Abstract
Purpose of review:
Subarachnoid neurocysticercosis (SUBNCC) is due to a morphologically unique proliferative form of Taenia solium involving the subarachnoid spaces. Prolonged therapy based upon the pathophysiology of SUBNCC and long-term follow-up have shed light on the course of disease and led to highly improved outcomes.
Recent findings:
SUBNCC has a prolonged incubation period of between 10–25 years characterized by cyst proliferation and growth and invasion of contiguous spaces leading to mass effect(Stage 1). With induction of the host immune responses cysts degenerate leading to a predominately inflammatory arachnoiditis(Stage 2) causing hydrocephalus, infarcts and other inflammatory based neurological manifestations. Inactive disease(Stage 3) may occur naturally but mostly is a result of successful treatment, which generally requires prolonged intensive anthelminthic and anti-inflammatory treatments. CSF cestode antigen or cestode DNA falling to non-detectable levels predicts effective treatment. Prolonged treatment with extended follow-up has resulted in moderate disability and no mortality. Repeated short intensive 8-14- day courses of treatment are also used, but long-term outcomes and safety using this strategy are not reported.
Summary:
SUBNCC gives rise to a chronic arachnoiditis. Its unique ability to proliferate and induce inflammatory responses requires long-term anthelmintic and anti-inflammatory medications.
Keywords: Neurocysticercosis, cysticercosis, cestode, biomarker, subarachnoid, brain inflammation, extraparenchymal
Introduction
Human infection
Neurocysticercosis (NCC) is an infection of the brain and spine with a larval form (cyst or metacestode) of the tapeworm Taenia solium. The pig is the usual intermediate host and is infected with larval cysts while humans are the definitive host and harbor tapeworms in the small intestine. Similar to the pig, humans can also be infected and develop cysticercosis after ingestion of infectious ova shed in the feces of a human tapeworm carrier (fecal-oral transmission). In man, most cysts develop in the muscle, subcutaneous tissue, and brain but almost all the disease manifestations are due to infection involving the brain or spinal cord (neurocysticercosis)(1).
Disease manifestations based on location
The clinical manifestations of NCC vary considerably based on the number, location and involved brain compartment, size, presence and degree of inflammation and cyst form (1). Parenchymal infection most commonly causes seizures and/or epilepsy. Extraparenchymal disease is caused by infection of cysts located in the ventricular system or subarachnoid spaces (SUBNCC). Ventricular NCC usually presents with signs and symptoms related to obstructing cysts impeding CSF flow ( hydrocephalus) and with frequently associated ventriculitis. The clinical presentation and initial approach to treatment is specific to ventricular NCC and differs from SUBNCC in most aspects(2).
Characterization of racemose and subarachnoid NCC
SUBNCC is caused by an anatomically abnormal, proliferative form of T. solium cyst (3) (4) (Figure 1, C–H) that is located within the subarachnoid spaces of the brain, most commonly the basilar cisterns, Sylvian fissures, and spinal cord(4). In contrast, normal T. solium cysts have a cysticercus cestode larval morphology characterized by a scolex invaginated into a fluid vesicle. These cysts are anlage of adult tapeworms (5) and can be found anywhere within the brain, but mostly occur within brain parenchyma, sulci or ventricles. Although sulci located cysts are within the subarachnoid space, in most aspects they resemble parenchymal cysts and are not classified as SUBNCC (4).
Figure 1. Radiology and histopathology of SUBNCC.
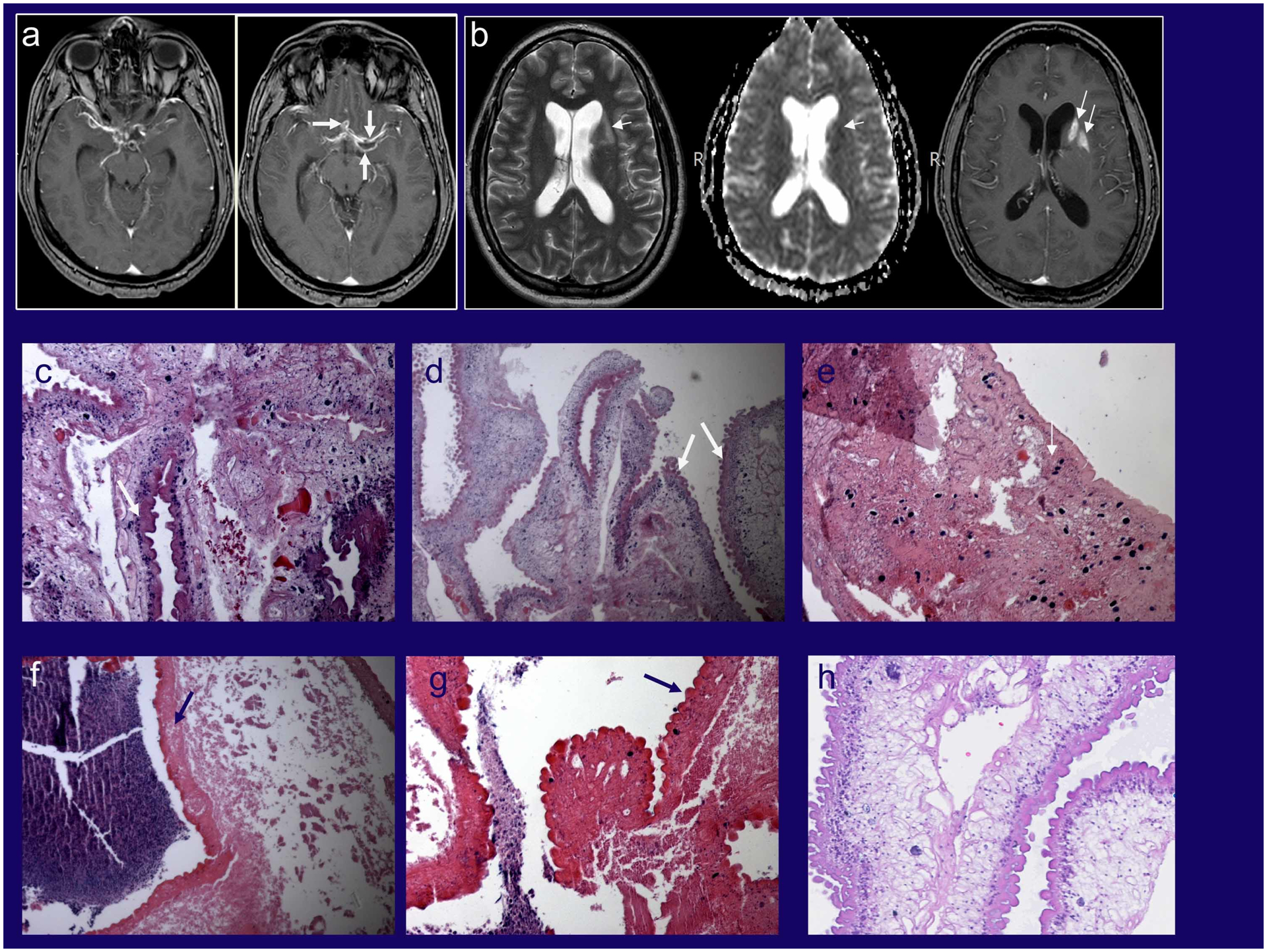
MRI imaging before treatment of a patient who presented primarily with arachnoiditis and lacunar infarcts who underwent brain biopsy for diagnosis (Stage 2) (11). (A) Post contrast T1-weighted images showing abnormal enhancement along the courses of the middle and anterior cerebral arteries bilaterally with associated cystic changes (white arrows). (B) Axial T2, apparent diffusion coefficient maps and axial post contrast T1-weighted images showing subacute infarcts in the left basal ganglia and white matter (black arrows). (C-G) Various histopathology brain biopsy samples from the patient demonstrating variable morphologies of the racemose form. (C, D) Viable parasite with tegument (wide white arrows) showing disorganized growth and a variety and different types and arrangements of subtegumental tissue elements including amorphous substance, parasite cells and calcareous corpuscles better shown in (E) (narrow white arrow). (E-G) Degenerated but still recognizable cestode tissue with associated adjacent necrotic host response (hematoxylin staining amorphous material). (H) Histopathology from another patient with racemose cysts showing a different morphology that resembles the cyst wall, which lacks calcareous corpuscles that are found the neck of the cyst. The presence of viable and non-viable residual parasite in an untreated patient is expected and one cause of long-lasting subarachnoid inflammation.
Source: Panels A and B from reference 11.
SUBNCC was first described by Virchow in 1860(6) who recognized grossly visible single or multicystic structures usually involving the base of the brain. However, Zenker in 1882 determined these were parasites and an unusual form of T.solium cysts {confirmed by recent molecular analysis(3)} after identification of cestode tegument and highly characteristic hooklets of T.solium within a sometimes present degenerated scolex. Zenker called this Cysticercus racemosus since the cystic structures resembled a bunch of grapes(7) (now commonly called racemose NCC) (Figure 1). Hennenberg in 1912(8) extensively reviewed the pathology and the pathophysiology of NCC. Even then, it was generally accepted that SUBNCC was caused by a proliferative form of T. solium manifested by unusual cystic growth with varying amounts of inflammation and fibrosis directed to residual degenerated parasite(9, 10) (11) (12). Although growth and proliferation of racemose forms have not been directly demonstrated in vitro, there is reasonable evidence indicating parasite growth (Table 1). It is the ability of this form of parasite to proliferate that gives rise to massive involvement and huge parasite burden, extensive and damaging subarachnoid located inflammation and the need for intensive prolonged or repeated treatments.
Table 1.
Evidence supporting racemose cysts proliferate
|
The Incubation period is decades
The incubation period is prolonged likely because of the slow growth of the parasite and accommodation of the subarachnoid space. Eventually the mass of the parasite becomes large enough to cause mass effects and/or inflammatory caused symptoms ensue. After leaving an endemic area, patients with SUBNCC develop symptoms about a decade after immigrating to the U.S(11). However, a more realistic estimate that incorporates exposure before immigration yielded a median of 22.2 years(11), which agrees with the prolonged incubation period of the limited number of documented travelers(13), the minimal incubation period of about 11 years for extra-parenchymal NCC in immigrants evaluated and treated in a U.S. hospital(14) and the course of a U.S. immigrant diagnosed at age 17 with asymptomatic SUBNCC who was serially imaged but untreated until diagnosed with extensive SUBNCC 19 years later(11).
The course of disease before diagnosis is commonly prolonged and frequently punctuated with intermittent symptoms
In addition to the long asymptomatic incubation period, the course of disease following the initial symptom is commonly chronic, lasting over a decade, episodic, and typically unrecognized or undiagnosed(15) (11) (16) (17). This includes sometimes severe, relatively transient symptoms and signs that are frequently undiagnosed, cryptic or never came to medical attention. For instance, real life examples include undiagnosed lacunar infarcts that occurred years before the diagnosis of extensive SUBNCC(11) or a prolonged 20 year course of undiagnosed spinal meningitis due to spinal SUBNCC(18).
Clinical course following the diagnosis
There are multiple series describing the clinical manifestations (19) (20) (21), complications, and short-term treatments but no adequately detailed studies describing long-term follow up and outcome. The diagnosis is considered when a patient with exposure in an endemic area presents with severe headaches, hydrocephalus, loss of consciousness with imaging demonstrating one or more cystic masses within the subarachnoid spaces of the brain and/or spinal cord. The classic description is the appearance of a bunch of grapes, racemose NCC(7), which is visualized best by Magnetic Resonance Imaging (MRI) steady-state sequences (e.g. Balanced Fast Field Echo [bFFE], Fast Imaging Employing Steady-state Acquisition Cycled Phases [FIESTA], three-dimensional constructive interference in steady state [3D CISS]), but can often also be visualized with T2 weighted sequences. These masses may be so large that they cause mass effects resulting in death if undiagnosed or untreated(22). The sensitivity of enzyme linked immunotransfer blot (EITB) in serum approaches 100%, whereas CSF EITB positivity is highly specific for SUBNCC but not very sensitive(23).The cestode CSF antigen is almost always positive (24,11). Serum cestode antigen is commonly positive but is generally less sensitive than the CSF(25). In the absence of identifiable cystic structures the radiological features are less suggestive and require presence of other findings of NCC, positive blood serology for antibodies, or cestode antigen or DNA in the serum or CSF to suggest the diagnosis (11). CSF testing for T. solium antigen (TsAg) and T. solium DNA are both highly sensitive and specific, whereas these tests are less sensitive in the serum(11, 24) (25). There are increasing reports of the diagnosis being made through the use of metagenomic sequencing from the CSF(18).
Natural history of SUBNCC
Documentation of clinical history and imaging prior to and following the advent of clinical symptoms as well as the long-term follow up post treatment reveals a typical course of disease, which can be described along an overlapping continuum of three stages (11) and are summarized in Table 2 and illustrated in Figure 2. Stage 1 is characterized by the presence of multicystic structures occupying one or more subarachnoid spaces typically causing mass effect. Stage 2 is characterized by symptoms caused by an inflammatory arachnoiditis. Cystic structures can persist or regrow but are not the main drivers of clinical symptoms. Hydrocephalus, nerve entrapments, lacunar infarcts, long tract signs and symptoms and other manifestations develop during this stage. Occasionally the cystic components are small or not present. The MRI shows a diffuse or localized arachnoiditis; aseptic meningitis sometimes with focal symptoms is a common clinical presentation. Stage 3 disease is inactive disease, which can occur spontaneously or more commonly as a consequence of effective treatment (11). At this stage there is no viable parasite that can continue to grow, but T. solium antigen may persist to varying degrees. Evidence of prior disease including old lacunar infarcts, subarachnoid scarring, or calcifications are still present but parasitic cystic structures are absent and enhancement on MRI (a measure of inflammation) is limited, decreased or absent.
Table 2.
Stage progression of SUBNCC
| Stage 1 |
|
| Stage 2 |
|
| Stage 3 |
|
Figure 2. Stages of subarachnoid neurocysticercosis.
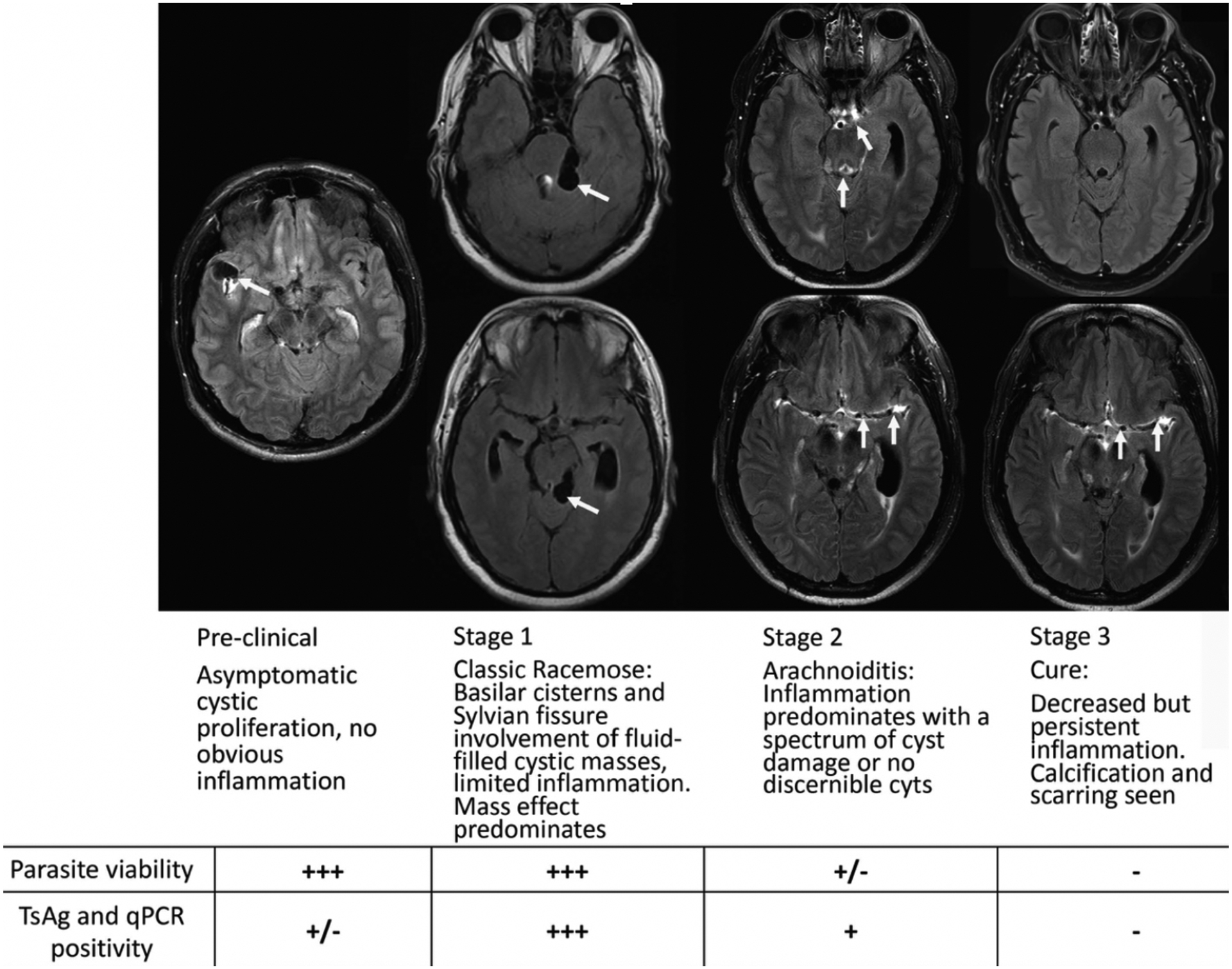
Post-contrast Fluid Attenuation Inversion Recovery (FLAIR) sequence Magnetic resonance imaging (MRI) images without gadolinium (Stage 1) and with gadolinium (Stages 2 and 3) are shown with pertinent findings highlighted by white arrows. The preclinical stage demonstrates an asymptomatic patient brought to medical attention after brain MRI following trauma showing cysts in the Sylvian fissure without surrounding enhancement or edema. Stages 1–3 show the same patient over the course of a 9-year period. Stage 1 shows bulky cysts in the quadrigeminal cistern causing mass effect but without enhancement or edema (not shown but evident in post gadolinium sequences). Stage 2 shows enhancement within the aqueduct (also had significant ventricular disease) and along the middle cerebral artery. Even at presentation there was significant enhancement along the middle cerebral arteries in gadolinium injected sequences. Commonly, different regions of brain have evolved at a slower or faster rate and are in a different Stage state. Pure Stage 1 with only cyst enlargement is not common, some inflammation is usually present. Stage 3 shows resolution of previous enhancement except along the middle cerebral artery. Parasite viability and T. solium antigen (TsAg) and qPCR positivity from the cerebral spinal fluid (CSF) from each stage are also shown. This patient’s course and imaging are detailed in reference 11.
2Symptoms reflect the stage, location and extent of involvement. Patients with a giant cyst (Stage 1), which commonly originate from one of the Sylvian fissures or the basilar cisterns commonly present in extremis with mass effects or compress structures such as the optic nerve resulting in vision loss. There are various sequelae of the inflammatory process typical of Stage 2 disease. These include lacunar infarcts (26), less commonly large vessel infarcts (27), hydrocephalus frequently requiring a shunt for decompression, nerve entrapments, etc. Accompanying parenchymal or ventricular disease may give rise to characteristic symptoms. Neurological deficits that are present upon entry into Stage 3 are typically non-reversible.
Prolonged treatments lead to excellent clinical outcomes
There are no randomized trials of treatment and until recently no adequately detailed reports of long-term outcomes, which is required to adequately assess well documented propensity for recurrences. The anthelminthic drugs used, their duration and use of accompanying corticosteroids were based on the preferences of the treating physicians. There are a number of reports using intensive 8–14 day courses of treatment (28) (29) (30) (31) Many employ high dose albendazole and corticosteroids with a taper. Response is gauged by change in size of one of more cysts. Even though cysts decrease in size or disappear, many times surprisingly quickly, recurrences are common. Two reports found a single short course of albendazole led to a complete response in 25.8% of patients(30) and a 36% rate of recurrence after two treatment courses(21).In addition, degeneration of cysts and decrease in size or volume post treatment as assessed by MRI examinations is the most common and usually the only method used to determine the effectiveness of treatment in published studies. The high rate of recurrences despite total cyst degeneration but with persisting CSF abnormalities suggest use of MRI as a sole indicator of treatment efficacy is often misleading(32). Although it has been suggested repeated short courses of treatment are effective and lead to inactive disease(33) there are no published reports of long-term follow up and clinical outcomes. Many patients with SUBNCC referred for treatment give a history of repeated failed courses of treatment of 8–30 days duration (11) suggesting prolonged treatment courses are required to prevent parasite regrowth and recurrence.
A recent paper employed long-term treatment with anthelmithics and anti-inflammatory agents to treat SUBNCC (11). The authors preferentially used combined albendazole and praziquantel, and employed high dosing of corticosteroids followed by a prolonged taper. To limit corticosteroid side effects, methotrexate and/or etanercept were added to the above regimen or used to in place of corticosteroids(11) (34) (35). There were prior studies that employed prolonged treatment courses. Proano et al. used this approach to treat unremoved 4th ventricular cysts(36) and later used it to treat giant subarachnoid cysts(22). In the recently published report, rather than treating for a specific duration, dose and level of immunosuppression, treatment decisions were made based upon multiple complimentary measures including decrease in cyst size and degree of enhancement by MRI, levels of CSF cestode antigen concentration, which reflects cyst burden and viability, and normalization of CSF pleocytosis. In general reassessments were scheduled every 3 months with evaluations earlier as required. Cestode antigen levels falling to undetectable predicted sustained inactive disease, likely cure. The use of CSF markers(37) (38) (32) and sometimes serum(39) to follow treatment response allowed an objective measure of effective treatment independent from change in cyst size, which even though a measure of treatment effect does not always predict cure. More recently, serial measurements of CSF T. solium DNA by qPCR were shown to be useful. Sensitivity to diagnose SUBNCC was about the same as cestode antigen and levels that became undetectable at least equally predicted inactive disease status or cure. Furthermore, this test may offer some predictive advantage (25). Since primers are more readily available than reagents for immune assays, this technique could be more readily available to treating physicians. A general drawback of prolonged and intensive therapy is the considerable increase in resources including medication and testing, invasive lumbar punctures, side effects of corticosteroid and expense. This highlights the need for randomized studies to determine the shortest and most effective treatments.
Role of inflammation and its control
Although the essential role of inflammation in disease pathogenesis was known over a century(8), the benefit and use of corticosteroids has never been studied(40) despite general agreement in the field of their usefulness to control inflammation in SUBNCC(23). Corticosteroids promptly alleviate symptoms and decrease enhancement, and symptoms and signs quickly return if corticosteroids are stopped too soon or the dose tapered too fast. However, the limitation and severity of corticosteroid side effects are well documented. More recently the empiric use of methotrexate(35) and etanercept (34) to control inflammation and limit corticosteroid side effects was reported. The potential efficacy of etanercept was supported by marked suppression of the usual proinflammatory cytokine release that occurs as a result of cysticidal treatment of naturally infected treated pigs(41). Since their use was found safe, likely beneficial and less morbid than corticosteroids, they were used as long as perceived necessary(34) and might have contributed to the limited morbidity reported. How to effectively and safely control inflammation remains one of the most important unstudied aspects of treatment in SUBNCC and for that matter other forms of NCC as well.
Measures of treatment response and stage 3 SUBNCC
The presence of cestode antigen in the CSF in and serum of humans indicates infection with T. solium. In general prior studies suggest a decrease in levels in response to treatment is a measure of treatment efficacy(37) (42, 43). However, except in one instance(39) follow up was relatively short or not reported and the ultimate clinical state, e.g whether there were recurrences, of patients unknown. A recent study (11) found CSF analysis to be useful and the fall in cestode antigen to undetectable levels predicted sustained inactive disease(11). The long follow up period of many treated subjects highly suggested they were cured. Measurement of T.solium DNA in the CSF appears to be similarly helpful in diagnosis (38) (43) (38, 44–46) but also suffers from lack of long term follow up. However, the presence and change of CSF cestode DNA in SUBNCC were retrospectively evaluated in the long-term followed cohort noted above using a newly developed very sensitive qPCR(25). The assay was 100% sensitive in the CSF in detecting viable disease and 93.3% specific in reverting to negative in those with sustained inactive disease, likely cured. These quantitative assays appear are an objective measure of treatment efficacy and reappearance confirms recurrent infection and regrowth. They were useful as an endpoint of anthelminthic treatment.
Long term disease and clinical status
In contrast the general dismal clinical outcomes of reports of SUBNCC (47) (48), the recent series that employed long-term treatments and prolonged follow up had no deaths and moderate overall morbidity (11). Roughly a quarter of the patients ended up with significant morbidities but none required institutionalization and all were able to care for themselves. Although it is impossible to assign a specific factor that led to the outstanding clinical results compared to earlier studies since a number of practice changes were made, the use of prolonged anthelminthic drugs, effective control of inflammation, reliance of biological markers and MRI imaging to define efficacy, and close follow up all contributed.
Conclusion
Use of intensive prolonged anthelminthic and anti-inflammatory treatments guided by objective measures of treatment efficacy led to probable cures in most patients and markedly improved clinical outcomes. However, treatment was not standardized so it is unclear which changes led to improved outcomes. There are a number of outstanding questions. A clearer understanding of how the parasite proliferates should lead to ways to control its growth. Although inflammation results in most of the complications, how to best control inflammation has barely been discussed or studied. Also, more effective drugs or drug combination are desperately needed. Lastly, randomized trials are required to define the shortest, most effective and safest regimens.
Main points.
SUBNCC is an aberrant proliferative form of T. solium cysts that slowly grows and has a long incubation period lasting 1–2 decades as it expands mostly within the subarachnoid spaces.
Because the parasite proliferates, the mass of the parasite and antigen load can be enormous necessitating intensive long-term or treatments to kill all proliferating cells and intensive immunosuppression to control inflammation directed to large quantities of residual antigen.
Initial disease is caused by mass effects with varying but usually limited amounts of inflammation and then transitions into a mostly chronic arachnoiditis. Inflammation is the cause of most morbidity and mortality.
The disease course can be divided into overlapping stages of 1) mostly cystic masses with limited inflammation causing mass effects; 2) chronic arachnoiditis with inflammation directed to prior cysts and the presence of residual or regrown cystic masses and; 3) inactive disease but presence of inflammation caused sequelae.
Long term intensive cysticidal and immunosuppressive treatments to suppress inflammation guided by normalization of CSF parameters and cestode antigen levels resulted in improved outcomes including likely cures in all but one patient in the series, There were no deaths and moderate morbidity.
Funding:
Intramural National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health
Financial support: Intramural NIAID, NIH
Footnotes
Conflicts of Interest: none
References and recommended reading:
- 1.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurology. 2014;13(12):1202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash TE, Ware JM, Mahanty S. Intraventricular Neurocysticercosis: Experience and Long-Term Outcome from a Tertiary Referral Center in the United States. Am J Trop Med Hyg. 2018;98(6):1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinojosa-Juarez AC, Sandoval-Balanzario M, McManus DP, Monroy-Ostria A. Genetic similarity between cysticerci of Taenia solium isolated from human brain and from pigs. Infection Genetics and Evolution. 2008;8(5):653–6. [DOI] [PubMed] [Google Scholar]
- 4.Rabiela MT, Rivas-Hernandez A, Rodriquez-Ibarra J, Castilo-Medina S, Cancino FdM. Anatomopathological Aspects of Human Brain Cysticercosis In: Flisser A, Willms k, Laclette JP, Larralde C, Ridaura C, Beltran F, editors. Cysticercosis: Present State of Knowledge and Prespectives. New York, New York: Academic Press; 1982. p. 179–200. [Google Scholar]
- 5.Rabiela MT, Rivas A, Flisser A. Morphological types of Taenia solium cysticerci. Parasitol Today. 1989;5(11):357–9. [DOI] [PubMed] [Google Scholar]
- 6.Virchow R Traubenhydatiden der weichen Hirnhaut. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1860;18(5–6):528–35. [Google Scholar]
- 7.Zenker FA. Über den Cysticercus racemosus des Gehirns. Beitr Anat Embryolo J Henle, Festg. 1882:119–40. [Google Scholar]
- 8.Henneberg R Die tierischen parasiten des zentralnerven-systems In: Lewandowsky M, editor. Handbuch Der Neurologie. Berlin: Verlag Von Julius Springer; 1912. p. 643–712. [Google Scholar]
- 9.Brown WJ, Voge M. Neuropathology of parasitic Infections: Oxford University Press; 1982. 229 p. [Google Scholar]
- 10.Pittella JE. Neurocysticercosis. Brain Pathol. 1997;7(1):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S. Natural History of Treated Subarachnoid Neurocysticercosis. Am J Trop Med Hyg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** An excellent series that gives insight into incubation periods, natural history, use of biomarkers to determine efficacy of treatment and when to cease therapy. The long term follow up allowed detection recurrences, assesment of likely cure and correlates of cure.
- 12.Cysticercosis Marquez-Monter H.. In: Marcial-Rojas RA, editor. Pathology of Protozoal and Helminthic Diseases. Baltimore: The Willimas & Wilkins Company; 1971. p. 592–617. [Google Scholar]
- 13.Del Brutto OH. Neurocysticercosis Among International Travelers to Disease-Endemic Areas. Journal of Travel Medicine. 2012;19(2):112–7. [DOI] [PubMed] [Google Scholar]
- 14.Serpa JA, Graviss EA, Kass JS, White AC Jr. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore). 2011;90(1):81–6. [DOI] [PubMed] [Google Scholar]
- 15.McCormick GF, Giannotta S, Zee C, Fisher M. Carotid occlusion in cysticercosis. Neurology. 1983;33(8):1078–80. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MR, O’Donovan BD, Gelfand JM, Sample HA, Chow FC, Betjemann JP, et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. Jama Neurology. 2018;75(8):947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Highlights the utility of metagenomic next generation sequening in the diagnosis of SUBNCC. 2 of 7 patients who did not present with cystic lesions ( Stage 2) and whose diagnosis was uncertain and were diagnosed using this sophisticated method.
- 17.Liu P, Weng X, Zhou J, Xu X, He F, Du Y, et al. Next generation sequencing based pathogen analysis in a patient with neurocysticercosis: a case report. BMC Infect Dis. 2018;18(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]; *One of several paper using next generation sequencing to diagnose chronic SUBNCC. The case is an example of SUBNCC presenting without any subarachnoid cysts, which initially did not suggest diagnosis of SUBNCC.
- 18.Beck ES, Ramachandran PS, Khan LM, Sample HA, Zorn KC, O’Connell EM, et al. Clinicopathology conference: 41-year-old woman with chronic relapsing meningitis. Ann Neurol. 2019;85(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A case presentation and discussion of a patient with long standing undiagnosed spinal disease finally diagnosed by metagenomic analysis of the CSF and subsequently successfully treated.
- 19.Bazan R, Hamamoto Filho PT, Luvizutto GJ, Nunes HR, Odashima NS, Dos Santos AC, et al. Clinical Symptoms, Imaging Features and Cyst Distribution in the Cerebrospinal Fluid Compartments in Patients with Extraparenchymal Neurocysticercosis. PLoS Negl Trop Dis. 2016;10(11):e0005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra MM, Arroyo M, Torres MC, Cruz NR, Hernandez FG, Taboada D, et al. Extraparenchymal neurocysticercosis: Demographic, clinicoradiological, and inflammatory features. Plos Neglected Tropical Diseases. 2017;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Review of Anti-Infective Therapy. 2011;9(1):123–33. [DOI] [PubMed] [Google Scholar]
- 22.Proano JV, Madrazo I, Avelar F, Lopez-Felix B, Diaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001;345(12):879–85. [DOI] [PubMed] [Google Scholar]
- 23.White AC Jr., Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, et al. Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2018;66(8):e49–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A current consensus of knowledge regarding diagnosis and treatmen of neurocysticercosis. A must read for those looking for guidance.
- 24.Garcia HH, Parkhouse RM, Gilman RH, Montenegro T, Bernal T, Martinez SM, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000;94(6):673–6. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB. A novel, highly sensitive qPCR assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing response to treatment. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A well performed study using a novel qPCR assay to quantify T. solium DNA in the CSF. Although detection of T. solium DNA is well documented, the advantage of this study is the use of patients treated and followed for years after cessation of treatment. Reaching undetectable levels predicated sustained inactive disease, likely cure.
- 26.Barinagarrementeria F, Del BO. Lacunar syndrome due to neurocysticercosis. Arch Neurol. 1989;46(4):415–7. [DOI] [PubMed] [Google Scholar]
- 27.Barinagarrementeria F, Cantu C. Frequency of cerebral arteritis in subarachnoid cysticercosis: An angiographic study. Stroke. 1998;29(1):123–5. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro L, Almeida-Pinto J, Stocker A, Sampaio-Silva M. Active neurocysticercosis, parenchymal and extraparenchymal: a study of 38 patients. J Neurol. 1993;241(1):15–21. [DOI] [PubMed] [Google Scholar]
- 29.Cardenas G, Carrillo-Mezo R, Jung H, Sciutto E, Hernandez JL, Fleury A. Subarachnoidal Neurocysticercosis non-responsive to cysticidal drugs: a case series. BMC Neurol. 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osorio R, Carrillo-Mezo R, Romo ML, Toledo A, Matus C, Gonzalez-Hernandez I, et al. Factors Associated With Cysticidal Treatment Response in Extraparenchymal Neurocysticercosis. J Clin Pharmacol. 2019;59(4):548–56. [DOI] [PubMed] [Google Scholar]; *The latest of a series of papers documenting responses to a 10 day high dose regimen of albendazole and corticosteroids. Response was determined decrease in volumn of cysts. Only about a quarter of the patient showed a complete response, which was associated with higher serum levels of albendazole sulfate.This is one of two papers documenting a dose response effect of albendazole in neurocysticercosis.
- 31.Gongora-Rivera F, Soto-Hernandez JL, Gonzalez Esquivel D, Cook HJ, Marquez-Caraveo C, Hernandez Davila R, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006;66(3):436–8. [DOI] [PubMed] [Google Scholar]
- 32.Fleury A, Garcia E, Hernandez M, Carrillo R, Govezensky T, Fragoso G, et al. Neurocysticercosis: HP10 Antigen Detection Is Useful for the Follow-up of the Severe Patients. Plos Neglected Tropical Diseases. 2013;7(3):e2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White AC, Fleury A. Optimal Treatment for Subarachnoid Neurocysticercosis: Closer, but Not There yet. Am J Trop Med Hyg. 2020;102(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash TE, Ware JM, Coyle CM, Mahanty S. Etanercept to Control Inflammation in the Treatment of Complicated Neurocysticercosis. American Journal of Tropical Medicine and Hygiene. 2019;100(3):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Summarizes the clinical course of a series of patients treated with etanerept to control inflammation in SUBNCC. Inflammation leads to most of the disease manifestations in SUBNCC and its control is essential to avoid complications. However corticosteroids cause many side effects. Etanercept was used with and in place of corticosteroids with few side effects and apparent efficacy. A randomized control study based on these promising anectodal results appear warranted
- 35.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44(4):549–53. [DOI] [PubMed] [Google Scholar]
- 36.Proano JV, Madrazo I, Garcia L, Garcia TE, Correa D. Albendazole and praziquantel treatment in neurocysticercosis of the fourth ventricle. J Neurosurg. 1997;87(1):29–33. [DOI] [PubMed] [Google Scholar]
- 37.Bobes RJ, Hernandez M, Marquez C, Fragoso G, Garcia E, Parkhouse RM, et al. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health. 2006;11(6):943–50. [DOI] [PubMed] [Google Scholar]
- 38.Yera H, Dupont D, Houze S, Ben M’Rad M, Pilleux F, Sulahian A, et al. Confirmation and Follow-Up of Neurocysticercosis by Real-Time PCR in Cerebrospinal Fluid Samples of Patients Living in France. Journal of Clinical Microbiology. 2011;49(12):4338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia HH, Pierre Dorny, Castillo Y, Pretell EJ, Rodriguez S, Lizardo Mija, et al. Antigen Levels Follow Post-Treatment Evolution of Subarachnoid Neurocysticercosis. Journal of Neuroparasitology 2010;1:1–3. [Google Scholar]
- 40.Nash TE, Mahanty S, Garcia HH, Cysticercosis Group in P. Corticosteroid use in neurocysticercosis. Expert Rev Neurother. 2011;11(8):1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahanty S, Orrego MA, Cangalaya C, Adrianzen MP, Arroyo G, Calcina J, et al. TNF-alpha blockade suppresses pericystic inflammation following anthelmintic treatment in porcine neurocysticercosis. Plos Neglected Tropical Diseases. 2017;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleury A, Hernandez M, Avila M, Cardenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78(9):970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L, et al. Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol. 2011;49(1):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez M, Gonzalez LM, Fleury A, Saenz B, Parkhouse RM, Harrison LJ, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102(4):317–23. [DOI] [PubMed] [Google Scholar]
- 45.Romo ML, Carpio A, Parkhouse RME, Cortez MM, Rodriguez-Hidalgo R. Comparison of complementary diagnostic tests in cerebrospinal fluid and serum for neurocysticercosis. Heliyon. 2018;4(12). [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors compared the sensitivity and specificity of two cestode antigens assays in serum, a commercial antigen assay in CSF, and PCR for T. solium DNA in CSF in parencymal and extraparenchymal neurocysticcercosis. Comparison of these more sophisticated diagnostic tests is uncommonly reported. Both PCR and an antigen assay in CSF were highly senstive and specific in the diagnosis of extraparenchymal disease( combined ventricular and SUBNCC).
- 46.Carpio A, Campoverde A, Romo ML, Garcia L, Piedra LM, Pacurucu M, et al. Validity of a PCR assay in CSF for the diagnosis of neurocysticercosis. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agapejev S, Da Silva MD, Ueda AK. Severe forms of neurocysticercosis: treatment with albendazole. Arq Neuropsiquiatr. 1996;54(1):82–93. [DOI] [PubMed] [Google Scholar]
- 48.Figueroa JJ, Davis LE, Magalhaes A. Extraparenchymal neurocysticercosis in Albuquerque, New Mexico. J Neuroimaging. 2011;21(1):38–43. [DOI] [PubMed] [Google Scholar]
- 49.Valkounova J, Zdarska Z, Slais J. Histochemistry of the racemose form of Cysticercus cellulosae. Folia Parasitol (Praha). 1992;39(3):207–26. [PubMed] [Google Scholar]
- 50.Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE, Cysticercosis Working Grp P. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology. 2012;78(18):1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield PJ, Evans NA. Parthenogenesis and Asexual Multiplication among Parasitic Platyhelminths. Parasitology. 1983;86(Apr):121–60. [DOI] [PubMed] [Google Scholar]


