Abstract
We present a case of recurrent cholangitis caused by Shewanella algae, a lethal, emerging pathogen that clinicians should be made aware of. An 86-year-old man with a history of gastrectomy for peptic ulcer disease and a cerebrovascular accident with known choledocholithiasis presented with recurrent episodes of cholangitis that failed conservative antibiotic treatment regimens. Shewanella has been described to have increasing resistance to piperacillin and tazobactam. Both S. algae and multidrug-resistant Escherichia coli were co-isolated in this patient, which required broader spectrum antibiotics for successful treatment and management. A high index of suspicion is required if the history is suggestive of marine or aquatic exposure, which could expose the patient to this lethal pathogen. Re-thinking and re-taking the history are important cornerstones in refining the diagnosis when faced with recurrent presentations of the same problem.
Keywords: general surgery, gastrointestinal surgery, hepatitis and other GI infections
Background
This case highlights the significance of Shewanella as a causative pathogen for difficult to treat cholangitis.
Cholangitis is most commonly caused by colonic bacteria.1 It can be caused by both gram-positive and gram-negative bacteria; however, Escherichia coli is the main gram-negative bacterium isolated, followed by Klebsiella and Enterobacter. Enterococcus is the most common gram-positive species.
The most common causes of biliary obstruction in patients with acute cholangitis without bile duct stents are biliary calculi (28%–70%), benign biliary stricture (5%–28%) and malignancy (10%–57%).2 We describe a patient with choledocholithiasis.
Case presentation
Our case is an 86-year-old man from a residential aged care facility with recurrent presentations of cholangitis with Shewanella algae isolated. His history included a partial gastrectomy for peptic ulcer disease, large left middle cerebral artery watershed infarct resulting in expressive aphasia, atrial fibrillation, benign prostatic hyperplasia, nephrolithiasis and hypercholesterolaemia.
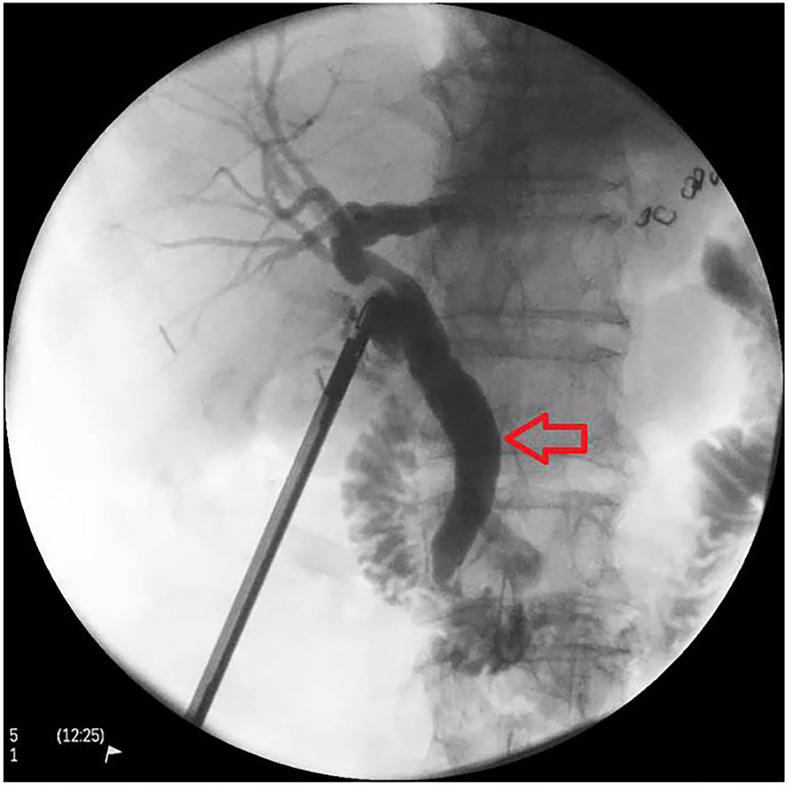
He had previously presented with acute cholecystitis in October 2015 and was commenced on intravenous antibiotics (ceftriaxone and metronidazole). His liver function tests (LFTs) were all within normal range. As he was taking clopidogrel prior to presentation, a decision was made to treat him with antibiotics and defer surgery unless he deteriorated. He improved clinically and was discharged with oral antibiotics and a planned elective laparoscopic cholecystectomy which he underwent in January 2016. His intraoperative cholangiogram showed a dilated common bile duct (CBD) but no filling defect (figure 1). He was discharged home on day 2 without complication. Histology showed chronic cholecystitis and cholelithiasis.
Figure 1.
Intraoperative cholangiogram from his laparoscopic cholecystectomy showing a dilated common bile duct (red arrow) with no filling defect.
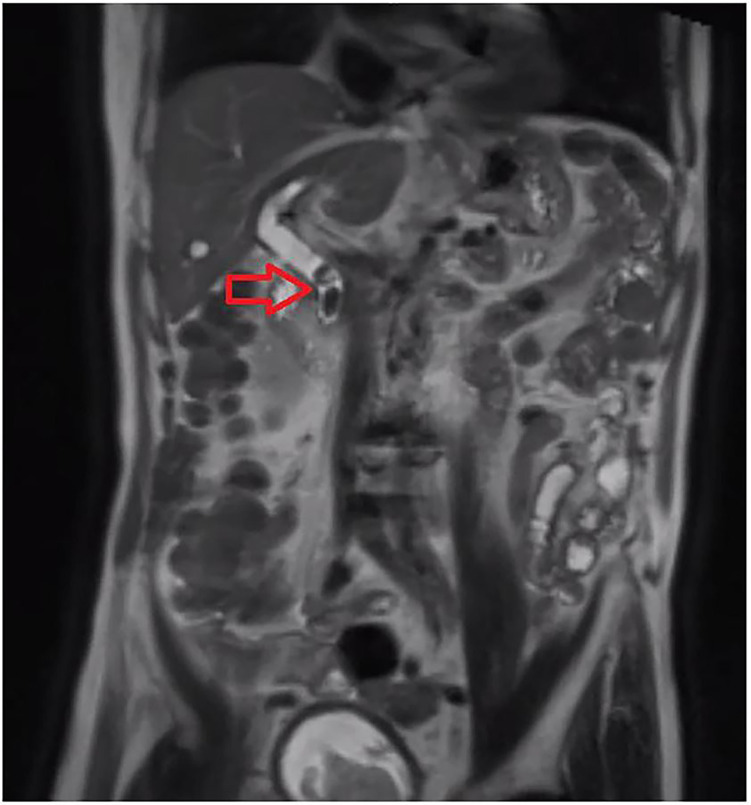
Two years later, he presented with cholangitis secondary to choledocholithiasis with an associated E. coli bacteraemia. His initial symptoms were dizziness and vomiting. He was febrile, tachycardic and hypotensive on arrival to the emergency department. LFTs showed a normal bilirubin of 8 μmol/L (normal range: <22 μmol/L), an elevated alkaline phosphatase of 228 IU/L (normal range: 30–110 IU/L) and gamma-glutamyl transpeptidase of 323 IU/L (normal range: 10–71 IU/L). His white cell count (WCC) was 5.5×109/L (normal range: 4×109/L–10×109/L) and C reactive protein (CRP) was 95 mg/L (normal range: <5 mg/L). E. coli was isolated on blood cultures in both aerobic and anaerobic bottles. It was sensitive to amoxycillin/clavulanate, gentamicin and meropenem, and resistant to cefazolin, amoxycillin and ceftriaxone. Magnetic resonance cholangiopancreatography (MRCP) confirmed two large CBD stones (figure 2). He was treated with piperacillin and tazobactam (Tazocin) and gentamicin on advice from the infectious diseases (ID) team. He underwent an attempted endoscopic retrograde cholangiopancreatography (ERCP), initially starting with a gastroscope to confirm the post-gastrectomy anatomy. Clean bile was seen flowing through the biliary limb of the gastrojejunostomy using a paediatric gastroscope. Unfortunately, the biliary limb was unable to be entered with a duodenoscope despite multiple attempts so the procedure was abandoned with a plan for percutaneous transhepatic cholangiography (PTC) if he were to deteriorate. Fortunately, he improved daily following 10 days of Tazocin. His inflammatory markers and LFTs improved, and he was eventually discharged home on 5 days of oral amoxycillin and clavulanic acid.
Figure 2.
Magnetic resonance cholangiopancreatography showing two common bile duct gallstones (red arrow).
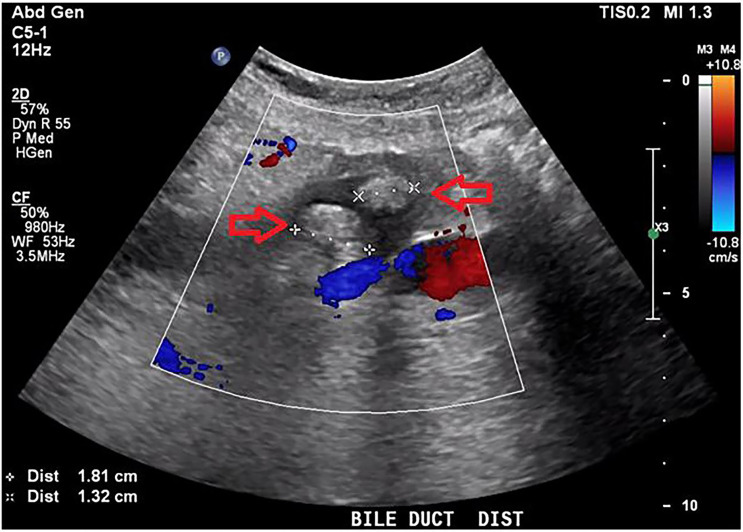
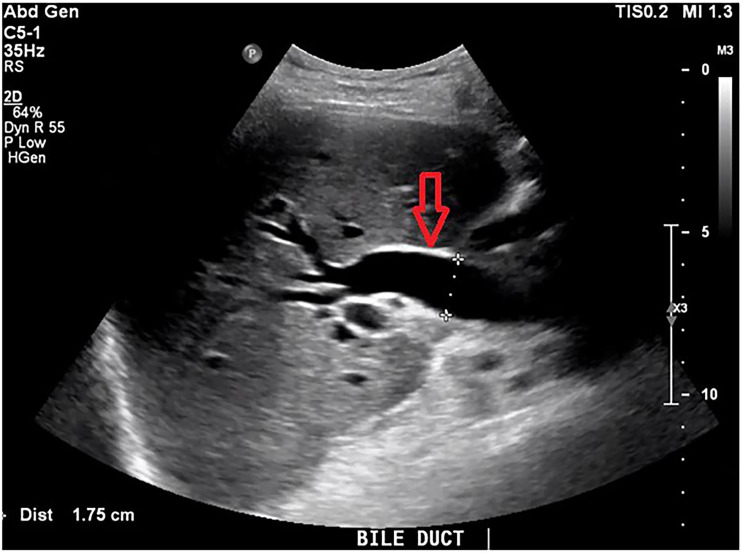
Two months later, he re-presented with fever, nausea, vomiting, hypotension and collapse. Bloods showed a cholestatic picture with a bilirubin of 49 μmol/L (normal range: <22 μmol/L), WCC of 16.8×109/L (4×109/L–10×109/L), CRP of 161 mg/L (normal range: <5 mg/L) and international normalised ratio of 2.3. E. coli and S. algae were both isolated on blood cultures in both aerobic and anaerobic bottles. E. coli was only sensitive to gentamicin and meropenem, and resistant to cephazolin, ciprofloxacin, amoxycillin, amoxycillin/clavulanate and ceftriaxone. S. algae was sensitive to ciprofloxacin, gentamicin, ceftriaxone and meropenem, and resistant to cefazolin, amoxycillin and amoxycillin/clavulanate. Abdominal ultrasound scan (USS) showed biliary dilatation of 25 mm in the distal CBD with associated choledocholithiasis (figure 3) and wall thickening of the CBD likely related to cholangitis (figure 4). PTC was discussed with the family and a decision was made not to proceed given he was improving clinically with current treatment. He was treated with 5 days of Tazocin and discharged home without any available effective oral antibiotic on advice of the ID team given the multidrug-resistant nature of E. coli.
Figure 3.
Abdominal ultrasound scan showing two gallstones in the distal common bile duct measuring 1.81 cm and 1.32 cm (red arrows).
Figure 4.
Abdominal ultrasound scan showing mild common bile duct wall thickening consistent with cholangitis (red arrow).
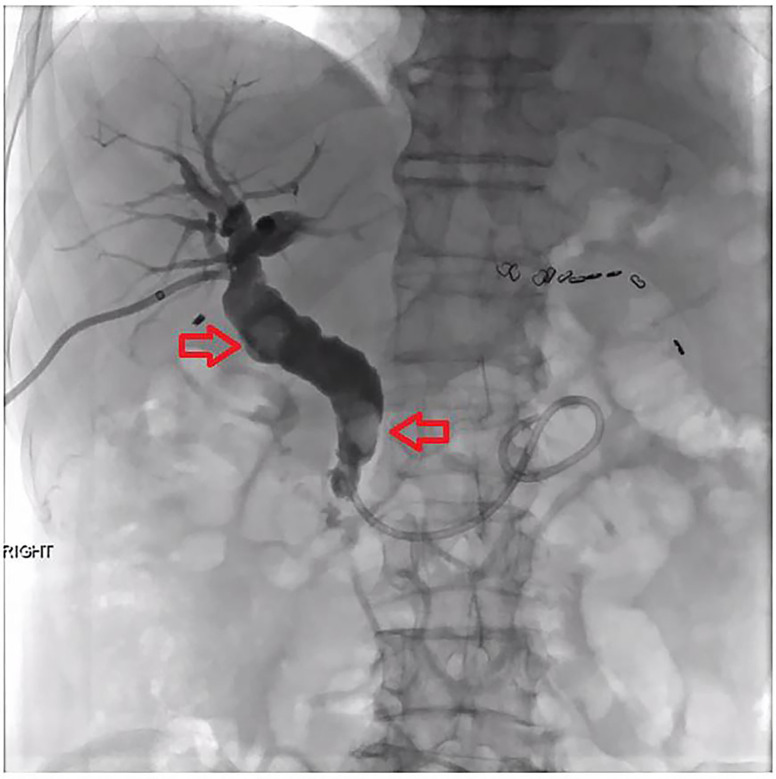
Sadly, he was re-admitted 2 days later with recurrent symptomatic cholangitis. On this admission, he was commenced on meropenem, a broad-spectrum carbapenem antibiotic. Additionally, he underwent PTC with biliary drainage where the biliary stones in the CBD were able to be crushed and pushed into the duodenum (figure 5). Repeat cholangiogram showed no evidence of filling defects. After 17 days of meropenem, he improved significantly, the biliary drain was removed and he was discharged home.
Figure 5.
Percutaneous transhepatic cholangiogram. Two gallstones are seen in the common bile duct (red arrows) before they are crushed and pushed into the duodenum.
DIFFERENTIAL DIAGNOSIS
Given he had previously undergone a laparoscopic cholecystectomy, acute cholecystitis could be ruled out. A biliary leak presenting 2 years after a laparoscopic cholecystectomy would be very rare and there was no evidence on CT imaging that this was the case. Lipase levels were never elevated with his recurrent presentations nor was there any radiographic evidence of focal or diffuse enlargement of the pancreas, thus excluding the possibility of acute pancreatitis. A liver abscess was certainly a possibility; however, there was no evidence on USS or CT imaging. Given his multiple presentations, as well as previous investigations (MRCP and USS) and a failed ERCP, this case was certainly concerning for untreated choledocholithiasis as a cause of acute cholangitis.
Outcome and follow-up
He has remained well since discharge and has not required admission to any health service for further management of cholangitis or any other medical problem.
Discussion
Shewanella spp are commonly associated with marine and aquatic environments. They are saprophytic, motile, gram-negative bacilli that are widely distributed in nature.3 The genus Shewanella has been categorised into three species, namely, S. putrefaciens, S. hanedai and S. benthica. S. putrefaciens is organised into four groups genotypically (I-IV) and strains in group IV are recognised as S. algae by some authors.4 Our patient grew up near a lake and enjoyed eating all kinds of seafood such as fish and prawns. He never ate raw seafood and spent a short period of his childhood near water before moving to the city areas.
Khashe and Janda5 reported that S. algae is the predominant human clinical isolate (77%), whereas S. putrefaciens represents the majority of non-human isolates (89%). Both were initially considered to be colonisers thriving on previously damaged tissue4; however, reports of their clinical significance are emerging. Shewanella has been isolated from wounds, urine, faeces, cerebrospinal fluid, bile and various other clinical samples.4
Shewanella spp are susceptible to carbapenems, aminoglycosides, quinolones, extended-spectrum cephalosporins and β-lactamase inhibitor combinations.6 7 A study by Vignier et al8 reported that 50% of Shewanella infections were polymicrobial in origin. Co-isolates, as demonstrated in our case, of both E. coli and S. algae being identified are clinically significant in the development of increased microbial resistance, resulting in limited treatment options and increased morbidity and mortality. There is recent epidemiological data showing increasing drug resistance especially to piperacillin–tazobactam and imipenem.9–12 This may explain the recurrent presentations of our patient.
Otsuka et al13 reported on a case of a 67-year-old Japanese man with primary sclerosing cholangitis who died from multi-organ failure from Shewanella. This was on a background of having had a distal gastrectomy for early gastric cancer 5 years prior. Several reports have demonstrated that Shewanella can cause lethal sepsis in patients with hepatobiliary disease.7 It would be interesting to research whether a distal gastrectomy and rearrangement of the gastrointestinal tract predisposes patients to developing Shewanella biliary infections as in our case and that reported by Otsuka et al13
Questions yet to be answered by the current body of research include whether Shewanella is contributing to more presentations of cholangitis. It would be also be interesting to investigate if Shewanella infections are contributing to increased morbidity and mortality, more intensive care admissions and increased hospital length of stay.
It is important to note that the presence of a foreign body such as a stone in the biliary tree can act as a nidus for bacterial colonisation. Bile taken from patients without biliary obstruction is sterile or almost sterile.14 Comparatively, approximately 70% of all patients with gallstones have evidence of bacteria in the bile.14 15 Our case highlights the development of biliary stones after a cholecystectomy, which were unable to be cleared by ERCP due to encountering difficult anatomy. It was not until the patient’s third presentation of recurrent cholangitis where the stones were able to be successfully removed and appropriate targeted antibiotics used. Subsequently, the patient remained well and did not require any further hospital admissions.
History taking remains the cornerstone to an accurate diagnosis and a thorough list of differential diagnoses. Noting any exposure to marine or aquatic environments or the consumption of raw fish may be helpful clues for recognising Shewanella in cholangitis. In this case, he had some exposure to an aquatic environment growing up which may be a risk factor for being colonised with Shewanella. Importantly, the medical community must be made aware of this emerging pathogen as a rare cause for cholangitis, particularly if there is a high index of suspicion because of its worrying morbid features. This case aims to convey this awareness. Although Shewanella was not isolated until his second admission, it may have been in fact an underlying contributor to his original presentation, but may have just been difficult to isolate.
In this case, a multidisciplinary approach to this patient was required. It involved expertise from a surgical team, interventional radiologist and ID team.
Patient’s perspective.
His daughter, next of kin, had the following comments from her perspective: ‘I am very appreciative of the medical care my father received over the course of multiple admissions. I am very grateful to the staff and their expertise. There were times when my father was very unwell and I am glad that the hospital and doctors were able to perform the necessary procedures to get him better and eventually discharge him back home’.
Learning points.
Shewanella and its spp are a rare but possibly emerging pathogen as a cause for cholangitis among other diseases. Shewanella is a potentially lethal organism, which can result in high morbidity and mortality. A high index of suspicion is required if the history is suggestive of exposure to marine or aquatic environments when seeing recurrent presentations of cholangitis.
This case report is aimed to highlight the importance of re-thinking the diagnosis and thoroughly re-taking the patient’s history to inform an accurate differential diagnosis in a patient with recurrent presentations of cholangitis. The identification of multidrug-resistant organisms can be a clue that a co-isolate, which may not be immediately identified, could be the cause of the recurrent presentations and an escalated treatment strategy may need to be employed earlier on.
Treatment of cholangitis not uncommonly requires a multidisciplinary approach as outlined in our case. This patient required input from a surgical team, interventional radiologist and infectious diseases team, which resulted in a positive outcome for the patient.
Footnotes
Contributors: All authors have made an individual contribution to the writing of the article and not just been involved with the patient’s care. JH and CS: conception and design. JH: acquisition of data or analysis and interpretation of data, and agreement to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved. JH, CS, EW and BK: drafting the article or revising it critically for important intellectual content, and final approval of the version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.van den Hazel SJ, Speelman P, Tytgat GN, et al. . Role of antibiotics in the treatment and prevention of acute and recurrent cholangitis. Clin Infect Dis 1994;19:279–86. 10.1093/clinids/19.2.279 [DOI] [PubMed] [Google Scholar]
- 2.Kimura Y, Takada T, Kawarada Y, et al. . Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007;14:15–26. 10.1007/s00534-006-1152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winn W, Allen S, Janda W, et al. . The non-fermentative gram-negative bacilli : Koneman’s color atlas and textbook of diagnostic microbiology. New York: Lippincott, 2006. [Google Scholar]
- 4.Simidu U, Kita-Tsukamoto K, Yasumoto T, et al. . Taxonomy of four marine bacterial strains that produce tetrodotoxin. Int J Syst Bacteriol 1990;40:331–6. 10.1099/00207713-40-4-331 [DOI] [PubMed] [Google Scholar]
- 5.Khashe S, Janda JM. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J Clin Microbiol 1998;36:783–7. 10.1128/JCM.36.3.783-787.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect 2005;11:347–52. 10.1111/j.1469-0691.2005.01108.x [DOI] [PubMed] [Google Scholar]
- 7.Liu P-Y, Lin C-F, Tung K-C, et al. . Clinical and microbiological features of Shewanella bacteremia in patients with hepatobiliary disease. Intern Med 2013;52:431–8. 10.2169/internalmedicine.52.8152 [DOI] [PubMed] [Google Scholar]
- 8.Vignier N, Barreau M, Olive C, et al. . Human infection with Shewanella putrefaciens and S. algae: report of 16 cases in Martinique and review of the literature. Am J Trop Med Hyg 2013;89:151–6. 10.4269/ajtmh.13-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Héritier C, Poirel L, Nordmann P. Genetic and biochemical characterization of a chromosome-encoded carbapenem-hydrolyzing Ambler class D beta-lactamase from Shewanella algae. Antimicrob Agents Chemother 2004;48:1670–5. 10.1128/AAC.48.5.1670-1675.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D-M, Kang C-I, Lee CS, et al. . Treatment failure due to emergence of resistance to carbapenem during therapy for Shewanella algae bacteremia. J Clin Microbiol 2006;44:1172–4. 10.1128/JCM.44.3.1172-1174.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poovorawan K, Chatsuwan T, Lakananurak N, et al. . Shewanella haliotis Associated with Severe Soft Tissue Infection, Thailand, 2012. Emerg Infect Dis 2013;19:1019–21. 10.3201/eid1906.121607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai M-S, You H-L, Tang Y-F, et al. . Shewanella soft tissue infection: case report and literature review. Int J Infect Dis 2008;12:e119–24. 10.1016/j.ijid.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 13.Otsuka T, Noda T, Noguchi A, et al. . Shewanella infection in decompensated liver disease: a septic case. J Gastroenterol 2007;42:87–90. 10.1007/s00535-006-1957-0 [DOI] [PubMed] [Google Scholar]
- 14.Csendes A, Becerra M, Burdiles P, et al. . Bacteriological studies of bile from the gallbladder in patients with carcinoma of the gallbladder, cholelithiasis, common bile duct stones and NO gallstones disease. Eur J Surg 1994;160:363–7. [PubMed] [Google Scholar]
- 15.Ohdan H, Oshiro H, Yamamoto Y, et al. . Bacteriological investigation of bile in patients with cholelithiasis. Surg Today 1993;23:390–5. 10.1007/BF00309495 [DOI] [PubMed] [Google Scholar]