Abstract
Breast abscesses are a common surgical problem, typically occurring secondary to lactation mastitis. Recurrent subareolar abscesses are rarely reported and may be poorly recognised as a presentation of squamous metaplasia of lactiferous ducts, known eponymously as ‘Zuska’s disease’. Other synonyms include subareolar breast abscess and lactiferous or mammary fistulas. Recognition of this painful entity is crucial for optimal outcomes since typical breast abscess management of recurrent aspiration or incision and drainage can lead to recurrence and chronic complications, such as fistula formation.
Keywords: breast surgery, general surgery, general practice / family medicine
Background
Typical breast abscesses are a common pathology in smokers. However, clinicians need to be wary of those presenting with recurrent subareolar abscesses or mammary fistulas. Failure to recognise Zuska’s disease can result in a protracted disease duration and disfiguration due to inappropriate management. Routine abscess management of antibiotics with drainage (either via aspiration or incision) is inadequate; only formal surgical excision can effectively resolve this chronic pathology with low recurrence rates and appropriate cosmesis.
Case presentation
A 29-year-old premenopausal Australian woman presented with an 8-month history of left nipple inversion with associated induration and cellulitis, on a background of subpectoral breast implants. She had no significant personal or family breast history and was an ex-smoker. She had been previously been reviewed by two different breast surgeons and was treated with multiple courses of oral antibiotics with transient resolution, but failure to resolve the nipple inversion and induration. A recognisable collection was never present clinically or on repeated ultrasounds.
Investigations
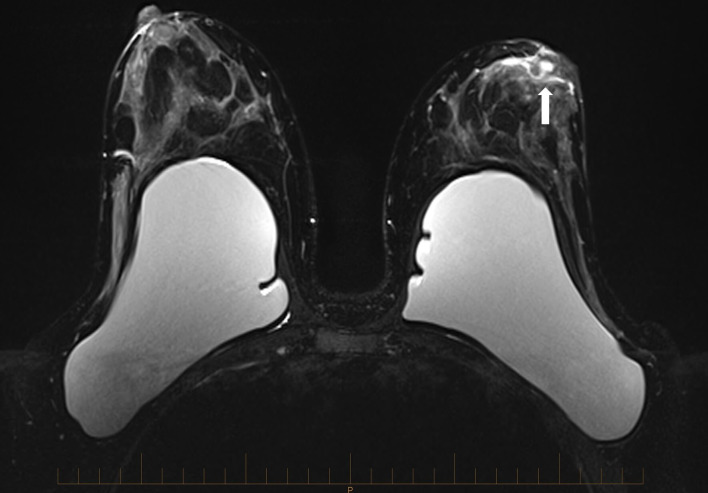
On our review, we were concerned regarding the potential for squamous metaplasia of lactiferous ducts (SMOLD) and arranged an MRI scan which identified a left periareolar lesion with shortening and widening of periareolar ducts (figure 1).
Figure 1.
Left breast retroareolar non-mass enhancement appearing centrally on several, mildly dilated ducts (arrow).
Differential diagnosis
Given the patient’s recurrent symptoms, coupled with its location and associated imaging features, the diagnosis of SMOLD was easily made.
Treatment
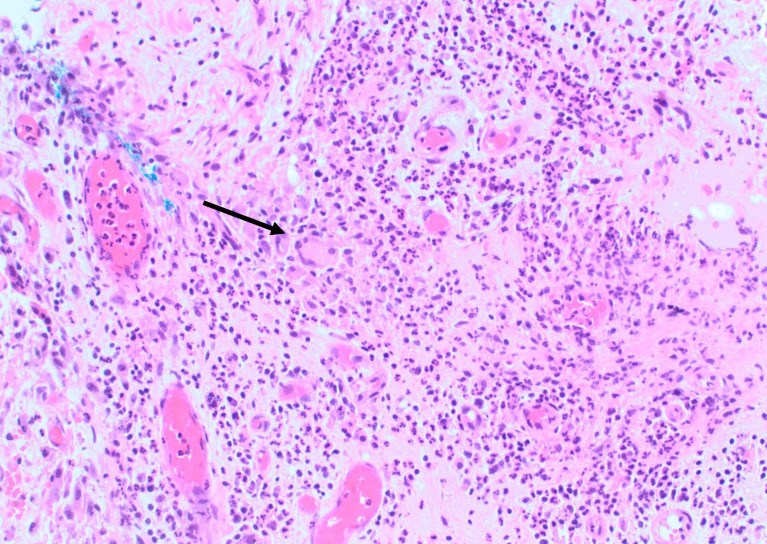
The patient underwent an excision of the left subareolar lesion and associated ducts via a periareolar incision. Histopathological examination showed lactiferous ducts and associated abscess containing foreign body giant cells and scattered anucleated squames (figure 2), a key diagnostic feature of SMOLD.
Figure 2.
Medium power H&E (200×) with giant cells (arrow) and surrounding mixed inflammatory cell infiltrate.
Outcome and follow-up
The patient had a complete recovery with no recurrence at 1 year with an excellent aesthetic outcome involving no distortion of the nipple-areolar complex.
Discussion
The primary risk factor for SMOLD (previously known as Zuska’s disease) is smoking in 90% of cases. Smokers have a sixfold risk of developing a primary breast abscess and are 15 times more likely to develop recurrence.1 The lactiferous ducts are physiologically lined by bilayer cuboidal glandular epithelium up to the ampulla and hypothetically cigarette byproducts cause either localised hypoxia or direct damage to the lactiferous ducts, resulting in keratinising squamous metaplasia proximal to the ampulla. This leads to keratin shedding with duct obstruction, rupture, associated inflammation and abscess formation. Mechanical factors may also play a role as SMOLD is more common in pierced or inverted nipples.
Once malignancy is excluded, an infective lesion should be treated accordingly. Failure of timely resolution should raise suspicions of SMOLD with early surgical intervention offered. Nipple or areolar discharge however is indicative of a fistula and patients should undergo immediate surgical excision. Surgeons may be unaware of this rare condition and therefore continue with inappropriate treatment. Pathologists may overlook the sometimes scant anucleate squames and keratin debris and misdiagnose the condition as a non-specific abscess in an incisional biopsy. The characteristic location and clinical history of SMOLD should trigger consideration of this entity by both surgeons and pathologists.
The recommended management of SMOLD is total surgical excision of the abscess, fistula and the obstructed ducts; coupled with smoking cessation. The surgical management of this pathology was initially proposed by Zuska et al in his seminal paper in 1951.2 In 1981, this opinion was challenged by Rosenthal et al who recommended aspiration and antibiotic therapy only.3 A single surgeon retrospective review of 67 cases suggested that needle aspiration or incision and drainage could resolve approximately 50% of subareolar abscesses, with definitive surgery only in abscess recurrence or fistula formation.4 However, Versluijs-Ossewaarde et al then reviewed 204 subareolar breast abscesses and proved that formal surgery, lactiferous duct excision, had a statistically significantly lower recurrence rate than no surgery (28% vs 79% respectively, p<0.001).5 Failure to adequately diagnose this condition and provide appropriate treatment can result in a protracted disease duration, with some reported durations of 1–2 years.6
Appropriate surgical procedures described include Meguid’s transverse,7 Hadfield’s curvilinear8 or Urban’s radial techniques.9 All three techniques isolate and resect the affected ducts and fistulas with low recurrence rates and high patient satisfaction rates on cosmesis.10 Given the rarity of this pathology, there are no randomised control trials to date comparing surgical techniques and their clinical and cosmetic outcomes.
Learning points.
Squamous metaplasia of lactiferous ducts (SMOLD) was previously known as Zuska’s disease.
SMOLD presents as recurrent breast abscesses and/or mammary fistulas due to pathological changes to the breast ducts.
The definitive cause of squamous metaplasia of the lactiferous ducts is uncertain; however, keratin shedding results in ductal obstruction resulting in chronic inflammation and abscess formation.
Due to the underlying pathology, antibiotic therapy will not cure SMOLD.
SMOLD is definitively managed with formal surgical excision only.
Footnotes
Contributors: All associated authors were involved in the writing of this case report. The patient was managed and treated by ED. The pathology was reviewed by SO. The preparation of the case report and discussion/consent with patient was obtained by AO. The write up was performed by AO with significant contributions by ED and SO.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gollapalli V, Liao J, Dudakovic A, et al. . Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg 2010;211:41–8. 10.1016/j.jamcollsurg.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 2.Zuska JJ, Crile G, Ayres WW. Fistulas of lactifierous ducts. Am J Surg 1951;81:312–7. 10.1016/0002-9610(51)90233-4 [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal LJ, Greenfield DS, Lesnick GJ. Breast abscess: management in subareolar and peripheral disease. N Y State J Med 1981;81:182–3. [PubMed] [Google Scholar]
- 4.Lannin DR. Twenty-Two year experience with recurring subareolar abscess andlactiferous duct fistula treated by a single breast surgeon. Am J Surg 2004;188:407–10. 10.1016/j.amjsurg.2004.06.036 [DOI] [PubMed] [Google Scholar]
- 5.Versluijs-Ossewaarde FNL, Roumen RMH, Goris RJA. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J 2005;11:179–82. 10.1111/j.1075-122X.2005.21524.x [DOI] [PubMed] [Google Scholar]
- 6.Kazama T, Tabei I, Sekine C, et al. . Subareolar breast abscess in male patients: a report of two patients with a literature review. Surg Case Rep 2017;3:128. 10.1186/s40792-017-0402-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meguid MM, Oler A, Numann PJ, et al. . Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery 1995;118:775–82. 10.1016/S0039-6060(05)80049-2 [DOI] [PubMed] [Google Scholar]
- 8.Hadfield J. Excision of the major duct system for benign disease of the breast. Br J Surg 1960;47:472–7. 10.1002/bjs.18004720504 [DOI] [PubMed] [Google Scholar]
- 9.Urban JA. Excision of the major duct system of the breast. Cancer 1963;16:516–20. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Grant CS, Degnim A, et al. . Surgical management of recurrent subareolar breast abscesses: Mayo clinic experience. Am J Surg 2006;192:528–9. 10.1016/j.amjsurg.2006.06.010 [DOI] [PubMed] [Google Scholar]