Highlights
-
•
Complete resection of the gastric GIST in a prompt matter must be done to avoid potentially deadly complications.
-
•
If there are the necessary tools to enhance the patient’s recovery, we shouldn’t hesitate to use them.
-
•
In emergencies, time is of essence, and surgical services must rise to the challenge.
Keywords: Gastrointestinal stromal tumors, Gastric GIST, Upper gastrointestinal bleeding, Covid-19, Cooperative approach
Abstract
Introduction
Upper gastrointestinal bleeding is one of the major manifestations of the stomach's gastrointestinal stromal tumors; when gastric GISTs bleed, they are associated with a poor prognosis and must be treated promptly to avoid dangerous complications. A worrisome side effect of the Covid-19 pandemic is the delay in surgical treatment for seriously ill patients, a rise in surgical complications due to delayed care, lack of access to the healthcare system, and patients' hesitancy to seek care due to fear of the virus. In Ecuador, where limitations were present even in the absence of a pandemic, we are yet to fully know the full extent of the damage this pandemic has caused to ourselves and our patients.
Presentation of case
We present the case of a 51-year-old female; she presented with upper gastrointestinal bleeding, and a gastric GIST was diagnosed. Due to the size and the symptoms, surgery was planned. Nonetheless, as Ecuador was hit hard by the Covid-19 pandemic to cope with the respiratory patients, surgeries were delayed or canceled. Our patient surgery was delayed for five months until she presented with severe gastrointestinal bleeding that required urgent action and care. Thankfully, she completely recovered.
Discussion and conclusions
This case is an example of the many complications we expect due to the pandemic; delay in treatment leads to troublesome complications. In these emergencies, time is of the essence, and surgical services must rise to the challenge; in a particular way, this case also proves that if there are the necessary tools to enhance the patient's recovery, we should hesitate to use them. Complete resection of the gastric GIST in a prompt matter must be done to avoid these potentially deadly complications.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are rare neoplasms of the gastrointestinal tract [1]. They are usually asymptomatic and detected incidentally by computed tomography (CT) or endoscopies [2,3]. When these tumors present with symptoms, they are generally vague and non-specific [1]. Upper gastrointestinal bleeding is the most common symptom when diagnosing a GIST; however, it is usually self-controlled or found incidentally in a positive fecal occult blood test [1,4]. On rare occasions, the tumor can cause severe bleeding leading to hypotension that must be controlled using surgery or endoscopic techniques [1,5]. We present a case of a 51-year-old female, a GIST was diagnosed, and surgery was planned.
Nonetheless, as the Covid-19 pandemic unfolded in Ecuador, her surgery was delayed. Suddenly, she experienced severe upper gastrointestinal bleeding that required urgent surgery and care. After a cooperative laparoscopic and endoscopic approach, she completely recovered, and on follow-ups, is doing well.
This work has been reported in line with the SCARE criteria [15].
2. Case report
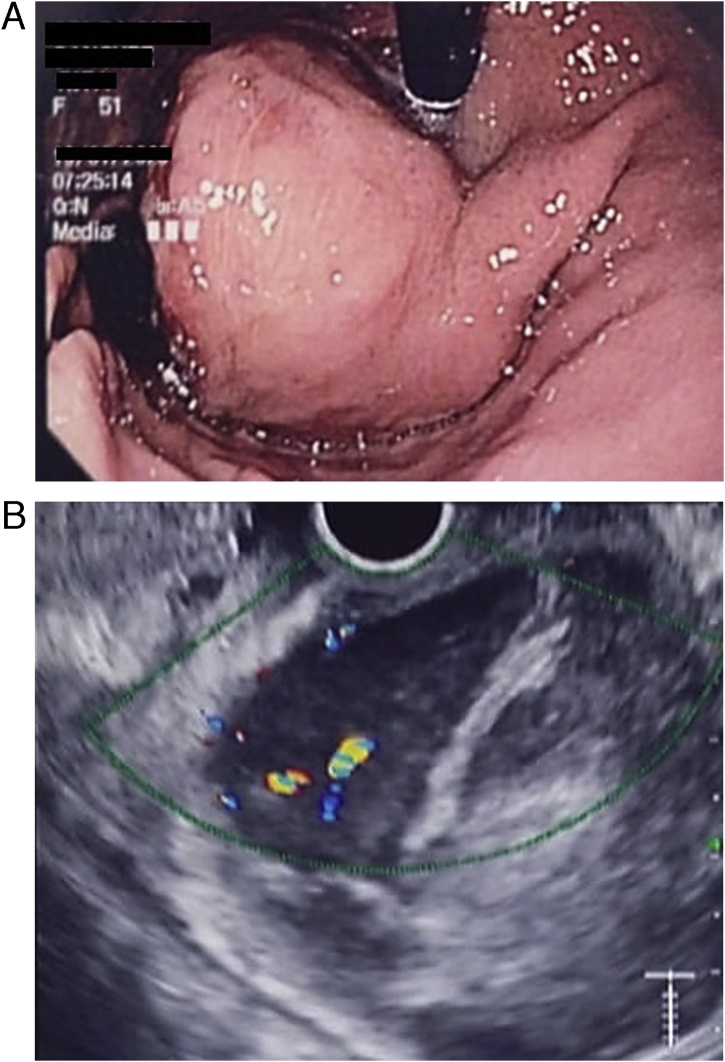
Patient is a 51-year-old female with a past medical history of hypothyroidism and pulmonary tuberculosis. Three years before seeking medical attention, she experienced mild episodes of upper abdominal pain associated with dark black, tarry feces. During the past year, these episodes became more frequent; thus, a gastroenterologist consultation was required. Anemia (Hb; 11 mg/dl) and fecal occult blood test was positive at that time. Due to this, an upper endoscopy was requested. A 103 × 46.6 mm submucosal mass in the lesser curvature 5 cm away from the cardias covered with normal mucosa was detected (Fig. 1A).
Fig. 1.
A; Upper endoscopy, the tumor is located on the lesser curvature. B; Endoscopic ultrasound the low-echo lesion had a regular and clear margin and was inside the submucosal layer.
To further investigate the submucosal tumor, endoscopic ultrasound (EUS) was done, revealing that the low-echo lesion had a regular and clear margin and was inside the submucosal layer. A color doppler image showed a vascular signal extending into the center of the low-echo lesion from the periphery. EUS fine-needle aspiration was achieved for pathological evaluation and was completed safely without any complications (Fig. 1B). Immunostaining was performed, revealing positivity for CD34 and CD 117, and was negative for S100 and anti-α-Sm-1. Gastric GIST was diagnosed. A contrast-enhanced abdominal computed tomography (CT) unveiled the 102 × 56.3 × 46.6 mm gastric mass. It also didn't reveal any masses or lymph nodes; thus, surgery was decided, and a preoperative check-up was scheduled.
Nonetheless, as the Covid-19 pandemic started to unfold in Ecuador, most surgeries were delayed or canceled. The outpatient clinic was closed, and the quarantine combined with the fear of Covid-19 caused anxiety in the general population and affected all our patients. In our case, the patient missed her appointments and remained untreated for five months.
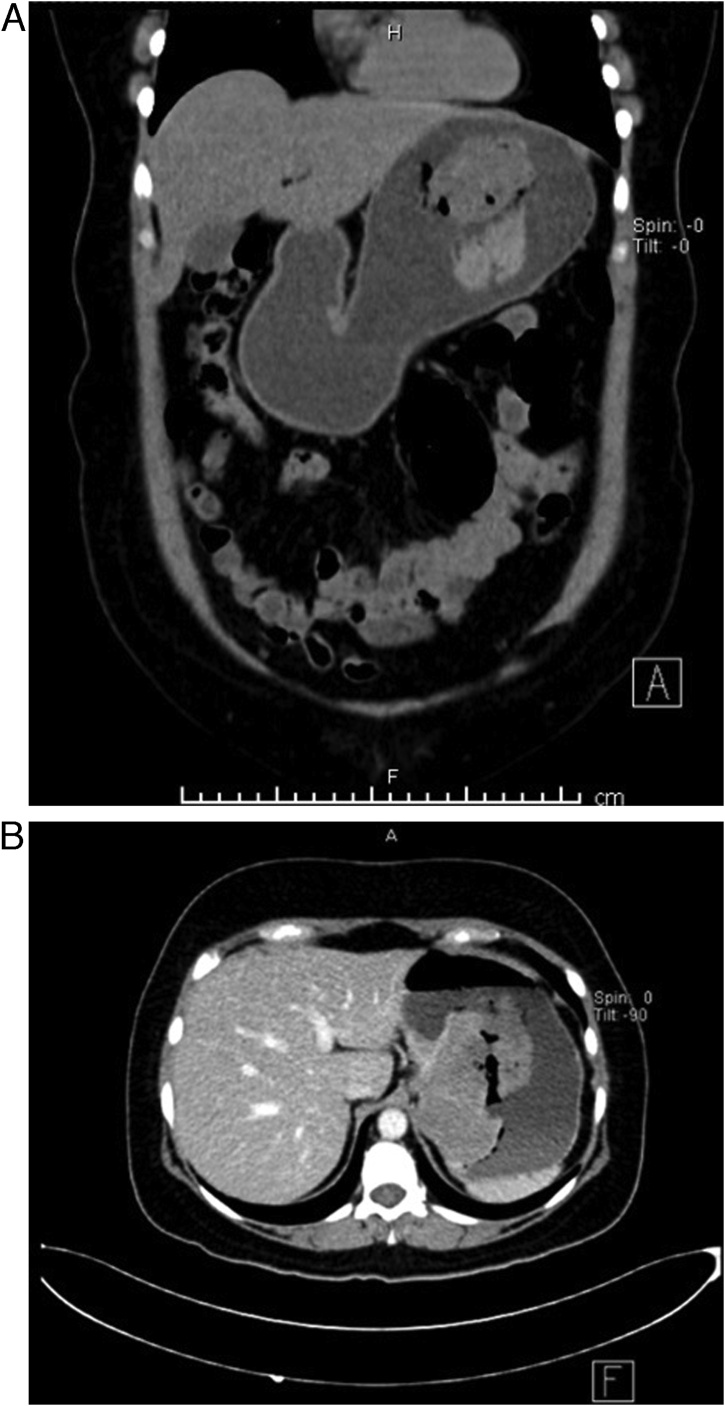
Since then, the patient endured the same symptoms; nonetheless, they became more severe as time passed. Suddenly, the patient underwent severe hematochezia, followed by fatigue and syncope; thus, she was brought immediately to the emergency room. On clinical evaluation, a hypotensive and diaphoretic patient was encountered. After reanimation, mild abdominal pain was detected in her upper abdomen. Complementary exams revealed severe anemia (Hb: 5.5 mg/dl), and a contrast-enhanced computed tomography revealed the previous 102 × 56.3 × 46.6 mm gastric tumor; however, contrast extravasation was seen from the tumor vessels into the gastric cavity (Fig. 2A & B). Two red cell concentrates were transfused, and surgery was decided. A laparoscopic and endoscopic cooperative approach was chosen. On laparoscopy, no masses, lymph nodes, or free liquid was seen. After this, three (10 mm, 10 mm, and 5 mm) gastrostomies were done using an ultrasound device (Harmonic, Ethicon, Johnson & Johnson), the trocars were placed through them, and the inner gastric surface was seen.
Fig. 2.
A: CT, the tumor is seen in the gastric wall. B: CT, showing contrast extravasation of the tumor.
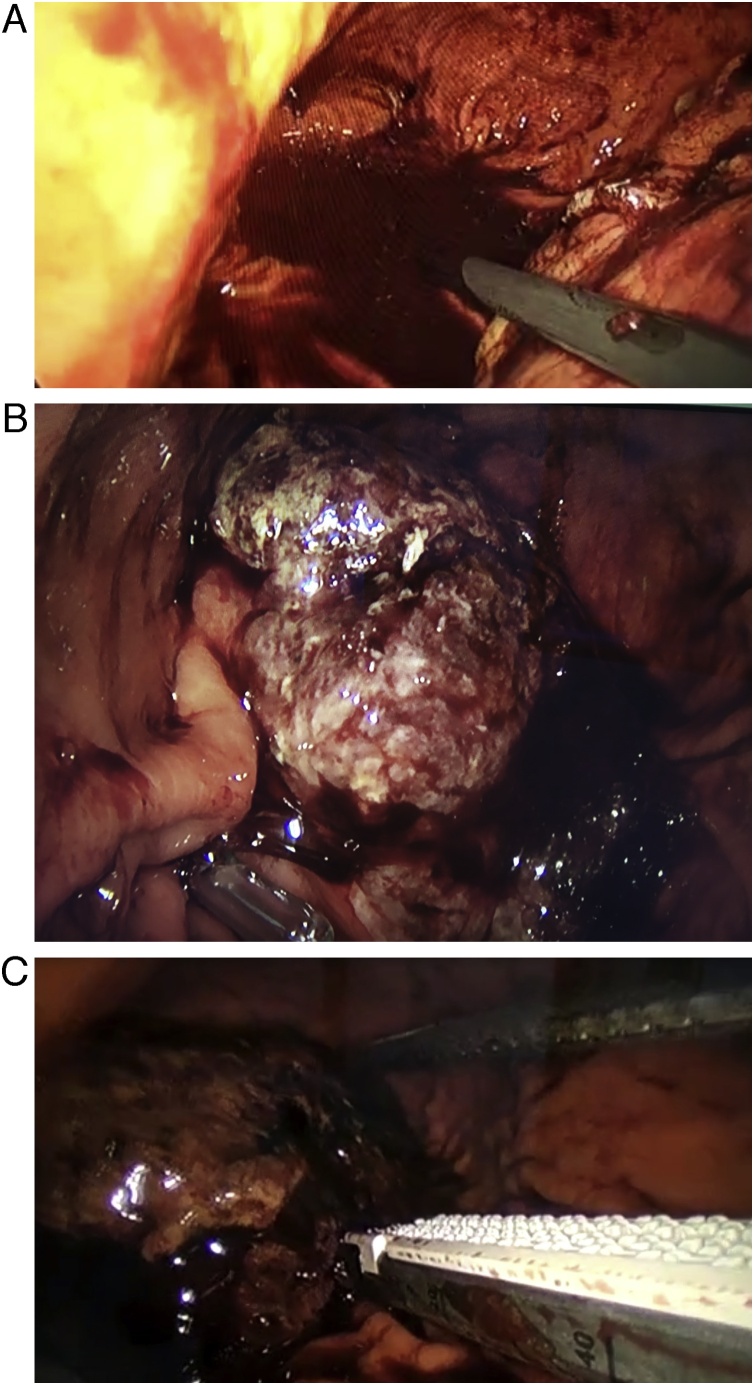
The bleeding gastric GIST was detected; it measured about 60 × 46 mm and had a 2 × 3 mm bleeding ulcer; the tumor was identified and grasped with the endoscope. After gaining adequate traction and using two consecutive 2.5 mm endo staplers (30 mm), the tumor was completely resected. After this, the tumor was extracted, the trocars were removed from the stomach, and the gastrostomies were closed using a 3-0 sterile synthetic absorbable monofilament. (PDS, Johnson & Johnson Medical) The rest of the procedure was completed without complications (Fig. 3A–C).
Fig. 3.
A: Blood is seen in the gastric cavity. B: Gastric GIST, with clot and ulcer. C: Resection of the Gastric GIST.
Pathology reported a total tumor excision; the tumors were composed of spindle cells with eosinophilic cytoplasm and low mitosis. Gastric GIST was the final diagnosis.
The postoperative course was uneventful; after blood transfusion and surgery, she remained stable, and liquids were initiated on the first postoperative day. She was discharged on postoperative day five without any complications. On follow-ups, two months later, the patient is doing well without any difficulties.
3. Discussion
Gastrointestinal stromal tumors (GISTs) are rare tumors that originate from the interstitial cells of Cajal; they usually express CD-117, a key antigen that triggers the growth mutation of these tumors [1,2]. They are generally rare, accounting for 1–2% of the gastrointestinal neoplasms, and are typically found in the stomach (56%) [1,2]. Nonetheless, they can be located in any part of the gastrointestinal tract (small bowel 32%, colon 6%, esophagus 0.7%). They regularly appear in middle-aged men with a peak incidence in the sixth decade of life [2,3].
GISTS are usually asymptomatic and found incidentally on CT scans, endoscopy, or other surgical procedures [3,4]. Nonetheless, they can present with a broad range of gastrointestinal symptoms according to their location or size, including abdominal pain, satiety, nausea, or abdominal mass [1]. In more unusual occasions, GISTs can perforate the bowel wall causing peritonitis and acute abdomen [1,2]. Gastrointestinal bleeding is the most common symptom of gastric GIST (54%), and in a few cases, the gastric GIST's acute bleeding is so severe that it may require embolization or surgical resection [4,5]. Cancer-related bleeding account for only 5% of all the causes of upper gastrointestinal bleeding; gastric GISTs tend to bleed when they are larger than 5 cm and are Ki-67 positive [[4], [5], [6]]. It is believed that as tumors grow, bleeding could be caused by a mucosal break or ulceration; Ki-67 also plays a role as it is a biomarker of tumor proliferation [5]. If Ki-67 is present and the tumor is more significant than 5 cm, surgical resection should be considered promptly [1,4].
In our case, the patient presented with gastrointestinal bleeding and anemia, a Gastric GIST was diagnosed, and surgery was planned.
When a submucosal tumor is discovered, Distinguishing between a benign and malignant tumor may be difficult since traditional endoscopy is insufficient. Leiomyoma, leiomyosarcoma, Schwannoma, lipoma, neurofibroma, and other tumors can be among the differential, and further investigation is needed to diagnose the patient accurately; endoscopic ultrasound, fine needle aspiration, computed tomography magnetic resonance imaging, or positron emission tomography can be used [13,14].
Histopathology was usually needed to confirm the diagnosis; nonetheless, since the introduction of endoscopic ultrasound in early 1980, the diagnosis of subepithelial tumors has improved considerably and is an invaluable tool for reaching a prompt diagnosis. Yet, histopathology is still the gold standard [1,4,5].
As typical for sarcomas, GISTs generally do not metastasize to the regional lymph nodes but instead can spread to the liver or metastasize to the peritoneum. Due to this unpredictable behavior, all GISTs must be treated as potentially malignant [13,14].
In our case, as the submucosal tumor was discovered, endoscopic ultrasound was performed to aid in the diagnosis; due to its findings, fine-needle aspiration and pathological were completed afterward, which confirmed our suspicion. For preoperative planning, a CT was done, which ruled out metastasis, masses, or lymph nodes.
GISTs have three different histologic findings, including spindle (70%), epithelioid (20%), or mixed type (10%) [2]. They are often misdiagnosed as leiomyoma or leiomyosarcoma before immunohistochemical analysis, and approximately 88% of GISTs stain positive for both CD117 and DOG-1 [1,3]. Many classification systems estimate the risk of metastases and recurrence, including the tumor's size, the mitotic count, and the tumor's location [1,2]. Surgical resection with free margins of tumor disease is the standard of care, and complete resection must be achieved to provide definite treatment [7,8]. With the rise of laparoscopic and endoscopic techniques, the surgical treatment of these rare tumors has improved considerably [9]. Cooperative or hybrid procedures using both endoscopy and laparoscopy have been successful in many reports and will be based on tumor location and size [10,11]. In our case, the tumor presented as severe bleeding, and surgery was required; since all the technical equipment and human personal were available at that time, successful treatment was achieved.
Adjuvant therapy with a tyrosine kinase inhibitor is recommended for a bigger tumor or in cases in which complete resection is not completed [1,4]. Also, gastric GIST bleeding is associated with worse outcomes as 5-year survival rates were significantly lower in the bleeding group than in the non-bleeding group [5,6].
When encountered with severe gastric GIST bleeding, embolization, endoscopy, or surgery are available to stop the bleeding [1,4]. Surgical resection seems more appropriate as it contains the bleeding and removes the lesion curatively [1,5]. As we completed in our patient.
As the Covid-19 pandemic continues to evolve, the full ramifications of this disease are yet to be seen; we have seen an increase in complications due to delayed surgical care and hesitancy of patients to seek care due to the fear of the virus, coordinated action is required to minimize the aftermath of this pandemic [12]. This case is an example of the many complications we expect due to the pandemic; delay in treatment leads to troublesome complications. In these emergencies, time is of essence, and surgical services must rise to the challenge.
4. Conclusions
A cooperative technique will depend exclusively on the team's expertise. The advantages will dramatically improve the patient's outcome as any technical difficulty can be weighted down and shared between the endoscopic and laparoscopic team, although further studies are required to assess this technique's implementation thoroughly. If there are the necessary tools available and a multidisciplinary team to enhance the patient's recovery, we should hesitate to use them. In any case, complete resection of the gastric GIST in a prompt matter must be done to avoid these potentially deadly complications.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
No funding.
Ethical approval
We have written consent of the patient to publish this article.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
-
1.
William G. Aguayo MD Writing the paper
-
2.
F. Xavier Moyon MD, Design
-
3.
Gabriel A. Molina MD, Writing the paper
-
4.
Miguel A. Moyon Study concept
-
5.
Daniela J. Rivadeneira Data collection
-
6.
Christian L. Rojas Interpretation
-
7.
B. Andrés Cárdenas Interpretation
-
8.
Maria Mercedes Cobo Data analysis
-
9.
Katherine Romero Writing the paper
Registration of research studies
Not applicable.
Guarantor
Gabriel A. Molina MD.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Contributor Information
William G. Aguayo, Email: williamaguayomd@hotmail.com.
F. Xavier Moyon, Email: xavomcons@hotmail.com.
Gabriel A. Molina, Email: gabomolina32@gmail.com.
Miguel A. Moyon, Email: mmoyon.surgeon@gmail.com.
Daniela J. Rivadeneira, Email: dany_ma13@hotmail.com.
Christian L. Rojas, Email: doctorbroco@hotmail.com.
B. Andrés Cárdenas, Email: andrescardenasp@gmail.com.
Maria M. Cobo, Email: mcobo@usfq.edu.ec.
A. Katherine Romero, Email: angelicakatherineromero@gmail.com.
References
- 1.Parab T.M., DeRogatis M.J., Boaz A.M., Grasso S.A., Issack P.S., Duarte D.A. Gastrointestinal stromal tumors: a comprehensive review. J. Gastrointest. Oncol. 2018;10(1):144–154. doi: 10.21037/jgo.2018.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pih G.Y., Jeon S.J., Ahn J.Y., Na H.K., Lee J.H., Jung K.W. Clinical outcomes of upper gastrointestinal bleeding in patients with gastric gastrointestinal stromal tumor. Surg. Endosc. 2019;34(2):696–706. doi: 10.1007/s00464-019-06816-9. [DOI] [PubMed] [Google Scholar]
- 3.Chetta N., Picciariello A., Nagliati C., Balani A., Martines G. Surgical treatment of gastric GIST with acute bleeding using laparoscopic sleeve gastrectomy: a report of two cases. Clin. Case Rep. 2019;7(4):776–781. doi: 10.1002/ccr3.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chetta N., Martines G., Picciariello A., Capuano P. Successful laparoscopic sleeve gastrectomy in emergency for a gastric gastrointestinal stomal tumor (GIST) with acute bleeding: a case report. Am. J. Case Rep. 2018;19:849–853. doi: 10.12659/ajcr.909798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo C., Canhoto C., Manata F., Bernardes A. Surgical treatment of giant gist with acute gastrointestinal bleeding: case report. Int. J. Surg. Case Rep. 2018;53:354–357. doi: 10.1016/j.ijscr.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catena F., Di Battista M., Fusaroli P., Ansaloni L., Di Scioscio V., Santini D. Laparoscopic treatment of gastric gist: report of 21 cases and literature’s review. J. Gastrointest. Surg. 2007;12(3):561–568. doi: 10.1007/s11605-007-0416-4. [DOI] [PubMed] [Google Scholar]
- 7.De Vogelaere K., Van Loo I., Peters O., Hoorens A., Haentjens P., Delvaux G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg. Endosc. 2012;26(8):2339–2345. doi: 10.1007/s00464-012-2186-7. [DOI] [PubMed] [Google Scholar]
- 8.Hiki N., Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: updated indications. Ann. Gastroenterol. Surg. 2019;3(3):239–246. doi: 10.1002/ags3.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ntourakis D. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: current status. World J. Gastroenterol. 2015;21(43):12482. doi: 10.3748/wjg.v21.i43.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiki N., Nunobe S., Matsuda T., Hirasawa T., Yamamoto Y., Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig. Endosc. 2015;27(2):197–204. doi: 10.1111/den.12404. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto H., Yaguchi Y., Kumano I., Takahata R., Ono S., Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J. Surg. 2011;36(2):327–330. doi: 10.1007/s00268-011-1387-x. [DOI] [PubMed] [Google Scholar]
- 12.Molina G.A., Rojas C.L., Aguayo W.G., Moyon C.M.A., Moyon F.X., Herrera J.M. COVID-19 in Ecuador, how the pandemic strained the surgical healthcare systems over the edge. Int. J. Surg. Open. 2020;26:106–107. doi: 10.1016/j.ijso.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponsaing L.G. Classification of submucosal tumors in the gastrointestinal tract. World J. Gastroenterol. 2007;13(24):3311. doi: 10.3748/wjg.v13.i24.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponsaing L.G. Diagnostic procedures for submucosal tumors in the gastrointestinal tract. World J. Gastroenterol. 2007;13(24):3301. doi: 10.3748/wjg.v13.i24.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]