Abstract
Background
In the curative-intent treatment of locally advanced lung cancer, significant morbidity and mortality can result from thoracic radiation therapy. Symptomatic radiation pneumonitis occurs in one in three patients and can lead to radiation-induced fibrosis. Local failure occurs in one in three patients due to the lungs being a dose-limiting organ, conventionally restricting tumour doses to around 60 Gy. Functional lung imaging using positron emission tomography (PET)/CT provides a geographic map of regional lung function and preclinical studies suggest this enables personalised lung radiotherapy. This map of lung function can be integrated into Volumetric Modulated Arc Therapy (VMAT) radiotherapy planning systems, enabling conformal avoidance of highly functioning regions of lung, thereby facilitating increased doses to tumour while reducing normal tissue doses.
Methods and analysis
This prospective interventional study will investigate the use of ventilation and perfusion PET/CT to identify highly functioning lung volumes and avoidance of these using VMAT planning. This single-arm trial will be conducted across two large public teaching hospitals in Australia. Twenty patients with stage III non-small cell lung cancer will be recruited. All patients enrolled will receive dose-escalated (69 Gy) functional avoidance radiation therapy. The primary endpoint is feasibility with this achieved if ≥15 out of 20 patients meet pre-defined feasibility criteria. Patients will be followed for 12 months post-treatment with serial imaging, biomarkers, toxicity assessment and quality of life assessment.
Discussion
Using advanced techniques such as VMAT functionally adapted radiation therapy may enable safe moderate dose escalation with an aim of improving local control and concurrently decreasing treatment related toxicity. If this technique is proven feasible, it will inform the design of a prospective randomised trial to assess the clinical benefits of functional lung avoidance radiation therapy.
Ethics and dissemination
This study was approved by the Peter MacCallum Human Research Ethics Committee. All participants will provide written informed consent. Results will be disseminated via publications.
Trials registration number
Keywords: radiation oncology, respiratory tract tumours, nuclear radiology, toxicity
Strengths and limitations of this study.
This is a novel trial interventional trial integrating Galligas/68Ga-macroaggregated albumin respiratory-gated (4-dimensional) ventilation/perfusion (positron emission tomography/CT) functional lung imaging into radiation treatment planning.
Volumetric modulated arc therapy (VMAT) planning used in this trial aims to allow for moderate dose escalation to the primary only, while respecting conventional normal tissue toxicity constraints.
This single-arm study will assess the technical feasibility of delivering personalised VMAT radiation.
Radiation plans will be personalised to an individual’s tumour location and spatial lung function.
This single arm feasibility study is unable to assess effectiveness of reduction in pulmonary toxicity or enhanced tumour control but will nonetheless provide valuable information about the feasibility of conducting a larger-scale randomised trial.
Introduction
In the curative treatment of locally advanced non-small cell lung cancer (NSCLC), significant treatment-related morbidity and even mortality can result from thoracic radiation therapy. The predominant mechanism behind treatment-related toxicity is radiation effects on the lung manifesting as symptomatic radiation pneumonitis which occurs in up to one in three patients with fatal pneumonitis occurring in 2% of patients.1 The risk of pneumonitis is related to the dose and volume of lung irradiated. Current lung dose volume constraints simplistically assume that the lungs are anatomically and functionally homogeneous. A number of factors increase pneumonitis risk further including the use of concurrent chemotherapy, interstitial lung disease and age.1 The need to limit lung dose and other organs for safety reasons may contribute to the high local failure rate in stage III disease in one in three patients.2–5
Integration of functional lung imaging into treatment planning
Lung imaging using inhaled Galligas and intravenously administered 68Ga- macroaggregated albumin (MAA) enables acquisition of four-dimensional (4D) ventilation and perfusion (V/Q) positron emission tomography (V/Q, positron emission tomography (PET)/CT).6 7 This provides a 3D map of lung V/Q.8 Previous work has demonstrated a relationship between radiation therapy dose and change in V/Q as visualised V/Q PET/CT.9 10 We have shown strong correlations between V/Q PET/CT functional volumes and pulmonary function test parameters.11
Planning studies conducted on participants in the observational GalliPET clinical trial have shown feasibility of radiation therapy treatment planning personalised to an individual’s lung functional distribution; it has been demonstrated that integrating V/Q PET/CT information in radiation therapy planning can significantly reduce dose to functional lung.12 13 Systematic review of the literature has demonstrated reductions in functional lung dose can be achieved with the mean functional volume receiving 20 Gy reduced by: 4.42% (95% CI 1.66% to 7.18%) for perfusion, 4.41% (95% CI 2.37% to 6.45%) for ventilation and 4.19% (95% CI 2.34% to 6.04%) overall when plans were optimised based on functional lung imaging.14 The mean functional lung dose was reduced by: 1.98 Gy (0.57: 3.39) for perfusion, 2.63 Gy (0.14: 5.12) for ventilation and 2.18 Gy (1.09: 3.26) overall when plans were optimised using functional lung imaging.14
Advances in radiation therapy planning
This functional map of lung can be integrated into volumetric modulated arc therapy (VMAT) advanced radiation therapy planning which can optimise radiation planning to avoid functional regions of lung. A number of studies have demonstrated improved dosimetry compared with other radiation therapy techniques for NSCLC.15 VMAT may enable safe dose escalation above the standard dose of 60 Gy with accurate normal tissue definition, motion management and functional avoidance. Another advance to VMAT planning is the introduction of more advanced techniques such as the use of non-coplanar arcs. This has been shown to significantly decrease heart dose by 20%–30% in patients with lower lobe tumours treated with 74 Gy in 37 fractions.16
Radiobiological basis for dose escalation
74 Gy in 37 fractions at 2 Gy per fraction has been established as the maximum tolerated safe dose in multiple phase 1/2 dose escalation studies.17 This was used as the basis for the phase 3 trial, RTOG 0617.2 This demonstrated inferior local control and worse survival in the dose escalation arm.2 18 Further analysis of the RTOG 0617 trial suggests that worse outcomes may relate to increased dose to organs at risk, especially the heart which may be able to be reduced with advanced planning techniques and a smaller volume being irradiated at a higher dose using a simultaneous-integrated boost technique.5 18 With the current standard of care dose of 60 Gy mediastinal nodal failure is rare.19 Therefore, there is renewed interest in escalating dose to the primary tumour which is a common site of failure while treating the nodal volumes to a standard dose of 60 Gy.20 As mediastinal nodes are centrally located, maintaining a standard dose to these regions has the added benefit of not increasing dose to the central organs at risk such as the heart, oesophagus and proximal bronchial tree. Further analysis of dose escalation trials suggest there may be an overall survival benefit to a dose escalated approach if it can be delivered without treatment prolongation.21 This form of dose escalation has not been tested with a consistent manner using advanced radiation therapy planning techniques.
Methods and analysis
This is a prospective single-arm interventional clinical trial assessing the technical feasibility of functionally adapted lung radiation therapy using V/Q PET/CT imaging and VMAT planning. The primary intervention is VMAT radiation therapy optimised to avoid regions of functional lung delivered to a total dose of 60 Gy in 30 fractions to the primary and nodal planning target volume. A simultaneous integrated boost will be delivered to the primary tumour to a total dose of 69 Gy in 30 fractions. The trial schema is demonstrated in figure 1. The trial plans to recruit a total of 20 patients over two tertiary teaching hospitals in Melbourne, Australia.
Figure 1.
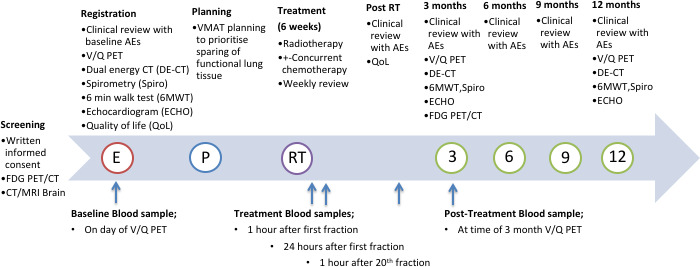
Demonstrates the trial schema from screening to the final assessment at 12 months post-treatment. AEs, adverse events; FDG, fluorodeoxyglucose; PET, positron emission tomography; RT, radiation therapy; VMAT, Volumetric Modulated Arc Therapy; V/Q, ventilation and perfusion.
Inclusion criteria
Age ≥18 years.
Informed consent documented and consent form signed.
Histologically or cytologically confirmed NSCLC.
ECOG performance status 0–2 within 2 weeks prior to registration.
Locally advanced disease (stage IIIA, IIIB, IIIC AJCC, eighth ed.) as confirmed on staging 18F-fluorodeoxyglucose (FDG)-PET/CT with no evidence of metastatic intracranial disease on CT brain with contrast or MRI.
Planned for treatment with curative intent.
Exclusion criteria
Participant is not able to tolerate supine position for imaging.
If history of a prior extrathoracic invasive malignancy (except non-melanomatous skin cancer) must be free from recurrence for a minimum of 3 years at the time of registration.
Prior radiation therapy to the lungs or mediastinum.
Prior known history of interstitial lung disease.
Trial objectives
The primary objective of this study is to assess the technical feasibility of the delivery of personalised functional lung radiation therapy using V/Q PET/CT. This study will be considered feasible if all criteria defined below are achieved for ≥15 out of 20 patients.
Feasibility will be considered to have been achieved for a given patient if all of the following criteria are me when functional plans are compared with conventional anatomical plans: (A) Reduction in mean functional lung dose of ≥2 Gy and functional lung volume receiving 20 Gy of ≥4%; (B) Mean heart dose is ≤30 Gy and relative heart volume receiving 50 Gy is <25%.
The secondary objectives are:
To determine the incidence of grade ≥2 clinical or radiological pneumonitis after high dose functionally adapted radiation therapy.
To determine the incidence of grade ≥2 acute and late toxicities.
To quantify regional ventilation loss and regional perfusion loss on post treatment V/Q PET/CT following functionally adapted lung radiation therapy and its relationship to spirometry and exercise tolerance.
To assess the relationships of cytokine release in patient’s plasma with grade ≥2 radiation pneumonitis.
-
To assess the associations between:
Ventilation PET/CT with inhale/exhale CT ventilation.
Perfusion PET/CT with dual energy CT iodine mapping (a surrogate for pulmonary perfusion).
To assess patient reported quality of life outcomes using the FACT-L quality of life questionnaire.
To assess incidence of complete metabolic response 3-month post-treatment FDG-PET/CT.
To assess progression-free survival at 12 months following completion of trial radiation therapy (defined by RECIST V.1.1).
To assess overall survival at 12 months following completion of trial radiation therapy.
The exploratory objectives are:
To assess the feasibility of conducting a radiation induced lymphocyte apoptosis assay.
To investigate the utility of mid-treatment cardiac biomarker testing and pretreatment coronary calcium scoring to predict patients at greater risk of radiation induced cardiac toxicity.
To correlate primary and nodal disease metabolic parameters seen on pretreatment and post-treatment FDG-PET/CT with dual energy CT iodine quantification.
To assess circulating tumour-DNA levels as a predictor of treatment response.
V/Q PET/CT procedure
The radiopharmaceuticals used in this study will be synthesised onsite by a qualified radio pharmacist using methods we have previously validated by our group.7 10 68Ga will be eluted from our 68Ge/68Ga generator and used to label the appropriate precursor. 68Ga-Galligas is prepared using a Technegas generator except that the radionuclide technetium-99m is replaced with gallium-68 in the carbon crucible inserted into the Technegas synthesis unit. 68Ga-MAA is prepared as follows: A commercially available kit of MAA is washed three times with sterile milli-Q water dispensed into 1 mL aliquots with each aliquot containing MAA particles of between 250 and 700 thousand particles. Gallium-68 obtained from the generator is buffered with acetate buffer to pH 6–7 before adding to the MAA aliquot. The suspension mixture is allowed to incubate for 1 min at 90°C after the addition of radioactivity for the radiolabeling process. Quality control tests was performed in accordance with the British Pharmacopoeia for radiolabelled MAA before the compound is released for clinical use.
The methodology of the V/Q PET/CT will be as follows:
A peripheral intravenous catheter is installed in the arm.
Participant inhales approximately 5 MBq of 68Ga-Galligas, in semi-supine position, using the same technique as for Technegas.
A chest 4D-CT acquisition is performed (140 kVp, 30–40 mA, axial scan time is breathing period +1 s.
Lung ventilation 3D list-mode respiratory gated PET acquisition is started (2–3 bed positions, 5 min per bed position). This acquisition will be reconstructed as both a respiratory gated and ungated scan.
Without moving, approximately 20–40 MBq 68Ga-MAA is injected intravenously, as a bolus, via the catheter. The syringe is then flushed with normal saline.
The lung perfusion 3D list-mode respiratory gated PET acquisition is started (2–3 bed positions, 5 min per bed position, exactly the same bed positions as for the ventilation study). This acquisition will be reconstructed as both a gated and ungated scan.
Personalised functional adapted VMAT radiation therapy
Technical description
Treatment planning will be done using the Varian Medical Systems, Palo Alto, California, Eclipse V.15.5 computerised radiation therapy planning system. At least two VMAT arcs will be used to deliver the radiation therapy. The 360° arcs are to be avoided to minimise dose to the contralateral lung. It is expected individual arc length will typically be between 180° and 240°.
Radiation therapy volumes
Target volumes will be defined as per standard practice including the primary tumour and nodal gross tumour volumes (GTV), internal target volumes (ITV) and planning target volumes (PTV). GTV incorporating respiratory motion on 4D CT, the internal gross tumour volume (IGTV) will be contoured for the primary tumour. ITV as defined by ICRU70 will be used to take into account tumour movement through respiration and a margin for subclinical spread.22 Target delineation and margins applied to primary tumour and nodal volumes are as per institutional protocol including mandatory use of 4D CT, FDG-PET/CT fusion and diagnostic contrast enhanced CT.
Boost volume definition: The proximal bronchial tree will have an isotropic 3 mm PRV expansion named proximal bronchial tree PRV. The boost volume (IGTV_6900) will be given to the IGTV of the primary tumour alone minus the proximal bronchial tree PRV. Normal tissue contouring will follow the RTOG 1106 contouring atlas.23
Functional lung volumes
Nuclear medicine physicians in conjunction with the radiation oncologists will define visually adapted functional lung subvolumes. The definitions and description of each functional lung grouping are described below. A 30% threshold was determined to separate regions of lung with higher potential function. On meta-analysis, this threshold was the most common threshold used when determining functional lung sub-volumes and similar to previously published thresholds using VQ PET.14 24
| Name (full) | Short name | Description |
| Perfused | Q Lung | Any Lung parenchyma containing 68Ga-MAA contoured using a visually adapted threshold that is confirmed by a physician. |
| Well Perfused | WQ Lung | Contour defined as 68Ga-MAA uptake greater than 30% of max. |
| Ventilated | Vent Lung | Any Lung parenchyma containing Galligas contoured using a visually adapted threshold that is confirmed by a physician. If there was significant clumping of the Galligas in the central airways was observed this was excluding activity four SD above the mean25 |
| Well Ventilated | WVent Lung | Contour defined as Galligas uptake greater than 30% of max. |
Galligas, 68Ga-carbon ultrafine aerosols; MAA, macroaggregates of human albumin.
These volumes are imported as DICOM structures into Eclipse. After visual verification, the radiation oncologist then uses these to generate the following opimisation structures. A graphical description of the components of all functional lung volumes is provided in figure 2.
Figure 2.
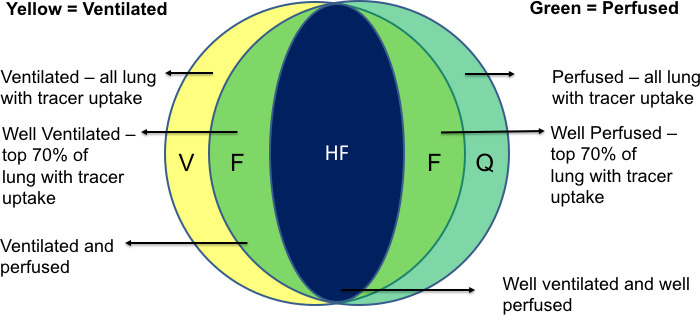
Demonstrates a spatial description of each of the lung subvolumes created by integrating functional information from the V/Q PET/CT is used to create optimisation functional lung subvolumes which are used in VMAT radiation therapy planning. PET, positron emission tomography; VMAT, Volumetric Modulated Arc Therapy; V/Q, ventilation and perfusion.
| Optimisation prioritisation | Name (full) | Name | Description |
| 1 | High functioning | Lung HF | Intersection of WVent lung and WQ lung, excluding PTV |
| 2 | Functioning | Lung F | Intersection of vent and Q contours excluding lung HF excluding PTV |
| 3 | Perfused | Lung P | Any lung parenchyma containing 68Ga-macroaggregated albumin contoured using a visually adapted threshold that is confirmed by a physician. Excluding lung HF, lung F, excluding PTV |
| 4 | Ventilated | Lung V | Any lung parenchyma containing Galligas contoured using a visually adapted threshold that is confirmed by a physician. Excluding lung HF, lung F, lung P, excluding PTV |
The expected outcome of these optimisation objectives is demonstrated in figure 3 where axial and coronal slices demonstrate the 20 Gy isodose line. In the functionally adapted plan there is less of the 20 Gy isodose line overlapping with high functioning and functioning lung segments.
Figure 3.
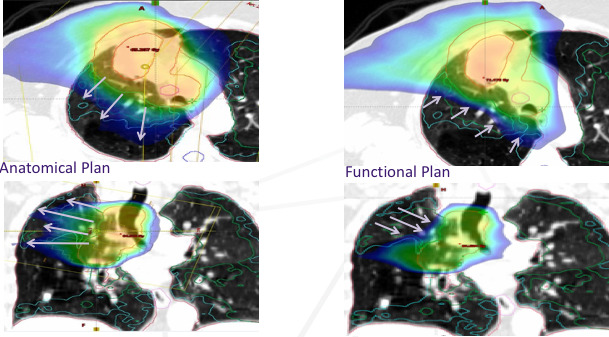
The arrows demonstrate the 20 Gy isodose line in radiation therapy plans optimised with conventional (anatomically based) lung constraints (left) and integrating functional information from V/Q PET/CT (right). PET, positron emission tomography; V/Q, ventilation and perfusion.
Treatment planning
The investigational treatment will be prescribed ensuring that 98% of the 60 Gy PTV is covered by 98% of the dose. The 69 Gy in 30 fractions (9 Gy) boost sub-volume (IGTV_6900) will aim to achieve 95% of the IGTV receives 100% of the boost dose (D95=100%). The maximum dose to PTV (PTV max) must be contained within the IGTV. This boost dose will be reduced if organ at risk planning constraints cannot be met. This will be at the discretion of the treating clinician. Dose constraints to organs at risk are described in table 1. If dose reduction is necessary, this event should be recorded and the D95% reported. Non-coplanar arcs may be considered if technically feasible and if this improves normal tissue sparing. It is expected that majority of patients will receive concurrent cytotoxic chemotherapy; the treating medical oncologist will determine the agents. Acceptable chemotherapy regimens include weekly carboplatin and paclitaxel and 3 weekly cisplatin and etoposide in accordance with Australian guidelines.26 27 Appropriate patients will also be offered adjuvant immunotherapy.28 This is not mandated by this study protocol.
Table 1.
This table demonstrates the normal tissue constraints used in the radiation therapy planning process
| Structure | Metric | Per protocol | Source | |
| Bony spinal canal | Max dose 0.03cc | ≤50 Gy | 13 | |
| Oesophagus | Max dose 0.03cc | <63 Gy | 13 | |
| Mean | <34 Gy | 13 | ||
| Heart | V40 | <30% | ||
| V50 | <25% | 33 | ||
| Mean | <20 Gy | 5 | ||
| Max dose 0.03cc | <70 Gy | 13 | ||
| Brachial plexus | Max dose 0.03cc | <63 Gy | 13 | |
| Proximal bronchial tree | Max dose 1.0cc | <64.5 Gy | 3 | |
| Great vessels (normal) | Max dose 0.03cc | <80 Gy | 34 | |
| Great vessels (tumour involved) | Max dose 0.03cc | <70 Gy | 34 | |
| Lung dose constraints | ||||
| Structure | Metric | Per protocol | Definition | Source |
| Lungs (anatomic) | Mean | <20 Gy | Mean dose to the whole anatomic lung | 13 |
| Left+right lung-IGTV | V30 | <30% | Volume of structure (%) receiving ≥30 Gy | 13 35 |
| V20 | <35% | Volume of structure (%) receiving ≥20 Gy | 13 35 | |
| V5 | <66% | Volume of structure receiving ≥5 Gy | 13 35 | |
Treatment delivery
All patients will have a pretreatment dosimetric quality assurance according to departmental VMAT quality assurance guidelines. Daily Cone Beam CT (CBCT) will be performed with online soft tissue matching will ensure that the target is within the PTV.
Statistical considerations
The study sample size is pragmatic and based on the clinically relevant number of patients needed to determine the technical feasibility of the functional lung sparing VMAT radiation therapy technique. Table 2 demonstrates the 95% CIs for different scenarios of feasibility rates.
Table 2.
This demonstrates shows the exact 95% CIs for different scenarios of feasibility rates
| No of feasible cases | Feasibility rate, % | Exact 95% CIs for rate estimate | |
| Lower limit, % | Upper limit, % | ||
| 15 | 75 | 51 | 91 |
| 16 | 80 | 56 | 94 |
| 17 | 85 | 62 | 97 |
| 18 | 90 | 68 | 99 |
| 19 | 95 | 75 | 100 |
| 20 | 100 | 83 | 100 |
The study follow-up duration was designed to be pragmatic for a feasibility study while having enough follow-up duration to detect clinically significant toxicity. In the context of NSCLC radiation therapy, late cardiac and lung toxicity typically presents within a 12-month time period. Our recent systematic review showed that functional lung imaging dose–response relationships plateau around 6–12 months post-treatment.14 29
Main analysis of the primary endpoint will be performed after all patients have been registered and completed their 3-month follow-up assessment. A final analysis will be performed at the completion of the study, which will be 12 months after the final patient completes treatment.
Monitoring programmes
Prior to commencement of radiation therapy, contours undergo peer review and sign off by a qualified radiation oncologist. All radiation therapy plans undergo review by a senior radiation therapist and senior medical physicist. Pretreatment dosimetric quality assurance is required prior to delivery of the first fraction.
All trial personnel are required to be qualified for their designated role with suitable training provided and credentials certified by the principal investigator. On treatment, weekly clinical reviews are performed a credentialed physician. At these assessments, offline CBCT assessment is performed to ensure adequate coverage of target volumes and review organs at risk. Post-treatment assessments must also be performed by a credentialed physician.
Independent trial monitoring
An independent data monitoring committee will be appointed and will convene with the purpose of:
Assessing quality issues related to radiation therapy.
Assessing the conduct and progress of the trial—accrual, non-eligibility, treatment toxicity and serious adverse events.
SAEs will be forwarded to the data monitoring committee. If three SAEs are recorded during the trial, the data monitoring committee must convene and determine the causal link between SAEs and the research. An assessment must be made regarding early termination of the trial, and recommendations forwarded to the Peter MacCallum Ethics Committee. The data monitoring committee should also convene at the completion of the trial to assess safety of this project.
Data collection and record retention
All data will be stored in reidentifiable form on Research Electronic Data Capture in a password-protected computer database.30 Any paper data will be stored in a locked filing cabinet in a secure building. Data will be stored for a minimum of 7 years after publication of study results, in accordance with Institutional guidelines and the Australian Code for the Responsible Conduct of Research. Patient confidentiality will be maintained at all times.
Ethics and dissemination
This study was approved by the Peter MacCallum Human Research Ethics Committee (HREC/18/PMCC/23). Prior studies have demonstrated safety of V/Q PET/CT.10 The stochastic and deterministic risks of radiation doses used in trial investigations and treatment have been carefully assessed and detailed in the trial participant information and consent form. The study results will be published in peer-reviewed journals.
Discussion
Advanced techniques such as VMAT functionally adapted radiation therapy may enable safe moderate dose escalation without treatment prolongation with an aim of improving local control and concurrently decreasing treatment related toxicity. The long-term results from the RTOG 0617 trial demonstrated worse survival in the dose-escalation arm. Long-term follow-up suggests this mechanism is primarily through radiation injury to the central structures of the heart and oesophagus.18 Advanced imaging and VMAT planning allow these structures to be precisely defined and using strict normal tissue constraints moderate dose escalation to the primary tumour alone may enable safe dose escalation without treatment prolongation.
The paradigm for the management of stage III lung cancer has changed significantly with the introduction of consolidation immunotherapy. Patients who receive consolidation durvalumab in the PACIFIC trial demonstrated an improvement in 3-year overall survival to 57.0% compared with 43.5% in the group randomised to placebo.28 Although this treatment has improved survival in this patient cohort, additional toxicities involved with immunotherapy have been documented with pneumonitis occurring more frequently in patients treated with immunotherapy.31 Prior radiation to the lungs is a known risk factor for this toxicity.32 Advanced functional imaging with V/Q PET/CT and planning to avoid highly functional regions of lung may minimise this risk of toxicity.
Currently, little is understood about why certain patients experience radiation induced toxicity. The pretreatment, mid-treatment and post-treatment imaging and blood biomarkers being investigated in this trial in an exploratory manner may provide useful hypotheses to enable further research into better understanding treatment related toxicity. If feasible, this technique will allow a prospective randomised trial to assess the clinical benefits of functional lung avoidance radiation therapy.
bmjopen-2020-042465supp001.pdf (3.4MB, pdf)
Supplementary Material
Acknowledgments
The authors would like to acknowledge the ongoing efforts of our study coordinator Lisa Selbie and the trials team lead by Dr Suzie Roache. We also acknowledge the interdepartmental collaboration between the departments of radiology, nuclear medicine, respiratory medicine and cardiology to facilitate performing multiple complex study measures in a single patient visit. We also acknowledge the ongoing research collaboration with Western Health and the Sunshine campus lead by Rebecca Height and the trials team in particular Angela Baugh and Heike Ranow.
Footnotes
Twitter: @NickWBucknell
Contributors: NWB, SS, MH, NH, JC, DB, TK, OM, BB, RL, MB, AI, BW, PE and DS contributed to the initial conception and design of the study. SS, GH, MH, JC and NWB were instrumental in securing funding and departmental support for the project. NWB led the development of the initial study protocol, which SS, NH, DB, TK, OM, BB, RL, MB, AI, BW, JC, PJ, MM, KB and DS reviewed and provided feedback on. NWB drafted the protocol manuscript and all other authors gave critical feedback. All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Funding: This work was supported by RANZCR grant number 2017/014, the Peter MacCallum Cancer Centre Foundation grant number 1709. NWB is the recipient of an Graduate Research Scholarship funded by the Australian Government. A Cancer Australia Priority-drive Collaborative CancerResearch Scheme Grant 2013, APP1060919, supported the development of V/Q PET/CT. This research was supported by the Cancer Trials Management Scheme Competitive Grants Programme, administered by Cancer Council Victoria.
Competing interests: None declared.
Patient and public involvement statement: Trial participant convenience, in particular, the number and timing of investigations were developed based on experiences and patient reports from our previous clinical trial.(9) Validated quality of life tools are performed before and after completion of treatment to enable assessment of meaningful measures of a patient’s treatment experience.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444–50. 10.1016/j.ijrobp.2012.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley JD, Hu C, Komaki RU, et al. Long-term results of RTOG 0617: a randomized phase 3 comparison of standard dose versus high dose conformal chemoradiation therapy +/- cetuximab for stage III NSCLC. Int J Radiat Oncol Biol Phys 2017;99:S105 10.1016/j.ijrobp.2017.06.250 [DOI] [Google Scholar]
- 3.Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 2013;31:4343–8. 10.1200/JCO.2013.51.5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99. 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non–small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. JCO 2017;35:1387–94. 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofman MS, Beauregard J-M, Barber TW, et al. 68Ga PET/CT ventilation-perfusion imaging for pulmonary embolism: a pilot study with comparison to conventional scintigraphy. J Nucl Med 2011;52:1513–9. 10.2967/jnumed.111.093344 [DOI] [PubMed] [Google Scholar]
- 7.Callahan J, Hofman MS, Siva S, et al. High-resolution imaging of pulmonary ventilation and perfusion with 68Ga-VQ respiratory gated (4-d) PET/CT. Eur J Nucl Med Mol Imaging 2014;41:343–9. 10.1007/s00259-013-2607-4 [DOI] [PubMed] [Google Scholar]
- 8.Le Roux P-Y, Hicks RJ, Siva S, et al. PET/CT lung ventilation and perfusion scanning using Galligas and Gallium-68-MAA. Semin Nucl Med 2019;49:71–81. 10.1053/j.semnuclmed.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 9.Siva S, Callahan J, Kron T, et al. A prospective observational study of Gallium-68 ventilation and perfusion PET/CT during and after radiotherapy in patients with non-small cell lung cancer. BMC Cancer 2014;14:740. 10.1186/1471-2407-14-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siva S, Hardcastle N, Kron T, et al. Ventilation/Perfusion Positron Emission Tomography--Based Assessment of Radiation Injury to Lung. Int J Radiat Oncol Biol Phys 2015;93:408–17. 10.1016/j.ijrobp.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Le Roux P-Y, Siva S, Steinfort DP, et al. Correlation of 68Ga ventilation-perfusion PET/CT with pulmonary function test indices for assessing lung function. J Nucl Med 2015;56:1718–23. 10.2967/jnumed.115.162586 [DOI] [PubMed] [Google Scholar]
- 12.Siva S, Devereux T, Ball DL, et al. Ga-68 MAA perfusion 4d-PET/CT scanning allows for functional lung avoidance using conformal radiation therapy planning. Technol Cancer Res Treat 2016;15:114–21. 10.1177/1533034614565534 [DOI] [PubMed] [Google Scholar]
- 13.Siva S, Thomas R, Callahan J, et al. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiother Oncol 2015;115:157–62. 10.1016/j.radonc.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 14.Bucknell NW, Hardcastle N, Bressel M, et al. Functional lung imaging in radiation therapy for lung cancer: a systematic review and meta-analysis. Radiother Oncol 2018;129:196–208. 10.1016/j.radonc.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Li T, Liu Y, et al. Planning analysis for locally advanced lung cancer: dosimetric and efficiency comparisons between intensity-modulated radiotherapy (IMRT), single-arc/partial-arc volumetric modulated Arc therapy (SA/PA-VMAT). Radiat Oncol 2011;6:140. 10.1186/1748-717X-6-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapet O, Khodri M, Jalade P, et al. Potential benefits of using non coplanar field and intensity modulated radiation therapy to preserve the heart in irradiation of lung tumors in the middle and lower lobes. Radiother Oncol 2006;80:333–40. 10.1016/j.radonc.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 2008;26:2457–63. 10.1200/JCO.2007.14.7371 [DOI] [PubMed] [Google Scholar]
- 18.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol 2020;38:706–14. 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg S, Gielda BT, Kiel K, et al. Patterns of locoregional failure in stage III non-small cell lung cancer treated with definitive chemoradiation therapy. Pract Radiat Oncol 2014;4:342–8. 10.1016/j.prro.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Men Y, Feng L, et al. A current review of dose-escalated radiotherapy in locally advanced non-small cell lung cancer. Radiol Oncol 2019;53:6–14. 10.2478/raon-2019-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong F-MS, Zhao J, Wang J, et al. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis 2014;6:336–47. 10.3978/j.issn.2072-1439.2014.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wambersie A, Landberg T. ICRU report 62: prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50. Bethesda, MD: International Commission on Radiation Units and Measurements, 1999. [Google Scholar]
- 23.Kong F-MS, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442–57. 10.1016/j.ijrobp.2010.07.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Roux P-Y, Siva S, Callahan J, et al. Automatic delineation of functional lung volumes with 68Ga-ventilation/perfusion PET/CT. EJNMMI Res 2017;7:82. 10.1186/s13550-017-0332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kipritidis J, Siva S, Hofman MS, et al. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med Phys 2014;41:011910. 10.1118/1.4856055 [DOI] [PubMed] [Google Scholar]
- 26.Cancer Institute NSW eviQ cancer treatments online protocol: non small cell lung cancer locally advanced definitive carboplatin and paclitaxel chemoradiation, 2018. Available: www.eviq.org.au/medical-oncology/respiratory/non-small-cell-lung-cancer/1436-nsclc-locally-advanced-definitive-carboplatin
- 27.Cancer Institute NSW eviQ cancer treatments online protocol: non small cell lung cancer locally advanced definitive cisplatin and etoposide chemoradiation, 2020. Available: www.eviq.org.au/medical-oncology/respiratory/non-small-cell-lung-cancer/379-nsclc-locally-advanced-definitive-cisplatin-an
- 28.Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC-Update from Pacific. J Thorac Oncol 2020;15:288–93. 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Ma J, Zhou S, et al. Radiation-Induced reductions in regional lung perfusion: 0.1-12 year data from a prospective clinical study. Int J Radiat Oncol Biol Phys 2010;76:425–32. 10.1016/j.ijrobp.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo J, Nishino M, Patel SP, et al. Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 2020;21:e435–44. 10.1016/j.cllc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 32.Manapov F, Roengvoraphoj O, Dantes M, et al. Pneumonitis in irradiated lungs after nivolumab: a brief communication and review of the literature. J Immunother 2018;41:96–9. 10.1097/CJI.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 33.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent Dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017;12:293–301. 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 34.RTOG 1106/ACRIN 6697, randomized phase II trial of individualized adaptive radiotherapy using during treatment FDG-PET/CT and modern technology in locally advanced non-small lung cancer (NSCLC), 2016RTOG; Available: https://www.nrgoncology.org/Clinical-Trials/Protocol/rtog-1106?filter=rtog-1106 [Accessed 04/2020]. [Google Scholar]
- 35.Vinod S, Choong C, Vial P, et al. Lung organ-at-risk volumes: a survey of practice and the need for a consistent definition in the 4DCT era. J Med Imaging Radiat Oncol 2020;64:120–6. 10.1111/1754-9485.12972 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042465supp001.pdf (3.4MB, pdf)


