Amiodarone is an antiarrhythmic drug that sometimes requires long‐term usage. However, amiodarone can cause not only amiodarone‐induced thyrotoxicosis but also thyroid storm.
Keywords: Amiodarone, induced thyrotoxicosis, thyroid storm, type 2 amiodarone
Abstract
Background
There are two types of amiodarone‐induced thyrotoxicosis (AIT). Type 1 AIT is increased synthesis of thyroid hormone, whereas type 2 AIT is excess release of thyroid hormone due to a destructive thyroiditis. However, cases leading to amiodarone‐induced thyroid storm are rare.
Case presentation
A 75‐year‐old man with a history of chronic heart failure, nonsustained ventricular tachycardia, and atrial fibrillation was treated with amiodarone from age 72. He was presented to the emergency department with edema of the legs and dyspnea on exertion for 3 weeks. He was diagnosed with thyroid storm associated with type 2 AIT on the basis of no pre‐existing thyroid disease and long‐term amiodarone administration without appropriate medical attention and thyroid function tests.
Conclusion
This case report suggests that amiodarone can cause not only AIT but also thyroid storm. Regular medical appointment and thyroid function tests can avoid this critical illness.
Introduction
Amiodarone is a potent class III antiarrhythmic drug used in clinical practice for the prophylaxis and treatment of many cardiac rhythm disturbances, ranging from paroxysmal atrial fibrillation to life‐threatening ventricular tachyarrhythmias. 1 Although highly effective in patients with arrhythmias, its use in clinical practice is associated with various adverse effects on the cornea, lungs, liver, skin, and thyroid. 1 Patients treated with amiodarone may manifest altered thyroid hormone profile without thyroid dysfunction, or they may present with clinically significant amiodarone‐induced hypothyroidism or amiodarone‐induced thyrotoxicosis (AIT). 2 AIT occurs in 2%–12% of patients on chronic amiodarone treatment and is divided into two types. 2 Type 1 AIT is associated with increased synthesis of thyroid hormone and occurs in patients with underlying thyroid pathology. Type 2 AIT is a result of amiodarone causing a subacute thyroiditis with the release of preformed thyroid hormones into the circulation. By contrast, thyroid storm occurs in 0.20 per 100,000 patients per year in hospitalized cases. 3 Although the mortality rate due to thyroid storm ranges from 20% to 30%, 4 , 5 the incidence of amiodarone‐induced thyroid storm remains unknown. We herein report the case of a patient with amiodarone‐induced thyroid storm associated with type 2 AIT.
Case report
A 75‐year‐old Japanese man presented to the emergency department of our hospital with edema of the legs and dyspnea on exertion for 3 weeks. The patient had been in his usual state of health until 3 weeks prior to admission. His medical history included mitral and aortic valve replacement, tricuspid valvuloplasty, chronic heart failure, nonsustained ventricular tachycardia, and bradycardia atrial fibrillation treated with a pacemaker at the age of 72. Since diagnosis, he had been taking medications including amiodarone with the dosage adjustments. From the day of admission, he was treated with amiodarone (200 mg), carvedilol (10 mg), and furosemide (10 mg) daily. He had taken the same medication and same dosage for at least 6 months without laboratory test.
On the day of admission, the patient had a temperature of 37.6°C, respiratory rate of 28 breaths per minute, heart rate of 71 beats per minute (irregularly irregular), blood pressure of 134/40 mmHg, and an oxygen saturation of 88% while breathing ambient air. His general appearance seemed agitated in acute distress. General physical examinations revealed sclerae icteric, no thyroid enlargement (without tenderness or bruit). Respiratory sounds were inspiratory coarse crackles bilaterally. His legs revealed slow pitting edema bilaterally. Laboratory analysis revealed normal complete blood count. Aspartate transaminase and alanine aminotransferase levels were 178 and 202 IU/L, respectively. Total‐bilirubin (T‐Bil), direct‐bilirubin (D‐Bil) an indirect‐bilirubin (ID‐Bil) were 5.0, 3.4, and 1.6 mg/dL, respectively. Alkaline phosphatase (ALP) and γ‐glutamyl transpeptidase (GTP) levels were normal. Free T3 and free T4 levels were higher than 11.40 pg/mL (reference value 2.30–4.00 pg/mL) and 6.99 ng/dL (reference value 0.90–1.70 ng/dL), respectively. Thyroid‐stimulating hormone (TSH) level was less than 0.015 μIU/mL (reference value 0.500–5.00 μIU/mL), and anti‐TSH receptor antibodies were negative. His electrocardiogram showed atrial fibrillation. Thyroid ultrasonography revealed normal size and hypovascularity without nodules. Chest radiograph showed bilateral lung congestion, 60% of cardiothoracic ratio, and implantation of pacemaker (Fig. 1A). Computed tomography of the abdomen demonstrated high density throughout the liver and dilation of the hepatic veins and inferior vena cava (Fig. 1B).
Fig. 1.
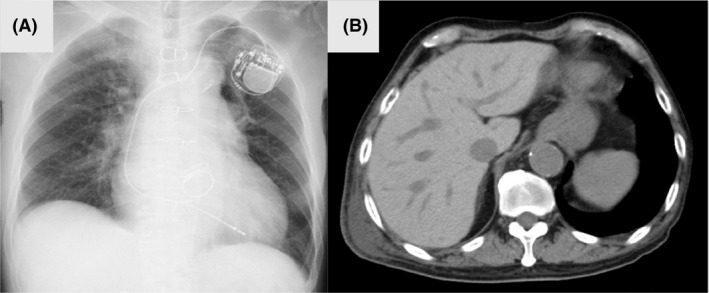
(A) Chest radiograph on the day of admission revealed pulmonary congestion bilateral, cardiothoracic ratio of 60%, and implanted pace maker. (B) Computed tomography of the abdomen demonstrated high density throughout the liver and dilation of the hepatic veins and inferior vena cava.
His physical examination and laboratory data revealed acute congestive heart failure, thyrotoxicosis, and hepatic dysfunction with hyperbilirubinemia. We suspected thyroid storm likely caused by AIT because of no pre‐existing thyroid disease and long‐term use of amiodarone. High‐density area throughout the liver on computed tomography suggested amiodarone administration (Fig. 1B). His hepatic dysfunction was caused by hyperthyroidism in addition to toxicity from amiodarone accumulation. Hepatic dysfunction related to hyperthyroidism encompasses abnormalities associated with the effects of thyroid hormone excess, drug‐related hepatic injury, and the presence of concomitant liver disease. 6 His Burch and Wartofsky’s score 7 was 55 (a score of 45 or more is highly suggestive of thyroid storm, a score of 25‐44 supports the diagnosis, and a score below 25 makes thyroid storm unlikely; Table 1). On the basis of these clinical features, he was diagnosed with thyroid storm associated with type 2 AIT. As a result, amiodarone was discontinued and he was treated with methimazole (60 mg), potassium iodine (200 mg), carvedilol (10 mg), and hydrocortisone (300 mg) daily. In addition, he was treated with furosemide for acute congestive heart failure and warfarin for atrial fibrillation. Following these treatment routines, his symptoms and laboratory findings improved gradually. The dosage of these medications for thyroid storm was gradually decreased once the euthyroid state had been achieved. He required no additional therapy for his arrhythmia, irrespective of whether or not amiodarone was stopped. He was discharged on day 28, and advised regular check‐ups with his family doctor.
Table 1.
Burch and Wartofsky’s score for the diagnosis of thyroid storm
| Thermoregulatory dysfunction | Score |
|---|---|
| Temperature (°C) | |
| 37.2–37.7 | 5 |
| 37.8–38.2 | 10 |
| 38.3–38.8 | 15 |
| 38.9–39.4 | 20 |
| 39.4–39.9 | 25 |
| ≧ 40.0 | 30 |
| Central nervous system effects | Score |
| Mild | |
| Agitation | 10 |
| Moderate | |
| Delirium | 20 |
| Psychosis | 20 |
| Extreme lethargy | 20 |
| Severe | |
| Seizure | 30 |
| Heart failure | Score |
| Mild | |
| Pedal edema | 5 |
| Moderate | |
| Bibasilar rales | 10 |
| Precipitant history | |
| Negative | 0 |
| Positive | 10 |
| Gastrointestinal–hepatic dysfunction | Score |
| Mild | |
| Diarrhea | 10 |
| Nausea/Vomiting | 10 |
| Abdominal pain | 10 |
| Severe | |
| Unexplained jaundice | 20 |
| Cardiovascular dysfunction | Score |
| Tachycardia | |
| 99–109 | 5 |
| 110–119 | 10 |
| 120–129 | 15 |
| 130–139 | 20 |
| ≧140 | 25 |
| Atrial fibrillation | 10 |
A score of 45 or more is highly suggestive of thyroid storm, a score of 25–44 supports the diagnosis, and a score below 25 makes thyroid storm unlikely. His score was 55 (temperature, 5; central nervous system effects, 10; gastrointestinal–hepatic dysfunction, 20; heart failure, 10; cardiovascular dysfunction, 10).
Discussion
Based on the patient’s background, it was important to distinguish the symptoms, as these could be from his hyperthyroidism or deterioration of chronic heart failure. Although his several symptoms may have been a result of exacerbation of chronic heart failure due to multiple cardiac disease, it was reasonable to assume that his hyperthyroidism was associated with thyroid storm.
Considering the background thyroid disease and amiodarone‐related hyperthyroidism, it is important to identify the type of AIT (1 or 2), and in this regard thyroid ultrasonography is useful. Type 1 AIT is associated with a high thyroidal blood flow, whereas type 2 AIT is characterized by a destructive thyroiditis with a low internal thyroidal blood flow. 8 In our case, the patient had no pre‐existing thyroid disease, was a long‐term user of amiodarone, no nodules, and hypovascularity on thyroid ultrasonography, and he was thus highly likely to have type 2 AIT. In medication therapy, although discontinuation of amiodarone is advisable, the arrhythmic status of the patient must be considered. Glucocorticoids are often effective in shortening the destructive process of type 2 AIT and controlling the thyrotoxicosis. 9 In this case, although thyroid storm was initially indicated, we could not confirm its association with amiodarone and thyrotoxicosis, so we administered methimazole for usual treatment on the day of admission. Type 2 AIT is difficult to predict and may develop at any time during treatment with amiodarone. For unclear reasons, the toxic effects of amiodarone may take 2–3 years to manifest. 9 Prolonged monitoring of thyroid function test is required in patients with AIT even if they become euthyroid, as there is a chance of them becoming hypothyroid. 10 In our case, based on his family doctor’s laboratory findings, he underwent no thyroid function tests in the last 6 months at least. It was possible that the thyroid storm could have been avoided if he had regular visits to his doctor and thyroid function test results checked. Thyroid function test should be checked every 3 months a year and thereafter every 6 months (i.e., during the follow‐up) in patients receiving amiodarone in order to avoid critical thyroid storm. 10
When physicians encounter a patient with thyroid storm, the patient’s medication history, as to whether it includes amiodarone, must be reviewed. It is important to distinguish between AIT 1 or 2, and consider specific therapy directed against the thyroid and supportive therapy. Treatment of any precipitating factors is essential because the mortality rate of thyroid storm is substantial.
Conclusion
We herein report the case of the patient with amiodarone‐induced thyroid storm associated with type 2 AIT. Physicians should be aware that the use of amiodarone can cause not only thyroid dysfunction but also thyroid storm. Regular thyroid function tests should be performed and results analyzed when prescribing amiodarone.
Disclosure
Approval of the research protocol: N/A.
Informed Consent: Informed consent was obtained from the patient for publication of this case report.
Registry and the Registration No. of the study/Trial: N/A.
Animal Studies: N/A.
Conflict of Interest: None declared.
Funding Information No Funding Information provided.
References
- 1. Ursella S, Testa A, Mazzone M, Gentiloni SN. Amiodarone–induced thyroid dysfunction in clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2006; 10: 269–78. [PubMed] [Google Scholar]
- 2. Loh K‐C. Amiodarone‐induced thyroid disorders: a clinical review. Postgrad. Med. J. 2000; 76: 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akamizu T, Satoh T, Isozaki O, et al Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 2012; 22: 661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tietgens ST, Leinung MC. Thyroid storm. Med. Clin. North Am. 1995; 79: 169–84. [DOI] [PubMed] [Google Scholar]
- 5. du Swee S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr. Pract. 2015; 21: 182–9. [DOI] [PubMed] [Google Scholar]
- 6. Khemichian S, Fong TL. Hepatic dysfunction in hyperthyroidism. Gastroenterol. Hepatol. (N Y). 2011; 7: 337–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Burch HB, Wartofsky L. Life–threatening thyrotoxicosis. Thyroid storm. Endocrinol. Metab. Clin. North Am. 1993; 22: 263–77. [PubMed] [Google Scholar]
- 8. Eaton SE, Euinton HA, Newman CM, et al Clinical experience of amiodarone–induced thyrotoxicosis over a 3–year period: role of colour–flow Doppler sonography. Clin. Endocrinol. 2002; 56: 33–8. [DOI] [PubMed] [Google Scholar]
- 9. Tsang W, Houlden R. Amiodarone–induced thyrotoxicosis: a review. Can J. Cardiol. 2009; 25: 421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajeswaran C, Shelton RJ, Gilbey SG. Management of amiodarone–induced thyrotoxicosis. Swiss Med. Wkly 2003; 133: 579–85. [DOI] [PubMed] [Google Scholar]


