Abstract
Toxic leukoencephalopathy (TL) is characterised by an insult to the myelin of the cerebral white manner which can be attributed to a number of offending agents, including drugs of abuse. We report a case of a fit and well young man presenting to hospital with an altered mental state. It was subsequently determined that the patient inhaled a significant volume of nitrous oxide recreationally. Nitrous oxide is easily accessible and the second most consumed drug among young adolescents (16–24 years old). Following extensive investigations and brain imaging, the patient was subsequently diagnosed with TL. After a prolonged hospital admission, he went on to make a complete neurological recovery.
Keywords: poisoning, drug misuse (including addiction), drug interactions, unwanted effects/adverse reactions, drugs misuse (including addiction)
Background
Toxic leukoencephalopathy (TL) was first identified in 1982 and refers to an insult in the brain white matter following exposure to leukotoxic agents.1 Altered mental status is the clinical hallmark of TL; however, the array of neurological symptoms can range from inattention, forgetfulness and personality changes to hemiparesis, dysarthria, dementia, stupor, coma and death.2 TL can present in both acute and chronic forms.
Although initially associated with heroin misuse, it is now apparent that it can arise from a wide spectrum of neurotoxins including radiation, therapeutic drugs, drugs of abuse and environmental toxins.3 The list of agents associated with drug misuse is increasing but includes toluene (and other inhalational solvents), ethanol, tobacco, heroin, morphine, fentanyl, cocaine, methylenedioxymethamphetamine, marijuana, amphetamines, methamphetamine, benzodiazepines and psylocibin.
The precise prevalence of TL is unknown. It was initially felt to be a rare event; however, as MRI has become more widely available, the incidence of the condition has increased.3 Autopsy data have demonstrated prominent myelin loss within cerebral white matter in the absence of any pathology within the cortical or subcortical grey matter.4 5 This has led to the conclusion that significant cognitive deficits observed in TL can be attributed to the selective toxic injury involving the white matter.6 It is hypothesised that the white matter is more susceptible to toxic insults due to low vascular perfusion of the regions and high metabolic demand of oligodendrocytes to maintain the myelin sheath.7 Furthermore, due to the lipophilic properties of toxins, they readily pass the blood–brain barrier and into the lipid-rich cerebral matter.8
Case presentation
We report a case of a 23-year-old man who was brought to the emergency department following concerns raised by his mother. On admission, he was in an irritable and psychotic state. He had a history of substance misuse with occasional cannabis consumption and substantial abuse of nitrous oxide (N2O) using up to 24 canisters per day (8 g/canister). His family and friends confirm he was agitated, unable to concentrate or perform habitual tasks and had a ‘zombie-like’ gait.
There was no medical history of note. His clinical observations revealed a heart rate of 111 beats/min, blood pressure 139/79 mm Hg, room-air oxygen saturation of 98% by pulse oximetry and a respiratory rate of 16 breaths per minute. His temperature was 36.8°C. He did not complain of any headaches or dizziness. However, he exhibited symptoms of auditory and visual hallucinations. His neurological examination confirmed that he was orientated to self, place and situation—but oblivious of the month, day and year. He was unable to concentrate or hold a coherent conversation. Cranial nerve functions and motor strength were all normal. He was moving all four limbs and had purposeful movement. Plantars were down-going.
His presentation raised concern for viral encephalitis and therefore was commenced on intravenous acyclovir pending results from his lumbar puncture. CT of the head was performed which showed subcortical white matter and external capsular abnormalities. Brain MRI showed abnormalities within the splenium of the corpus callosum as well as in the subcortical white matter involving the frontal and parietal lobes bilaterally (figure 1).
Figure 1.
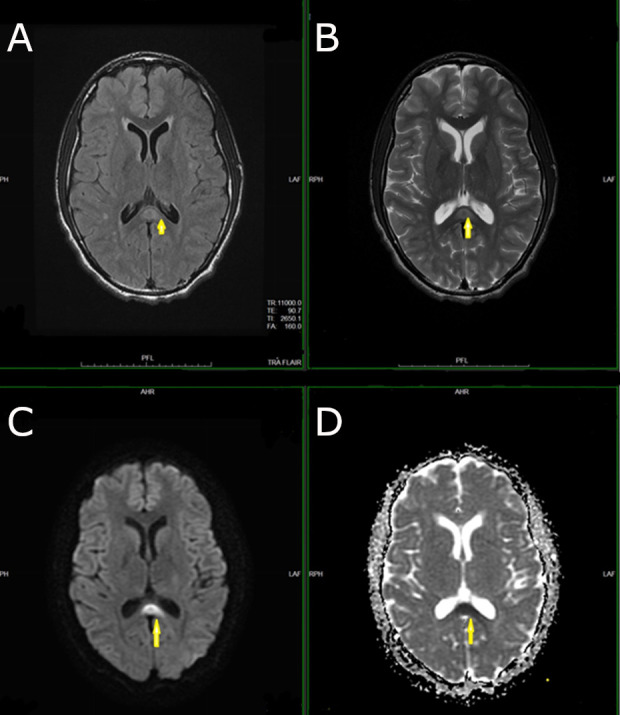
Axial FLAIR (A) and axial T2-weighted images (B) demonstrate a high signal in the splenium of the corpus callosum. The lesion shows restricted diffusion, that is, high signal on the axial diffusion-weighted image (C) and low signal on the corresponding apparent diffusion coefficient map images (D).
During his admission, the patient was extremely agitated and demonstrated paranoid thoughts. He also had pressured speech. He required personal security to ensure his personal safety was maintained. Given the MRI findings, he was commenced on hydroxocobalamin 1 mg intramuscularly once a day for 1 week. Following psychiatric evaluation, he was commenced on quetiapine though continued to require intermittent administration of parenteral haloperidol. Following completion of his investigations he was subsequently transferred to a mental health facility.
Within the care of the psychiatric team, he was treated with lorazepam and zopiclone with the management plan focused on a period of observation to monitor progress without anti-psychotics in view of possible residual effects of drug use, while awaiting the results of further investigations including an autoimmune screen—which were negative. Owing to his continuing erratic and distressed presentation, he was commenced on 5 mg olanzapine once a day with as required haloperidol and promethazine to control restless and combative behaviour. His olanzapine was increased to 15 mg after one week. This was effective in the management of his symptoms. The patient made a gradual improvement in his cognition and behaviour symptoms. After 6 weeks, the patient was well enough to go on day leave. His improvement continued, and 9 weeks on from his medical admission, he was able to be discharged from hospital.
Investigations
On admission, the white cell count was 10.5×10−9/L and C reactive protein was 11. His creatine kinase was 1240 IU/L, but his renal function was within normal parameters. Blood toxicology screen was performed by high-resolution mass spectrometry which demonstrated the presence of cannabis metabolite (carboxy-tetrahydrocannabinol). Additional studies, including thyroid-stimulating hormone, folate, HbA1C, HIV and hepatitis panel, were all normal. Cerebral spinal fluid (CSF) analysis was normal (glucose was 2.4 mmol/L and protein 0.16 g/L), and there were no red blood cells or white blood cells. CSF PCR was negative for herpes simplex virus type 2, varicella zoster, enterovirus and parechovirus. An autoantibody screen was also negative including rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibody. Tests for autoimmune encephalitis, including anti–voltage-gated potassium channel complex (anti-VGKC), N-methyl-D-aspartate (NMDA) receptor antibodies, contactin-associated protein-2 (CASPR2) antibody and leucine-rich glioma inactivated 1 (LGI1) antibody, were also negative. Vasculitis screening was also performed and was negative. His folate was 10.3 mg/L and vitamin B12 304 mg/L. His homocysteine concentration was raised at 128.8 µmol/L (normal 4–16 µmol/L).
CT performed on admission revealed multi-focal non-specific ill-defined areas of low attenuation in the subcortical white matter within the frontal lobes, left parietal lobe and external capsule. Subsequent MRI demonstrated diffusion restriction within the splenium of the corpus callosum with T2 hyperintensities in the subcortical white matter in the frontal and parietal lobes bilaterally in keeping with acute toxic leukoencephalopathy (ATL) (figure 1).
Outcome and follow-up
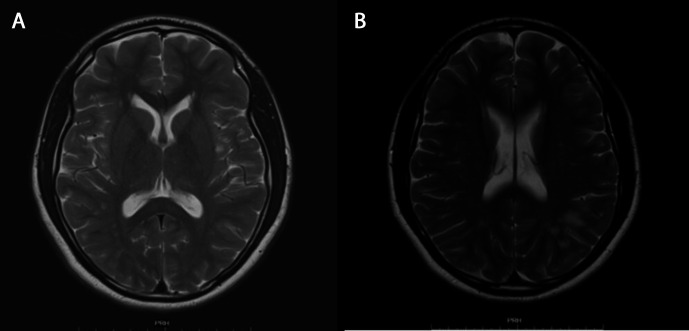
The patient proceeded to a full and complete neurological recovery within 4 months following his discharge. He subsequently attended an outpatient clinic following repeat MRI imaging. The scans showed resolution of the focal T2 hyperintensities with improvements in the changes in the splenium of corpus callosum (figure 2).
Figure 2.
Follow-up MRI images after 4 months show complete resolution of the lesion in the splenium of the corpus callosum on axial T2-weighted images (A). Extensive patchy T2 hyperintensities are noted in the deep and subcortical white matter (B) and likely to represent cerebral vascular disease in the context of illicit drug use.
Discussion
The patient presented a diagnostic challenge to the acute medical team at time of presentation due to his acute neurobehavioral state. The patient was known to be a student at an institution of Higher Education, who was fit and well without a medical history of significance. The bizarre behaviour initially led to a wide differential diagnosis to be considered. In addition, symptoms which relate to drug intoxication would have been expected to have resolved during the hospital admission due to abstinence. However, following exploration of his collateral history, it was found that the patient did consume cannabis occasionally, but there was a significant nitrous oxide misuse over the preceding 4 months. The amount of nitrous oxide was at least one box per day (containing 24 canisters of 8 grams each).
Recreational use of nitrous oxide is known for its euphoric effects.9 Its use and supply is legal in the UK for a number of applications such as a propellant for whipped cream, foam creations like espuma and mousse desserts. It is also used as a packaging gas to protect food from oxidation and spoilage as well as within medical practice for its analgesia and anaesthetic properties. However, in 2016, its sale for misuse as a psychoactive substance was prohibited with the introduction of the Psychoactive Substances Act (2016). Despite this, its recreational use remains common. The 2017/2018 Crime Survey for England and Wales estimated that 2.3% of adults aged between 16 and 59 had used the drug (725 000). Despite the 2016 legislation, there has been no significant reduction in its misuse and that young adults (16–24 years) were more likely to access the drug than the older population.
Owing to the clinical course of the illness, the absence of other positive findings, as well as the known physiochemical properties of nitrous oxide, we hypothesised that the diagnosis for this patient is that of a nitrous oxide–induced ATL.
Nitrous oxide is an NMDA receptor antagonist, with activity also on endogenous opiate receptors.10 It is known to antagonise the NMDA receptor, a non-selective ion channel which is involved in synaptic plasticity and memory formation. It is also known that N2O can disrupt vitamin B12 levels and thus cause neurobehavioural symptoms.11 Furthermore, it is hypothesised that N2O can inhibit methylcobalamin production by oxidation of the B12 cobalt ion from a 1+ to 3+ valence state. This may have a number of effects on a variety of biochemical pathways—most important of which is the prevention of methionine synthase in converting homocysteine to methionine. This results in a reduction in myelin formation and raised homocysteine levels.12 13 Similarly, cobalamin deficiency may also result in cytokine and growth factor insults.14 Owing to the oxidised and non-functional vitamin B12, despite normal vitamin B12 levels, some clinicians recommend supplementation, though such practice presently has little evidence to support it.
Such hypotheses appear to be backed up in animal studies.15 However, whether such mechanisms are involved in ATL remains unclear. Given the inhalational nature of the substance misuse, it has been postulated that psychosis may be attributed to cerebral anoxia due to raised homocysteine levels. Homocysteine is an NMDA agonist which is associated with oxidative stress and mitochondrial disruption via an intracellular calcium release.16 This would suggest that following inhalation of nitrous oxide, it dissolves in the blood causing a dilution of the volume of the oxygen within the alveolus and thus lessening the alveolar oxygen tension leading to decreased oxygen delivery to the brain. The subsequent cerebral anoxia may well be a precipitating factor for the white matter changes seen in TL. Removal of the precipitating factor appears to be a significant feature in the resolution of the conditions.
The majority of reported cases of neurotoxicity secondary to nitrous oxide misuse relates to the development of subacute combined degeneration (SACD) of the spinal cord and ataxia. However, the hallmark features observed in the MRI of our patient make a compelling case of ATL, given the insult to the white matter which resulted in widespread, bilateral, confluent vacuolation of the white matter known as a spongiform degeneration.
Given that nitrous oxide has been implicated as a causative agent in SACD, it is averred that in the absence of other causative agents, it is implicated as the substance responsible for the symptoms exhibited in our patient.
The initial presentation of the patient was challenging to the medical team from a medical and psychiatric perspective. Involvement of the multidisciplinary team was crucial to ensure a steady recovery and the best possible outcome for the patient. Preliminary treatment was with benzodiazepines (lorazepam); however, more long-term use of atypical antipsychotics produced the better response in reducing the behavioural disturbances of the patient as well as allowing the patient to come to terms with the frightening events which were occurring. The patient did reveal to the team following his discharge and recovery that he had no insight or recollection of the events during his admission.
Following a MEDLINE literature review, this appears to be the first reported case of inhaled nitrous oxide ATL. However, given the significant misuse of nitrous oxide among young adolescents and adults, clinicians should be aware of the potential complications of nitrous oxide abuse and TL.
Learning points.
Nitrous oxide–induced toxic leukoencephalopathy is an uncommon scenario with nitrous oxide abuse.
In patients presenting with neurobehavioural complaints, a detailed drug misuse history must be undertaken.
Investigations include MRI which can show diffusion restriction within the cerebral white matter.
Treatment is mainly supportive though may require the use of antipsychotics to control the symptoms.
Like other cases of acute toxic leukoencephalopathy, the prognosis was good and patients can make a clinical recovery.
Footnotes
Contributors: RA wrote the case report, performed a literature review and contacted the patient to obtain written consent. PGM assisted with the investigations of the case report and preparing the figures. NL provided guidance in preparing the article and contributed substantially to revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ellis WG, Sobel RA, Nielsen SL. Leukoencephalopathy in patients treated with amphotericin B methyl ester. J Infect Dis 1982;146:125–37. 10.1093/infdis/146.2.125 [DOI] [PubMed] [Google Scholar]
- 2.Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med Overseas Ed 2001;345:425–32. 10.1056/NEJM200108093450606 [DOI] [PubMed] [Google Scholar]
- 3.Kumar Y, Drumsta D, Mangla M, et al. . Toxins in Brain! Magnetic Resonance (MR) Imaging of Toxic Leukoencephalopathy – A Pictorial Essay. Pol J Radiol 2017;82:311–9. 10.12659/PJR.901791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, et al. . Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol 1988;23:611–4. 10.1002/ana.410230614 [DOI] [PubMed] [Google Scholar]
- 5.Keogh CF, Andrews GT, Spacey SD, et al. . Neuroimaging features of heroin inhalation toxicity: "chasing the dragon". AJR Am J Roentgenol 2003;180:847–50. 10.2214/ajr.180.3.1800847 [DOI] [PubMed] [Google Scholar]
- 6.Wolters EC, van Wijngaarden GK, Stam FC, et al. . Leucoencephalopathy after inhaling "heroin" pyrolysate. Lancet 1982;2:1233–7. 10.1016/S0140-6736(82)90101-5 [DOI] [PubMed] [Google Scholar]
- 7.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging 2011;32:1341–71. 10.1016/j.neurobiolaging.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JB, Blain PG. Neurotoxicology: what the neurologist needs to know. J Neurol Neurosurg Psychiatry 2004;75 Suppl 3:iii29–34. 10.1136/jnnp.2004.046318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randhawa G, Bodenham A. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth 2016;116:321–4. 10.1093/bja/aev297 [DOI] [PubMed] [Google Scholar]
- 10.Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog 2007;54:9–18. 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garakani A, Jaffe RJ, Savla D, et al. . Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict 2016;25:358–69. 10.1111/ajad.12372 [DOI] [PubMed] [Google Scholar]
- 12.Safari A, Emadi F, Jamali E, et al. . Clinical and MRI manifestations of nitrous oxide induced vitamin B12 deficiency: a case report. Iran J Neurol 2013;12:111–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Nunn JF. Clinical aspects of the interaction between nitrous oxide and vitamin B12. Br J Anaesth 1987;59:3–13. 10.1093/bja/59.1.3 [DOI] [PubMed] [Google Scholar]
- 14.Hathout L, El-Saden S. Nitrous oxide-induced B₁₂ deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B₁₂. J Neurol Sci 2011;301:1–8. 10.1016/j.jns.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Misra UK, Kalita J, et al. . Nitrous oxide related behavioral and histopathological changes may be related to oxidative stress. Neurotoxicology 2015;48:44–9. 10.1016/j.neuro.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 16.Savage S, Ma D. The neurotoxicity of nitrous oxide: the facts and "putative" mechanisms. Brain Sci 2014;4:73–90. 10.3390/brainsci4010073 [DOI] [PMC free article] [PubMed] [Google Scholar]