Abstract
Calorie restriction (CR) has been considered the most effective non-pharmacological intervention to counteract aging-related diseases and improve longevity. This intervention has shown beneficial effects in the prevention and treatment of several chronic diseases and functional declines related to aging, such as Parkinson's, Alzheimer's, and neuroendocrine disorders. However, the effects of CR on cognition show controversial results since its effects vary according to intensity, duration, and the period of CR. This review focuses on the main studies published in the last ten years regarding the consequences of CR on cognition in different neurological diseases and conditions of experimental animals. Also, possible CR mimetics are discussed. These findings highlight the potential beneficial effects of CR of up to 40 % on cognition when started early in life in non human animals.
Keywords: Calorie intake, Memory, Cognitive function, Aging, Neurodegenative diseases
1. Introduction
Calorie restriction (CR) consists of reducing the daily calorie intake while maintaining essential nutrients for health without malnutrition (Bales and Kraus, 2013; Han and Ren, 2010). It can be considered moderate when there is a reduction in daily calorie intake between 20–40 % of measured intake of an animal when it is given ad libitum (AL) food (Speakman and Mitchell, 2011). The first study about CR date from 1935, when McCay et al. reported that CR retarded the growth but increased the life span of both male and female rats (McCay et al., 1935).
Recently, it has been reported that CR attenuates the levels of biomarkers involved with the aging process, such as sirtuins. Sirtuins are a class of proteins that play important roles in DNA repair, telomerase function, genomic stability, cellular senescence and other factors related to inflammation, oxidative stress and aging (López-Otín et al., 2013; Salvestrini et al., 2019; Zhang et al., 2016). Long term CR studies with humans are difficult since they present confounding factors such as their distinct daily physical activities and food intake control.
Although studies with humans are difficult to perform due to the impracticality of carrying out randomized studies, controlled by diet and long-term application, some studies have been agreed and alternative results have been reported for a beneficial effect of CR on the cardiovascular system and CR metabolism lipids and glucose, as a reduction in blood pressure, improves the lipid profile and greater insulin sensitivity (Fontana, 2007; Fontana et al., 2004; Heilbronn et al., 2006; Larson-Meyer et al., 2006; Meyer et al., 2006; Racette et al., 2006; Redman et al., 2007; Walford et al., 2002; Weiss et al., 2006). One of the studies carried out showed the results found in studies with rodents and monkeys, showing a serum reduction in the concentration of triiodothyronine (T3) (Fontana et al., 2006).
Although the above studies demonstrate beneficial effects on the cardiovascular and metabolic profile, some studies with humans have shown that CR can be harmful, which can cause anemia, loss of muscle mass, reduced bone mineral density, weakness and irritability, depending on the physiological characteristics of individuals and the degree of CR applied (Fontana, 2007; Villareal et al., 2006).
According to Martin, Mattson and Maudsley, CR can improve brain health, impinging on the fundamental metabolic and cellular signaling pathways that regulate life span (Martin et al., 2006). However, regarding the effect on cognitive function, studies are less conclusive, since depending on the protocol performed, it can lead to opposite effects on cognition (Ingram and Cabo, 2017; Mattson, 2014, 2012). Memory is one of the most important cognitive functions. Cognition is a mental action of understanding something through your own experience and senses through the thought and, thus, a process of assimilating knowledge. Memory loss and cognitive impairment are the main features of dementia (Cassilhas et al., 2016). Several studies show that CR can have beneficial effects or not, depending on its intensity, the period in which it starts, and its duration. One of the widely discussed questions is whether the CR can mitigate aging-related cognitive decline. Thus, the purpose of this review was to present the main findings of studies carried out with CR over the past ten years focusing on the effect of CR on cognition of experimental animals.
2. Methodology
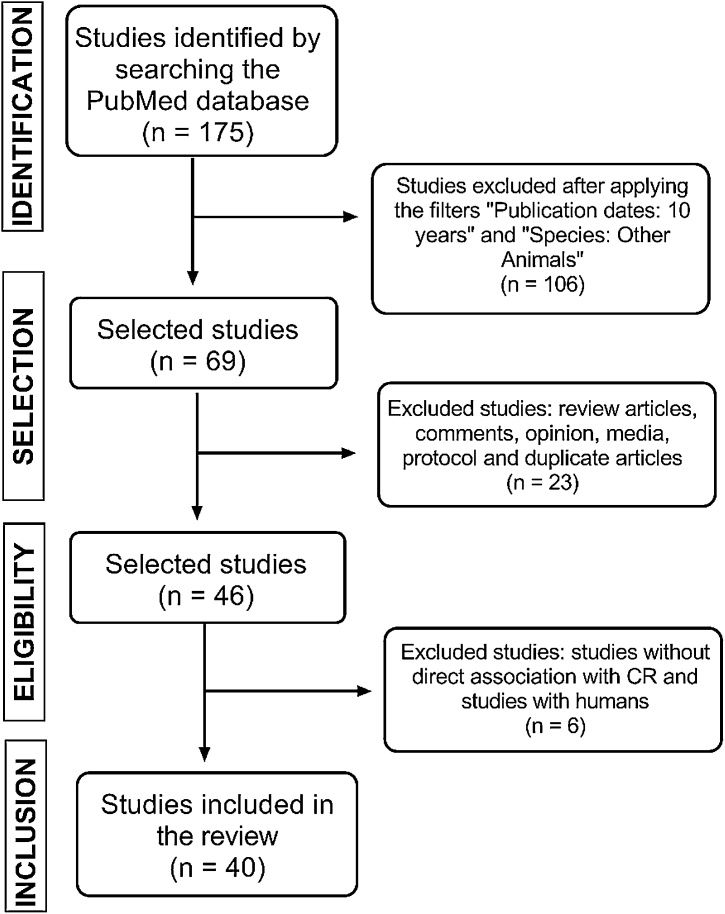
This review article was developed to answer the following guiding question: What are the effects of CR on cognition of experimental animals? The search was carried out in the months of October and November 2019, using the electronic database PubMed, through the medical subject headings (MeSH) combinations: ("caloric restriction" OR "calorie restriction") AND (cognition OR "cognitive function" OR "clinical evaluation of dementia" OR "cognitive psychotherapies" OR "Mental Status and Dementia Tests" OR "cognitive therapies"). We identified 175 studies initially. Then, we decided to select only studies published between 2009 and 2019, in order to analyze a reasonable sample of works and more recent ones. Thus, were exclude studies not published in the last ten years and performed in vitro and with humans (n = 106), remaining only studies performed with experimental animals in vivo (n = 69). Publications such as reviews, comments, media, protocols, as well as duplicate articles, were excluded, resulting in the selection of 46 publications manuscripts. Then, we excluded studies addressing CR effects on humans, which still remained selected due to some error in the algorithm. Thus, 40 studies were analyzed in this review (Fig. 1).
Fig. 1.
Methodological flowchart.
3. Results
Of the 40 studies included in this review, 34 were performed using animal models of laboratory rodents, 6 with other animal models, including flies, gray mouse lemurs, baboons and underground rodents. One study was carried out with CR of 15 %, 1 with CR of 16 %, 2 with CR of 20 %, 12 with CR of 30 %, 5 with CR of 40 %, 1 with CR of 44 %, 2 with CR of 50 %, 1 with CR of 60 %, 1 with CR of 70 %, 3 with CR of intermittent fasting, 1 with CR reducing the initial weight of the animals by 35 %, 1 keeping the initial weight of the animals at 65 % and 1 maintaining the initial weight of the animals in 75 % and 85 %. Twenty-one studies presented experiments with CR up to 1 year, 12 were carried out with elderly animals, and 10 studies presented experiments with pathological models. Table 1 shows the main results of the studies analyzed.
Table 1.
Studies included in the review and main results.
| Studies included in the review | |
|---|---|
| Main results | |
| Authors/Year | |
| Martin et al., 2016 | CR caused significant regional effects on the energy metabolism of the hippocampus, such as decreased activity of GSK3b and PGC1α. |
| Mahmoud A. Alomari et al., 2016. | CR associated with voluntary and forced exercise increases BDNF and memory. CR alone did not change BDNF levels. |
| Dong et al., 2016 | CR increased learning and memory capacity, decreased GFAP, mTOR, S6K levels, and raised the expression of LC3B. |
| Burger et al., 2010. | CR led to the extension of the life span in Drosophila, but the decline in aversive learning performance associated with aging has not slowed. |
| Rich et al., 2010. | CR decreased cortical lesion size after traumatic brain injury, increased spatial memory, and BDNF expression. |
| Rumani Singh et al., 2015. | Intermittent fasting CR improved motor coordination, learning, decreased oxidative damage of proteins, recovery in the expression of energy regulating neuropeptides. Moreover, CR decreased the levels of nuclear factor kappa, cytochrome c, and positively regulated the expression of synaptophysin. |
| Villain et al., 2016. | Animals submitted to CR showed lower learning performance after restraint but maintained their locomotor abilities. |
| Navarro-Cruz et al., 2017. | Chronic resveratrol administration maintained the cytoarchitecture of the CA1 and CA2 regions of the rat hippocampus and improved cognitive performance. |
| Fu et al., 2017. | Mice submitted to chronic CR had impaired spatial recognition memory. Acute CR had positive and negative effects on memory. |
| Rühlmann et al., 2016. | CR had increased levels of plasma and brain FGF-21, increased level of brain phospho- FGFR1c, ERK1/2 and AMPK, decreased brain levels of mTOR, and phosphor-tau, increased synaptic plasticity and improved cognitive performance. |
| Parikh et al., 2016. | CR had neurovascular improvement, which has been shown to play an important role in vascular, cognitive, and mental health. |
| Kim et al., 2016 | CR increased insulin sensitivity, decreased blood-brain barrier loss and glial activation induced by a high-fat diet and improved memory, reversed neurogranin expression induced by a high-fat diet and activation of Ca-dependent protein kinase II (2 +) / calmodulin and calpain. |
| Sarker et al., 2015. | CR and curcumin improved cognitive flexibility, suggesting a positive effect on frontal cortical functions. |
| Chen et al., 2015. | DNJ alleviated age-related disturbances such as declined sensorimotor capacity, anxiety, spatial and non-spatial memory, decreased serum insulin level; increased levels of insulin-like growth factor 1 receptor, synaptotagmin-1 presynaptic protein and astrocyte activation; decreased levels of insulin receptor, neurotrophic factor derived from the brain, pre-synaptic protein syntaxin-1 and acetylation of histones H4 in lysine 8 in the dorsal hippocampus. |
| Kaptan et al., 2015. | Low-calorie diet in adult female rats improved learning and spatial memory, increased proliferative cells and number of neurons in the hippocampal dentate gyrus and BDNF in the hippocampus and prefrontal cortex. |
| Kishi et al., 2015. | CR decreased insulin, systolic blood pressure, fasting blood glucose, adiponectin and oxidative stress in the hippocampus, increased BDNF in the hippocampus, and improved cognitive performance in model mice of metabolic syndrome. |
| Schleich et al., 2015. | Immunologically challenged animals had their spatial learning capabilities affected, increasing errors, and the time required to reach the goal of a complex maze. There was no effect of CR or interaction between factors. |
| Brownlow et al., 2014. | CR caused improvement in learning and short-term memory. Motor performance, spatial memory and total or phospho-tau levels were not affected by the intervention. |
| Yang et al., 2014. | CR decreased hippocampal BDNF levels, mTOR signaling, PI3K expression and Akt phosphorylation, delayed the decline in age-related autophagy and prevented the increase in poly-ubiquitinated proteins with aging. |
| Kuhla et al., 2013. | Lifelong CR exacerbated spontaneous locomotor activity and anxiety in mice, but improved cognitive performance resulting in an improvement in spatial memory. |
| Grayson et al., 2014. | Weight loss after CR, were associated with improvements in metabolic health and hippocampal-dependent learning. Rats treated with VSG exhibited deficit in spatial learning tasks in the Morris water maze. VSG animals showed high hippocampal inflammation comparable to that of obese controls. The RYGB and calorie-restricted controls exhibited an improvement in inflammation. Ghrelin treatment did not alleviate the inflammation of the hippocampus. |
| Gräff et al., 2013. | CR retarded neurodegeneration and synaptic dysfunction, increased the expression of SIRT1 protein regulating life expectancy. |
| Dhurandhar et al., 2013. | Long-term administration of ghrelin agonist improved performance in the water maze, reduced amyloid beta (Aβ) and inflammation (microglial activation) in a similar manner to CR. |
| Kishi and Sunagawa, 2012. | Physical training avoided the cognitive decline, which was evaluated by the Morris water maze test. Physical training associated with CR caused greater protection against cognitive decline than just physical training or CR. |
| Porquet et al., 2013. | Resveratrol supplements increased life expectancy. Resveratrol reduced cognitive impairment and plays a neuroprotective role, reducing amyloid load and reducing tau hyperphosphorylation. |
| Lee et al., 2012. | The facilitation of NQO1 activity by feeding β-lapachone (βL), prevented the age-related decline in motor and cognitive function in elderly mice. |
| Rodriguez et al., 2012. | Maternal CR in baboons caused improvement in the learning of female puppies and impaired learning in male puppies, which also showed increased impulsivity. |
| Rumani Singh et al., 2012. | In rats, Intermittent fasting improved motor coordination and cognitive abilities, decreased oxidative damage and improved IV activity of the mitochondrial complex. |
| Dal-Pan et al., 2011. | Both CR and resveratrol supplementation (RSV) increased spontaneous locomotor activity and improved working memory in the spontaneous alternation task. RSV supplementation increased the performance of spatial memory in the circular platform task, but not the CR. |
| Steinman et al., 2011. | In females, CR can cause differential effects on acquisition and learning, depending on the photoperiod. |
| Chouliaras et al., 2011. | The age-related increase in DNA methyltransferase 3a (Dnmt3a) -IR in CA3 and CA1−2 cells in type I cells was mitigated by CR, but not by overexpression of SOD. The density of Dnmt3a type II immunoreactive cells decreased with age, without significant effects of CR and SOD. |
| McEwen and Paterson, 2010. | 30 % CR administered from global ischemia did not decrease brain damage or improve long-term recovery. |
| Liu et al., 2017. | Short-term CR after mTBI improved mTBI-induced cognitive dysfunction, increased autophagy, and suppressed astrocyte activation. |
| Jeon et al., 2016. | CR reduced fatty liver and insulin resistance in diabetic mice induced by obesity (ob / ob), increased levels of O-linked N-acetylglucosamine in the hippocampus (O-GlcNAc) and GlcNAc transferase and decreased protein expression calcium / calmodulin-dependent kinase II, lipocalin-2 and phosphorylated tau. CR reduced the learning deficits normally seen in ob/ob mice. |
| Delic et al., 2015. | CR was not able to restore the decrease in the activity of complex I, respiratory rate stimulated by ADP and the increase in the potential of the mitochondrial membrane that occurs in the mitochondria of mice that express human P301 l (Tg4510). CR led to a genotype-independent reduction in F0F1-ATPase mitochondrial activity. |
| Akitake et al., 2015. | Moderate maternal RC induced intrauterine growth retardation (IUGR). Offspring had low birth weight and a delay in the development of physical and coordinated movements. IUGR's children exhibited mental deficiencies. |
| Babenko and Shakhova, 2014. | The sensitive redox-neutral SMase was important in interrupting the renewal of sphingolmyelin (SM) in the hippocampus and neocortex in old age, and CR prevented accumulation of age-dependent ceramide through the neutral targeting of the SMase. |
| Wei et al., 2014. | The rats in the d-gal group exhibited deficits in spatial reference memory in the Morris water maze test, while the rats in the d-gal group submitted to CR exhibited almost normal cognitive function, indicating that the CR protects the learning and memory of the harmful effects induced by d-gal. |
| Park et al., 2013. | CR increased cell divisions in the dentate gyrus of female mice, suggesting that CR increases the number of divisions by which neural and progenitor stem cells suffer in the aging brain. |
| Jin et al., 2016. | Metformin rescued cells from mutant hunting-induced toxicity (HTT) and maintained levels of ATP in cells expressing mutant HTT and prevented depolarization of the mitochondrial membrane and excess fission and modulated the disturbed mitochondrial dynamics in DH cells. |
3.1. Studies with rodents
3.1.1. Effects of CR during development and aging on cognition
Several studies have been carried out with CR to investigate its effects on cognition during development and aging, particularly on learning and memory. Akitake et al. demonstrated through the object recognition test that 30 % CR in pregnant mice caused intrauterine growth retardation (IUGR) leading to impaired cognitive function in the offspring (Akitake et al., 2015). Fu et al. conducted a study to examine the effects of acute and chronic CR with different intensities on spatial recognition memory through the Y-maze test in developing male mice. Three groups received 70 %, 50 % or 30 % of food consumed by the ad libitum control. The chronic CR was performed from the 25th after birth (P25) to the 74th day (P74). The data suggest that chronic CR (50 % e 30 %) impaired spatial recognition memory in mice depending on the individual intensity and tolerability. Acute CR positively or negatively affected memory retention (Fu et al., 2017). Parikh et al. also showed that 40 % CR initiated in the 14th week up to the age of 5–6 months of mice did not lead to significant changes in learning and spatial memory assessed by the radial arm water maze (RAWM). 18–20 months mice showed preserved learning and memory in the same test, and significantly lower levels of anxiety compared to animals of the same age fed ad libitum (Parikh et al., 2016).
Kaptan et al. observed that a CR of 15 % in adolescent rats improved the learning and spatial memory at adulthood (Kaptan et al., 2015). Kuhla et al. used 4-week-old female mice calorie-restricted in 40 % for 4, 20, and 74 weeks. The cognitive performance assessed through the Morris water maze decreased with aging, nevertheless, the 74-week CR group significantly improved the animals' spatial memory. The authors also performed a late-onset CR in 66-week mice for 12 weeks. CR was not able to improve memory (Kuhla et al., 2013). Wei et al. demonstrated that 30 % CR improved the performance of aged mice by d-gal injections in the Morris water maze test, suggesting that the CR protects against the decline in learning and memory (Wei et al., 2014). Yang et al. conducted a study demonstrating that 30 % CR in 12-month and 20-month-old male mice improved the age-dependent cognitive deficits using the open field test and the Morris water maze (Yang et al., 2014).
These studies reveal that depending on the intensity, duration, and period of life in which it is started, CR can lead to different learning and memory. However, it can be speculated that a mild or moderate CR (≤40 %), lead to beneficial effects on learning and memory when initiated in the early stages of life. More severe or later-onset CR tends to be less effective in preventing aging-related memory and learning deficits, as demonstrated by Fu et al. (2017) and Kuhla et al. (2013), respectively.
3.1.2. Effects of CR on brain neuroplasticity during development and aging
The decrease in neurogenesis can lead to age-related cognitive deficits. Thus, the generation of new neurons from stem cells is crucial for maintaining cognitive function (Park et al., 2013). The mechanisms of neuronal survival and synaptic plasticity induced by CR are still not clear, but several studies have shown a relationship between CR effects and neurotrophin signaling, autophagy, and oxidative metabolism. Park et al. demonstrated that female mice subjected to 40 % CR showed increase in the total number of dividing cells, the number of neural stem cells and progenitor cells in the dentate gyrus of the hippocampus (Park et al., 2013). In a study conducted by Kaptan et al., it was observed that 15 % CR in adolescent rats increased cell proliferation and neuron labeling in the dentate gyrus of the hippocampus and increased BDNF levels in the hippocampus and prefrontal cortex (Kaptan et al., 2015).
BDNF is an important neurotrophin related to neurogenesis and neuronal survival during development (Lee et al., 2002; Linnarsson et al., 2000). Although some studies show increase in BDNF levels in animals submitted to CR (Kaptan et al., 2015; Kishi et al., 2015), Yang et al. demonstrated that 30 % CR in male mice aged 12 and 20 months decreased BDNF levels compared to animals fed ad libitum. This reduction in BDNF levels resulted in impaired mTOR signaling, PI3K expression, and Akt phosphorylation in young and elderly mice. CR also delayed the decline in age-related autophagy and inhibited the progressive increase in polyubiquitylated proteins with aging, since mTOR is one of the most important inhibitors of autophagy. The study also demonstrated that CR improved the age-dependent cognitive deficits assessed by open field and Morris water maze test (Yang et al., 2014).
Ceramide is a constituent of sphingolipids and an important molecule in the regulation of several cellular processes, including cell growth, differentiation and apoptosis. The loss of neurons and synapses in the neocortex, hippocampus and other brain regions in Alzheimer's disease is associated with age-induced accumulation of ceramide, which regulates the processing of amyloid precursor protein and the generation of beta amyloids (Costantini et al., 2005; Puglielli et al., 2003b, 2003a). Babenko and Shakhova demonstrated that long-term CR of 60 % reduces the content of ceramide and neutral SMase activity, a ceramide-regulating enzyme, in the hippocampus of elderly rats, demonstrating that CR can prevent the accumulation of age-dependent ceramides (Babenko and Shakhova, 2014).
3.1.3. Effects of CR on mitochondrial energy metabolism
Metabolism involves anabolic and catabolic reactions that consume and produce ATP (Curi and Procópio, 2009). Oxygen is essential for the production of energy for cellular metabolism, however, a small amount of oxygen consumed is reduced producing reactive oxygen species (ROS), which can cause damage to tissues, cellular organelles, nucleic acids, lipids and proteins (Comhair and Erzurum, 2002; Valko et al., 2007). The production of ROS by the mitochondrial electron transport chain acts in the aging process and in the pathogenesis of neurodegenerative diseases (Liu et al., 2002). One of the hypotheses for the beneficial effects of CR is that there is a reduction in ROS since less glucose will be available to the mitochondria and, consequently, attenuating oxidative damage mediated by free radicals (Dubey et al., 1996; Sohal and Weindruch, 1996).
PGC1α is a member of the family of gene transcription coactivators that is highly inducible when there an increase in oxidative metabolism. These co-activators play an important role in controlling mitochondrial function, and their expression is related to cell metabolism homeostasis (Llimona, 2011; Spiegelman and Heinrich, 2004). A study by Martin et al. demonstrated that with the aging precess PGC1α levels are reduced.
Using aged mice, it was found that 16 % CR for 18 months, induced a distinct metabolic state, wich the levels of PGC1α and GSK3b, a regulator of PGC1α, were lower in the restricted group and there was no effect of CR on AMPK levels (Martin et al., 2016).
3.1.4. Effects of CR in models of neurodegenerative diseases
Several studies have been carried out using CR in models of neurodegenerative diseases, such as Alzheimer's (AD), Huntington's disease (HD), and Parkinson's disease (PD) (Bayliss et al., 2016). CR has been shown to reduce amyloid deposition in models of transgenic mice with Alzheimer's disease, leading to an improvement in cognitive deficits (Halagappa et al., 2007; Patel et al., 2005). In a study with 4-week-old ApoE-deficient mice (neurodegeneration model), Rülmann et al. demonstrated that 40 % CR led to an improvement in spatial memory assessed by the Morris water maze. It has also been shown that CR increased AMPK phosphorylation, reduced mTOR activity, Tau phosphorylation, and also increased hepatic mRNA expression of fibroblastic growth factor type 21 (FGF-21), an important protein for adapting metabolic states (Rühlmann et al., 2016).
Brownlow et al. conducted a study to investigate the effects of CR (keeping animals at 65 % of their initial body weight) for 3 months on cognitive deficits in a TAU protein deposition transgenic mouse model (Tg4510). CR led to better performance of Tg4510 mice in the object recognition test, suggesting an improvement in short-term memory, but it did not prevent neuronal loss, and the activation of astrocytes, microglia, or TAU levels (Brownlow et al., 2014). Delic et al. demonstrated that CR (reducing the animals' body weight by 35 %) for 4 months did not restore the decreased activity of the mitochondrial electron transport chain complex I and the increased potential of the mitochondrial membrane, but decreased the mitochondrial activity of F0F1-ATPase in the brain of mice Tg4510 model for deposition of tau (Delic et al., 2015).
Dhurandhar et al. conducted a study comparing animals calorie restricted in 20 % or treated with a ghrelin agonist, to investigate whether only the restriction would be able to improve cognition in mouse model of Alzheimer's disease. The long-term treatment of the ghrelin agonist was sufficient to improve the animals' performance in the water maze test and to reduce the levels of beta amyloid and inflammation compared to the control group. This result was similar to the effects of CR (Dhurandhar et al., 2013). Huntington's disease is caused by the pathological elongation of CAG repeats in the huntingtin gene (MacDonald et al., 1993). Jin et al. demonstrated that metformin, a drug that mimics the various metabolic effects of CR, prevented cell toxicity induced by mutant huntingtin (HTT), activated AMPK, prevented depolarization of the mitochondrial membrane and excess fission in the cells in HD model of mice (Jin et al., 2016).
3.1.5. Brain effects of CR in models of obesity and diabetes
High-calorie diet (HFD) causes obesity and the development of diseases such as diabetes, which increases the risk of neurodegenerative disturbances such as Alzheimer's disease (Arnoldussen et al., 2014). Studies have been conducted using CR as a treatment for obesity in order to prevent or delay cognitive decline. Kim et al. investigated the effects of CR on memory deficits induced by diabetes in 40 weeks aged obese mice. Obese mice were subjected to 30 % CR on the HFD for 12 weeks and, and subsequently to the Morris water maze test. The results showed that mice submitted to CR improved the learning and spatial memory deficits induced by HFD. It has also been shown that CR reduced HFD-induced Iba-1 expression, reduced the number of microglial cells, HFD-induced IgG and VEGF levels, indicating that CR can decrease glial activation and leakage of the blood-brain barrier. Moreover, levels of phosphorylated GSK-3b were increased in animals submitted to CR, as well as neurogranin signaling, a synaptic function regulator (Kim et al., 2016).
Kishi et al. conducted a study with obesity-prone and hypertensive rats as a model of metabolic syndrome to investigate whether 30 % CR protected animals from cognitive decline. Compared to ad lubitum group, Cognitive performance assessed from the Morris water maze was significantly better in restricted animals, and this benefit was attenuated by the administration of a tyrosine kinase B receptor (TrkB) antagonist, showing a relationship between BDNF and its receptor with the protection induced by CR on cognitive decline. Compared to control animals, restricted ones also showed a significant increase in BDNF levels, even when administered its antagonist. Together, these results suggest a role for CR in BDNF signaling via the TrkB receptor. It was also observed that the levels of TBARS, an indicator of oxidative stress, were significantly lower in restricted animals (Kishi et al., 2015). In a study by Dong et al. comparing the learning ability of six-week-old male mice submitted to 30 % CR or a HFD for ten months, it was observed that the high calorie diet impaired the learning and spatial memory assessed by the Morris water maze, while in the restricted group, these parameters were improved. Mice fed with high-calorie diet showed increased levels of GFAP (used to determine the number, volume, and morphology of astrocytes), mTOR, and S6K (protein through which mTOR inhibits autophagy), while all these protein levels were decreased in restricted animals. Also, in restricted mice, the levels of LC3B, a biomarker of autophagy, were increased, while in high-fat diet mice, these levels were decreased (Dong et al., 2016).
GlcNAcilation is an important post-translational modification in the regulation of several cellular processes, including signaling, cell cycle, and transcription. Several glcNAcylated proteins are involved in diabetes since hyperglycemia increases the glcNAcylation of proteins of the insulin signaling pathway favoring insulin resistance (Dias and Hart, 2007). These proteins are also involved in AD, and negative regulation of O-GlcNAcilation leads to hyperphosphorylation of Tau (Liu et al., 2009). Jeon et al. demonstrated that 44 % CR in mutant mice diabetes models increased the levels of O-linked N-acetylglucosamine in the hippocampus (O-GlcNAc) and GlcNAc transferase, and reduced the expression of calcium-calmodulin-dependent protein kinase II, lipocaine-2 and phosphorylated tau. CR in diabetic mice also decreased learning deficits measured through the Morris water maze (Jeon et al., 2016).
In order to eliminate weight and improve health, the main alternative to improve comorbidities related to metabolic syndrome is lifestyle modifications. Currently, bariatric surgery is one of the most effective methods for significant weight loss (Sjöström et al., 2004). In view of this, Grayson et al. conducted a study comparing CR with two bariatric surgeries (Roux-en-Y gastric bypass - RYGB and vertical sleeve gastrectomy - VSG) on cognitive function of rats. The study showed that the three interventions resulted in weight loss and were associated with widespread improvements in metabolic health and hippocampal-dependent learning, measured through the radial arm maze and spontaneous alternation tests. The rats submitted to VSG showed deficits in spatial learning tasks, measured through the Morris water maze, and increased inflammation compared to other interventions (Grayson et al., 2014).
3.1.6. Effects of CR on traumatic brain injury models
Traumatic brain injury (TBI) can significantly affect cognitive, behavioral, and emotional function. Blunt injuries to the head can result in diffuse injury, affecting the frontal and temporal regions producing a distinct pattern of deficits that vary according to the location and severity of injury (Draper and Ponsford, 2008; Ponsford et al., 2008). Currently, strategies for treating moderate to severe injuries are limited, which stimulates researches for preventive and alternatives methods, which help to reduce the severity of deficits resulting from the injury (Rich et al., 2010). Liu et al. investigated the effects of 30 % CR vs. ad libitum diet vs. HFD on the cognitive function of mice after mild traumatic brain injury (mTBI). The Morris water maze test demonstrated that the animals under CR had less latency to find the platform than the control groups, and the HFD group a higher number of platform crossings than the other groups. It has also been shown that CR decreased GFAP, mTOR levels, and increased LC3B levels, demonstrating that CR can improve cognitive deficits due to mTBI (Liu et al., 2017).
Rich et al. conducted a study testing the hypothesis that CR could improve spatial memory in a model of traumatic brain injury in two-month-old male mice. The animals in the restricted group were submitted to 30 % CR. The study revealed significant increases in BDNF of the cortical region around the injury site and in the hippocampus of animals submitted to CR (Rich et al., 2010).
3.1.7. Effects of CR on DNA
DNA methylation may be implicated in age-related changes in cognition and gene expression. Thus, a study investigated whether CR and transgenic overexpression of normal superoxide dismutase 1 (SOD) would attenuate age-related changes in DNA methyltransferase 3a (Dnmt3a), which catalyzes DNA methylation and is related to memory formation and changes in neural and synaptic plasticity in the hippocampus of rats. The results showed that CR attenuated the age-related increase in type I cells immunoreactive to Dnmt3a in the CA3 and CA1−2 regions of the hippocampus. Type II cells immunoreactive to Dnmt3a reduced with age, and CR or SOD did not cause any significant effect (Chouliaras et al., 2011).
3.1.8. Effects of CR associated with physical exercise
In addition to CR, physical exercise is another intervention capable of improving cognitive functions (Jee et al., 2008; Kim et al., 2010). Kishi and Sunagawa investigated whether physical exercise associated with 30 % CR causes synergistic protection against cognitive decline. Exercise training associated with CR led to greater protection from cognitive decline than exercise training alone. The study also showed that exercise associated with CR increased BDNF levels compared to the other groups. On the other hand, only CR was not able to change the BDNF levels (Kishi and Sunagawa, 2012).
3.2. Studies using other animal models
Although the largest number of studies with CR have been conducted using rodents models, other animal models have also been used in studies with CR, as demonstrated by Burger, Buechel, and Kawecki (Burger et al., 2010). In this study, the authors analyzed the effects of yeast content in the diet on an aversive learning task in young and old Drosophila melanogaster, and showed that dietary restriction did not affect cognitive decline related to aging, despite extending lifespan of flies (Burger et al., 2010).
Although CR is one of the most studied interventions to alleviate cognitive deficits in aging, there are still some challenges when it comes to studies with humans. Thus, animal models are important for understanding issues related to CR mechanisms, especially non-human primate models (Mattison et al., 2017; Yamada et al., 2018). In a study by Villain et al. it was evaluated the impact of 40 % CR on the associative learning ability in males of grey mouse lemurs, a small primate, during 19 days. It was demonstrated that the animals showed lower learning performance after the CR, and the effects of restriction on memory recovery varied depending on the metabolism of each animal. The restricted animals showed no difference in the rotaroad task, demonstrating that CR did not interfere with the animals' physical capacity (Villain et al., 2016). Dal-Pan et al. conducted a study comparing the effects of chronic CR (30 %) and oral supplementation with resveratrol on the cognitive performance of grey mouse lemurs. In both treatments, working memory was improved, demonstrated by a spontaneous alternation task. In the circular platform task, only the treatment with resveratrol improved the performance in spatial memory (Dal-Pan et al., 2011). Pifferi et al. demonstrated that chronic 30 % CR prolonged life by 50 %, decreased diseases associated with aging, and preserved the loss of white matter in different regions of the brain. However, this intervention accelerated the loss of gray matter in the brain, but did not affect cognition and behavior. A study by Rodriguez et al. investigated the effects of CR on baboons born from mothers submitted to 30 % CR during pregnancy and lactation. The pups (males and females) of mothers subjected to the CR showed reduced motivation and the learning outcomes varied by sex. The female's pups of restricted mothers making fewer mistakes than the females of the control ones. Also the male's pups of restricted mothers making more errors than the males of the control ones (Rodriguez et al., 2012).
Models of subterranean rodents are also used in studies with CR, since the behavior of these animals involves subterranean exploration and, therefore, developed spatial skills are necessary for a precise orientation inside the dens and galleries (Busch et al., 2000). Schleich, Zenuto and Cutrera showed that immunologically challenged underground rodents of the Ctenomys talarum species were affected when assessed for spatial learning capacities and mild calorie (maintaining the animals' weight at 85 % of the initial body weight) or severe CR (maintaining the animals' weight at 75 % of initial body weight) had no effect as well as no interaction between factors (Schleich et al., 2015). Another study with subterranean rodents showed that CR of 30 % did not decrease brain damage or improve long-term recovery in Mongolian squirrels with ischemia, assessed by open field test and neuron count in the hippocampus (McEwen and Paterson, 2010).
3.3. CR and possible mimetics
Investigations on possible substances that can mimic the effects of CR are an important area of study. One of the most well-documented mimetics is resveratrol, a natural polyphenolic compound found in grape skins. Resveratrol has antioxidant properties and regulates glucose, insulin production, fat metabolism, and cell survival through SIRT1 activation, imitating the extension of the Sir2-dependent life span, seen in CR (Baur et al., 2006; Baur and Sinclair, 2006; Lagouge et al., 2006). A study by Gräff et al. demonstrated that SIRT activating compounds could mimic the effects of CR in transgenic mice model that allows temporal and spatially controlled onset of neurodegeneration. Mice were restricted by 30 % for 3 months and or received 30 mg/kg of SRT3657 for 6 weeks. The authors observed that CR improved associative and object recognition memories (Gräff et al., 2013). Porquet et al. examined the effect of resveratrol on the diet of SAMP8 mice, a model of Alzheimer's disease. The object recognition test revealed that mice treated with resveratrol showed no memory impairment, had higher levels of phosphorylated SIRT1 and AMPK, decreased Aβ and phospho-TAU (pTau) levels, which was correlated with increased levels of GSK3b (Porquet et al., 2013). Navarro-Cruz et al. demonstrated that the administration of resveratrol for 8 months maintained the integrity of the cytoarchitecture of the CA1 and CA2 regions in the hippocampus of male rats and also improved cognitive performance, analyzed through the object recognition test (Navarro-Cruz et al., 2017).
Curcumin has also been focused in recent years due to its potential as a treatment for obesity-related comorbidities and neurodegenerative disorders (Bar-Sela et al., 2009; Begum et al., 2008; Gupta et al., 2013). Sarker et al. demonstrated that both dietary curcumin and a 30 % CR improved learning, spatial memory, and cognitive flexibility in male mice (Sarker et al., 2015).
Another substance that has been investigated due to its effects on glucose modulation and insulin metabolism is 1-deoxinojirimicina (DNJ), an alkaloid of the mulberry leaf that inhibits intestinal α-glucosidase and suppresses glucose absorption, showing itself a new therapeutic agent for diabetes (Kimura et al., 2007; Kong et al., 2008; Li et al., 2011). Chen et al., using a mouse model for aging (SAMP8) investigated the role of DNJ in behavioral and biochemical changes related to aging. The dorsal hippocampus of elderly control mice exhibited significant deficits in sensory-motor capacity, spatial and non-spatial memory, anxiety and biochemical changes related to aging, including reduced serum insulin levels, increased insulin-like growth factor 1 (IGF1) receptor and presynaptic protein synaptotagmine, activation of astrocytes, decreased levels of insulin receptors, BDNF, presynaptic protein syntax 1 and acetylation of histones H4 in lysine 8. Chronic treatment for 6 months of DNJ prevents these changes, and the group treated with higher doses of DNJ showed more significant improvement (Chen et al., 2015).
Recent studies have shown that NQO1 (NAD(P)H quinone reductase) modulates the cellular NAD+/ NADH ratio associated with aging and age-related disorders. The enzymatic function of NQO1 is reduced during aging and improved by CR, thus, researchers have been dedicated to finding substances that stimulate NQO1 activity (Hyun et al., 2006; Ying, 2008). Lee et al. demonstrate that β-lapachone (βL), a facilitator of NQO1, prevented the age-dependent decline in cognitive function of adult rats. Compared to the control group, both CR animals (30 %) and the group treated with βL showed improved associative memory capacity in the fear conditioning test (Lee et al., 2012).
3.4. CR and photoperiod
Food availability and photoperiod are environmental factors that can interfere with spatial learning (Steinman et al., 2011). Galea et al. demonstrated that short days increase spatial learning in female mice of the genus Peromyscus and have the opposite effect in males (Galea et al., 1994). Steinman, Crean, and Trainor investigated whether the length of the day could influence the impact of CR on the cognition of female mice. The mice were housed on long and short days and subjected to 20 % CR. Tests in Barnes maze demonstrated that the effects of CR depend on the photoperiod, differing in the acquisition parameters versus reverse learning. Mice housed on short days increased the expression of synapsin I in the hippocampus, which is involved with neurotransmitters release and synaptic formation (Greengard et al., 1993). CR reversed this effect. No effects of diet or photoperiod on the hippocampal expression of glial GFAP biomarker were observed (Steinman et al., 2011).
4. Final considerations and conclusions
It is possible to find a wide range of studies that have been carried out with CR in order to investigate its beneficial effects and mechanisms of aging in experimental animals. Here we present some of the results of the last ten years of studies with CR at different stages of life on neurodegenerative diseases such as Alzheimer. Some investigations associating CR with physical exercise have also been presented. Together this association is the main non-pharmacological strategy to prolong longevity and quality of life. We also presented some substances that mimic the effects of CR and would be potential drugs to mimic the beneficial effects of CR in individuals with some restrictions on intervention. Several studies have also been carried out not only in conventional laboratory animals but also in some wild models since CR can be an environmental factor that interferes with the survival and perpetuation of species. Thus, this information can show the different effects of CR on cognition depending on the period in wich it is initiated, its intensity and duration in different animal models, and how it can interfere with the quality of life of individuals. These studies contribute to a better understanding of the mechanisms related to CR in cognition and support future studies with humans. Thus CR may be a potential alternative to the treatment of comorbidities related to mental healthy and cogntition. We conclude that CR between 20 and 40 % initiated in the first month of life would attenuate age-related cognitive declines in experimental animals, in healthy and pathological aging, such as in Alzheimer's disease. CR may not reverse the harmful effects of aging on cognition if starts later. In addition, CR also attenuates cognitive deficits resulting from obesity and brain injuries such as traumatic brain. Finally, CR can improve cognition, when performed with moderate intensity and early in life. When performerd intensely and later in experimental animals, the CR may be deleterious for cognition.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
For the financial support, the authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant number 438498/2018-6; Chamada Universal) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), grant number APQ-03855-16. For the academic support, the authors are grateful to Department of Physical Education – Universidade Federal dos Vales do Jequitinhonha e Mucuri (UVFJM), and Laboratório Experimental de Exercício Físico (LETFIs), UFVJM.
References
- Akitake Y., Katsuragi S., Hosokawa M., Mishima K., Ikeda T., Miyazato M., Hosoda H. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr. Res. 2015;35:76–87. doi: 10.1016/j.nutres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Arnoldussen I.A., Kiliaan A.J., Gustafson D.R. 2014. Obesity and Dementia: Adipokines Interact With the Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko N.A., Shakhova E.G. Long-term food restriction prevents aging-associated sphingolipid turnover dysregulation in the brain. Arch. Gerontol. Geriatr. 2014;58:420–426. doi: 10.1016/j.archger.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Bales C.W., Kraus W.E. Caloric restriction: implications for human cardiometabolic. J. Cardiopulm. Rehabil. Prev. 2013;33:201–208. doi: 10.1097/HCR.0b013e318295019e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sela G., Epelbaum R., Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr. Med. Chem. 2009;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., Pistell P.J., Poosala S., Becker K.G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K.W., Spencer R.G., Lakatta E.G., Le Couteur D., Shaw R.J., Navas P., Puigserver P., Ingram D.K., De Cabo R., Sinclair D.A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss J.A., Lemus M.B., Stark R., Santos V.V., Thompson A., Rees D.J., Galic S., Elsworth J.D., Kemp B.E., Davies J.S., Andrews Z.B. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J. Neurosci. 2016;36 doi: 10.1523/JNEUROSCI.4373-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum A.N., Jones M.R., Lim G.P., Morihara T., Kim P., Heath D.D., Rock C.L., Pruitt M.A., Yang F., Hudspeth B., Hu S., Faull K.F., Teter B., Cole G.M., Frautschy Sally A. Curcumin structure-function, bioavailability, and efficacy in models of Neuroinflammation and alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow M.L., Joly-Amado A., Azam S., Elza M., Selenica M.L., Pappas C., Small B., Engelman R., Gordon M.N., Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 2014;271:79–88. doi: 10.1016/j.bbr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Burger J.M.S., Buechel S.D., Kawecki T.J. Dietary restriction affects lifespan but not cognitive aging in Drosophila melanogaster. Aging Cell. 2010;9:327–335. doi: 10.1111/j.1474-9726.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- Busch C., Antinuchi D.C., Vallle J.C.Del, Kittlein M.J., Malizia A.I., Vassallo A.I., Zenuto R.R. University of Chicago Press; Chicago: IL: 2000. Population Ecology of Subterranean Rodents, Life Underground: The Biology Of Subterranean Rodents. [DOI] [Google Scholar]
- Cassilhas R.C., Tufik S., De Mello M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016;73:975–983. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.H., Tong J.J., Wang F., Hu X.Q., Li X.W., Tao F., Wei Z.J. Chronic adjunction of 1-deoxynojirimycin protects from age-related behavioral and biochemical changes in the SAMP8 mice. Age (Omaha) 2015;37 doi: 10.1007/s11357-015-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L., van den Hove D.L.A., Kenis G., Dela Cruz J., Lemmens M.A.M., van Os J., Steinbusch H.W.M., Schmitz C., Rutten B.P.F. Caloric restriction attenuates age-related changes of DNA methyltransferase 3a in mouse hippocampus. Brain Behav. Immun. 2011;25:616–623. doi: 10.1016/j.bbi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Comhair S.A.A., Erzurum S.C. Antioxidant responses to oxidant-mediated lung diseases. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2002 doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- Costantini C., Weindruch R., Della Valle G., Puglielli L. A TrkA-to-p75 NTR molecular switch activates amyloid β-peptide generation during aging. Biochem. J. 2005;391:59–67. doi: 10.1042/BJ20050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi R., Procópio J. 2009. Fisiologia Básica. [Google Scholar]
- Dal-Pan A., Pifferi F., Marchal J., Picq J.L., Aujard F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delic V., Brownlow M., Joly-Amado A., Zivkovic S., Noble K., Phan T.A., Ta Y., Zhang Y., Bell S.D., Kurien C., Reynes C., Morgan D., Bradshaw P.C. Calorie restriction does not restore brain mitochondrial function in P301L tau mice, but it does decrease mitochondrial F0F1-ATPase activity. Mol. Cell. Neurosci. 2015;67:46–54. doi: 10.1016/j.mcn.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Dhurandhar E.J., Allison D.B., van Groen T., Kadish I. Hunger in the absence of caloric restriction improves cognition and attenuates alzheimer’s disease pathology in a mouse model. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias W.B., Hart G.W. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol. Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- Dong W., Wang R., Ma L.N., Xu B.L., Zhang J.S., Zhao Z.W., Wang Y.L., Zhang X. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin. Exp. Res. 2016;28:303–311. doi: 10.1007/s40520-015-0398-0. [DOI] [PubMed] [Google Scholar]
- Draper K., Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- Dubey A., Forster M.J., Lal H., Sohal R.S. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Fontana L. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L., Meyer T.E., Klein S., Holloszy J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Klein S., Holloszy J.O., Premachandra B.N. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fu Y., Chen Y., Li L., Wang Y., Kong X., Wang J. Food restriction affects Y-maze spatial recognition memory in developing mice. Int. J. Dev. Neurosci. 2017;60:8–15. doi: 10.1016/j.ijdevneu.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Galea L.A.M., Kavaliers M., Ossenkopp K.P., Innes D., Hargreaves E.L. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Gräff J., Kahn M., Samiei A., Gao J., Ota K.T., Rei D., Tsai L.H. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J. Neurosci. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B.E., Fitzgerald M.F., Hakala-Finch A.P., Ferris V.M., Begg D.P., Tong J., Woods S.C., Seeley R.J., Davidson T.L., Benoit S.C. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int. J. Obes. 2014;38:349–356. doi: 10.1038/ijo.2013.100. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A.J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science (80-.) 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Gupta S.C., Kismali G., Aggarwal B.B. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- Halagappa V.K.M., Guo Z., Pearson M., Matsuoka Y., Cutler R.G., LaFerla F.M., Mattson M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Han X., Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharmacol. Sin. 2010;31:1111–1117. doi: 10.1038/aps.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn L.K., de Jonge L., Frisard M.I., DeLany J.P., Larson-Meyer D.E., Rood J., Nguyen T., Martin C.K., Volaufova J., Most M.M., Greenway F.L., Smith S.R., Deutsch W.A., Williamson D.A., Ravussin E., Pennington CALERIE Team Effect of 6-Month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals. JAMA. 2006;295:1539. doi: 10.1001/jama.295.13.1539. for the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun D.-H., Emerson S.S., Jo D.-G., Mattson M.P., De Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D.K., Cabo Rde. Calorie restriction in rodents: caveats to consider. Ageing Res. Rev. 2017;39:15–18. doi: 10.1016/j.arr.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee Y.S., Ko I.G., Sung Y.H., Lee J.W., Kim Y.S., Kim S.E., Kim B.K., Seo J.H., Shin M.S., Lee H.H., Cho H.J., Kim C.J. Effects of treadmill exercise on memory and c-Fos expression in the hippocampus of the rats with intracerebroventricular injection of streptozotocin. Neurosci. Lett. 2008;443:188–192. doi: 10.1016/j.neulet.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Jeon B.T., Heo R.W., Jeong E.A., Yi Cok, Lee J.Y., Kim K.E., Kim H., Roh G.S. Effects of caloric restriction on O-GlcNAcylation, Ca2+ signaling, and learning impairment in the hippocampus of ob/ob mice. Neurobiol. Aging. 2016;44:127–137. doi: 10.1016/j.neurobiolaging.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Jin J., Gu H., Anders N.M., Ren T., Jiang M., Tao M., Peng Q., Rudek M.A., Duan W. Metformin protects cells from mutant huntingtin toxicity through activation of AMPK and modulation of mitochondrial dynamics HHS Public Access. Neuromolecular Med. 2016;18:581–592. doi: 10.1007/s12017-016-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptan Z., Akgün-Dar K., Kapucu A., Dedeakayoʇullari H., Batu Ş., Üzüm G. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; Hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res. 2015;1618:194–204. doi: 10.1016/j.brainres.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Ko I.G., Kim B.K., Shin M.S., Cho S., Kim C.J., Kim S.H., Baek S.S., Lee E.K., Jee Yong-Seok Y.S. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp. Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim H., Kang H., Heo R.W., Jeon B.T., Yi C.O., Shin H.J., Kim J., Jeong S.Y., Kwak W., Kim W.H., Kang S.S., Roh G.S. Caloric restriction improves diabetes-induced cognitive deficits by attenuating neurogranin-associated calcium signaling in high-fat diet-fed mice. J. Cereb. Blood Flow Metab. 2016;36:1098–1110. doi: 10.1177/0271678X15606724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Nakagawa K., Kubota H., Kojima Y., Goto Y., Yamagishi K., Oita S., Oikawa S., Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007;55:5869–5874. doi: 10.1021/jf062680g. [DOI] [PubMed] [Google Scholar]
- Kishi T., Sunagawa K. Exercise training plus calorie restriction causes synergistic protection against cognitive decline via up-regulation of BDNF in hippocampus of stroke-prone hypertensive rats. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. 2012:6764–6767. doi: 10.1109/EMBC.2012.6347547. [DOI] [PubMed] [Google Scholar]
- Kishi T., Hirooka Y., Nagayama T., Isegawa K., Katsuki M., Takesue K., Sunagawa K. Calorie restriction improves cognitive decline via Up-Regulation of brain-berived neurotrophic Factor. Int. Heart J. 2015;56:110–115. doi: 10.1536/ihj.14-168. [DOI] [PubMed] [Google Scholar]
- Kong W.H., Oh S.H., Ahn Y.R., Kim K.W., Kim J.H., Seo S.W. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem. 2008;56:2613–2619. doi: 10.1021/jf073223i. [DOI] [PubMed] [Google Scholar]
- Kuhla A., Lange S., Holzmann C., Maass F., Petersen J., Vollmar B., Wree A. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1a. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer D.E., Heilbronn L.K., Redman L.M., Newcomer B.R., Frisard M.I., Anton S., Smith S.R., Alfonso A., Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, -Cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Duan W., Mattson M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J., Park A.H., Lee Sang-Hee, Lee Seoung-Hoon, Kim J.-H., Yang S.-J., Yeom Y.Il, Kwak T.H., Lee D., Lee S.-J., Lee C.-H., Kim J.M., Kim D. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.G., Ji D.F., Zhong S., Lv Z.Q., Lin T.B., Chen S., Hu G.Y. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in allox. J. Ethnopharmacol. 2011;134:961–970. doi: 10.1016/j.jep.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Linnarsson S., Willson C.A., Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Mol. Brain Res. 2000;75:61–69. doi: 10.1016/S0169-328X(99)00295-8. [DOI] [PubMed] [Google Scholar]
- Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Liu F., Shi J., Tanimukai H., Gu Jinhua, Gu Jianlan, Grundke-Iqbal I., Iqbal K., Gong C.-X. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. BRAIN A J. Neurol. 2009;132:1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang R., Zhao Z., Dong W., Zhang X., Chen X., Ma L. Short-term caloric restriction exerts neuroprotective effects following mild traumatic brain injury by promoting autophagy and inhibiting astrocyte activation. Behav. Brain Res. 2017;331:135–142. doi: 10.1016/j.bbr.2017.04.024. [DOI] [PubMed] [Google Scholar]
- Llimona F. 2011. Co-ativador De Transcrição Gênica PGC-1 Na Pancreatite Aguda. [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L., Barnes G., Taylor S.A., James M., Groot N., MacFarlane H., Jenkins B., Anderson M.A., Wexler N.S., Gusella J.F., Bates G.P., Baxendale S., Hummerich H., Kirby S., North M., Youngman S., Mott R., Zehetner G., Sedlacek Z., Poustka A., Frischauf A.M., Lehrach H., Buckler A.J., Church D., Doucette-Stamm L., O’Donovan M.C., Riba-Ramirez L., Shah M., Stanton V.P., Strobel S.A., Draths K.M., Wales J.L., Dervan P., Housman D.E., Altherr M., Shiang R., Thompson L., Fielder T., Wasmuth J.J., Tagle D., Valdes J., Elmer L., Allard M., Castilla L., Swaroop M., Blanchard K., Collins F.S., Snell R., Holloway T., Gillespie K., Datson N., Shaw D., Harper P.S. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- Martin B., Mattson M.P., Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res. Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.A., Demuth T.M., Miller K.N., Pugh T.D., Polewski M.A., Colman R.J., Eliceiri K.W., Beasley T.M., Johnson S.C., Anderson R.M. Regional metabolic heterogeneity of the hippocampus is nonuniformly impacted by age and caloric restriction. Aging Cell. 2016;15:100–110. doi: 10.1111/acel.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison J.A., Colman R.J., Beasley T.M., Allison D.B., Kemnitz J.W., Roth G.S., Ingram D.K., Weindruch R., De Cabo R., Anderson R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017;8:1–12. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Interventions that improve body and brain bioenergetics for parkinson’s disease risk reduction and therapy. J. Parkinsons Dis. 2014;4:1–13. doi: 10.3233/JPD-130335. [DOI] [PubMed] [Google Scholar]
- McCay C.M., Crowell M.F., Maynard L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McEwen B.R., Paterson P.G. Caloric restriction provided after global ischemia does not reduce hippocampal cornu ammonis injury or improve functional recovery. Neuroscience. 2010;166:263–270. doi: 10.1016/j.neuroscience.2009.11.076. [DOI] [PubMed] [Google Scholar]
- Meyer T.E., Kovács S.J., Ehsani A.A., Klein S., Holloszy J.O., Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Navarro-Cruz A.R., Ramírez Y Ayala R., Ochoa-Velasco C., Brambila E., Avila-Sosa R., Pérez-Fernández S., Morales-Medina J.C., Aguilar-Alonso P. Effect of chronic administration of resveratrol on cognitive performance during aging process in rats. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/8510761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh I., Guo J., Chuang K.H., Zhong Y., Rempe R.G., Hoffman J.D., Armstrong R., Bauer B., Hartz A.M.S., Lin A.L. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany. NY). 2016;8:2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-H., Glass Z., Sayed K., Michurina T.V., Lazutkin A., Mineyeva O., Velmeshev D., Ward W.F., Richardson A., Enikolopov G. Calorie restriction alleviates age-related decrease in neural progenitor cell division in the aging brain. Eur. J. Neurosci. 2013;37:1987–1993. doi: 10.1111/ejn.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N.V., Gordon M.N., Connor K.E., Good R.A., Engelman R.W., Mason J., Morgan D.G., Morgan T.E., Finch C.E. Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol. Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Ponsford J., Draper K., Schönberger M. Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J. Int. Neuropsychol. Soc. 2008;14:233–242. doi: 10.1017/S1355617708080272. [DOI] [PubMed] [Google Scholar]
- Porquet D., Casadesús G., Bayod S., Vicente A., Canudas A.M., Vilaplana J., Pelegrí C., Sanfeliu C., Camins A., Pallàs M., Del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Omaha) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglielli L., Ellis B.C., Saunders A.J., Kovacs D.M. Ceramide stabilizes β-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid β-peptide biogenesis. J. Biol. Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer’s disease: the cholesterol connection. Nat. Neurosci. 2003 doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Racette S.B., Weiss E.P., Villareal D.T., Arif H., Steger-May K., Schechtman K.B., Fontana L., Klein S., Holloszy J.O. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman L.M., Heilbronn L.K., Martin C.K., Alfonso A., Smith S.R., Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J. Clin. Endocrinol. Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich N.J., VanLandingham J.W., Figueiroa S., Seth R., Corniola R.S., Levenson C.W. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J. Neurosci. Res. 2010;88:2933–2939. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.S., Bartlett T.Q., Keenan K.E., Nathanielsz P.W., Nijland M.J. Sex-dependent cognitive performance in baboon offspring following maternal caloric restriction in pregnancy and lactation. Reprod. Sci. 2012;19:493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühlmann C., Wölk T., Blümel T., Stahn L., Vollmar B., Kuhla A. Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging (Albany. NY). 2016;8:2777–2789. doi: 10.18632/aging.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvestrini V., Sell C., Lorenzini A. Obesity may accelerate the aging process. Front. Endocrinol. 2019;1:266. doi: 10.3389/fendo.2019.00266. www.frontiersin.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M.R., Franks S., Sumien N., Thangthaeng N., Filipetto F., Forster M. Curcumin mimics the neurocognitive and anti-inflammatory effects of caloric restriction in a mouse model of midlife obesity. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich C.E., Zenuto R.R., Cutrera A.P. Immune challenge but not dietary restriction affects spatial learning in the wild subterranean rodent Ctenomys talarum. Physiol. Behav. 2015;139:150–156. doi: 10.1016/j.physbeh.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Sjöström L., Lindroos A.-K., Peltonen M., Torgerson J., Bouchard C., Carlsson B., Dahlgren S., Larsson B., Narbro K., David Sjöström C., Sullivan M., Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996 doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R., Mitchell S.E. Caloric restriction. Mol. Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M., Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004 doi: 10.1016/j.cell.2004.09.037. [DOI] [PubMed] [Google Scholar]
- Steinman M.Q., Crean K.K., Trainor B.C. Photoperiod interacts with food restriction on performance in the Barnes maze in female California mice. Eur. J. Neurosci. 2011;33:361–370. doi: 10.1111/j.1460-9568.2010.07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Villain N., Picq J.L., Aujard F., Pifferi F. Body mass loss correlates with cognitive performance in primates under acute caloric restriction conditions. Behav. Brain Res. 2016;305:157–163. doi: 10.1016/j.bbr.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Villareal D.T., Fontana L., Weiss E.P., Racette S.B., Steger-May K., Schechtman K.B., Klein S., Holloszy J.O. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch. Intern. Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- Walford R.L., Mock D., Verdery R., MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-Year period. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.B211. [DOI] [PubMed] [Google Scholar]
- Wei S., Shi W., Li M., Gao Q. Calorie restriction down-regulates expression of the iron regulatory hormone Hepcidin in normal and d-galactose-induced aging mouse brain. Rejuvenation Res. 2014;17:19–26. doi: 10.1089/rej.2013.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E.P., Racette S.B., Villareal D.T., Fontana L., Steger-May K., Schechtman K.B., Klein S., Holloszy J.O. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am. J. Clin. Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Kemnitz J.W., Weindruch R., Anderson R.M., Schoeller D.A., Colman R.J. Caloric restriction and healthy life span: frail phenotype of nonhuman Primates in the wisconsin national primate research center caloric restriction study. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:273–278. doi: 10.1093/gerona/glx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Chu X., Yin M., Liu X., Yuan H., Niu Y., Fu L. MTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav. Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxidants Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Zhang N., Li Z., Mu W., Li L., Liang Y., Lu M., Wang Zhuoran, Qiu Y., Wang Zhao. Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-kB signaling. Cell Cycle. 2016;15:1009–1018. doi: 10.1080/15384101.2016.1152427. [DOI] [PMC free article] [PubMed] [Google Scholar]