets for genes differentially expressed in the hif-1(ia4) background, suggesting that differences between hif-1(ia4) and wild-type in individual datasets may largely reflect strain-specific effects rather than HIF-1-dependent transcription under normoxia (Fig. 3b).
Fig. 3.
A subset of genes are antagonistically regulated by egl-9 and vhl-1. a Overlap between genes that are differentially expressed in both Leiser and Sternberg datasets for vhl-1(ok161) and egl-9(sa307) along with differentially expressed genes from Sternberg rhy-1(ok1402). b Overlap between differentially expressed genes in hif-1(ia4) in Leiser and Sternberg datasets. c Correlation of expression levels of differentially expressed genes in egl-9(sa307) and vhl-1(ok161) in Leiser and Sternberg datasets
Table 1.
Core hypoxic response genes. List of 38 genes that were differentially regulated in rhy-1(ok1402) (Sternberg only), egl-9(sa307) and vhl-1(ok161) mutants in both Leiser and Sternberg RNA-seq datasets
| ORF | Gene | Description |
|---|---|---|
| K08C7.5 | fmo-2 | FMO5 (flavin containing dimethylaniline monoxygenase 5) ortholog |
| T05B4.2 | nhr-57 | Nuclear hormone receptor family transcription factor |
| C14C6.5 | C14C6.5 | Predicted role in innate immune response |
| F22D6.10 | col-60 | Collagen structural protein in cuticle |
| F26H9.5 | F26H9.5 | PSAT1 (phosphoserine aminotransferase 1) ortholog |
| C08E8.3 | C08E8.3 | Unknown function |
| K06A4.7 | K06A4.7 | Unknown function |
| F15B9.1 | far-3 | Fatty acid-binding protein |
| F38B2.4 | F38B2.4 | AK1 (adenylate kinase 1) ortholog |
| Y37A1B.5 | Y37A1B.5 | SELENBP1 (selenium binding protein 1) ortholog |
| F38A3.2 | ram-2 | Structural protein in cuticle |
| F28F8.2 | acs-2 | ACSF2 (acyl-CoA synthetase family member 2) ortholog |
| F08G5.4 | col-130 | Collagen structural protein in cuticle |
| T10D4.13 | ins-19 | Predicted insulin protein |
| C31C9.2 | C31C9.2 | PHGDH (phosphoglycerate dehydrogenase) ortholog |
| F22B5.4 | F22B5.4 | Unknown function |
| F10D2.9 | fat-7 | SCD5 (stearoyl-CoA desaturase 5) ortholog |
| R08E5.3 | R08E5.3 | Methyltransferase orthologs |
| Y22F5A.4 | lys-1 | Lysozyme activity, predicted role in innate immune response |
| C05E7.1 | C05E7.1 | Unknown function |
| Y58A7A.1 | Y58A7A.1 | SLC31A1 (solute carrier family 31 member 1) ortholog |
| M05D6.6 | taap-1 | FAM162A/B (family with sequence similarity 162 member A/B) ortholog |
| W07A12.7 | rhy-1 | Negative regulator of HIF-1 |
| M199.4 | clec-190 | C-type lectin predicted to have carbohydrate binding activity |
| B0222.6 | col-144 | Collagen structural protein in cuticle |
| M05D6.5 | M05D6.5 | HIGD2A/B (HIG1 hypoxia inducible domain family member 2A/B) ortholog |
| F09A5.9 | ttr-34 | Predicted transthyretin-like protein |
| W07A12.6 | oac-54 | Predicted amino-acyl transferase protein |
| F02H6.5 | sqrd-1 | SQOR (sulfide quinone oxidoreductase) ortholog |
| ZK637.13 | glb-1 | Predicted role in heme/oxygen binding and carrying |
| F54C9.4 | col-38 | Collagen structural protein |
| Y22F5A.5 | lys-2 | Lysozyme activity, predicted role in innate immune response |
| F37B1.8 | gst-19 | HPGDS (hematopoietic prostaglandin D synthase) ortholog |
| T03F1.11 | T03F1.11 | Predicted for CIBs (calcium and integrin binding family member) ortholog |
| VF13D12L.3 | VF13D12L.3 | Predicted oxidoreductase protein |
| C04G6.2 | C04G6.2 | Unknown function |
| K10B3.7 | gpd-3 | GAPDH (glyceraldehyde-3-phosphate dehydrogenase) ortholog |
| R02E4.3 | R02E4.3 | Unknown function |
We next plotted β coefficients of differentially expressed genes shared between vhl-1(ok161) and egl-9(sa307) in Leiser and Sternberg datasets (Fig. 3c). Both datasets produce a similar pattern, with egl-9(sa307), and vhl-1(ok161) causing highly correlated changes in expression for most shared differentially expressed genes. Both datasets also contained EGL-9/VHL-1 antagonistic genes, which were either upregulated in vhl-1(ok161) and downregulated in egl-9(sa307) (Fig. 3c, quadrant II), or upregulated in egl-9(sa307) or rhy-1(ok1402) and downregulated in vhl-1(ok161) (Fig. 3c, quadrant IV). The genes in quadrants II and IV, which we hypothesized could play a role in different outcomes between vhl-1(ok161) and egl-9(sa307) or rhy-1(ok1402) strains are listed in Supplementary Table 5. Together, our and the Sternberg lab’s results show that HIF-1 stabilization through loss of its negative regulators has many common effects and a smaller number of opposing effects, depending on which negative regulator is mutated.
Knockdown of EGL-9 target genes rescues lifespan of egl-9(sa307);vhl-1(ok161) mutants
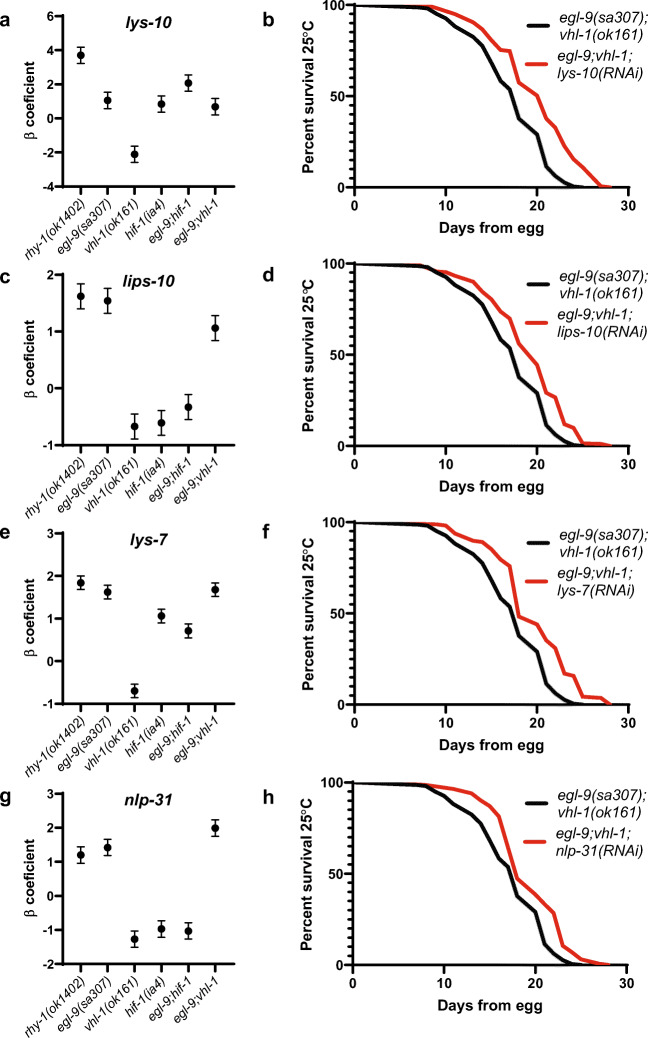
We next tested the hypothesis that EGL-9/VHL-1 antagonistic genes regulate longevity. Previous results suggest that individual longevity-pathway-target-genes often have small effects on lifespan, and longevity increases are more likely than longevity decreases to reflect modulation of the aging process as a whole (Murphy et al. 2003). Thus, we identified candidate EGL-9/VHL-1 antagonistic genes whose downregulation in vhl-1(ok161) mutants was reversed in egl-9(sa307);vhl-1(ok161) mutants and determined whether RNAi targeting them could extend life lifespan of egl-9(sa307);vhl-1(ok161) mutants (Fig. 4a, c, e, g; Supplementary Table 6).
Fig. 4.
VHL-1/EGL-9 antagonistic HIF-1 targets rescue lifespan in egl-9(sa307);vhl-1(ok161) mutants. a, c, e, g Expression levels of selected transcripts from RNA-seq analyses. b–h Treatment with nlp-31(RNAi), lys-7(RNAi), lys-10(RNAi) and lips-10(RNAi) increases lifespan of egl-9(sa307);vhl-1(ok161) mutants (p < .05 by log-rank). Lifespan data are aggregated from at least three experiments and are significant (p < .05 by log-rank with Bonferroni correction) in at least 3 of 5 individual trials (Supplementary Table 2)
EGL-9 and VHL-1 have distinct roles in pathogen resistance, with egl-9(sa307) mutants exhibiting HIF-1-dependent resistance to fast killing by Pseudomonas aeruginosa, while vhl-1(ok161) mutants do not (Luhachack et al. 2012; Shao et al. 2010). We noticed that our list of EGL-9/VHL-1 antagonistic genes contained several genes with reported or predicted roles in pathogen response, including nlp-31, which encodes five neuropeptide-like proteins with functions in defense against fungal pathogens and gram-negative pathogenic bacteria, lys-7, which encodes a lysozyme with a reported role in defense against gram-negative bacteria, lys-10, which encodes another lysozyme, and lips-10, which encodes an enzyme that, like lysozymes, has hydrolase activity (Couillault et al. 2004; Harris et al. 2010; Marsh et al. 2011; Nathoo et al. 2001). We found that treatment with lys-10(RNAi), lips-10(RNAi), lys-7(RNAi), and nlp-31(RNAi) each extended longevity of egl-9;vhl-1 mutants (Fig. 4b, d, f, h).
We also tested treatment with RNAi targeting the ferritin genes, ftn-1 and ftn-2, which are predicted oxidative and heavy metal response genes that have been identified as EGL-9/VHL-1 antagonistic genes in multiple studies (Ackerman and Gems 2012; Romero-Afrima et al. 2020). We found slight but significant increases in lifespan in egl-9(sa307);vhl-1(ok161) animals treated with ftn-1(RNAi) and ftn-2(RNAi); however, the magnitude of these changes was small and they were not consistent across individual replicates (significant in 2 of 4 trials) (Supplementary Figure 2; Supplementary Table 2). These data are consistent with ferritins playing a minor role in modulation of lifespan by the hypoxic response.
Taken together, these results are consistent with a model in which EGL-9 activity promotes longevity during HIF-1 stabilization through VHL-1-independent inhibition of target genes, including multiple genes with predicted functions in defense against pathogens.
Discussion
Collectively, our results show that, while reduction in RHY-1 and EGL-9 activity can increase lifespan via the hypoxic response, RHY-1 and EGL-9 activity also promote longevity downstream of HIF-1 stabilization by vhl-1 mutation (Fig. 5). We further demonstrate that RHY-1 and EGL-9 activity are required to control the direction of differential expression of numerous transcripts in vhl-1 mutants and that EGL-9-dependent suppression of nlp-31, lys-7, lys-10, and lips-10 promotes longevity in vhl-1 mutants.
Fig. 5.
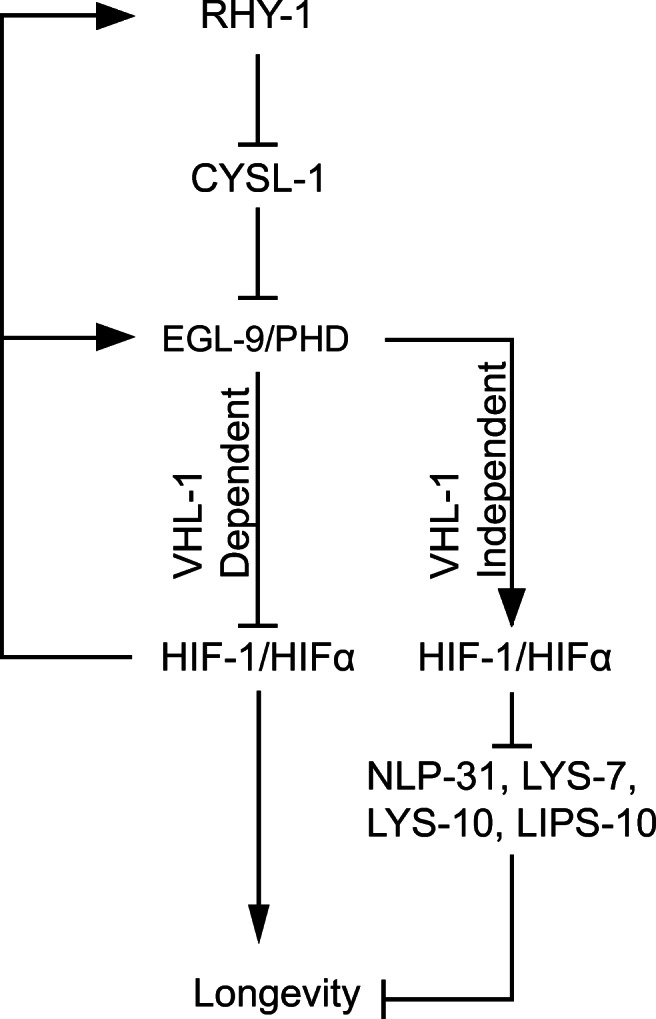
Epistatic model of lifespan regulation by VHL-1, RHY-1, and EGL-9. RHY-1 and EGL-9 act in the same pathway to inhibit HIF-1 activity through both VHL-1-dependent and VHL-1-independent mechanisms. When HIF-1 is stabilized through loss of VHL-1, expression of RHY-1 and EGL-9 is increased, driving reductions in expression of longevity reducing target genes including NLP-31, LYS-7, LYS-10, and LIPS-10 through a HIF-dependent and VHL-1-independent mechanism. Inhibition of EGL-9 activity causes upregulation of genes including NLP-31, LYS-7, LYS-10, and LIPS-10, inhibiting longevity
Genetic interactions of vhl-1, egl-9, and rhy-1
While HIF has emerged as a key regulator of longevity, it was previously unknown how hypoxic signaling pathway components interact to influence longevity. HIF-1 hydroxylation is required for its interaction with VHL-1, so in theory, we might expect egl-9 mutant phenotypes to be epistatic to vhl-1 mutant phenotypes. However, reports that (1) EGL-9 and RHY-1 have VHL-1-independent roles in the expression and tissue distribution of hypoxic response genes, (2) EGL-9 interacts with other proteins through proline-hydroxylase-activity-dependent and -independent mechanisms to influence phenotype, and (3) EGL-9 and RHY-1 are transcriptionally upregulated when HIF-1 is stabilized make these phenotypic interactions difficult to predict (Luhachack et al. 2012; Shao et al. 2009; Shao et al. 2010; Shen et al. 2005; Shen et al. 2006).
We found that rhy-1(ok1402) blocked lifespan extension by vhl-1(RNAi) and vhl-1(ok161), while egl-9(sa307) blocked lifespan extension by vhl-1(RNAi) and partially blocked lifespan extension by vhl-1(ok161). While these results are broadly consistent with a model where RHY-1 regulates lifespan through its known EGL-9 modulating activity, it is interesting that rhy-1 mutation has a stronger effect on the longevity of vhl-1(ok161) mutants than the egl-9(sa307) mutation. This could be explained by the reportedly more robust HIF-1 stabilization and upregulation of pro-longevity HIF-1 target genes in egl-9(sa307) relative to rhy-1(ok1402), or by an additional, HIF-1-independent role for RHY-1 in longevity determination (Shen et al. 2006).
We confirmed the surprising, previously reported, result that the rhy-1(ok1402) mutation does not extend lifespan, despite stabilizing HIF-1. However, interestingly, rhy-1(RNAi) extends lifespan through a mechanism that is partially dependent on its established interactions with CYSL-1 and HIF-1. While off-target effects of RNAi are a concern when mutant and RNAi phenotypes differ, the observation that the rhy-1(RNAi)-mediated lifespan increase is fully abrogated in a rhy-1(ok1402) mutant background strongly suggests that modulation of RHY-1 is the primary factor influencing lifespan in this context. It is worth noting that, while cysl-1(ok762) and hif-1(ia4) mutations reduce the longevity promoting effect of rhy-1(RNAi), neither completely abrogates it. This suggests that RHY-1 may have secondary, HIF-1-independent roles that influence longevity. Published reports showing that rhy-1;hif-1 compound mutants have a synthetic deleterious effect on fertility and that RHY-1 modulates hydrogen sulfide resistance in a HIF-1-independent manner also suggest interesting HIF-1-independent roles for RHY-1 (Horsman et al. 2019; Shen et al. 2006).
A published mechanism explaining the interaction between RHY-1 and HIF-1 suggests that rhy-1 mutants should largely phenocopy egl-9 mutants (Ma et al. 2012). While interpretation of egl-9 phenotypes is complicated by the lack of a viable egl-9 null mutant, published results do suggest that egl-9(n571), a point mutant that is predicted to affect splicing, and egl-9(RNAi) have more robust longevity promoting phenotypes than the strong loss-of-function mutant, egl-9(sa307), a deletion in the EGL-9 catalytic domain (Darby et al. 1999; Miller et al. 2017; Shao et al. 2009; Trent et al. 1983). These results are consistent with EGL-9 also having longevity promoting and limiting functions, with the mutant phenotype depending on the severity of the loss in activity. These data are consistent with a model in which EGL-9 and RHY-1 act in the same pathway to both limit wild-type longevity by destabilizing HIF-1 and increase longevity when HIF-1 is stabilized.
VHL-1-independent EGL-9 targets modulate lifespan
Along with other groups, we identified a substantial subset of targets that are transcriptionally regulated in opposite directions by EGL-9 and VHL-1 (Ackerman and Gems 2012; Angeles-Albores et al. 2018). This suggests that upregulation of EGL-9 and RHY-1 when HIF-1 is stabilized has substantial effects on the hypoxic transcriptome in addition to possible feedback regulation of HIF-1. Previous publications have established that EGL-9 has VHL-1-independent activities that can increase or reduce resistance to various pathogens (Luhachack et al. 2012; Shao et al. 2010). Here, we find that several RNAi clones targeting genes with reported or likely functions in innate immunity, nlp-31(RNAi), lys-7(RNAi), lys-10(RNAi), and lips-10(RNAi), extend lifespan in egl-9(sa307);vhl-1(ok161) mutants, suggesting that EGL-9-dependent downregulation of these genes promotes longevity in vhl-1(ok161) mutants.
The mechanisms underlying VHL-1-independent transcriptional regulation by EGL-9 have not been fully established. Previous studies report that egl-9(sa307) and hif-1(ia4) loss of function mutants cause transcriptional upregulation of the direct HIF-1 transcriptional target ftn-1, while vhl-1 loss of-function mutants and overexpression of non-hydroxylatable HIF-1 (P621A) suppress ftn-1 expression (Ackerman and Gems 2012; Romero-Afrima et al. 2020). These results are consistent with a model in which binding of hydroxylated and non-hydroxylated HIF-1 may have opposite effects on the ftn-1 promoter region, with vhl-1(ok161) mutation causing increases in hydroxylated HIF-1, while hif-1(ia4) and egl-9(sa307) mutation both eliminate hydroxylated HIF-1 (Ackerman and Gems 2012; Romero-Afrima et al. 2020). A recent analysis from the Sternberg group showed expression patterns consistent with a role for hydroxylated HIF-1 in expression of a larger set of transcripts that are oppositely affected by VHL-1 and EGL-9 (Angeles-Albores et al. 2018).
Other reports suggest that EGL-9 may trigger VHL-1-independent transcriptional responses through more complex mechanisms. Multiple labs report that EGL-9 affects gene expression through mechanisms that are independent of its hydroxylation activity (Luhachack et al. 2012; Shao et al. 2009). One study suggests that EGL-9 represses HIF-1 activity through a mechanism that requires physical interaction between EGL-9/PHD and the WD-repeat containing protein SWAN-1 (Shao et al. 2010). EGL-9 might also affect gene expression via hydroxylation of substrates other than HIF-1, with one study indicating that LIN-10 is a target of EGL-9 hydroxylation (Park et al. 2012). Mechanistic biochemical studies to (1) identify EGL-9 hydroxylation targets and protein-protein interaction partners and (2) establish whether hydroxylated and non-hydroxylated HIF-1 interact with distinct transcriptional complexes will be needed to fully understand the complex biological function of EGL-9/PHD.
Constitutive sterile activation of the innate immune response increases during mammalian aging and is a key driver of many age-related pathologies (Franceschi and Campisi 2014). A trade-off between constitutive immune activation and longevity has also been established in Drosophila (Libert et al. 2006). In C. elegans, immune-related signaling genes contribute to the longevity phenotypes of long-lived insulin signaling mutants; however, to our knowledge, this is the first evidence of antagonism between immune activation and non-pathogen-exposed survival in C. elegans (Murphy et al. 2003). Further mechanistic studies of the connection between pathogen resistance genes and accelerated aging-related phenotypes in tractable model systems may yield insights and interventions that can be translated to mammalian inflammatory aging pathologies.
Conclusion
Drugs that inhibit PHD activity are currently in clinical trials for anemia, and animal studies have suggested that they may have efficacy in models of neurodegenerative conditions, indicating that modulation of the hypoxic response to treat age-related disorders in humans may be on the horizon (Ashok et al. 2017; Haase 2017; Li et al. 2018; Mehta et al. 2009). However, the role of HIF-1 activity in modulating aging and age-related pathologies is highly complex. The finding that RHY-1 and EGL-9/PHD modulate aging by a VHL-1-independent mechanism indicates that further studies to illuminate the mechanisms underlying non-canonical roles of proline-hydroxylases in influencing transcription, aging, and disease will be critical to support effective translation of compounds that modulate the hypoxic response.
Methods
Worm culture and RNAi
Standard procedures for C. elegans strain maintenance and handling were used (Ahringer 2006; Stiernagle 2019). Experiments were performed on animals grown on Escherichia coli HT115 expressing empty vector or target RNAi from the Ahringer library. Nematode strains N2, rhy-1(ok1402), egl-9(sa307); vhl-1(ok161), hif-1(ia4), cysl-1(ok762) and egl-9(sa307);vhl-1(ok161) were obtained from the Caenorhabditis Genetics Center (CGC). rhy-1(ok1402) was outcrossed 5 times to a recently unfrozen CGC N2 strain, and rhy-1(ok1402);vhl-1(ok161) was made from outcrossed rhy-1(ok1402) using standard methods.
Lifespans
Lifespans were performed as described previously with minor modifications (Amrit et al. 2014). Animals were cultured on standard RNAi plates (NGM + 4 ml 1 M IPTG/L) inoculated with empty vector RNAi or the clone being tested, at the temperature of the assay, for at least two generations prior to measuring lifespans. Synchronized populations were transferred to lifespan plates (NGM + 100 mg Carbenicillin 4 ml 1 M IPTG/l + 660 μl 150 mM FUdR/L) at adulthood. Animals with age-related vulval integrity defects were included; animals that left the plate were not considered (Leiser et al. 2016). The authors are aware that FUdR can be confounding effector when interpreting survival epistasis. While previous studies have not shown an interaction between the hypoxic response and FUdR, the results presented in this study remain to be validated in the absence of FUdR. Replicates and statistics are included in Supplementary Table 1.
RNA isolation, sequencing, and analysis
Worm strains were synchronized by treating gravid adult worms with sodium hypochlorite and collecting ~ 1000 offspring per genetic condition. Once the offspring reached young adulthood, they were collected in M9 buffer and immediately flash-frozen in liquid nitrogen. RNA was extracted following Invitrogen’s TRIZOL RNA extraction protocol. Before library preparation, the samples were analyzed on an Agilent 2100 BioAnalyzer. Only samples with an RNA integrity numbers (RIN) equal to or greater than 9.0 were used in downstream analyses. Single-strand reverse transcription, library preparation, and sequencing were performed on an Illumina machine. Read alignments were mapped using Kallisto, and differential expression analyses were performed using Sleuth. We used a q-value cutoff of < .05 for downstream comparisons across conditions as it is adjusted for multiple hypothesis testing (Bray et al. 2016; Pimentel et al. 2017).
Electronic supplementary material
rhy-1-mediated effects of lifespan. A-C) Individual lifespan experiments with N2 and rhy-1(ok1402) animals treated with empty vector or vhl-1(RNAi). D). Additional lifespan trial comparing N2 and rhy-1(ok1402) lifespans on empty vector RNAi. E. Aggregated data from A-C. (PNG 262 kb)
Effects of additional VHL-1/EGL-9 antagonistic HIF-1 targets on lifespan. A, C) expression levels of selected transcripts from RNA-seq analyses. B-D) Treatment with ftn-1(RNAi), and ftn-2(RNAi) increases lifespan of egl-9(sa307);vhl-1(ok161) mutants (p < 0.05 by log-rank). Lifespan data are aggregated from at least three experiments, and are significant (p < 0.05 by log-rank with Bonferroni correction) in 2 out of 4 individual trials (Supplementary Table 2). (PNG 153 kb)
(XLSX 15 kb)
(XLSX 18 kb)
(XLSX 11 kb)
(XLSX 39 kb)
(XLSX 18 kb)
(XLSX 19 kb)
Acknowledgments
We thank David Angeles and the Sternberg lab for sharing their Kallisto analyses and expertise.
Funding information
This work was supported by NIH R01AG058717 and the Glenn Foundation for Medical Research. HAM was supported by NIH F31AG060663. Additionally, the University of Washington Nathan Shock Center provided support of gene expression analysis (NIH P30AG013280). Strains were provided by the Caenorhabditis Genetics Center that is funded by the NIH ORIP (P40OD010440).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ackerman D, Gems D. Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet. 2012;8(3):e1002498. doi: 10.1371/journal.pgen.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahringer, J. (2006). Reverse genetics WormBook: The Online Review of C. elegans Biology [Internet]: WormBook.
- Amrit FRG, Ratnappan R, Keith SA, Ghazi A. The C. elegans lifespan assay toolkit. Methods. 2014;68(3):465–475. doi: 10.1016/j.ymeth.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Angeles-Albores D, Robinson CP, Williams BA, Wold BJ, Sternberg PW. Reconstructing a metazoan genetic pathway with transcriptome-wide epistasis measurements. Proc Natl Acad Sci. 2018;115(13):E2930–E2939. doi: 10.1073/pnas.1712387115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok BS, Ajith TA, Sivanesan S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin Exp Pharmacol Physiol. 2017;44(3):327–334. doi: 10.1111/1440-1681.12717. [DOI] [PubMed] [Google Scholar]
- Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang G-W, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275(33):25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou J-F, Kohara Y, Ewbank JJ. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5(5):488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- Darby C, Cosma CL, Thomas JH, Manoil C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc Natl Acad Sci. 1999;96(26):15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chen ELT, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5(5):e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Series A. 2014;69(Suppl_1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Haase VH. Therapeutic targeting of the HIF oxygen-sensing pathway: lessons learned from clinical studies. Exp Cell Res. 2017;356(2):160–165. doi: 10.1016/j.yexcr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TW, Antoshechkin I, Bieri T, Blasiar D, Chan J, Chen WJ, et al. WormBase: a comprehensive resource for nematode research. Nucleic Acids Res. 2010;38(suppl_1):D463–D467. doi: 10.1093/nar/gkp952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman JW, Heinis FI, Miller DL. A novel mechanism to prevent H2S toxicity in Caenorhabditis elegans. Genetics. 2019;213(2):481–490. doi: 10.1534/genetics.119.302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci. 2001;98(14):7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Kaeberlein M. The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging. Biol Chem. 2010;391(10):1131–1137. doi: 10.1515/bc.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011;10(2):318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Jafari G, Primitivo M, Sutphin GL, Dong J, Leonard A, Fletcher M, Kaeberlein M. Age-associated vulval integrity is an important marker of nematode healthspan. Age. 2016;38(5–6):419–431. doi: 10.1007/s11357-016-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cui X-X, Chen Y-J, Wu T-T, Xu H, Yin H, Wu Y-C. Therapeutic potential of a prolyl hydroxylase inhibitor FG-4592 for Parkinson’s diseases in vitro and in vivo: regulation of redox biology and mitochondrial function. Front Aging Neurosci. 2018;10:121. doi: 10.3389/fnagi.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFκB signaling. Aging Cell. 2006;5(6):533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Luhachack LG, Visvikis O, Wollenberg AC, Lacy-Hulbert A, Stuart LM, Irazoqui JE. EGL-9 controls C. elegans host defense specificity through prolyl hydroxylation-dependent and-independent HIF-1 pathways. PLoS Pathog. 2012;8(7). [DOI] [PMC free article] [PubMed]
- Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73(5):925–940. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EK, van den Berg MC, May RC. A two-gene balance regulates Salmonella typhimurium tolerance in the nematode Caenorhabditis elegans. PLoS One. 2011;6(3):e16839. doi: 10.1371/journal.pone.0016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324(5931):1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Fletcher M, Primitivo M, Leonard A, Sutphin GL, Rintala N, Kaeberlein M, Leiser SF. Genetic interaction with temperature is an important determinant of nematode longevity. Aging Cell. 2017;16(6):1425–1429. doi: 10.1111/acel.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-U, Fabretti F, Zank S, Burst V, Benzing T, Schermer B. The von Hippel Lindau tumor suppressor limits longevity. J Am Soc Nephrol. 2009;20(12):2513–2517. doi: 10.1681/ASN.2009050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, Hart AC. Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci. 2001;98(24):14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Ghose P, Shao Z, Ye Q, Kang L, Xu XS, et al. Hypoxia regulates glutamate receptor trafficking through an HIF-independent mechanism. EMBO J. 2012;31(6):1379–1393. doi: 10.1038/emboj.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A review. Curr Mol Med. 2018;18(6):343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- Pimentel H, Bray NL, Puente S, Melsted P, Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat Methods. 2017;14(7):687–690. doi: 10.1038/nmeth.4324. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan SK, Shah YM. Role of intestinal HIF-2α in health and disease. Annu Rev Physiol. 2016;78:301–325. doi: 10.1146/annurev-physiol-021115-105202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Afrima L, Zelmanovich V, Abergel Z, Zuckerman B, Shaked M, Abergel R, et al. Ferritin is regulated by a neuro-intestinal axis in the nematode Caenorhabditis elegans. Redox Biol. 2020;28:101359. doi: 10.1016/j.redox.2019.101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhang Y, Powell-Coffman JA. Two distinct roles for EGL-9 in the regulation of HIF-1-mediated gene expression in Caenorhabditis elegans. Genetics. 2009;183(3):821–829. doi: 10.1534/genetics.109.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhang Y, Ye Q, Saldanha JN, Powell-Coffman JA. C. elegans SWAN-1 binds to EGL-9 and regulates HIF-1-mediated resistance to the bacterial pathogen Pseudomonas aeruginosa PAO1. PLoS Pathog. 2010;6(8). [DOI] [PMC free article] [PubMed]
- Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem. 2005;280(21):20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- Shen C, Shao Z, Powell-Coffman JA. The Caenorhabditis elegans rhy-1 gene inhibits HIF-1 hypoxia-inducible factor activity in a negative feedback loop that does not include vhl-1. Genetics. 2006;174(3):1205–1214. doi: 10.1534/genetics.106.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of Caenorhabditis elegans. African Scientist. 2019;16(2):55–68. [Google Scholar]
- Treinin M, Shliar J, Jiang H, Powell-Coffman JA, Bromberg Z, Horowitz M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol Genomics. 2003;14(1):17–24. doi: 10.1152/physiolgenomics.00179.2002. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104(4):619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS One. 2009;4(7). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rhy-1-mediated effects of lifespan. A-C) Individual lifespan experiments with N2 and rhy-1(ok1402) animals treated with empty vector or vhl-1(RNAi). D). Additional lifespan trial comparing N2 and rhy-1(ok1402) lifespans on empty vector RNAi. E. Aggregated data from A-C. (PNG 262 kb)
Effects of additional VHL-1/EGL-9 antagonistic HIF-1 targets on lifespan. A, C) expression levels of selected transcripts from RNA-seq analyses. B-D) Treatment with ftn-1(RNAi), and ftn-2(RNAi) increases lifespan of egl-9(sa307);vhl-1(ok161) mutants (p < 0.05 by log-rank). Lifespan data are aggregated from at least three experiments, and are significant (p < 0.05 by log-rank with Bonferroni correction) in 2 out of 4 individual trials (Supplementary Table 2). (PNG 153 kb)
(XLSX 15 kb)
(XLSX 18 kb)
(XLSX 11 kb)
(XLSX 39 kb)
(XLSX 18 kb)
(XLSX 19 kb)