Highlights
-
•
Cognitive-motor function affects the functionality from initial dependency in IADL.
-
•
Walk plus cognitive demand impacts IADL more than only physical or cognitive tasks.
-
•
Dual-task and spatial navigation can be useful for screening IADL ability in Dementia.
Keywords: Alzheimer’s disease, Mild cognitive impairment, Cognition, Mobility, Dual-task, Spatial navigation
Abstract
Background
Instrumental activities of daily living (IADLs) ability impairments are clearly related to cognitive and motor decline, as well as to the progression of Dementia. However, more low-cost assessments are necessary to better understand the process of IADL in patients with Dementia.
Objective
To compare cognitive, motor and cognitive-motor performance at different stages of dependence on IADL in patients with Dementia.
Methods
Dementia patients (n = 53, age range: 63–94) and healthy older adults (n = 39, age range: 62–97) were included, and those with Dementia were separated into IADL 1 (n = 18), IADL 2 (n = 17), IADL 3 (n = 18). All groups performed cognitive (Trail making test A, semantic verbal fluency, and Stroop test), motor (sit to stand, aerobic steps, and 8-foot up-and-go), and cognitive-motor tests (dual-task, and spatial navigation). One-way ANOVA, Kruskal-Wallis, and Bonferroni post-hoc tests were used to compare groups. Also, an effect size (ES) has been applied to evaluate differences among the dementia groups while the healthy older adults were used as a reference group.
Results
Only cognitive-motor and cognitive tests showed significant differences among groups (IADL 1 x IADL 2 x IADL 3). Compared with the healthy group, the ES analysis exposed that patients in different stages of IADL showed the worst performance on tests combining motor and cognitive demand, but not for motor and cognitive function separately.
Conclusion
Poor dual-task and spatial navigation abilities are present in partial dependence in IADL, and these tasks should be considered as a functionality screening tool in patients with Dementia.
1. Introduction
The management of Dementia symptoms and treatments still requires knowledge around the declines present during the neuropathological evolution. Cognitive and functional impairment are the core symptoms of the Dementia syndrome and these functions become worse during the continuum stages of the disease (American Psychiatric Association, 2014). Several tasks and scales have been used for screening cognitive decline and loss of ability to perform activities of daily living (ADL) in Dementia. Different scales are frequently applied to measure ADLs (e.g., Katz, Barthel, and Lawton), with similar questions about eating, toileting, shopping, and others that are asked to caregivers (Katz, 1983; Lawton and Brody, 1969; Wade and Collin, 1988). Njegovan et al. (2001) defined ADLs as instrumental (IADL), linked to complex self-management tasks (e.g., traveling), whereas others are related to the basic self-care (BADL) (e.g. dressing). Mao et al. (2018), showed that the IADL scale can screen older adults for Dementia with 0.82 of accuracy, and 0.92 of the area under the curve in low-income countries. Thereby, the evaluation of IADL is crucial to detect Dementia, especially if we consider the interference of low education on cognitive assessment in low and middle-income countries.
However, in clinical practice, several studies have shown biases caused by caregiver burnout, which can influence these results, under and overestimating patient functional capability (Anderson et al., 1995; Pfeifer et al., 2013). To reduce this bias, objective measures have been proposed, such as the Direct Assessment of Functional Status-Revised (DAFS-R) that assesses functional abilities by requiring the participant to carry out the different tasks (Loewenstein et al., 1989). In this context, physical tests can predict functional disability (Boyle et al., 2002), but are not used to assess dependency in IADL of patients with Dementia in clinical practice. It is important to highlight that functionality in ADL is related to several factors, such as sensory system, physical capacity, and cognitive function (Prince et al., 2011; Zidan et al., 2012). For example, physical capacity impacts the IADL through the strength, aerobic capacity, and mobility that are needed to conduct everyday activities (Gnosa et al., 2019; Hesseberg et al., 2016). Cognitive functions such as attention, working memory, and inhibitory control are present in the executive functions and are also related to performed daily instrumental functions (Putcha and Tremont, 2016; Rodriguez-Bailon et al., 2015).
Moreover, cognitive-motor function is the characteristic present in the IADLs that requires a higher ability to perform, such as dual-tasks and spatial navigation. Dual-task condition refers to performing two different tasks simultaneously (cognitive-motor or motor-motor tasks), and spatial navigation refers to determining and maintaining a trajectory from one place to another (self-centered or non-self-centered navigation) (Committeri et al., 2020; Verghese et al., 2007). Both are complex functions that include multiple cognitive, motor, and perceptual processes. Also, disorientation in a familiar environment and the cognitive interference in gait performance can reflect deficits in different components of daily function (Agmon et al., 2014; Dingova et al., 2016). Neuroimage studies have shown the relationship between brain areas related to IADLs and cognitive-motor performance. For example, Hippocampal and frontotemporoparietal gray cortical matter atrophy is associated with IADL decline (Cahn-Weiner et al., 2007; Mioshi et al., 2013). The volume loss in these specific brain areas are also related to spatial navigation (Aguirre et al., 1998) and dual-task impairments (Kahya et al., 2019) in patients with Dementia.
Furthermore, it is important to note that IADL scales categorize older adults into independent, partial dependent, and totally dependent. However, this original division already has cognitive and motor characteristics established in literature regarding patients with Dementia (Boyle et al., 2002; Lindbergh et al., 2016; Martyr and Clare, 2012). Therefore, we need to clarify what happens in the partial dependence continuum regarding the behavior of motor, cognitive, and cognitive-motor functions. It is necessary to better understand objective measures related to dependency in IADL in order to facilitate the screening, clinical evolution, and manning of a specific treatment for each patient with Dementia. Thus, our objective was to compare cognitive, motor, and cognitive-motor performance at different stages of dependence on IADL task in patients with Dementia. We hypothesize that impairments combining motor and cognitive demands are higher than separate cognitive or motor tasks for the IADL partial dependence group.
2. Material and methods
2.1. Study design and setting
The data collection presented in this cross-sectional study was performed between 2014 and 2018. Diagnosis was performed by the medical staff at the Center for Alzheimer's Disease and other Mental Disorders in Older Persons at the Psychiatry Institute of the Federal University of Rio de Janeiro (IPUB / UFRJ). Through structured interview ICD-10 (World Health, 2004), DSM-IV (Bell, 1994), and Clinical Dementia Rating (CDR) (Black et al., 2009) for the analysis of mental disorders. All participants have been treated in the Psychiatry Institute and signed informed consent forms. The study also was approved by the Research Ethics Committee of IPUB-UFRJ under the CAAE registry: 24904814.0.0000.5263 and is part of a larger research project entitled "Physical exercise efficacy in the treatment of Major Depression, Alzheimer's Disease and Parkinson's Disease."
2.2. Participants
Dementia patients (n = 53) and healthy older adults (n = 39) were included, and those with Dementia were separated into IADL 1 (n = 18), IADL 2 (n = 17), IADL 3 (n = 18). Participants were included according to the following criteria: individuals aged over 60 years, of both sex, residents of the city of Rio de Janeiro, had a clinical diagnosis of Dementia – mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Healthy elderly (HE) people also were examined to discard the presence of mental illness diagnosis only to obtain normative data for reference analysis. Exclusion criteria were: illiterate elderly, functional classes III and IV according to New York Heart Association (NYHA) criteria, with psychological or physical comorbidities that impairs the performance during the tests, as well as critical visual and/or auditory impairments, cerebrovascular infarction, use of treatments such as electroconvulsive therapy, psychotherapy, and diagnosis of severe stage (CDR-3) in AD.
2.3. Procedure
All included subjects underwent clinical tests in the Psychiatry Institute. At the first visit to the Psychiatry Institute, patients or caregivers were informed about the research procedures and answered the anamnesis and cognitive tests battery. On the second visit, the participants performed the motor and cognitive-motor tests. After the data collection, we separate Dementia patients in different groups based on their IADL performance.
The Mini-Mental State Exam (MMSE) and the CDR were used to evaluate the global cognition, behavior, and ability to manage daily life (Black et al., 2009; Brucki et al., 2003). Lawton scale (Lawton and Brody, 1969) was used to score the subject's ability in IADL. Seven basic tasks (using the telephone, transport, shopping, food preparation, housekeeping, medication, and finances) present in everyday activities were used to rank the individual among totally dependent (score = 7), partial dependent (score between 8 and 20), and independent (score = 21). Each question proceeds to the analysis on three levels (“without help,” “with partial help,” and “cannot perform”), with the score calculation, 3, 2, and 1 point, respectively. To analyze the continuum of the partial dependent rank, we divided the dementia group according to their functionality in three levels. Therefore, a tercile range was performed in different stages of partial dependence, from the higher to the lower functional capability: IADL 1 (higher Lawton tercile score = 20 to 16), IADL 2 (medium Lawton tercile score = 16 to 12), IADL3 (lower Lawton tercile score = 12 to 8).
2.4. Outcomes
Motor functions were evaluated with three assessments by the senior fitness test battery (Rikli and Jones, 2001), the sit to stand test (STS), aerobic functional test (STEP), and 8-foot up and go test (8UG). The reliability and validity of the motor tests for older adults are, respectively: STS r = 0.89, (CI95 % = 0.79-0.93) and r = 0.77; STEP r = 0.90, (CI95 % = 0.84-0.93) and r = 0.73; and 8UG r = 0.95, (CI95 % = 0.92-0.97) and r = not described (Rikli and Jones, 1999).
Cognitive functions were assessed by the Trail Making Test – part A (TMTA) (Ashendorf et al., 2008), semantic verbal fluency (VF) with animal categorical (Lopes et al., 2009), and Stroop test – color card (ST_color) (Stroop, 1935). The reliability and validity of the cognitive tests for older adults are, respectively: TMTA r = 0.53-0.64, (CI95 %=not described) and r = not described; VF r = 0.56, (CI95 %= -7.6-10.5) and r = 0.66-0.71; and ST r = 0.74, (CI95 %=not described) and r = 0.33-0.56 (Strauss et al., 2006).
Cognitive-motor functions were measured by the 8UG performance while talking the major number of different animals on a dual-task condition (8UGDT) (Bridenbaugh and Kressig, 2015), and spatial navigation analysis with the Floor Maze Test immediate time (FMT_IT), and delay time (FMT_DT) (Sanders et al., 2008). The reliability and validity of the dual-task test for older adults with dementia are, respectively: ICC = 0.51-0.88, (CI95 %=not described) and r = not described (Lemke et al., 2017). The reliability and validity of the FMT for older adults were not found.
2.5. Statistical analyses
Normality and homoscedasticity of the data were analyzed by the Kolmogorov-Smirnov and Levene tests, respectively. A chi-square analysis was performed to assess the difference in percentages of sex and CDR. One-way ANOVA, Kruskal-Wallis test, and Bonferroni post-hoc test were applied to compare groups (IADL 1 x IADL 2 x IADL 3). The effect size (ES) was calculated for all groups with the following formula: ES = (Mean of IADL group – mean of healthy elderly control group)/Pooled standard deviation. We interpreted the magnitude of the ES as suggested for Cohen (1988) (>0.20 small; >0.50 moderate; >0.80 large). The statistical packages used were SPSS® version 19.0 (IBM Corporation, New York, USA), GraphPad Prism version 5.01 (GraphPad Software, San Diego, USA), and the level of significance accepted in the study was p ≤ 0.05.
3. Results
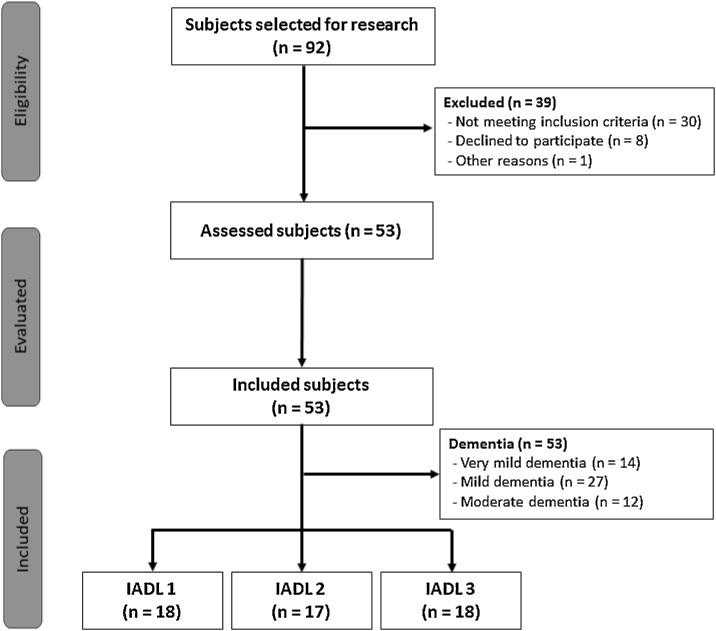
We selected 92 subjects for the research, 39 were excluded, and 53 Dementia patients were included. A flowchart with the selection of participants was presented in Fig. 1.
Fig. 1.
Flowchart of the subject selection.
3.1. Descriptive data
As expected, we founded a significant difference in MMSE (p < 0.001) and CDR (p = 0.023) among groups (IADL 1, IADL 2, and IADL 3). The other characteristics (age, sex, schooling, BMI, history of falls, comorbidity, and medications) did not present significant differences among groups. See descriptive data in Table 1.
Table 1.
Descriptive analysis.
| IADL 1 (n = 18) | IADL 2 (n = 17) | IADL 3 (n = 18) | F / X² | p | |
|---|---|---|---|---|---|
| Age, y | 80.88 ± 4.65 | 79.38 ± 7.13 | 78.40 ± 9.44 | 0.142 a | 0.868 |
| Female sex, n (%) | 9 (50) | 12 (71) | 8 (44) | 0.472 c | 0.492 |
| Schooling, y | 12 (7.25) | 11 (9.00) | 12 (11.00) | 0.526 b | 0.526 |
| MMSE, score | 26.33 ± 3.80 | 21.84 ± 2.67 | 18.46 ± 4.88 | 17.853 a | <0.001 #, d, e |
| BMI, kg/m2 | 26.83 ± 3.97 | 26.11 ± 4.71 | 25.88 ± 3.62 | 0.562 a | 0.574 |
| History of falls, n | 0 (1.00) | 0 (2.00) | 0 (1.00) | 0.546 b | 0.717 |
| Comorbidity, n | 2 (1.00) | 2 (1.00) | 1 (3.00) | 0.276 b | 0.704 |
| Medications, n | 4 (2.25) | 4 (2.50) | 2 (5.00) | 0.518 b | 0.580 |
| CDR, n (%) | |||||
| Very mild dementia | 13 (72) | 1 (6) | 0 (0) | 7.509 c | 0.023 # |
| Mild dementia | 4 (22) | 15 (88) | 9 (50) | ||
| Moderate dementia | 1 (6) | 1 (6) | 9 (50) |
IADL 1 = instrumental activities of daily living (tercile 1, higher Lawton score in partial dependence); IADL 2 = instrumental activities of daily living (tercile 2, medium Lawton score in partial dependence); IADL 3 = instrumental activities of daily living (tercile 3, lower Lawton score in partial dependence); MMSE = Mini Mental State Exam; BMI = Body Mass Index; CDR = Clinical Dementia Rating.
a F = one-way ANOVA; Mean ± standard deviation.
b X² = Kruskal-Wallis test; Median (interquartile range).
c Chi-square.
#p ≤ 0.05.
Post hoc analysis:
d Significant difference between IADL 1 and IADL 3;
e Significant difference between IADL 1 and IADL 2;
f Significant difference between IADL 2 and IADL 3.
3.2. Motor, cognitive, and cognitive-motor performance
Motor tests did not show a significant difference among groups in STEP (F = 1.062; p = 0.353), STS (X2 = 0.424; p = 0.807), and 8UG (X2 = 3.572; p = 0.097). On cognitive tests, we found a significant difference in TMTA (X2 = 6.723; p = 0.004), and VF (X2 = 6.611; p = 0.002), but not in ST_color (X2 = 5.791; p = 0.052). Executive functions characteristics present in the cognitive tests, showed differences in attention (TMTA), and cognitive flexibility (VF) tests. However inhibitory control (ST) does not show differences in IADL deterioration. The post-hoc analysis showed differences among groups in TMTA and VF (IADL 1 > IADL 3). Also, dual-task and spatial navigation conditions present in cognitive-motor tests showed significant difference in 8UGDT (X2 = 2.890; p = 0.047), FMT_IT (X2 = 5.360; p = 0.006), and FMT_DT (X2 = 5.601; p = 0.010). The post-hoc analysis was in the same cognitive function direction and showed a difference among groups in 8UGDT (IADL 1 > IADL 3), FMT_IT (IADL 1 > IADL 3), and FMT_DT (IADL 1 > IADL 3). Finally, the tercile-based group allocation exposed that IADL 3 (lower Lawton score in partial dependence) present a worse performance than IADL 1 (higher Lawton score in partial dependence) in attention, memory, dual-task and spatial navigation conditions. Also, IADL 2 (medium Lawton score in partial dependence) did not show a significant difference between the cited groups (Table 2).
Table 2.
Analysis of motor, cognitive, and cognitive-motor tests in IADL groups.
| IADL 1 (n = 18) | d | IADL 2 (n = 17) | d | IADL 3 (n = 18) | d | F / X² | p | |
|---|---|---|---|---|---|---|---|---|
| Motor tests | ||||||||
| STEP, n | 67.58 ± 20.37 | 0.96 | 67.93 ± 23.32 | 0.97 | 60.07 ± 30.51 | 1.24 | 1.062 a | 0.353 |
| STS, n | 11 (4.00) | 0.76 | 11 (1.75) | 0.98 | 10 (6.00) | 1.06 | 0.424 b | 0.807 |
| 8UG, s | 7.08 (2.00) | 0.96 | 6.98 (2.00) | 0.74 | 8.13 (4.00) | 1.47 | 3.572 b | 0.097 |
| Cognitive tests | ||||||||
| TMTA, s | 66.00 (53.04) | 0.03 | 96.81 (47.49) | 0.83 | 177.00 (136.23) | 1.46 | 6.723 b | 0.004 #, d |
| VF, n | 16 (9.50) | 0.67 | 11 (3.50) | 1.24 | 8 (3.50) | 2.17 | 6.611 b | 0.002 #, d |
| ST_color, s | 47.47 (19.31) | 0.97 | 47.30 (21.27) | 1.03 | 80.48 (53.97) | 2.08 | 5.791 b | 0.052 |
| Cognitive-motor tests | ||||||||
| 8UGDT, s | 9.57 (4.00) | 1.22 | 11.24 (6.00) | 1.78 | 11.31 (5.00) | 1.94 | 2.890 b | 0.047 #, d |
| FMT_IT, s | 50.54 (45.18) | 0.80 | 68.39 (143.48) | 1.39 | 112.00 (119.63) | 2.17 | 5.360 b | 0.006 #, d |
| FMT_DT, s | 40.02 (54.13) | 0.88 | 104.90 (129.13) | 1.85 | 178.19 (106.72) | 2.46 | 5.601 b | 0.010 #, d |
IADL 1 = instrumental activities of daily living (tercile 1, higher Lawton score in partial dependence); IADL 2 = instrumental activities of daily living (tercile 2, medium Lawton score in partial dependence); IADL 3 = instrumental activities of daily living (tercile 3, lower Lawton score in partial dependence); STEP = aerobic functional test; STS = sit to stand test; 8UG = 8-foot up and go; TMTA = trail making test A; VF = verbal fluency; ST_ color = stroop test color card; 8UGDT = 8-foot up and go with dual-task; FMT_IT = Floor maze test_ immediate time; FMT_DT = Floor maze test_ delay time.
d = effect size.
a F = one-way ANOVA; Mean ± standard deviation.
b X² = Kruskal-Wallis test; Median (interquartile range).
#p ≤ 0.05.
Post hoc analysis:
d Significant difference between IADL 1 and IADL 3;
e Significant difference between IADL 1 and IADL 2;
f Significant difference between IADL 2 and IADL 3.
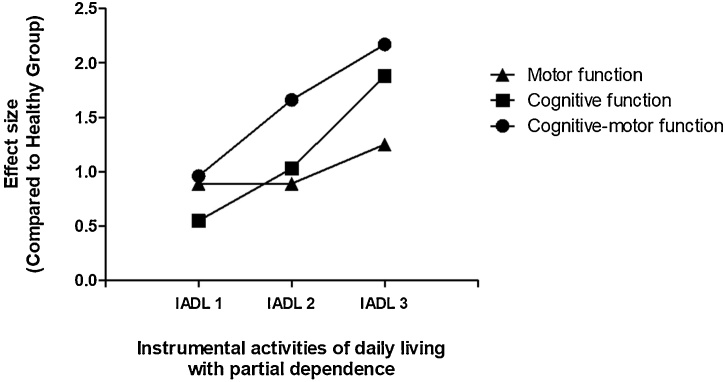
The ES was evaluated on the motor, cognitive, and cognitive-motor tests in each group (Table 2). Also, a line graph with ES comparisons between functions and IADL groups against the reference group was performed (Fig. 2). The HE group used to these analyses did not show a significant difference in the following descriptive data (mean ± standard deviation): 74.95 ± 8.94 years-old, 85 % females, and 13.03 ± 5.50 years of schooling. The ES results (Fig. 2) showed that, comparing to HE, the motor tests present the same ES between IADL 1 (SMD = 0.89; CI95 % = 0.55, 1.23) and IADL 2 (SMD = 0.89; CI95 % = 0.55, 1.24), but show a worst performance (higher ES magnitude) in IADL 3 (SMD = 1.25; CI95 % = 0.90, 1.60). Cognitive tests exhibited an IADL 1 (SMD = 0.55; CI95 % = 0.00, 1.10) with better performance (lower ES magnitude) than motor tests in IADL 1, but poor performance in IADL 2 (SMD = 1.03; CI95 % = 0.68, 1.38), and IADL 3 (SMD = 1.88; CI95 % = 1.43, 2.33). Cognitive-motor tests present the worst performance among all tests, starting with IADL 1 (SMD = 0.96; CI95 % = 0.62, 1.30), evolving to IADL 2 (SMD = 1.66; CI95 % = 1.28, 2.04), and IADL 3 (SMD = 2.17; CI95 % = 1.77, 2.57). Comparing each tests between IADL groups (Table 2), the 8UGDT showed the poorest performance (higher ES magnitude) in the IADL 1 (SMD = 1.22; CI95 % = 0.62, 1.82), while the FMT_DT presented the worst performance in the IADL 2 (SMD = 1.85; CI95 % = 1.19, 2.52) and IADL 3 (SMD = 2.46; CI95 % = 1.74, 3.18).
Fig. 2.
Effect size of motor, cognitive, and cognitive-motor performance at different stages of partial dependence on IADL in dementia.
4. Discussion
The present study aimed to compare cognitive, motor and cognitive-motor performance at different levels on IADL dependence in patients with Dementia. Cognitive-motor performance impairments are more eminent in partial dependence on IADL than in cognitive or motor tests, confirming our hypothesis. Walking while performing a cognitive demand interfered with the functional capability of patients with Dementia more than performing an individual physical or cognitive task. Thus, our results suggest that dual-task and spatial navigation can be used as complementary tools for screening ability in IADL in Dementia.
Although there is a clear relationship between human movement and mental health, the use of motor assessment in clinical evaluation is still rare (Deschamps, 2018). In neurocognitive disorders, gait parameters (de Oliveira Silva et al., 2019a), dual-task (Ferreira et al., 2019), handgrip strength (Teixeira et al., 2019), and spatial navigation (Zanco et al., 2018) have been investigated to differentiate healthy older adults, MCI and AD, as well as different stages of Dementia (de Oliveira Silva et al., 2019b; Placido et al., 2019). However, our results showed a reduced ES magnitude in motor tests, compared to cognitive and cognitive-motor parameters for distinguishing groups according to functionality. In the present study, patients with Dementia showed similar motor performance between higher and medium Lawton scores and worse performance in cognitive tests in the first stage of IADL partial dependence. These results might suggest that in the early stage of dependence, patients with Dementia maintain their impaired motor ability and start to show loss in functional capability only after advanced cognitive function declines. Lindbergh et al. (2016) propose that these motor and cognitive processes are related to IADL dysfunction in patients with Dementia. Also, Giebel et al. (2015) showing that in the earlier phases of IADL dysfunction, motor decline can be compensated by cognitive function. Thus, this compensatory strategy or accommodation, reduces the partial dependence evolution in motor functions.
Neuroimaging studies showed that executive function could predict the IADL decline independently within the course of Dementia (Cahn-Weiner et al., 2007). The ES in the cognitive battery showed a constant deterioration among the three stages of IADL partial dependence. These results corroborate a meta-analysis conducted by Martyr and Clare (2012), showing that executive functions follow IADL impairment in the earlier stages of Dementia. Also, Njegovan et al. (2001) explained the link between executive function and the ability to perform IADL when it is defined that both require a higher level of brain control to work. The loss of grey matter volume in cortical regions covering the frontal, medial temporal, occipital lobes, cingulate cortex, and precuneus was associated with lower cognitive and IADL scores (Jutten et al., 2019; Slachevsky et al., 2019). These brain areas are also related to cognitive-motor functions and might corroborate that cognitive deficits increase cognitive-motor decline (Kahya et al., 2019; Wagshul et al., 2019). Therefore, substituting some cognitive tests for cognitive-motor tests could serve to decrease testing-time and perhaps reduce the bias caused by poor education in middle-income countries when screening for dementia risk.
Wang et al. (2015) describe that cognitive-motor functions are interrelated to IADL preservation. Present in everyday function, declines in dual-task and spatial navigation performance could produce interference in IADL. Thus, the higher this interference, the greater the IADL dependence becomes for patients with dementia. Sunderaraman et al. (2019) showed that executive functions, processing speed, and verbal memory were associated with dual-task performance (walking while talking test) in Dementia. In this context, Zanco et al. (2018) also verified that verbal memory and global cognition were associated with spatial navigation. In our study, the ES in cognitive and cognitive-motor tasks showed constant deterioration among the three stages of IADL. Is important to note that the dual-task test presents a higher rate of decline in the early stage of dependence, and the delayed time in spatial navigation revealed a major impairment in the continuum phases of the IADL deterioration. Thus, the dual-task and spatial navigation tests present higher performance decline and can be an important parameter to evaluate IADL deterioration and the progress of Dementia.
Considering the importance of the objective measures related to dependency in IADL to facilitate the assessment of older adults with Dementia, understanding the cognitive-motor characteristics during different stages of functionality could be helpful to improve prognosis and treatment of the disease. This study presents some limitations such as the cross-sectional design that cannot demonstrate a causal relationship; the generalization of the results is limited to patients with Dementia in partial dependence on IADL; and the considerable variability present in cognitive-motor test performance.
5. Conclusion
Poor dual-task and spatial navigation abilities are present in IADL partial dependence, and these tasks should be considered as a functional screening tool in patients with Dementia.
Consent
Informed consent was obtained from all individual participants involved in the study.
Ethical statement
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Research Ethics Committee of IPUB-UFRJ under the CAAE registry: 24904814.0.0000.5263) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” under Grant (CNPq-303474/2019-0); “Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro” under Grant (FAPERJ-E26/202.523/2019).
Footnotes
The study was conducted at the Institute of Psychiatry, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
References
- Agmon M., Kodesh E., Kizony R. The effect of different types of walking on dual-task performance and task prioritization among community-dwelling older adults. The Scientific World Journal. 2014;2014 doi: 10.1155/2014/259547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre G.K., Zarahn E., D’Esposito M. Neural components of topographical representation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:839–846. doi: 10.1073/pnas.95.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, A . 5a edição ed. Artmed; Porto Alegre: 2014. DSM-5: Manual Diagnóstico e Estatístico de Transtornos Mentais. [Google Scholar]
- Anderson C.S., Linto J., Stewart-Wynne E.G. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26:843–849. doi: 10.1161/01.str.26.5.843. [DOI] [PubMed] [Google Scholar]
- Ashendorf L., Jefferson A.L., O’Connor M.K., Chaisson C., Green R.C., Stern R.A. Trail making test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 2008;23:129–137. doi: 10.1016/j.acn.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.C. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272:828–829. [Google Scholar]
- Black R., Greenberg B., Ryan J.M., Posner H., Seeburger J., Amatniek J., Resnick M., Mohs R., Miller D.S., Saumier D., Carrillo M.C., Stern Y. Scales as outcome measures for Alzheimer’s disease. Alzheimers Dement. 2009;5:324–339. doi: 10.1016/j.jalz.2009.05.667. [DOI] [PubMed] [Google Scholar]
- Boyle P.A., Cohen R.A., Paul R., Moser D., Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int. J. Geriatr. Psychiatry. 2002;17:164–169. doi: 10.1002/gps.539. [DOI] [PubMed] [Google Scholar]
- Bridenbaugh S.A., Kressig R.W. Motor cognitive dual tasking: early detection of gait impairment, fall risk and cognitive decline. Zeitschrift fur Gerontologie und Geriatrie. 2015;48:15–21. doi: 10.1007/s00391-014-0845-0. [DOI] [PubMed] [Google Scholar]
- Brucki S.M., Nitrini R., Caramelli P., Bertolucci P.H., Okamoto I.H. [Suggestions for utilization of the mini-mental state examination in Brazil] Arquivos de neuro-psiquiatria. 2003;61:777–781. doi: 10.1590/s0004-282x2003000500014. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner D.A., Farias S.T., Julian L., Harvey D.J., Kramer J.H., Reed B.R., Mungas D., Wetzel M., Chui H. Cognitive and neuroimaging predictors of instrumental activities of daily living. J. Int. Neuropsychol. Soc. 2007;13:747–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. L. Erlbaum Associates; Hillsdale, N.J: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Committeri G., Sebastiani V., de Pasquale F., Stocchi M., Fini C. Functional autonomy affects elderly spatial perception in body-centered coordinates. J. Aging Res. 2020;2020:1–8. doi: 10.1155/2020/5694790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Silva F., Ferreira J.V., Plácido J., Chagas D., Praxedes J., Guimarães C., Batista L.A., Laks J., Deslandes A.C. Gait analysis with videogrammetry can differentiate healthy elderly, mild cognitive impairment, and Alzheimer’s disease: A cross-sectional study. Exp. Gerontol. 2019 doi: 10.1016/j.exger.2019.110816. [DOI] [PubMed] [Google Scholar]
- de Oliveira Silva F., Ferreira J.V., Plácido J., Chagas D., Praxedes J., Guimarães C., Batista L.A., Marinho V., Laks J., Deslandes A.C. Stages of mild cognitive impairment and Alzheimer’s disease can be differentiated by declines in timed up and go test: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2019;85 doi: 10.1016/j.archger.2019.103941. [DOI] [PubMed] [Google Scholar]
- Deschamps T. Is Psychiatry Ready to Move? Psychiatry Investig. 2018;15:3–5. doi: 10.4306/pi.2018.15.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingova D., Fazekas T., Okuliarova P., Strbova J., Kucera M., Hrabovska A. Low plasma cholinesterase activities are associated with deficits in spatial orientation, reduced ability to perform basic activities of daily living, and low body mass index in patients with progressed Alzheimer’s disease. J. Alzheimers Dis. 2016;51:801–813. doi: 10.3233/JAD-151060. [DOI] [PubMed] [Google Scholar]
- Ferreira J.V., Araujo N.Bd., Oliveira Fd., Plácido J., Anna P.S., Monteiro-Junior R.S., Marinho V., Laks J., Deslandes A. Dual task in healthy elderly, depressive and Alzheimer s disease patients. J. Brasileiro de Psiquiatria. 2019;68:200–207. [Google Scholar]
- Giebel C.M., Challis D., Montaldi D. Understanding the cognitive underpinnings of functional impairments in early dementia: a review. Aging Ment. Health. 2015;19:859–875. doi: 10.1080/13607863.2014.1003282. [DOI] [PubMed] [Google Scholar]
- Gnosa K., Marczak A., Binder J., Adler G. Impact of physical fitness on cognitive performance in patients at a memory clinic. Dement. Geriatr. Cogn. Dis. Extra. 2019;9 129+ [Google Scholar]
- Hesseberg K., Bergland A., Rydwik E., Brovold T. Physical fitness in older people recently diagnosed with cognitive impairment compared to older people recently discharged from hospital. Dement. Geriatr. Cogn. Dis. Extra. 2016;6:396–406. doi: 10.1159/000447534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutten R.J., Dicks E., Vermaat L., Barkhof F., Scheltens P., Tijms B.M., Sikkes S.A.M. Impairment in complex activities of daily living is related to neurodegeneration in Alzheimer’s disease-specific regions. Neurobiol. Aging. 2019;75:109–116. doi: 10.1016/j.neurobiolaging.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Kahya M., Moon S., Ranchet M., Vukas R.R., Lyons K.E., Pahwa R., Akinwuntan A., Devos H. Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: a systematic review. Exp. Gerontol. 2019;128 doi: 10.1016/j.exger.2019.110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- Lawton M., Brody E. Assessment of older people self-maintaining and instrumental activities of daily living. Soc. Biol. Psychiatry. 1969:179–186. [PubMed] [Google Scholar]
- Lemke N.C., Wiloth S., Werner C., Hauer K. Validity, test-retest reliability, sensitivity to change and feasibility of motor-cognitive dual task assessments in patients with dementia. Arch. Gerontol. Geriatr. 2017;70:169–179. doi: 10.1016/j.archger.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Lindbergh C.A., Dishman R.K., Miller L.S. Functional disability in mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol. Rev. 2016;26:129–159. doi: 10.1007/s11065-016-9321-5. [DOI] [PubMed] [Google Scholar]
- Loewenstein D.A., Amigo E., Duara R., Guterman A., Hurwitz D., Berkowitz N., Wilkie F., Weinberg G., Black B., Gittelman B. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. J. Gerontol. 1989;44:P114–121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- Lopes M., Brucki S.M.D., Giampaoli V., Mansur L.L. Semantic verbal fluency test in dementia: preliminary retrospective analysis. Dement. Neuropsychol. 2009;3:315–320. doi: 10.1590/S1980-57642009DN30400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H.F., Chang L.H., Tsai A.Y., Huang W.W., Tang L.Y., Lee H.J., Sun Y., Chen T.F., Lin K.N., Wang P.N., Shyu Y.L., Chiu M.J. Diagnostic accuracy of Instrumental Activities of Daily living for dementia in community-dwelling older adults. Age Ageing. 2018;47:551–557. doi: 10.1093/ageing/afy021. [DOI] [PubMed] [Google Scholar]
- Martyr A., Clare L. Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dement. Geriatr. Cogn. Disord. 2012;33:189–203. doi: 10.1159/000338233. [DOI] [PubMed] [Google Scholar]
- Mioshi E., Hodges J.R., Hornberger M. Neural correlates of activities of daily living in frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 2013;26:51–57. doi: 10.1177/0891988713477474. [DOI] [PubMed] [Google Scholar]
- Njegovan V., Hing M.M., Mitchell S.L., Molnar F.J. The hierarchy of functional loss associated with cognitive decline in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M638–643. doi: 10.1093/gerona/56.10.m638. [DOI] [PubMed] [Google Scholar]
- Pfeifer L., Drobetz R., Fankhauser S., Mortby M.E., Maercker A., Forstmeier S. Caregiver rating bias in mild cognitive impairment and mild Alzheimer’s disease: impact of caregiver burden and depression on dyadic rating discrepancy across domains. Int. Psychogeriatr. 2013;25:1345–1355. doi: 10.1017/S1041610213000562. [DOI] [PubMed] [Google Scholar]
- Placido J., Ferreira J.V., de Oliveira F., Sant’Anna P., Monteiro-Junior R.S., Laks J., Deslandes A.C. Association among 2-min step test, functional level and diagnosis of dementia. Dement. Neuropsychol. 2019;13:97–103. doi: 10.1590/1980-57642018dn13-010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Acosta D., Ferri C.P., Guerra M., Huang Y., Jacob K.S., Jotheeswaran A.T., Liu Z., Rodriguez J.J., Salas A., Sosa A.L., Williams J.D. The association between common physical impairments and dementia in low and middle income countries, and, among people with dementia, their association with cognitive function and disability. A 10/66 dementia research group population-based study. Int. J. Geriatr. Psychiatry. 2011;26:511–519. doi: 10.1002/gps.2558. [DOI] [PubMed] [Google Scholar]
- Putcha D., Tremont G. Predictors of independence in instrumental activities of daily living: amnestic versus nonamnestic MCI. J. Clin. Exp. Neuropsychol. 2016;38:991–1004. doi: 10.1080/13803395.2016.1181716. [DOI] [PubMed] [Google Scholar]
- Rikli R.E., Jones C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 1999;7:129–161. [Google Scholar]
- Rikli R.E., Jones C.J. 2001. Senior Fitness Test Manual Human Kinetics, Champaign, IL. [Google Scholar]
- Rodriguez-Bailon M., Montoro-Membila N., Garcia-Moran T., Arnedo-Montoro M.L., Funes Molina M.J. Preliminary cognitive scale of basic and instrumental activities of daily living for dementia and mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2015;37:339–353. doi: 10.1080/13803395.2015.1013022. [DOI] [PubMed] [Google Scholar]
- Sanders A.E., Holtzer R., Lipton R.B., Hall C., Verghese J. Egocentric and exocentric navigation skills in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1356–1363. doi: 10.1093/gerona/63.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slachevsky A., Forno G., Barraza P., Mioshi E., Delgado C., Lillo P., Henriquez F., Bravo E., Farias M., Munoz-Neira C., Ibanez A., Parra M.A., Hornberger M. Mapping the neuroanatomy of functional decline in Alzheimer’s disease from basic to advanced activities of daily living. J. Neurol. 2019;266:1310–1322. doi: 10.1007/s00415-019-09260-w. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New York: 2006. Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Sunderaraman P., Maidan I., Kozlovski T., Apa Z., Mirelman A., Hausdorff J.M., Stern Y. Differential associations between distinct components of cognitive function and mobility: implications for understanding aging, turning and dual-task walking. Front. Aging Neurosci. 2019;11:166. doi: 10.3389/fnagi.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira I.A., Silva Fd.O., Ferreira J.V.A., Plácido J., Marinho V., Deslandes A.C. Utility of handgrip strength cut-offs for identification of weakness and disability in community-dwelling older people with mild cognitive impairment and Alzheimer s disease. J. Bras. Psiquiatr. 2019;68:208–214. [Google Scholar]
- Verghese J., Kuslansky G., Holtzer R., Katz M., Xue X., Buschke H., Pahor M. Walking while talking: effect of task prioritization in the elderly. Arch. Phys. Med. Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D.T., Collin C. The Barthel ADL Index: a standard measure of physical disability? Int. Disabil. Stud. 1988;10:64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- Wagshul M.E., Lucas M., Ye K., Izzetoglu M., Holtzer R. Multi-modal neuroimaging of dual-task walking: structural MRI and fNIRS analysis reveals prefrontal grey matter volume moderation of brain activation in older adults. NeuroImage. 2019;189:745–754. doi: 10.1016/j.neuroimage.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pi Y., Chen P., Liu Y., Wang R., Chan C. Cognitive motor interference for preventing falls in older adults: a systematic review and meta-analysis of randomised controlled trials. Age Ageing. 2015;44:205–212. doi: 10.1093/ageing/afu175. [DOI] [PubMed] [Google Scholar]
- World Health O. 2nd ed. World Health Organization; Geneva: 2004. ICD-10 : International Statistical Classification of Diseases and Related Health Problems : Tenth Revision. [Google Scholar]
- Zanco M., Placido J., Marinho V., Ferreira J.V., de Oliveira F., Monteiro-Junior R., Barca M., Engedal K., Laks J., Deslandes A. Spatial navigation in the elderly with Alzheimer’s disease: a cross-sectional study. J. Alzheimers Dis. 2018;66:1683–1694. doi: 10.3233/JAD-180819. [DOI] [PubMed] [Google Scholar]
- Zidan M., Arcoverde C., de Araujo N.B., Vasques P., Rios A., Laks J., Deslandes A. Motor and functional changes in different stages of Alzheimer’s disease. Rev. Psiq. Clin-Brazil. 2012;39:161–165. [Google Scholar]