We report a case of an elderly woman with no history of diabetes mellitus who experienced emphysematous cholecystitis after the successful treatment of a heat stroke. Emergency cholecystectomy was performed on day 2.
Keywords: Digestive system disorder, emphysematous cholecystitis, heat stroke, shock
Abstract
Background
During a heat stroke, microvascular injury may occur as a result of thermal damage and systemic hypoperfusion. We present a case of an older woman who experienced emphysematous cholecystitis during a treatment of heat stroke.
Case presentation
A 91‐year‐old woman presented unconscious with a blood pressure, pulse, and core temperature of 73/48 mmHg, 135 bpm, and 39.8°C, respectively. The patient was diagnosed with heat stroke. Twenty‐two hours after arrival, the patient fell into septic shock. We diagnosed emphysematous cholecystitis and performed an emergency cholecystectomy. As the bile culture was positive for Clostridium perfringens, meropenem was administered. The patient was transferred for rehabilitation 32 days after admission.
Conclusions
Emphysematous cholecystitis can present during a treatment of heat stroke. An abdominal X‐ray examination should be performed during treatment of heat stroke in the acute phase regardless of the physical assessment.
Introduction
Emphysematous cholecystitis possibly begins with acute cholecystitis followed by ischemia or gangrene of the gallbladder wall and infection by gas‐forming microorganisms. 1 The condition occurs most frequently in older men and patients with diabetes mellitus but has not been reported in patients with heat stroke. During a heat stroke, microvascular injury may occur as a result of thermal damage and systemic hypoperfusion. 2 Furthermore, endotoxin leakage in the digestive tract causes a decrease in blood flow, resulting in multiple organ dysfunction. 3 We report a case of an older woman with no history of diabetes mellitus who experienced emphysematous cholecystitis during a treatment of heat stroke.
Case report
A 91‐year‐old woman presented unconscious after experiencing a heat stroke while working outside. Her health was normal before the onset of heat stroke; she was transferred to our hospital 5 h after the onset. She was undergoing hyperlipidemia treatment with 5 mg/day of atorvastatin calcium hydrate. Physical examination of the patient revealed a deep coma; the blood pressure, pulse, and respiratory rate were 73/48 mmHg, 135 bpm, and 24 breaths/min, respectively. Her axillary body temperature was 40.7°C, while the core temperature was 39.8°C in the bladder; she weighed 45 kg. Laboratory investigations revealed elevated lactate levels and disseminated intravascular coagulation (DIC) due to heat stroke; however, infection‐related tests, such as white blood cell count, C‐reactive protein, and procalcitonin were normal (Table 1). A computed tomography (CT) scan of the head, thorax, abdomen, and pelvis with contrast indicated cerebral atrophy and dilatation of the gallbladder and digestive tract; it did not identify emphysematous changes. Consequently, the patient was diagnosed with a heat stroke.
Table 1.
Laboratory findings on admission
| Complete blood count | Biochemistry | ||
| White blood cell | 4,210/μL | Total protein | 5.8 g/dL |
| Red blood cell | 337 × 104/μL | Albumin | 3.1 g/dL |
| Hemoglobin | 9.8 g/dL | Aspartate transaminase | 75 IU/L |
| Hematocrit | 29.9% | Alanine transaminase | 30 IU/L |
| Platelet | 14.6 × 104/μL | Lactate dehydrogenase | 380 IU/L |
| Creatine kinase | 985 IU/L | ||
| Coagulation status | Creatinine | 1.57 mg/dL | |
| Activated partial thromboplastin time | 32.5 s | Blood urea nitrogen | 25.1 mg/dL |
| Prothrombin time (PT) | 68% | Total bilirubin | 0.7 mg/dL |
| PT international normalized ratio | 1.22 | Triglyceride | 42 mg/dL |
| Fibrinogen | 246 mg/dL | Total cholesterol | 125 mg/dL |
| Fibrin/fibrinogen degradation products | 69.3 μg/ml | Sodium | 140 mmol/L |
| Potassium | 5.6 mmol/L | ||
| Arterial blood gas (under the intubation) | Chloride | 5.6 mmol/L | |
| FiO2 | 1.0 | C‐reactive protein | 0.10 mg/dL |
| pH | 7.429 | Procalcitonin | 2.33 ng/dL |
| PaCO2 | 27 mmHg | ||
| PaO2 | 339 mmHg | ||
| HCO3 ‐ | 17.5 mmol/l | ||
| Base excess | −5.5 mmol/L | ||
| Lactate | 51 mg/dL | ||
FiO2, fraction of inspired oxygen; HCO3 ‐, bicarbonate; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
We immediately performed tracheal intubation and cooled the body by spraying cold water and cold fluid infusion. The patient’s core temperature lowered to <38°C, while the systolic pressure recovered to >100 mmHg for 90 min. Although the immediate cooling and stabilization of circulation was successful, the lactate level improved only marginally. We treated her with massive fluid infusion and anti‐DIC therapy (17,100 U/day of thrombomodulin alfa and 1,500 U/day of antithrombin III) due to the prolonged shock and DIC. The patient was treated for 24 h with 6,200 mL of fluid infusion; the total urinary output was 1,800 mL with acute diarrhea (Fig. 1).
Fig. 1.
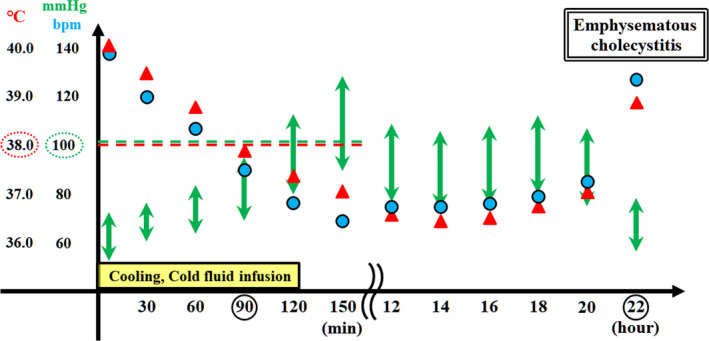
Patient’s clinical course during the initial 24 h after arrival. The core temperature was lowered to 38°C (red dotted oval), while the systolic pressure recovered to above 100 mmHg (green dotted oval) for 90 min following cooling and cold fluid infusion. Although the immediate cooling and stabilization of circulation for heat stroke was successful, improvement in the lactate (Lac) level was limited. Because of the prolonged shock and disseminated intravascular coagulation (DIC), she was treated by massive fluid infusion and anti‐DIC therapy (17,100 U/day of thrombomodulin alfa and 1,500 U/day of antithrombin III). Acute diarrhea was observed 10–20 h after arrival. The patient progressed into hypotension and hyperthermia 22 h after her arrival, and she was diagnosed with septic shock caused by emphysematous cholecystitis. Laboratory investigations revealed elevated Lac and procalcitonin (PCT) levels, DIC, and decreased white blood cell (WBC) count. CRP, C‐reactive protein; EC, emphysematous cholecystitis, Lac, lactate, PCT, procalcitonin, WBC, white blood cell.
After 22 h of arrival at our hospital, the patient’s blood pressure decreased to 72/50 mmHg, while her fever increased to 40.6°C (Fig. 1). Although the physical examination of right upper quadrant abdominal pain and signs of peritoneal irritation was unclear, an abdominal X‐ray examination indicated emphysematous cholecystitis (Fig. 2). We diagnosed the patient with septic shock resulting from emphysematous cholecystitis and performed emergency cholecystectomy. During the surgery, the gallbladder revealed ischemic changes, including edema, dark red coloration, and emphysematous changes on its wall. Inflammation of the gallbladder affected the neighboring liver and duodenum (Fig. 2).
Fig. 2.
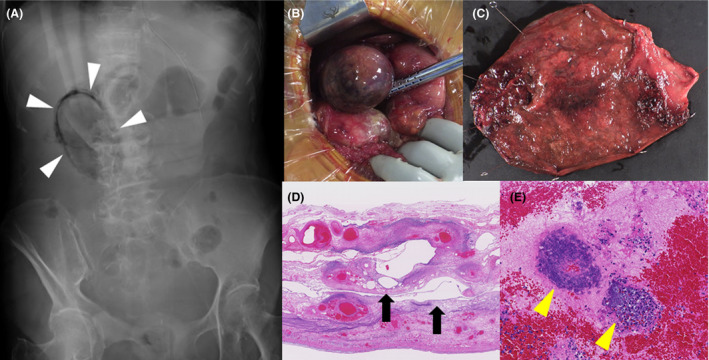
Emergency cholecystectomy performed following patient diagnosis with emphysematous cholecystitis (EC) on day 2. A, EC (white triangle heads) was identified using an abdominal X‐ray examination. B and C, The gallbladder X‐ray revealed ischemic changes, including enlargement, edema, dark red coloration, and emphysematous changes on the gallbladder wall. Inflammation of the gallbladder affected the neighboring liver and duodenum. The bile culture indicated presence of Clostridium perfringens. D and E, Histological findings of gallbladder using hematoxylin and eosin stain showed amorphous cystic spaces (black arrows), possibly resulting from proliferation of gas‐forming bacteria, and hemorrhage and necrotic changes with bacterial colonies (yellow triangle heads).
Although stable following the cholecystectomy, the patient experienced disuse syndrome. She suffered from DIC and acute kidney and hepatic injury presented as complications of heat stroke. She recovered as a result of anti‐DIC therapy and massive fluid infusion until day 5 of hospitalization. As the bile culture was positive for Clostridium perfringens, we continued to administer 1.5 g/day of meropenem starting from the day after cholecystectomy to day 7 and 9 g/day of sulbactam/ampicillin until day 14. On day 12, the patient indicated signs of Clostridium difficile colitis and was administered 0.5 g/day of oral vancomycin, which was continued until day 25. On day 13, mechanical ventilation management was terminated; on day 32, the patient was transferred for rehabilitation.
Discussion
This case demonstrates two important clinical issues: (i) emphysematous cholecystitis can present during the treatment of heat stroke, and (ii) abdominal X‐ray examination should be performed during treatment of heat stroke in the acute phase, regardless of the physical assessment.
During a heat stroke, microvascular injury may occur as a result of thermal damage and systemic hypoperfusion. 2 In our patient, ischemic and necrotic changes in the gallbladder might have occurred due to microvascular injuries from the heat stroke, leading to the development of emphysematous cholecystitis. 4 Although no necrosis was observed in the digestive tract, the acute diarrhea might have resulted from microvascular injuries and mucosal damage due to systemic hypoperfusion and reperfusion. The reason the ischemic and necrotic changes appeared only in the gallbladder is unclear.
Previous studies reported the occurrence of bowel ischemia 5 and colon perforation 6 during the treatment of heat stroke. Furthermore, older patients and those undergoing hemodialysis with arteriosclerosis as an underlying disease may experience ischemic and necrotic changes after recovery from shock. 7 In this case, the patient was elderly and had arteriosclerosis. She could have had asymptomatic acalculous cholecystitis and developed emphysematous cholecystitis due to the onset of heat stroke. 4 Although she immediately recovered from hypotension and hyperthermia, emphysematous cholecystitis and watery diarrhea occurred because of the heat stroke lasting for 5 days. Patients experiencing heat stroke in the acute phase are mostly unconscious due to sedative and analgesic management during mechanical ventilation. Therefore, their digestive system disorders, including emphysematous cholecystitis, may go unrecognized during the treatment of heat stroke. Clostridium difficile colitis could occur due to heat stroke and the continuous use of carbapenem.
The patient developed emphysematous cholecystitis with septic shock despite effective cooling and mass fluid infusion for heat stroke. The physical examination for emphysematous cholecystitis was not definitive. In addition, we could not perform CT to examine the septic shock because transferring the patient under unstable condition was challenging. The ischemic changes in abdominal organs may have occurred due to the dilatation of gallbladder and digestive tract observed on the CT scan on admission. Therefore, we performed chest and abdominal X‐ray examinations and diagnosed emphysematous cholecystitis. An abdominal X‐ray examination might help to screen for ischemia and necrotic changes in the digestive systems during treatment of heat stroke. Therefore, it should be performed during treatment of heat stroke in the acute phase, regardless of the physical assessment.
Conclusion
Emphysematous cholecystitis can present during the treatment of heat stroke. Abdominal X‐ray examination should be performed during treatment of heat stroke in the acute phase, regardless of the physical assessment.
Disclosure
Informed consent: Written informed consent was obtained from the patient’s legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
Conflict of interest: Authors declare no conflicts of interest for this article.
Author contributions
NY, HO, TM, YK, TF, RY, MM, YH, HT, and SY treated the patient. NY wrote the manuscript. SO supervised this report. All authors have read and approved the final manuscript.
Acknowledgements
The authors thank the paramedical crew for the data.
Funding information
No funding information provided.
Data Availability Statement
The data set analyzed in this study is available from the corresponding author on reasonable request.
References
- 1. Greenberger NJ, Paumgartner G et al Disease of the gallbladder and bile ducts In: Fauci AS, Braunwald E, Loscalzo J. (eds). Harrison’s Principles of Internal Medicine, 17th edn, p. 1996 New York: McGraw Hill, 2008. [Google Scholar]
- 2. Roberts GT, Ghebeh H, Bouchama A et al Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heat stroke: a study in baboon model. Arterioscler. Thromb. Vasc. Biol. 2008; 28: 1130–6. [DOI] [PubMed] [Google Scholar]
- 3. Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. 2010; 109: 1980–8. [DOI] [PubMed] [Google Scholar]
- 4. McChesney JA, Northup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion: a case series and review of pathophysiology. Dig. Dis. Sci. 2003; 48: 1960–7. [DOI] [PubMed] [Google Scholar]
- 5. Masood U, Sharma A, Syed W, Manocha D. Bowel ischemia from heat stroke: a rare presentation of an uncommon complication. Case Rep. Med. 2016; 2016: 5217690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsai MK, Chen IH, Wang CC, Shiang JC. Colon perforation as a critical complication of exertional heat stroke. Intern. Med. 2010; 49: 2473–6. [DOI] [PubMed] [Google Scholar]
- 7. Liao CY, Tsai CC, Kuo WH et al Emphysematous cholecystitis presenting as gas‐ forming liver abscess and pneumoperitoneum in a dialysis patient: a case report and review of the literature. BMC Nephrol. 2016; 17: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set analyzed in this study is available from the corresponding author on reasonable request.


