Abstract
Perivascular epithelioid cell tumours are a novel histological description of mesenchymal tumours consisting of perivascular and epithelioid cells. Angiomyolipomas are one of the commoner types of this tumour group. They are typically associated with the inherited condition tuberous sclerosis (TS). In TS they are often seen arising in the kidneys and brain, although much more rarely can be seen in the liver. While usually thought of as benign tumours there is little evidence to predict whether they will progress to malignancy. Currently, there is no recommended best management strategy between resection and surveillance. We report two patients with TS seen in our centre with these described PEComa liver lesions histologically, however only one required a resection. On review of the literature, features such as increased size, rapid growth and vascular invasion would be concerning for possible malignant potential and therefore merit resection, as well as significant symptoms.
Keywords: paediatric oncology, pathology, liver disease
Background
Perivascular epithelioid cell tumours (PEComas) are often found in children with tuberous sclerosis (TS), more rarely in the liver. There is no current recommended best management strategy. After we had two similar patients at our centre it was felt that a relevant review of the literature to ascertain the current guidance and experience with these types of tumour was necessary.
Case presentation
A 9-year-old boy with known TS had been noted to have some increasing lethargy, weight loss and decreased appetite over a few months. He was found to have a hepatic mass that was increasing in size significantly over 6 months on serial ultrasound scans (measuring 12×8×10.5 cm on the last ultrasound). His blood tests were unremarkable with normal liver function and Alpha feta protein (AFP), CA-199 had always been normal. He was known to already have multiple angiomyolipomas (AMLs) in the kidneys, lungs and brain (including a subependymal giant cell astrocytoma and left retinal astrocytoma) and was under regular radiological surveillance.
The hepatic mass raised suspicion due to the rapid growth so it was resected. At resection the mass measured 13 cm in length. The histology revealed epithelioid cells with inflammatory cells and thick-walled blood vessels in between. The tumour stained positive for HMB-45 (human melanoma black) and smooth muscle actin, immunohistochemical features found suggestive of PEComa. However, due to some nuclear atypia, evidence of necrosis and the larger size of the mass some concerns were raised about its malignant potential. While there was no capsular breech and the tumour resection margins were >10 mm away, the noted histological features warranted a close follow-up.
In our histology database we found one other case of PEComa—also a child with TS. A 16-year-old asymptomatic girl was found to have a 5 cm liver lesion on routine follow-up scans, along with some smaller satellite lesions in the liver. She underwent a CT guided biopsy which revealed histological features also consistent with AML (PEComa). The histology demonstrated a tumour composed of spindle and epithelioid cells mixed with blood vessels and adipose tissue. No mitotic activity or cellular atypia or necrosis were identified. As there was no interval size change it was decided not to remove it but to keep under a regular imaging surveillance instead—this was planned for initially 6 monthly ultrasounds by the local team.
Investigations
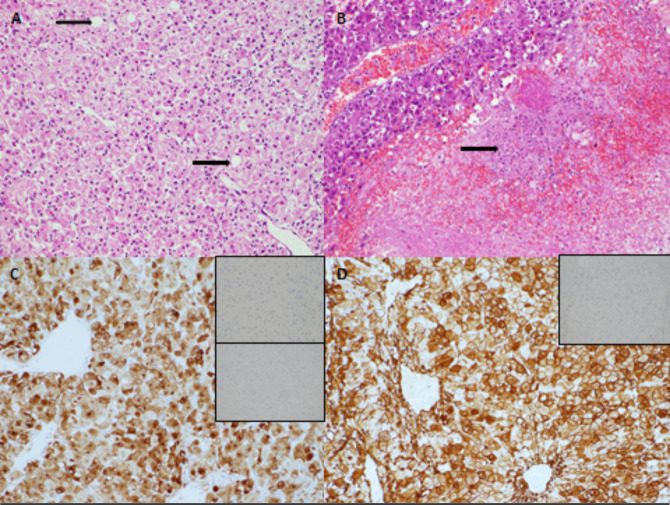
Histology after resection for case 1 (figure 1).
Figure 1.
Histology from resection of liver lesion in first case. Large polygonal tumour cells with abundant cytoplasm were arranged around ectatic vessels. occasional fat-containing cells were present (arrow) (A, H&E ×200 magnification). Fewer than 1 mitosis was identified per 10 high power fields. Foci of tumour necrosis were present (B, arrow, ×100 magnification). The tumour expressed HMB-45 (C, main image, HMB-45 immunostain ×200 magnification) and also expressed smooth muscle actin (D, main image, SMA immunostain ×200 magnification). There was no expression of S100 (C, upper inset, S100 immunostain ×200 magnification), no expression of OCH1E5 (C, lower inset, OCH1E5 immunostain ×100 magnification) and no expression of desmin (D, inset, desmin immunostain ×100 magnification).
Treatment
The first case had a resection of the liver mass and subsequent radiological monitoring. The second case had no treatment but was kept under regular radiological surveillance.
Outcome and follow-up
Both patients have been seen to have progression of growth and number of all of their existing lesions in the brain, kidneys and the second case also has lesions now in the spleen. However, neither patient has had any tumours that have progressed to become malignant currently.
The patient in the first case has notably had a right retinal astrocytoma develop subsequently, and the renal lesions have grown up to 35 mm in size. He has recently been started on Everolimus to limit further growth of these.
Discussion
We describe two unusual liver tumours developing in the background of TS, requiring different clinical management approaches.
PEComas are mesenchymal tumours composed of histologically and immunohistochemically distinctive perivascular epithelioid cells.1 Typically, PEComas are multiple and have radiological appearances consistent with lipomatous hepatic lesions distinct from the adjacent normal liver tissue. Histologically, the tumour is composed of three components: mature fat, blood vessels and epithelioid-spindle cells which are seen to radiate around the vascular lumina.2 They are distinctive in their immunohistochemical profile for displaying positivity for both melanocytic and smooth muscle markers.3 They typically stain positive for HMB-45, Melan A, microphthalmia transcription factor, smooth muscle actin, pan muscle actin and calponin, while they are negative for cytokeratin and S100 protein.
There is a strong association with TS, and the lesions can arise in multiple places, most typically the kidney. Hepatic PEComas in TS are much less common, with an incidence between 6%4 and 15%.3 5 This is opposed to 80% of patients with TS having identified renal PEComas. However, the prevalence of renal lesions were found to be much higher and age at occurrence lower in patients with hepatic lesions.5 Most liver lesions are small and are asymptomatic, a minority grow, but with no clinical consequences reported.5 PEComas represent 0.4% of primary liver tumours. PEComas can also be found in patients with no evidence of TS.
More atypical lesions, up to 20 cm in diameter and associated with rapid growth have been described in some of the literature.6 These are more likely to be symptomatic, associated with weight loss, abdominal pain, vomiting and therefore often requiring treatment such as resection, in contrast to smaller/multiple lesions which are usually just routinely monitored. The likelihood of anything other than a benign tumour in such patients is considered very low.7 Conversely, if there is no evidence of TS, the lesion is larger or growing rapidly, there should be a concern for hepatocellular carcinoma or other malignant neoplasm and resection options should be considered early. Interestingly, there was one adult patient (with TS) documented who was reported to have shown a significant tumour reduction after 6 months of Tamoxifen to shrink the tumour size medically instead of surgical management.8
A recent paper investigating the malignant potential of hepatic AMLs, included 81 cases of which 6% are patients with TS. Pathological features of the more aggressive ones included hepatocyte necrosis, high mitotic figures and vascular invasion. Initially, no patient had metastases, but on follow-up there was a 10% rate of recurrence or metastases.9 Another paper has suggested that the malignant potential could be determined using features such as diameters exceeding 5 cm, infiltrative margins, high-grade nuclear atypia, a mitotic count >1/50 high power fields, vascular invasion and necrosis. Of note, our first patient who had his lesion resected, had a tumour with diameter >5 cm, nuclear atypia and hepatocyte necrosis, which was in keeping with the clinical evolution.10
Of increasing interest is the suggestion that immunohistochemical studies may assist in the differentiation between benign and malignant neoplasms. While AMLs uniformly express melanocytic markers such as Melan-A and HMB-45, it is proposed that immunohistochemical staining for CD117 may predict clinical behaviour.11 Makhlouf et al first demonstrated that both the epithelioid and spindle cell component of AMLs show diffuse cytoplasmic positivity for CD117.12 While subsequent studies have confirmed this finding in benign AMLs, down-regulation or loss of CD117 expression has been observed in a proportion of the malignant cases providing a potential prognostic marker.11 13
We have tried to illustrate clinical difficulties in managing patients with TS and focal hepatic lesions. Our first patient had a tumour which was growing fairly rapidly and we felt its resection was justified due to the symptoms and subsequent histological features as discussed. In the second patient we opted for conservative and expectant approach as there were no clinical features to suggest its malignant potential, agreeing with similar findings previously documented.14 In any case, close surveillance with ultrasound scan (USS) and MRI is required and necessary both in the sporadic cases and those associated with TS. The progressive lesions need radiological monitoring even after effective resection.
Learning points.
Hepatic perivascular epithelioid cell tumours (PEComas) are tumours seen more frequently in tuberous sclerosis, although their incidence is still much less frequent than in their renal counterparts
PEComas are usually benign tumours, although there have been reported recurrences/metastases in up to 10% in one study.
Features such as increased size, rapid growth and vascular invasion would be concerning for possible malignant potential and therefore merit resection, as well as other reasons such as significant symptoms.
Acknowledgments
The authors would like to thank Dr Anne-Marie Grima and the paediatric and adult gastroenterology teams in Malta for liasing with the patient in the second case.
Footnotes
Contributors: MJ gathered the case information together and wrote the article. AH added the histopathology information and slides. MD gave advice and guidance on the histopathology sections and NDH gave oversight and guidance to the whole article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours : Pathology and genetics of soft tissue and bone. IARC Press, 2002. [Google Scholar]
- 2.Chen W, Liu Y, Zhuang Y, et al. . Hepatic perivascular epithelioid cell neoplasm: a clinical and pathological experience in diagnosis and treatment. Mol Clin Oncol 2017;6:487–93. 10.3892/mco.2017.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fricke BL, Donnelly LF, Casper KA, et al. . Frequency and imaging appearance of hepatic angiomyolipomas in pediatric and adult patients with tuberous sclerosis. AJR Am J Roentgenol 2004;182:1027–30. 10.2214/ajr.182.4.1821027 [DOI] [PubMed] [Google Scholar]
- 4.Kechaou I, Cherif E, Ben Hassine L, et al. . Liver involvement in tuberous sclerosis. BMJ Case Rep 2014;2014. 10.1136/bcr-2013-201650. [Epub ahead of print: 26 Mar 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jóźwiak S, Sadowski K, Borkowska J, et al. . Liver angiomyolipomas in tuberous sclerosis complex-their incidence and course. Pediatr Neurol 2018;78:20–6. 10.1016/j.pediatrneurol.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 6.Maebayashi T, Abe K, Aizawa T, et al. . Improving recognition of hepatic perivascular epithelioid cell tumor: case report and literature review. World J Gastroenterol 2015;21:5432–41. 10.3748/wjg.v21.i17.5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black ME, Hedgire SS, Camposano S, et al. . Hepatic manifestations of tuberous sclerosis complex: a genotypic and phenotypic analysis. Clin Genet 2012;82:552–7. 10.1111/j.1399-0004.2012.01845.x [DOI] [PubMed] [Google Scholar]
- 8.Lenci I, Angelico M, Tisone G, et al. . Massive hepatic angiomyolipoma in a young woman with tuberous sclerosis complex: significant clinical improvement during tamoxifen treatment. J Hepatol 2008;48:1026–9. 10.1016/j.jhep.2008.01.036 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Zhang C-W, Hong D-F, et al. . Primary hepatic epithelioid angiomyolipoma: a malignant potential tumor which should be recognized. World J Gastroenterol 2016;22:4908–17. 10.3748/wjg.v22.i20.4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol 2010;41:1–15. 10.1016/j.humpath.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TT, Gorman B, Shields D, et al. . Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol 2008;32:793–8. 10.1097/PAS.0b013e3181607349 [DOI] [PubMed] [Google Scholar]
- 12.Makhlouf HR, Remotti HE, Ishak KG. Expression of kit (CD117) in angiomyolipoma. Am J Surg Pathol 2002;26:493–7. 10.1097/00000478-200204000-00012 [DOI] [PubMed] [Google Scholar]
- 13.Fukuda Y, Omiya H, Takami K, et al. . Malignant hepatic epithelioid angiomyolipoma with recurrence in the lung 7 years after hepatectomy: a case report and literature review. Surg Case Rep 2016;2:31. 10.1186/s40792-016-0158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calleja-Stafrace D, Vella C. Tuberous sclerosis associated with multiple hepatic lipomatous tumours. Images Paediatr Cardiol 2016;18:1–4. [PMC free article] [PubMed] [Google Scholar]