Summary
Among laboratory mouse strains many genes are differentially expressed in the same cell population. As consequence, gene targeting in 129-derived embryonic stem cells (ESCs) and backcrossing the modified mice onto the C57BL/6 background can introduce passenger mutations in the close proximity of the targeted gene. Here, we demonstrate that several transgenic mice carry a P2rx7 passenger mutation that affects the function of T cells. By the example of P2rx4tm1Rass we demonstrate that P2X4ko T cells express higher levels of P2X7 and are more sensitive toward the P2X7 activators ATP and NAD+, rendering these cells more vulnerable toward NAD-induced cell death (NICD) compared with wild type (WT). The enhanced NICD sensitivity confounded functional assays e.g. cytokine production and cell migration. Our results need to be considered when working with P2rx4tm1Rass mice or other 129-based transgenic strains that target P2rx7 neighboring genes.
Subject Areas: Genetics, Immunology
Graphical Abstract
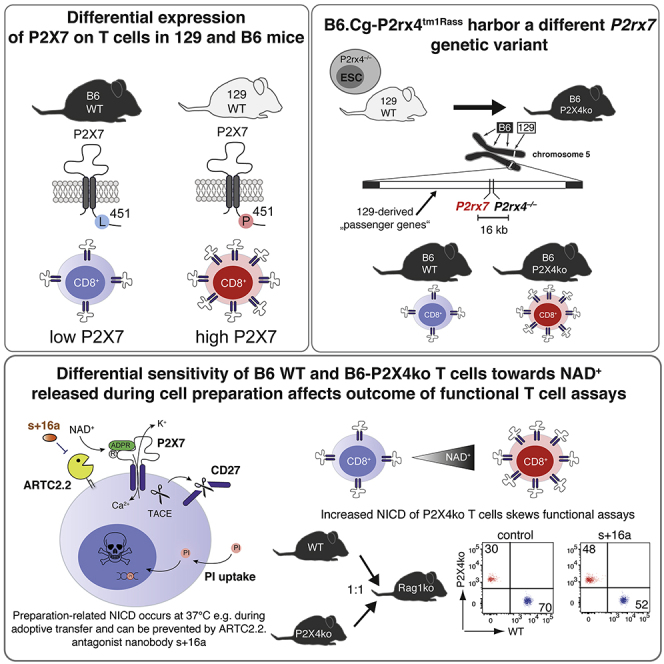
Highlights
-
•
T cells from 129 mice express higher level of P2X7 compared with T cells from B6 mice
-
•
P2rx4tm1Rass T cells express high level of P2X7 due to a P2rx7 passenger mutation
-
•
P2rx4tm1Rass T cells are highly susceptible to NAD-induced cell death (NICD)
-
•
NICD susceptibility of P2rx4tm1Rass T cells confounds the outcome of functional assays
Genetics; Immunology
Introduction
In the pre-CRISPR-Cas9 era, gene targeting, in order to generate “knockout” or “knockin” mice, was often conducted in embryonic stem cells (ESCs) derived from 129-originating mouse strains. The obtained transgenic mice were then backcrossed on C57BL/6, the most widely used strain in immunological research. Because of genetic linkage, however, flanking regions of the targeted gene are unlikely to be exchanged by backcrossing, and the likelihood of exchange decreases the closer these regions are located to the targeted gene (Lusis et al., 2007). Genetic distances are often given in centimorgans (cM), with 1 cM distance representing the equivalent to 1% probability of two loci being separated during homologous recombination in the context of meiosis. In the mouse, 1 cM is, on average, equivalent to 2,000 kilobases; however, the rate of equivalence can vary greatly due to numerous factors (Silver, 1995). This implicates a probability of about 90% that upon backcrossing for 10 generations; a flanking region with a distance of 1 cM to the targeted gene is still of donor origin (Berghe et al., 2015; Lusis et al., 2007). Gene expression can vary in cells from different laboratory mouse strains and some mouse strains even constitute “natural knockouts” for certain genes e.g. if mutations or SNPs introduce premature STOP codons (Mostafavi et al., 2014). If such a differentially expressed or inactive gene is in close proximity of a targeted gene in 129 ESCs, backcrossing to C57BL/6 will introduce an experimental bias, because the differentially expressed/inactive gene most likely will be retained. A prominent example is the 129-derived Casp1 knockout mouse (e.g. Casp1tm1Flvw). The 129 mouse strains carry a defective Casp11 gene in the close proximity of Casp1, and the strong resistance to lethal lipopolysaccharide (LPS) injection found in Casp1−/− mice was mainly due to the defective Casp11 neighboring gene that was carried along as passenger mutation (Kayagaki et al., 2011). A later study could show that targeting of other Casp11 neighboring genes, such as Panx1, also led to a resistance toward lethal LPS injection, if ESCs were derived from 129 mice, but not when B6-derived ESCs were used (Berghe et al., 2015). These two examples illustrate the potential impact of 129-derived passenger mutations and possible consequences, if they remain undetected.
P2X7 is a homotrimeric, adenosine triphosphate (ATP)-gated ion channel expressed on the cell surface of many immune cells (Bartlett et al., 2014). During tissue damage and inflammation ATP is released into the extracellular space where it serves as damage-associated molecule (DAMP). ATP-induced activation of P2X7 in macrophages and microglia triggers the formation of the NACHT-, LRR-, and PYD-domain-containing protein 3 (NLRP3) inflammasome, consisting of NLRP3, the adaptor protein apoptosis-associated speck-like protein-containing CARD (ASC), and caspase 1. The NLRP3 inflammasome catalyzes the processing of pro-interleukin 1 beta (pro-IL-1β) into its active form IL-1β (Ferrari et al., 1997, 2006). In the mouse, certain T cell populations have been identified as “high P2X7” expressers: these are regulatory T cells (Treg) (Aswad et al., 2005; Hubert et al., 2010), cytotoxic lymphocytes (CTL) from the lamina propria (Heiss et al., 2008), natural killer T cells (NKT), follicular helper T cells (Tfh) (Proietti et al., 2014), and tissue-resident memory T cells (Trm) (Rissiek et al., 2018b). Autocrine ATP release followed by activation of P2X7 and other P2X receptors plays a role in various T cell functions, including in IL-2 secretion, metabolic fitness, and cell migration (da Silva et al., 2018; Ledderose et al., 2018; Rissiek et al., 2015; Schenk et al., 2008). High concentrations of ATP and prolonged activation of P2X7 on T cells, however, is a strong trigger of T cell death (Scheuplein et al., 2009). In contrast to human T cells, mouse T cells exhibit an alternative way of P2X7 activation, which is triggered by ecto-ADP-ribosyltransferase C2.2 (ARTC2.2). ARTC2.2 utilizes nicotinamide adenine dinucleotide (NAD+), which is also released as DAMP during tissue damage, to ADP-ribosylate a variety of cell surface proteins such as CD25 (Teege et al., 2015), CD8β (Lischke et al., 2013), FcγR1, FcγR2b (Rissiek et al., 2017), and P2X7 (Seman et al., 2003). ADP-ribosylation of arginine 125 in the extracellular loop of P2X7 (Adriouch et al., 2008) acts like a covalently bound P2X7 agonist (Schwarz et al., 2009). Of note, 10-fold lower concentrations of NAD+ compared with ATP are needed to activate T cell P2X7 receptors, and its ADP-ribosylation ultimately leads to NICD (Seman et al., 2003). Our own studies revealed that NAD+ is also released during preparation of primary T cells from mouse organs such as spleen and lymph nodes (Rissiek et al., 2014; Scheuplein et al., 2009). Interestingly, ARTC2.2 is active at 4°C; however, ADP-ribosylation-mediated gating of P2X7 needs temperatures above 30°C (Scheuplein et al., 2009). This implicates that T cells prepared freshly on ice appear vital, but incubation at 37°C can trigger cell death in T cells that co-express high level of ARTC2.2 and P2X7 skewing the results of cytokine secretion assays as has been demonstrated for Trm, NKT, and Tfh (Borges da Silva et al., 2019; Georgiev et al., 2018; Rissiek et al., 2018b). The problem of P2X7 ADP-ribosylation during cell preparation can be solved by injecting an ARTC2.2-blocking nanobody (clone s+16a) into the mice 30 min before harvesting the mouse organs (Hubert et al., 2010; Koch-Nolte et al., 2007; Rissiek et al., 2014).
In the mouse, the P2rx7 gene is located on chromosome 5 with 95 other characterized, protein-coding genes being within a distance of 2 megabases (Mb) upstream and downstream of P2rx7 (Table S1). For P2rx7, a SNP (rs48804829) introduces a change of proline to leucine at amino acid position 451 in the cytosolic C-terminal tail of P2X7. The 129 and BALB/c mouse strains harbor the P2X7451P variant, whereas C57BL/6 (B6) mice express the loss-of-function P2X7451L variant. Studies revealed that P2X7451P and P2X7451L differ in their capacity to induce the formation of a P2X7-associated membrane pore (Adriouch et al., 2002) but not in P2X7-triggered calcium influx (Le Stunff et al., 2004; Sorge et al., 2012). No comparative P2X7 expression studies among mouse strains that express P2X7451P and P2X7451L have been performed. In this study, we analyzed P2X7 expression on T cell populations from different mouse strains. We show that CTL and helper T cells (Th) from strains that harbor P2X7451P express much higher P2X7 levels than cells derived from P2X7451L strains. As 129 mice express P2X7451P whereas B6 mice express P2X7451L, we hypothesized that a 129-derived P2rx7 gene could introduce an experimental bias when passed along as passenger mutation in congenic mice. Here, we demonstrate that in P2rx4tm1Rass mice Th and CTLs are much more prone to NICD compared with their wild-type (WT) counterparts. As consequence, these T cells appear less potent in terms of cytokine production and migration. However, when ARTC2.2 is blocked during cell preparation, the functional deficits of B6-P2X4ko T cells vanish. We identify other congenic mouse strains with targeted P2rx7 neighboring genes that also carry the 129-derived P2rx7 passenger mutation. Our study emphasizes the importance of checking the ESC origin of transgenic mice and analyzing them for passenger mutations in order to prevent misinterpretation of experimental results.
Results
P2X7 Expression Levels on T cells in Laboratory Mouse Strains Are Associated with the P2X7 451P/L Polymorphism
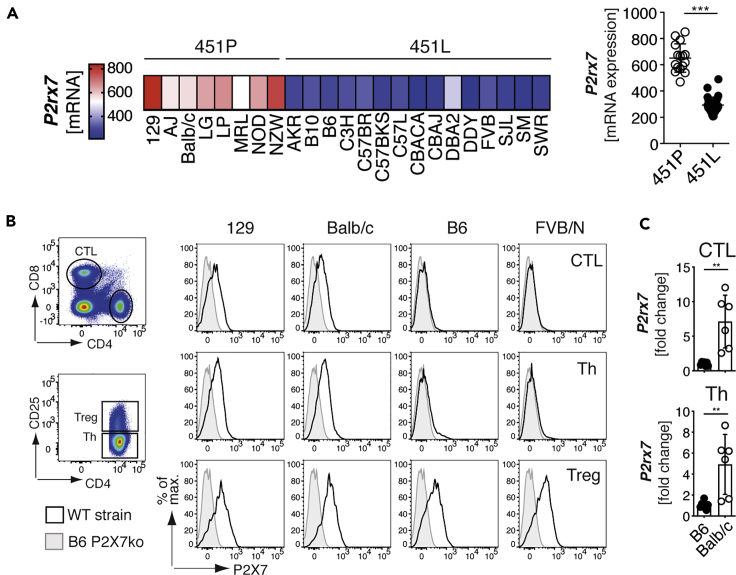
The well-characterized SNP rs48804829 leads to a proline (451P) to leucine (451L) exchange at amino acid position 451 in the P2X7 protein. B6 mice express the P2X7451L variant, whereas 129 and BALB/c mice express the P2X7451P variant. This SNP affects P2X7 pore formation (Adriouch et al., 2002; Sorge et al., 2012). In order to determine whether this SNP is associated with P2X7 expression levels, we analyzed the RNA sequencing dataset provided by Mostafavi et al. to compare P2rx7 mRNA expression levels of CD4+ T cells from 23 different mouse strains (Mostafavi et al., 2014). The results show that 451P strains express higher P2rx7 levels in CD4+ T cells, compared with 451L strains (Figure 1A). We next analyzed 129, BALB/c, B6, and FVB/N T cells for their P2X7 expression using the P2X7-specific monoclonal antibody Hano44 (Adriouch et al., 2005) and flow cytometry. In agreement with the RNA expression analysis, the results show higher cell surface levels of P2X7 on CD8+ cytotoxic T lymphocytes (CTL) and CD4+ helper T cells (Th) from 129 and BALB/c mice than from B6 and FVB/N mice (Figure 1B). Of note, regulatory T cells (Treg) display high levels of P2X7 on the cell surface in all analyzed mouse strains. Because naive and effector memory T cells were described to exhibit different P2X7 expression levels (Romagnani et al., 2020), we also analyzed P2X7 expression on naive (CD62L+CD44low) and effector/memory (CD62L–CD44high, TEM) CD4+ T cells. Here, we could show that P2X7 expression was higher in naive CD4+ T cells from 129 and BALB/c mice compared with B6 and FVB/N mice, whereas TEM from all analyzed strains expressed similarly high level of P2X7 (Figure S1). Comparison of P2rx7 mRNA levels from CTL and Th cells of B6 and BALB/c mice confirmed that both BALB/c cell population expressed about 5-fold higher P2rx7 mRNA levels (Figure 1C). Hence, our data show that the 451P/L P2rx7 polymorphism is associated with higher/lower P2X7 expression in two major T cell populations.
Figure 1.
P2X7 Expression Levels in T cells Are Associated with the 451P/L Polymorphism
(A) Comparison of P2rx7 mRNA expression on CD4+ T cells from individual mouse strains expressing P2X7 451P (white) or 451L (black). P2rx7 mRNA data were pool among 451L and 451P strains and compared. mRNA expression data were obtained from www.imgen.org (Heng et al., 2008; Mostafavi et al., 2014).
(B) Flow cytometric analyses of cell surface P2X7 expression on CD8+ cytotoxic T cells (CTL), CD4+CD25– helper T cells (Th) and CD4+CD25+ regulatory T cells (Treg) of 129, BALB/c, B6, and FVB/N mice.
(C) P2rx7 mRNA expression was analyzed in CTL and Th from B6 and BALB/c mice (n = 5–6). Data are represented as mean +/−SD. Statistical comparison of two groups was performed by using the Student's t test (p < 0.05 = ∗/p < 0.01 = ∗∗/p < 0.001 = ∗∗∗, ns = no significant).
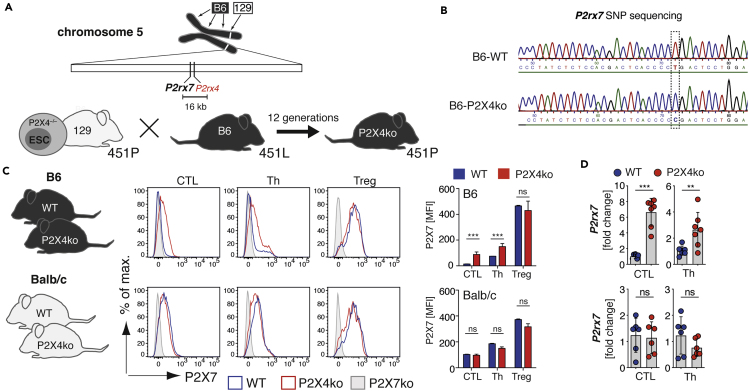
P2rx4tm1Rass Mice Carry the 129-Originating P2rx7 Gene as Passenger Mutation
One of the first P2X4-deficient mouse lines, P2rx4tm1Rass, was generated by the Rassendren lab in 2006 (Sim et al., 2006). Here, P2rx4 was targeted in ESCs of the 129 mouse strain and the generated P2X4ko mice were then backcrossed onto the B6 background. Because P2rx4 and P2rx7 are adjacent genes on mouse chromosome 5, we hypothesized that the 129-derived P2X7451P is still present in B6-P2X4ko mice (Figure 2A). We confirmed this hypothesis by sequencing an amplified fragment flanking rs48804829 (Figure 2B). B6-WT and B6-P2X4ko thus differ in both P2rx4 and P2rx7. In order to obtain mice that differ only in P2rx4 but not P2rx7, we backcrossed P2X4ko mice onto the BALB/c background for 13 generations. This way, we obtained Balb/c-WT and Balb/c-P2X4ko mice, which both express P2X7451P in addition to B6-WT and B6-P2X4ko mice that express P2X7451L and P2X7451P. We next compared P2X7 expression on T cells of B6 and BALB/c P2X4ko mice and the corresponding WT strains. Here, we observed that CTL and Th, but not Treg, from B6-P2X4ko mice expressed higher level of P2X7 when compared with B6-WT T cells (Figure 2C). Again, when separating naive CD4+ Th and CD4+ TEM, P2X7 expression was higher in naive CD4+ T cells from B6-P2X4ko mice compared with B6-WT mice, and TEM from both strains expressed comparable level of P2X7 (Figure S2A). Further, BALB/c P2X4ko and WT T cells exhibited comparable P2X7 expression levels (Figure 2C). Finally, we isolated mRNA from CTL and Th from all four strains and analyzed P2rx7 mRNA expression. The results show that B6-P2X4ko CTL and Th express 3- to 6-fold higher level of P2rx7 mRNA compared with B6-WT T cells. There was no statistically significant difference in P2rx7 mRNA expression in BALB/c P2X4ko and WT CTL and Th (Figure 2D). Of note, frequencies of T cells in blood, lymph nodes, and spleen were similar in B6-WT and P2X4ko mice and Balb/c-WT and Balb/c-P2X4ko mice, with a slight but significantly reduced frequency of CTLs in lymph nodes and spleen of B6-P2X4ko mice (Figure S2B). Taken together, B6-P2X4ko mice carry the P2X7451P variant as passenger mutation, which is associated with a higher P2X7 expression on T cells compared with corresponding WT cells.
Figure 2.
P2X4ko Mice Harbor a P2rx7 Passenger Mutation
(A) P2rx7 and P2rx4 are neighboring genes on mouse chromosome 5. A non-synonymous SNP in the P2rx7 gene introduces a single amino acid change in the P2X7 protein: 129 mice express a proline at position 451 (451P), whereas B6 mice express a leucine at position 451 (451L). P2X4ko mice were generated by deleting exon 1 of P2rx4 in embryonic stem cells derived from a 129 mouse strain. These mice express the 451P variant of P2X7. Backcrossing of P2X4ko onto the B6 background leads to the generation of B6-P2X4ko mice that express the 451P variant, due to a high degree of genetic linkage.
(B) Sequencing of cDNA obtained from isolated immune cell mRNA confirmed the 451P passenger mutation in the B6-P2X4ko mice.
(C) Flow cytometric analyses of cell surface P2X7 expression on CTL, Th, and Treg of WT and P2X4ko mice on the B6 and BALB/c background. The mean fluorescence intensity (MFI) of P2X7 on the different T cell populations from WT and P2X4ko mice (n = 3) was compared.
(D) P2rx7 mRNA of FACS-sorted WT and P2X4ko CTL, Th, and Treg (B6 and BALB/c) was analyzed (n = 6–7). Data are represented as mean +/−SD. Statistical comparison of two groups was performed by using the Student's t test (p < 0.05 = ∗/p < 0.01 = ∗∗/p < 0.001 = ∗∗∗, ns = no significant).
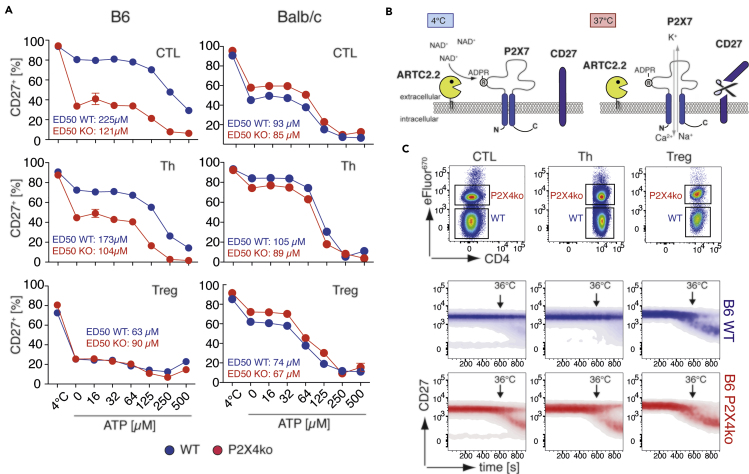
B6-P2X4ko T Cells Are More Sensitive to ATP and NAD+ Compared with B6-WT T Cells
P2X7 expression levels likely influence the cellular response to adenosine triphosphate (ATP). To analyze this, we generated stably transfected HEK cell lines that express P2X7451L or P2X7451P, each in the two major splice variants (the canonical P2X7a and P2X7k) (Nicke et al., 2009; Schwarz et al., 2012). We first compared ATP-induced calcium responses of these variants by real-time flow cytometry. P2X7k451L HEK cells were labeled with eFluor450 and mixed with unlabeled P2X7k451P HEK cells. Mixed cells were loaded with Fluo4 and were analyzed in parallel. In order to adjust for cell surface levels of P2X7, cells were stained with AF647-conjugated Hano44 and the analysis gate was adjusted on the basis of P2X7 mean fluorescence intensity (MFI). All cell lines responded with a comparably strong and ATP dose-dependent Ca2+ influx (Figure S3A). Shifting the analysis gates in P2X7451L HEK cells toward a higher P2X7 MFI and that in P2X7451P HEK cells toward a lower P2X7 MFI resulted in increased and decreased calcium signals, respectively (Figure S3B). This suggests that the level of cell surface P2X7 influences ATP-induced calcium signals and likely also downstream functions. Because CTL and Th cells from B6-P2X4ko mice exhibit higher P2X7 expression levels, it is likely that they also react more sensitive to stimulation with ATP. To test this hypothesis, we incubated splenocytes from B6-WT and B6-P2X4ko mice with rising concentrations of ATP for 10 min at 37°C and measured the loss of CD27 from the cell surface, a well-known readout for P2X7 activation on T cells (Rissiek et al., 2018a). Strikingly, the majority of B6-P2X4ko CTL and Th responded already to incubation at 37°C with a massive loss of CD27 from their cell surface, in contrast to their B6-WT counterparts (Figure 3A). In contrast, Treg cells from B6-WT and B6-P2X4ko mice both responded with a strong loss of CD27, when incubated at 37°C. Likewise, B6-P2X4ko CTL and Th were more sensitive to addition of exogenous ATP, with an ED50 of 120μM (P2X4ko) and 225μM (WT) for CTL and 100μM (P2X4ko) and 170μM (WT) for Th cells. Of note, a similar experiment with BALB/c P2X4ko and BALB/c WT splenocytes showed no significant difference in ATP sensitivity between P2X4ko and WT T cells.
Figure 3.
B6 P2X4ko T cells Show an Increased Response to ATP and NAD+-mediated P2X7 Activation When Compared with B6 WT T cells
(A) CTL, Th, and Tregs from WT (blue) and P2X4ko mice (red) on the B6 and BALB/c background were incubated at 37°C in the presence of 0–500 μM ATP or left at 4°C. CD27 shedding was assessed as surrogate readout for P2X7 activation. ED50 for ATP stimulation was calculated.
(B) Ecto-ADP-ribosyltransferase ARTC2.2 can ADP-ribosylate P2X7 at arginine (R) 125. Extracellular NAD+, which is released during cell preparation, can serve as substrate for ARTC2.2, even if cells are prepared at 4°C. Bringing the cells back to 37°C triggers gating of P2X7 inducing shedding of CD27.
(C) B6 P2X4ko splenocytes were labeled with eFluor670 and mixed with unlabeled B6 WT splenocytes. Mixed cells were stained with anti-CD27, and cell surface CD27 was monitored over time on CTL, Th, and Treg while increasing the temperature up to 36°C.
The loss of CD27 during incubation at 37°C is most likely due to activation of P2X7 by ADP-ribosylation in response to extracellular NAD+ released during cell isolation (Figure 3B). This effect has been well described for Treg, natural killer T cells (NKTs), tissue-resident memory T cells (Trm), and T follicular helper cells (Georgiev et al., 2018; Rissiek et al, 2014, 2018b; Scheuplein et al., 2009), which co-express P2X7 and ARTC2.2 (Glowacki et al., 2002). Although ADP-ribosylation usually occurs at 4°C, ADP-ribosylation-induced gating of P2X7, however, needs temperatures above 30°C (Scheuplein et al., 2009). To better visualize the temperature dependence of this process, we labeled B6-P2X4ko splenocytes with eFluor670, mixed them with unlabeled B6-WT splenocytes at 4°C, and then monitored the loss of CD27 on CTL, Th, and Treg cells at elevating temperatures using real-time flow cytometry. During the measurement, the sample was brought to 36°C while the CD27 signal was continuously monitored. Once the cells reached temperatures close to 36°C, the CD27 signal decreased in a fraction of cells (Figure 3C). Apparently, this loss of CD27 was more prominent in CTL and Th from B6-P2X4ko compared with corresponding B6-WT cells (Figure 3C). Again, Tregs from both B6-P2X4ko and B6-WT mice reacted with a pronounced loss of CD27 in this experimental setup. Of note, ARTC2.2 expression levels were comparable among individual T cell populations from P2X4ko and corresponding WT mice (Figure S4), as determined by flow cytometry.
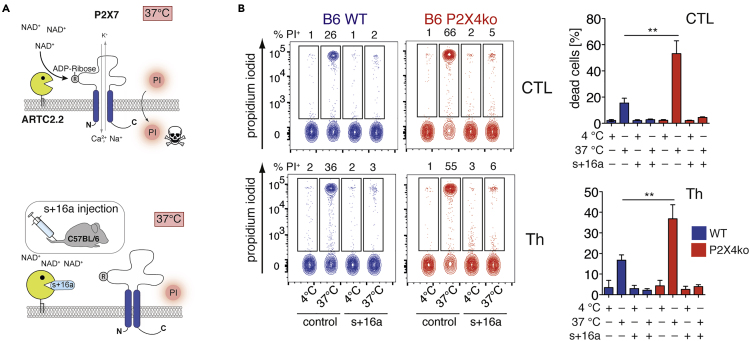
The Increased Sensitivity of B6-P2X4ko T cells toward NICD Confounds Results from Functional Assays
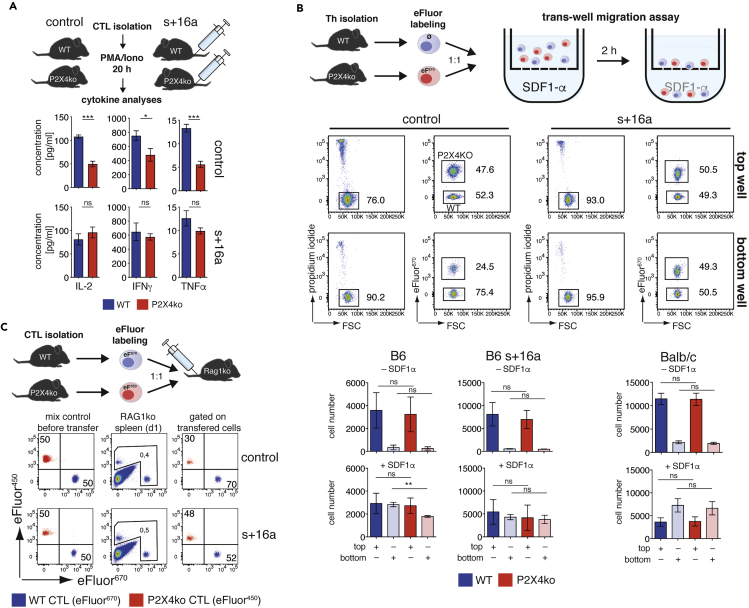
The ultimate consequence of ADP-ribosylation-mediated P2X7 activation is NICD (Seman et al., 2003). Cell-preparation-related ADP-ribosylation of P2X7 on T cells can be prevented by injecting the ARTC2.2 blocking nanobody s+16a prior to sacrificing the mice (Figure 4A) (Koch-Nolte et al., 2007; Rissiek et al., 2014). Because CTL and Th from B6-P2X4ko mice are sensitive to P2X7-induced CD27 shedding at 37°C, we next tested whether this also results in an increased susceptibility toward NICD of FACS-sorted CTL and Th from mice that had been treated or not with s+16a. As a readout for cell death, we measured staining of cells with propidium iodide (PI). We observed that only a minor fraction of B6-WT CTL and Th (20%–30%) underwent NICD (Figure 4B). In contrast, 40%–60% of B6-P2X4ko CTL and Th were PI+ after 2 h of incubation at 37°C. Of note, injection of s+16a prevented NICD of B6-WT and B6-P2X4ko CT and Th.
Figure 4.
B6-P2X4ko T cells Exhibit Enhanced NICD
(A) B6 WT and B6 P2X4ko mice were injected or not with anti-ARTC2.2 nanobody s+16a in order to prevent P2X7 ADP-ribosylation during cell preparation.
(B) CTL and Th from spleen were FACS sorted, and obtained cells were incubated at 37°C for 2 h or left at 4°C. Cell vitality was measured by propidium iodide (PI) uptake (n = 3). Data are represented as mean +/−SD. Statistical comparison of two groups was performed by using the Student's t test (p < 0.05 = ∗/p < 0.01 = ∗∗/p < 0.001 = ∗∗∗, ns = no significant).
Next, we compared cytokine production and migration of CTL and Th from mice that had been treated or not with s+16a. For cytokine analyses, we stimulated FACS-sorted CTL with phorbole-12-myristate-13-acetate (PMA)/ionomycin. When comparing CTL from untreated B6-P2X4ko and B6-WT mice, it appeared that P2X4-deficiency dampened secretion of IL-2, IFNγ, and TNFα. However, when ADP-ribosylation during cell preparation was prevented by s+16a injection, CTL from both mouse lines produced comparable amounts of all three cytokines (Figure 5A). Because P2X4 has been attributed a role in T cell migration (Ledderose et al., 2018), we next compared B6 WT and P2X4ko Th in an in vitro migration assay using a trans-well chamber and SDF1α (CXCL12) as chemoattractant in the bottom chamber. In order to compare WT and P2X4ko Th side by side, we labeled P2X4ko Th with eFluor450, mixed them with unlabeled WT Th, and measured the ratio of vital (PI-negative) cells in the top and bottom well (Figure 5B). The presence of SDF1α markedly increased the migration of Th cells from the top to the bottom wells; however, B6-P2X4ko Th cells seemed to migrate less compared with B6-WT Th (Figure 5B). This difference in migration was not observed with cells from mice that had been treated with the ARTC2.2-blocking nanobody s+16a. This suggests that increased NICD susceptibility rather than a P2X4-mediated effect on migratory capacity causes the difference in Th migration. This conclusion is supported by the finding that WT and P2X4ko Th cells from BALB/c mice, which do not differ in NICD susceptibility, also did not differ in their migration capacity toward SDF1α (Figure 5B). Finally, we performed in vivo adoptive transfer experiments where we mixed differentially labeled B6-WT and B6-P2X4ko CTL harvested from mice that had been treated or not with s+16a in a 1:1 ratio and transferred them into RAG1ko mice. Twenty-four hours after adoptive transfer, spleens of the injected RAG1ko mice were analyzed for the presence of the injected CTLs. Here, we retrieved about 2-fold more B6-WT than B6-P2X4ko CTL from the RAG1ko spleens, again suggesting a deficit in migration capacity of the B6-P2X4ko CTL (Figure 5C). However, when B6-WT and B6-P2X4ko CTL were harvested from s+16a treated mice, B6-WT and B6-P2X4ko CTL were retrieved at a ratio of almost 1:1. The results with these three functional assays demonstrate increased P2X7 expression rather than loss of P2X4 alters several functions of Th and CTL on the B6 background.
Figure 5.
The enhanced NICD of B6-P2X4ko T cells Can Lead to Misinterpretation of Functional Assays
(A) B6 WT and P2X4ko mice were injected (s+16a) or not (control) with anti-ARTC2.2 nanobody s+16a. 5 × 104 CTL were FACS sorted and stimulated ex vivo with PMA/ionomycin for 20 h. IL-2, IFNγ, and TNFα in the supernatants were measured by cytometric bead array.
(B) 1 × 105 eFluor670 labeled Th from P2X4ko mice (B6, B6 treated with s+16a or BALB/c) and 1 × 105 unlabeled Th from corresponding WT mice were mixed and transferred to the top well of a transwell chamber in order to perform a comparative migration assay toward SDF1α, which was used as chemoattractant in the bottom well. Number of vital (PI–) Th in top and both wells was determined after 2 h of incubation at 37°C.
(C) Comparative adoptive transfer of CTL obtained from B6 WT and P2X4ko mice that have been treated or not with s+16a was performed. Cells were differentially labeled with eFluor670 and eFluor450, and 4 × 105 cells mixed in a 1:1 ratio were i.v. injected into RAG1ko mice. Spleens were analyzed after 24 h for the presence of transferred mixed cells.
Data are represented as mean +/−SD. Statistical comparison of two groups was performed by using the Student's t test (p < 0.05 = ∗/p < 0.01 = ∗∗/p < 0.001 = ∗∗∗, ns = no significant).
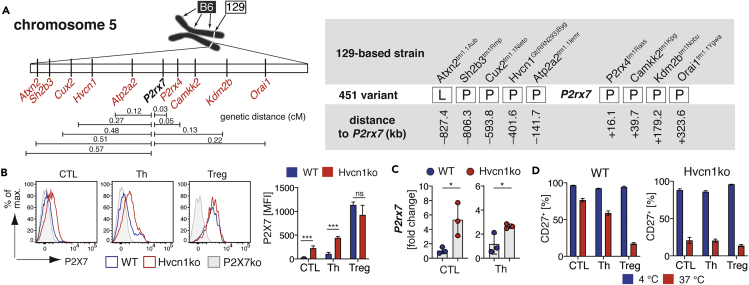
The P2rx7 Passenger Mutation Can Be Found in Other Transgenic Mice
A genetic distance of 2 Mb in the mouse genome is, on average, equivalent to 1 centiMorgan (cM) (Bryant et al., 2018). This implicates that for 129-derived transgenic mice genes in a 2 Mb maximum distance of the targeted gene are retained with a theoretical probability of 90% after 10 generations of backcrossing. P2rx4 is with a distance of only 16 kb the nearest neighbor gene of P2rx7. However, there are 94 other characterized, protein-coding genes within 2 megabases (Mb) upstream and downstream of P2rx7. A search in the Mouse Genome Informatics (MGI) database resulted in 96 mouse strains generated from 129-derived ESCs in which genes within +/−2MB of P2rx7 had been targeted (Table S1). To test our hypothesis that the P2rx7 passenger mutation will affect T cell functions in mice of targeted neighboring genes, we analyzed nine other mouse strains with targeted P2rx7 neighboring genes on the B6 background for the presence of the 129-derived P2rx7 passenger mutation. Results of the SNP rs48804829 sequencing revealed that eight of the nine analyzed strains harbor the 129-derived P2rx7 passenger mutation (Figure 6A). Only in the Atxn2tm1.1Aub mouse strain the 129-derived P2X7451P was exchanged by the B6-derived P2X7451L. In order to reproduce the phenotypical and functional consequences of the P2rx7 passenger mutation described for P2rx4tm1Rass, we evaluated T cells from B6-Hvcn1Gt(RRRN293)Byg (B6-Hvcn1ko) mice for the expression of P2X7. Here, we obtained a similar result as for P2rx4tm1Rass: the P2X7 cell surface expression was significantly higher on B6-Hvcn1ko CTL and Th compared with B6-WT (Figure 6B). Similarly, P2rx7 mRNA levels were increased in B6-Hvcn1ko CTL and Th in comparison to B6-WT CTL and Th (Figure 6C). As a consequence, B6-Hvcn1ko CTL and Th responded more pronounced to preparation-related P2X7 ADP-ribosylation, as indicated by a massive shedding of CD27 upon incubation at 37°C, when compared with B6-WT T cells (Figure 6D).
Figure 6.
The 129-derived P2rx7 Passenger Mutation Is Present in Other Transgenic Mouse Strains
(A) Nine congenic mouse strains with targeted genes in close proximity of P2rx7 were analyzed for the presence of the SNP leading to the 451P/L polymorphism.
(B) P2X7 expression was determined on CTL, Th, and Treg from B6-Hvcn1ko carrying the 129-derived P2rx7 passenger mutation (n = 3).
(C) P2rx7 mRNA expression was analyzed in CTL and Th from B6-Hvcn1ko and B6-WT mice.
(D) CTL, Th, and Treg from Hvcn1ko and B6-WT were incubated at 4°C and 37°C for 15 min in order to measure the impact of P2X7 ADP-ribosylation by shedding of CD27. Data are represented as mean +/−SD. Statistical comparison of two groups was performed by using the Student's t test (p < 0.05 = ∗/p < 0.01 = ∗∗/p < 0.001 = ∗∗∗, ns = no significant).
Discussion
Passenger mutations are a phenomenon occurring in genetically modified mice when the genetic background of the used ES cells and the genetic background of the line in which the modified gene was backcrossed are not identical. Even after backcrossing for more than 12 generations, the flanking regions of the modified gene often contain multiple foreign genes of ES cell origin, some of which can be differentially expressed between the transgenic strain and the supposed WT strain. Further, functionally inactive 129-derived passenger genes can introduce an experimental bias, as in the example of the Caspase-1 knockout mouse (Casp1tm1Flvw). Here, the 129-derived ES cells used to generate Casp1tm1Flvw (Kuida et al., 1995) introduced a defective Casp11 gene in close proximity of the knocked out Casp1 gene, thereby transferring resistance to otherwise lethal LPS injection to Casp1tm1Flvw mice. The role of the inactive Casp11 passenger gene in LPS resistance was unnoticed for more than 15 years until Kayagaki and colleagues revealed this important fact (Kayagaki et al., 2011). The defective Casp11 gene was also found in other transgenic mice generated with 129-derived ES cells, such as Panx1tm1.1Vshe (Dvoriantchikova et al., 2012), again conferring these transgenic mice resistance to LPS (Berghe et al., 2015). These examples illustrate the importance of screening transgenic mice with a congenic background for passenger mutations in order to avoid experimental bias.
In this study we demonstrate that the expression of P2X7 on conventional helper T cells as well as on cytotoxic T cells differs among laboratory mouse strains. Interestingly, P2X7 expression was high on T cells from strains carrying the 451P variant of P2X7, whereas P2X7 expression was low on T cells from strains carrying the 451L variant resulting from the SNP rs48804829. Despite the clear correlation between the rs48804829 SNP and P2rx7 expression levels in T cells, it appears unlikely that the rs48804829 SNP alone is responsible for the difference in P2X7 expression levels. According to the Wellcome Sanger Institute SNP database, there are 172 SNPs within the P2rx7 gene (+/− 1 kbp), which differ in B6 and 129 mice (including the rs48804829 SNP). Further, 129 mice harbor a 249 bp intronic deletion close to exon 12 of P2rx7 (Keane et al., 2011). Both SNPs and the deletion could affect accessibility of the P2rx7 locus or the binding of certain transcription factors. Therefore, the reason(s) for higher P2rx7 expression in CD4 and CD8 T cells in 129 mice compared with B6 remain to be determined.
Regarding T cells, the 451L P2X7 variant of B6 mice is often referred to as less sensitive or loss-of-function variant, compared with the e.g. the BALB/c 451P P2X7 variant (Bartlett et al., 2014; de Campos et al., 2012; Proietti et al., 2014). However, the lower cell surface levels of P2X7 on B6 T cells compared with BALB/c T cells also contribute to the reduced ATP/NAD+ sensitivity. This is underlined by our findings with P2X7-transfected HEK cells, which show comparable ATP-induced calcium influx of 451L and 451P variants when adjusted for P2X7 expression levels.
Because T cells from 129 mice (451P) and B6 mice (451L) mice differ in their P2X7 expression level, we hypothesized that this difference would be passed on as passenger mutation to the B6 background from 129-based mice with targeted genes in the P2rx7 genetic neighborhood. For B6 mice in which the nearest neighbor gene of P2rx7, P2rx4, had been targeted in 129 ES cells, we found that these B6 P2X4ko mice (1) carry P2rx7 451P as passenger gene, (2) express higher levels of P2X7 on CD4 and CD8 T cells, when compared with B6 WT, (3) are more sensitive to treatment with exogenous ATP and NAD+, and (4) are more prone to ADP-ribosylation of P2X7 during cell preparation, resulting in NAD-induced cell death (NICD) in a higher fraction of cells compared with B6 WT. Increased sensitivity of NICD is receiving more and more attention, because important T cell populations that co-express high level of P2X7 and ARTC2.2, such as Tregs, NKTs, Tfh, and Trm, are highly affected by ADP-ribosylation during cell preparation (Georgiev et al., 2018; Hubert et al., 2010; Proietti et al., 2014; Rissiek et al, 2014, 2018b; Stark et al., 2018). The analyses and functional characterization of these cell populations has been greatly improved by the use of the ARTC2.2-blocking nanobody s+16a, which prevents ADP-ribosylation of P2X7 during cell preparation and preserves the vitality of the prepared cells in functional assays and during adoptive transfer (Koch-Nolte et al., 2007; Rissiek et al., 2014). Similarly, injection of s+16a into P2X4ko mice preserved the vitality of the NAD-sensitive P2X4ko T cells. Otherwise, the higher loss of P2X4ko T cells during functional assays in vivo and in vitro, due to NICD, skews experimental results, as demonstrated by our cytokine expression and migration analyses. This likely holds also for previously reported studies with T cells from P2rx4tm1Rass mice, e.g. where polyclonally activated P2X4ko T cells exhibited less proliferation and granzyme B expression compared with T cells from B6 WT mice (Ventre et al., 2017).
We found that eight of nine other congenic strains on the B6 background derived from 129 ES cells with targeted genes in the P2rx7 neighborhood carried the 129-derived P2rx7 passenger gene. We had further access to one of these strains, B6-Hvcn1Gt(RRRN293)Byg mice, and were able to reproduce the phenotype of P2rx4tm1Rass T cells, suggesting that our findings can be extrapolated to other congenic mice carrying the P2rx7 passenger gene. Orai1, for example, plays an important role in T cell function (Nohara et al., 2015) and is also a neighboring gene of P2rx7. Studies have demonstrated that genetic ablation of Orai1 in mice on a pure B6 background, e.g. Orai1tm1Rao or Orai1tm1Fesk, leads to a diminished T cell cytokine production (Kim et al., 2014; McCarl et al., 2010), underlining the importance of Orai1 in T cell function. In our study, we could identify the 451P P2rx7 passenger mutation in the 129-derived Orai1tm1.1Ygwa strain. These mice were used in a study to generate T-cell-specific B6 Orai1ko mice by crossing them with CD4-Cre mice. Interestingly, CD8 T cells from these T-cell-specific B6-Orai1ko were less potent in killing peptide-loaded EL-4 tumor cells in vitro compared with CD8 T cells from B6-WT mice (Kim et al., 2017). Further, T cells from the 129-based Orai1Gt(XL922)Byg mouse also exhibited reduced IL-2 and IFNγ production in response to polyclonal T cell receptor stimulation (Vig et al., 2008). It is conceivable that these mice also harbor the 129-derived P2rx7 passenger gene, and their T cells are consequently also more sensitive to NICD than the WT controls. It would be interesting to determine whether treatment with the ARTC2.2 blocking nanobody s+16a has any impact on the outcome of these experimental settings.
Further, it is worth noting that when working with conditional knockouts, the control group can have a great impact on the results and interpretation when it comes to the impact of passenger mutations: a comparison of Cre – floxed and Cre + floxed mice would “silence” the impact of a passenger mutation, because it is present in both mice. In contrast, a comparison of Cre + floxed and Cre + WT or pure WT mice can introduce an experimental bias triggered by a passenger mutation.
In conclusion, our results emphasize the importance of a thorough analysis of the genetic neighborhood of 129-based transgenic mice on the B6 background. As demonstrated for P2rx4tm1Rass, a passenger mutation in the neighboring P2rx7 gene can introduce an experimental bias. Apart from T cells, which were the focus of this study, it would be worthwhile to evaluate the impact of the P2rx7 passenger mutation on other P2X7 expressing cell populations such as astrocytes, neurons, or adipocytes.
Limitations of the Study
Our work clearly demonstrates the impact of the P2rx7 passenger mutation on the vitality of T cell from B6-P2X4ko mice. However, one has to keep in mind that a transgenic strain in one animal facility can carry a certain passenger mutation, whereas the same strain bred in another animal facility might have successfully replaced the passenger mutation during backcrossing. Therefore, it is highly recommended to check for possible passenger mutations when working with newly imported mouse strains in order to avoid experimental bias.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Björn Rissiek (b.rissiek@uke.de).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank the following colleagues for providing genomic DNA samples of transgenic mice: Georg Auburger(Atxn2tm1.1Aub), Satoshi Takaki and Meena S Madhur (Sh2b3tm1Rmp), Marta Nieto and Elia Marcos Grañeda (Cux2tm1.1Nieto), Carmella Evans-Molina (Atp2a2tm1.1Iemr), David Carling (Camkk2tm1Kpg), Nobuaki Yoshida and Manabu Ozawa (Kdm2btm1Nobu), and Yousang Gwack (Orai1tm1.1Ygwa). The authors would like to thank the HEXT FACS Core Facility (Hamburg, Germany) for cell sorting. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation–Project-ID: 335447717—SFB1328, A06 and A16 to MAF, A10 and Z02 to FK-N, A13 to TM, and A15 to AN), the ‘‘Hermann und Lilly Schilling-Stiftung für Medizinische Forschung’’ to TM, and a faculty grant to BR (FFM, NWF-17/07).
Authors Contributions
BR and ME-L performed all experiments and analyzed and interpreted the data. YD generated P2X7 stably transfected HEK cells. FR, LU, FU, and MAF provided essential material. AN, FK-N, and TM supervised the experiments and assisted with data interpretation and manuscript preparation. BR assembled the figures and wrote the manuscript, which has been reviewed by all authors.
Declaration of Interests
FK-N receives royalties from sales of antibodies developed in the lab via MediGate GmbH, a 100% subsidiary of the University Medical Center, Hamburg. All other authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101870.
Supplemental Information
References
- Adriouch S., Bannas P., Schwarz N., Fliegert R., Guse A.H., Seman M., Haag F., Koch-Nolte F. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2008;22:861–869. doi: 10.1096/fj.07-9294com. [DOI] [PubMed] [Google Scholar]
- Adriouch S., Dox C., Welge V., Seman M., Koch-Nolte F., Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J. Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Adriouch S., Dubberke G., Diessenbacher P., Rassendren F., Seman M., Haag F., Koch-Nolte F. Probing the expression and function of the P2X7 purinoceptor with antibodies raised by genetic immunization. Cell Immunol. 2005;236:72–77. doi: 10.1016/j.cellimm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Aswad F., Kawamura H., Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J. Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- Bartlett R., Stokes L., Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- Berghe T.V., Hulpiau P., Martens L., Vandenbroucke R.E., Van Wonterghem E., Perry S.W., Bruggeman I., Divert T., Choi S.M., Vuylsteke M. Passenger mutations confound interpretation of all genetically modified congenic mice. Immunity. 2015;43:200–209. doi: 10.1016/j.immuni.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H., Wang H., Qian L.J., Hogquist K.A., Jameson S.C. ARTC2.2/P2RX7 signaling during cell isolation distorts function and quantification of tissue-resident CD8+ T cell and invariant NKT subsets. J. Immunol. 2019;202 doi: 10.4049/jimmunol.1801613. ji1801613–j1802163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C.D., Ferris M.T., De Villena F.P.M., Damaj M.I., Kumar V., Mulligan M.K. Reduced complexity cross design for behavioral genetics. In: Gerlai R.T., editor. Molecular-Genetic and Statistical Techniques for Behavioral and Neural Research. Elsevier; 2018. pp. 165–190. [Google Scholar]
- da Silva H.B., Beura L.K., Wang H., Hanse E.A., Gore R., Scott M.C., Walsh D.A., Block K.E., Fonseca R., Yan Y. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8 + T cells. Nature. 2018;5:1. doi: 10.1038/s41586-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos N.E., Marques-da-Silva C., Corrêa G., Castelo-Branco M.T.L., de Souza H.S.P., Coutinho-Silva R. Characterizing the presence and sensitivity of the P2X7 receptor in different compartments of the gut. J. Innate Immun. 2012;4:529–541. doi: 10.1159/000336628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G., Ivanov D., Barakat D., Grinberg A., Wen R., Slepak V.Z., Shestopalov V.I. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 2012;7:e31991. doi: 10.1371/journal.pone.0031991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Chiozzi P., Falzoni S., Hanau S., Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Pizzirani C., Adinolfi E., Lemoli R.M., Curti A., Idzko M., Panther E., Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Georgiev H., Ravens I., Papadogianni G., Malissen B., Förster R., Bernhardt G. Blocking the ART2.2/P2X7-system is essential to avoid a detrimental bias in functional CD4 T cell studies. Eur. J. Immunol. 2018;48:1078–1081. doi: 10.1002/eji.201747420. [DOI] [PubMed] [Google Scholar]
- Glowacki G., Braren R., Firner K., Nissen M., Kühl M., Reche P., Bazan F., Cetkovic-Cvrlje M., Leiter E., Haag F., Koch-Nolte F. The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss K., Jänner N., Mähnss B., Schumacher V., Koch-Nolte F., Haag F., Mittrücker H.-W. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J. Immunol. 2008;181:3861–3869. doi: 10.4049/jimmunol.181.6.3861. [DOI] [PubMed] [Google Scholar]
- Heng T.S.P., Painter M.W., Immunological genome project consortium The immunological genome project: networks of gene expression in immune cells. Nat. Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Hubert S., Rissiek B., Klages K., Huehn J., Sparwasser T., Haag F., Koch-Nolte F., Boyer O., Seman M., Adriouch S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J. Exp. Med. 2010;207:2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Keane T.M., Goodstadt L., Danecek P., White M.A., Wong K., Yalcin B., Heger A., Agam A., Slater G., Goodson M. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-D., Bae S., Capece T., Nedelkovska H., de Rubio R.G., Smrcka A.V., Jun C.-D., Jung W., Park B., Kim T.-I., Kim M. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat. Commun. 2017;8:15365. doi: 10.1038/ncomms15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-D., Srikanth S., Tan Y.-V., Yee M.-K., Jew M., Damoiseaux R., Jung M.E., Shimizu S., An D.S., Ribalet B. Calcium signaling via Orai1 is essential for induction of the nuclear orphan receptor pathway to drive Th17 differentiation. J. Immunol. 2014;192:110–122. doi: 10.4049/jimmunol.1302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Nolte F., Reyelt J., Schössow B., Schwarz N., Scheuplein F., Rothenburg S., Haag F., Alzogaray V., Cauerhff A., Goldbaum F.A. Single domain antibodies from llama effectively and specifically block T cell ecto-ADP-ribosyltransferase ART2.2 in vivo. FASEB J. 2007;21:3490–3498. doi: 10.1096/fj.07-8661com. [DOI] [PubMed] [Google Scholar]
- Kuida K., Lippke J.A., Ku G., Harding M.W., Livingston D.J., Su M.S., Flavell R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Le Stunff H., Auger R., Kanellopoulos J., Raymond M.-N. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J. Biol. Chem. 2004;279:16918–16926. doi: 10.1074/jbc.M313064200. [DOI] [PubMed] [Google Scholar]
- Ledderose C., Liu K., Kondo Y., Slubowski C.J., Dertnig T., Denicoló S., Arbab M., Hubner J., Konrad K., Fakhari M. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Invest. 2018;128:3583–3594. doi: 10.1172/JCI120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke T., Schumacher V., Wesolowski J., Hurwitz R., Haag F., Koch-Nolte F., Mittrücker H.-W. CD8-β ADP-ribosylation affects CD8(+) T-cell function. Eur. J. Immunol. 2013;43:1828–1838. doi: 10.1002/eji.201243231. [DOI] [PubMed] [Google Scholar]
- Lusis A.J., Yu J., Wang S.S. The problem of passenger genes in transgenic mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:2100–2103. doi: 10.1161/ATVBAHA.107.147918. [DOI] [PubMed] [Google Scholar]
- McCarl C.-A., Khalil S., Ma J., Oh-hora M., Yamashita M., Roether J., Kawasaki T., Jairaman A., Sasaki Y., Prakriya M., Feske S. Store-Operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J. Immunol. 2010;185:5845–5858. doi: 10.4049/jimmunol.1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S., Ortiz-Lopez A., Bogue M.A., Hattori K., Pop C., Koller D., Mathis D., Benoist C., Consortium T.I.G. Variation and genetic control of gene expression in primary immunocytes across inbred mouse strains. J. Immunol. 2014;193:4485–4496. doi: 10.4049/jimmunol.1401280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A., Kuan Y.-H., Masin M., Rettinger J., Marquez-Klaka B., Bender O., Górecki D.C., Murrell-Lagnado R.D., Soto F. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J. Biol. Chem. 2009;284:25813–25822. doi: 10.1074/jbc.M109.033134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara L.L., Stanwood S.R., Omilusik K.D., Jefferies W.A. Tweeters, woofers and horns: the complex orchestration of calcium currents in T lymphocytes. Front. Immun. 2015;6:234. doi: 10.3389/fimmu.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti M., Cornacchione V., Rezzonico Jost T., Romagnani A., Faliti C.E., Perruzza L., Rigoni R., Radaelli E., Caprioli F., Preziuso S. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Rissiek B., Danquah W., Haag F., Koch-Nolte F. Technical Advance: a new cell preparation strategy that greatly improves the yield of vital and functional Tregs and NKT cells. J. Leukoc. Biol. 2014;95:543–549. doi: 10.1189/jlb.0713407. [DOI] [PubMed] [Google Scholar]
- Rissiek B., Haag F., Boyer O., Koch-Nolte F., Adriouch S. P2X7 on mouse T cells: one channel, many functions. Front. Immun. 2015;6:204. doi: 10.3389/fimmu.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissiek B., Lukowiak M., Haag F., Magnus T., Koch-Nolte F. Monitoring the sensitivity of T cell populations towards NAD+ released during cell preparation. Methods Mol. Biol. 2018;1813:317–326. doi: 10.1007/978-1-4939-8588-3_22. [DOI] [PubMed] [Google Scholar]
- Rissiek B., Lukowiak M., Raczkowski F., Magnus T., Mittrücker H.-W., Koch-Nolte F. In vivo blockade of murine ARTC2.2 during cell preparation preserves the vitality and function of liver tissue-resident memory T cells. Front. Immun. 2018;9:1580. doi: 10.3389/fimmu.2018.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissiek B., Menzel S., Leutert M., Cordes M., Behr S., Jank L., Ludewig P., Gelderblom M., Rissiek A., Adriouch S. Ecto-ADP-ribosyltransferase ARTC2.1 functionally modulates FcγR1 and FcγR2B on murine microglia. Sci. Rep. 2017;7:16477. doi: 10.1038/s41598-017-16613-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani A., Rottoli E., Mazza E.M.C., Rezzonico Jost T., De Ponte Conti B., Proietti M., Perotti M., Civanelli E., Perruzza L., Catapano A.L. P2X7 receptor activity limits accumulation of T cells within tumors. Cancer Res. 2020;80:3906–3919. doi: 10.1158/0008-5472.CAN-19-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk U., Westendorf A.M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signaling. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- Scheuplein F., Schwarz N., Adriouch S., Krebs C., Bannas P., Rissiek B., Seman M., Haag F., Koch-Nolte F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J. Immunol. 2009;182:2898–2908. doi: 10.4049/jimmunol.0801711. [DOI] [PubMed] [Google Scholar]
- Schwarz N., Drouot L., Nicke A., Fliegert R., Boyer O., Guse A.H., Haag F., Adriouch S., Koch-Nolte F. Alternative splicing of the N-terminal cytosolic and transmembrane domains of P2X7 controls gating of the ion channel by ADP-ribosylation. PLoS One. 2012;7:e41269. doi: 10.1371/journal.pone.0041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., Fliegert R., Adriouch S., Seman M., Guse A.H., Haag F., Koch-Nolte F. Activation of the P2X7 ion channel by soluble and covalently bound ligands. Purinergic Signal. 2009;5:139–149. doi: 10.1007/s11302-009-9135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seman M., Adriouch S., Scheuplein F., Krebs C., Freese D., Glowacki G., Deterre P., Haag F., Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/s1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- Silver L.M. Oxford University Press on Demand; 1995. Mouse Genetics. [Google Scholar]
- Sim J.A., Chaumont S., Jo J., Ulmann L., Young M.T., Cho K., Buell G., North R.A., Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J. Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Trang T., Dorfman R., Smith S.B., Beggs S., Ritchie J., Austin J.-S., Zaykin D.V., Vander Meulen H., Costigan M. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R., Wesselink T.H., Behr F.M., Kragten N.A.M., Arens R., Koch-Nolte F., van Gisbergen K.P.J.M., van Lier R.A.W. TRMmaintenance is regulated by tissue damage via P2RX7. Sci. Immunol. 2018;3:eaau1022. doi: 10.1126/sciimmunol.aau1022. [DOI] [PubMed] [Google Scholar]
- Teege S., Hann A., Miksiewicz M., MacMillan C., Rissiek B., Buck F., Menzel S., Nissen M., Bannas P., Haag F. Tuning IL-2 signaling by ADP-ribosylation of CD25. Sci. Rep. 2015;5:8959. doi: 10.1038/srep08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre E., Rozières A., Lenief V., Albert F., Rossio P., Laoubi L., Dombrowicz D., Staels B., Ulmann L., Julia V. Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72:1212–1221. doi: 10.1111/all.13118. [DOI] [PubMed] [Google Scholar]
- Vig M., DeHaven W.I., Bird G.S., Billingsley J.M., Wang H., Rao P.E., Hutchings A.B., Jouvin M.-H., Putney J.W., Kinet J.-P. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat. Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.