Highlights.
This ESMO Clinical Practice Guideline provides key recommendations on the management of breathlessness in patients with cancer.
Authorship includes a multidisciplinary group of experts from different institutions and countries in Europe and worldwide.
Key treatment recommendations are provided, including levels of evidence and grades of recommendation where applicable.
Routine assessment of breathlessness and its impact facilitates timely interventions.
Key non-pharmacological measures include fan, breathing retraining, mobility aids, education and pulmonary rehabilitation.
The main pharmacological option is opioids. Supplemental oxygen, non-invasive ventilation and high flow may be considered.
Introduction
Breathlessness, dyspnoea and shortness of breath are synonymous terms to describe the ‘subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity’.1 2 Chronic breathlessness syndrome was recently defined by an international consensus as breathlessness ‘that persists despite optimum treatment for the underlying pathophysiology and causes disability’.3 4 The pathophysiology and characterisation of breathlessness are described in further details in the supplemental material available at ESMO Open online. Breathlessness is often cited as the most distressing symptom experienced by patients with cancer.5 A 2019 systematic review highlighted six major areas of concerns for patients living with breathlessness refractory to disease-modifying treatments, including physical, emotional, spiritual, social, control and context, unifying these under the concept of total breathlessness.6 In addition to the significant functional limitation, breathlessness can have devastating effects on patients’ quality of life (QoL) and is associated with a poor prognosis.7
esmoopen-2020-001038supp001.pdf (146.1KB, pdf)
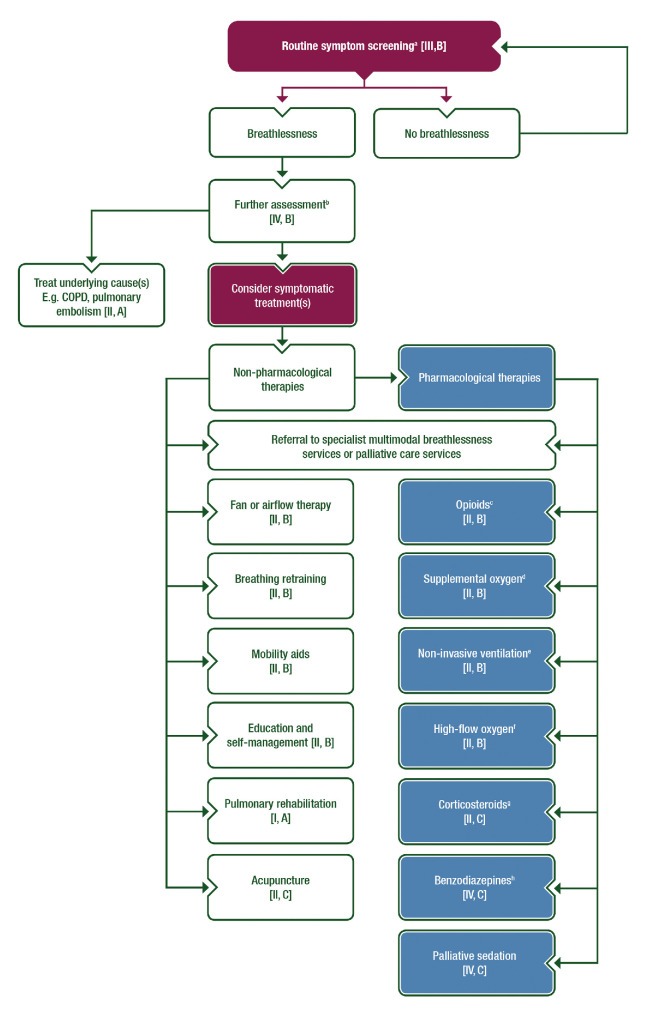
This ESMO Clinical Practice Guideline provides an up-to-date, evidence-based approach on the assessment and management of breathlessness in patients with cancer (figure 1). Proper assessment of breathlessness involves screening and in-depth evaluation to characterise the symptom and direct management. Treatments include interventions aimed at reversing any underlying causes, non-pharmacological and pharmacological therapies to palliate breathlessness, and multimodal approaches. Although the focus of this guideline is on patients with cancer, the evidence base for breathlessness in the oncology setting is relatively limited; thus, we have included studies conducted in other patient populations, such as chronic obstructive pulmonary disease (COPD), if the findings are applicable to the oncology setting.
Figure 1.
Management of breathlessness in cancer patients in outpatient or inpatient settings aBreathlessness intensity and functional impact. bAssessment for causes, severity, episodic nature, emotional and functional impact and caregiver support. cIf favourable benefit–risk ratio. dIf SpO2 <90%, however, palliative oxygen is not recommended in patients with resting SpO2 ≥90% [II, D]. eIf hypercapnic respiratory failure. fIf hypoxaemic respiratory failure. gIf other therapies have failed. hEspecially for anxiety and after trial of other agents.COPD, chronic pulmonary obstructive disease; SpO2, peripheral oxygen saturation.
Prevalence
Breathlessness is highly prevalent, affecting between 20% and 70% of patients with cancer,8 particularly among patients with thoracic malignancies, with advanced stages of disease, and in the last weeks of life.9–11
Assessment
Breathlessness is a subjective experience and thus the gold standard for breathlessness assessment is based on patient self-report.2 Breathlessness is easily overlooked when relying only on observation of the patient at rest (such as in clinic or on the ward) where they can appear comfortable. Breathlessness is intimately related to physical and emotional exertion and patients usually avoid activity causing a vicious cycle of deconditioning. The response to ‘are you short of breath’ is often therefore ‘no’, rendering the breathlessness ‘invisible’.12 Enquiry should include the activities that the patient has reduced or given up because of breathlessness.
Commonly used objective physiological measures only have a weak association with subjective sensations of breathlessness.13 However, in patients unable to self-report breathlessness, vital signs are useful to indicate breathlessness distress as well as underlying pathology.14 In general, lung function tests correlate poorly with the symptom of breathlessness and relatively normal values do not obviate enquiry about breathlessness.
The multi-faceted experience of breathlessness represents another challenge. There is no one tool which captures the full experience of breathlessness. Screening for breathlessness should include an assessment of intensity with unidimensional tools (eg, Numerical Rating Scale (NRS)), and functional impact (eg, modified Medical Research Council Breathlessness Scale). Patients with a positive screen would require further assessment for potential causes, pattern and severity of breathlessness (perception), distress due to breathlessness (emotional response), functional impairment, and impact on social, family, financial and spiritual domains. Patients with episodic breathlessness should be asked about the average frequency and duration of episodes, the intensity of these episodes and potential triggers.
Over 40 patient-reported outcomes for breathlessness were identified in the 2007–2008 reviews.15–18 Many of these questionnaires have multiple questions and are designed more for research. A rapid review (Medline database; search terms relating to breathlessness/dyspnoea and neoplasms/cancer/malignancy and measurement/patient-reported outcome measures; date limits 2009–2019; single reviewer) failed to show a more recent systematic review of breathlessness measurement tools usable in cancer. The recommendations therefore are unchanged: breathlessness assessment should include a unidimensional measure of severity, a measure of multidimensional functional impact and patient interview. Details of assessments can be found in supplemental material available at ESMO Open online.
Recommendations
Patient-reported outcomes are the gold standard for assessment of breathlessness. Physiological assessments may complement but not replace patient reports (III, B*).
All patients should be screened for breathlessness routinely at all inpatient and outpatient clinical encounters (III, B*).
Routine screening for breathlessness should include a unidimensional scale of choice and activities that patients have stopped or reduced because of breathlessness (III, B*).
Patients identified as having chronic breathlessness should have a fuller assessment which includes potential causes, pattern and severity of breathlessness, distress due to breathlessness and functional impairment (IV, B*).
For patients with episodic breathlessness, clinicians should ask about the intensity, frequency, duration and impact of these episodes along with potential triggers (IV, B*).
Management
Treat the underlying cause
Breathlessness may be related to cancer progression, cancer therapies and/or acute complications such as infections or pulmonary embolism. Pre-existing comorbidities such as COPD and heart failure may also contribute to breathlessness. One of the key strategies in the management of breathlessness is to identify and treat any potential underlying cause(s).19 However, breathlessness is often not the primary outcome in randomised clinical trials (RCTs) evaluating interventions to treat any underlying causes, and validated patient-reported outcomes are rarely used to assess breathlessness as a secondary outcome. In clinical trials, the Common Toxicity Criteria are often used to assess breathlessness instead of patient-reported outcomes. A detailed review is beyond the scope of this guideline. Table 1 includes management strategies for selected conditions, with further explanations provided in supplemental material section 'Treatment of underlying causes' available at ESMO Open online.
Table 1.
Management strategies of selected conditions contributing to breathlessness
| Condition | Management strategies |
| Anaemia (symptomatic) | Consider transfusion if haemoglobin <70–80 g/L to keep haemoglobin above 70–80 g/L |
| Asthma/COPD exacerbation | Medical optimisation |
| Cachexia | Consider referral to palliative care, dietician and/or physical therapy |
| Central airway obstruction | For proximal lesions, consider endobronchial interventions, such as bronchoscopy with mechanical debridement, tumour ablation and airway stent placement For distal lesions, consider radiotherapy |
| Cytotoxic chemotherapy-induced pulmonary toxicities | Withhold treatment and consider corticosteroids |
| Immunotherapy-induced pulmonary toxicities | Withhold treatment and consider corticosteroids |
| Heart failure exacerbation | Medical optimisation |
| Lymphangitic carcinomatosis | Treatment of underlying malignancy. Consider corticosteroids (anecdotal) |
| Malignant ascites | Paracentesis with or without indwelling catheter |
| Malignant pleural effusions | For patients with a short-life expectancy (<3 months), consider simple thoracentesis For patients with longer life expectancy, consider tunnelled pleural catheter or chemical pleurodesis; both are reasonable options |
| Malignant pericardial effusion/tamponade | Pericardiocentesis, pericardiectomy with or without pericardial window |
| Metabolic acidosis | Identify and treat the underlying cause |
| Pneumonia | Anti-infective agents |
| Pulmonary embolism | Anticoagulation |
| Radiation-induced pneumonitis or fibrosis | Consider corticosteroids |
| Superior vena cava syndrome | Treatment of underlying malignancy. Consider corticosteroids (anecdotal) |
| Tumour embolism | Treatment of underlying malignancy |
COPD, chronic obstructive pulmonary disease.
Non-pharmacological symptomatic interventions
Several non‐pharmacological interventions offer first-line treatment options for the management of breathlessness and complement use of pharmacological interventions in advanced disease. Although studies of sole interventions are lacking in cancer, direct evidence exists in chronic respiratory disease, and the interventions below are core components in most studies of multimodal services (see the 'Multimodal interventions section').20
Fan or airflow
Using a hand-held fan to increase airflow towards the face represents a simple intervention that patients can use to self-manage their breathlessness. Although there is a paucity of studies on the use of a fan in patients with hypoxaemia and it is unclear if a fan would provide additional benefit in patients already on supplemental oxygen (ie, delivering airflow), the guideline panel supports the use of a fan irrespective of the patient’s oxygen saturation given its potential benefit and lack of harm. The fan is a cheap, easily-obtained, light, portable and non-stigmatising piece of equipment.21 Plausible mechanisms of action include cooling nasal receptors and moderating afferent signals to the respiratory centre, and increasing self-efficacy, particularly around unexpected episodes of breathlessness that patients can find difficult to manage.22
A recent review summarised 10 studies of fan therapy (median duration 5 min) in 344 patients (159 with cancer). Six studies reported a statistically significant improvement in breathlessness whereas four did not. Some non-significant findings may relate to underpowered study designs; however, no meta-analysis was undertaken to increase statistical power. The authors concluded limited experimental evidence of efficacy, calling for larger trials in more diverse populations and settings.23
A secondary multimethod analysis of qualitative interview data explored benefits of a hand-held fan as perceived by patients with breathlessness (n=133, n=21 with cancer) and their carers (n=72). Most patients with usable data (91/111) perceived some or substantial benefit, described in terms of shorter recovery time, especially after physical activity. Negative perceptions of a few patients included dislike of the cooling sensation and embarrassment in public.21 An exploratory review of uncontrolled pre-to-post measures in 16 studies (n=929) where airflow was used either as an intervention (eg, fan) or comparator (eg, as a sham in an oxygen trial), found hand-held fan at rest, airflow via nasal cannula at rest and airflow during exercise all led to reductions in breathlessness intensity.24
Breathing retraining
Altered breathing patterns, including increased respiratory rate, apical breathing, excessive accessory muscle recruitment and/or dynamic hyperinflation, can reduce efficiency of ventilation, increase work of breathing and cause or exacerbate breathlessness.25 Breathing retraining techniques aim to improve a patient’s control over their breathing to counter these changes. Common techniques include pursed-lip breathing, to produce pressure to support the airways and improve expiratory flow; diaphragmatic breathing, to reduce accessory muscle use, and breathing control or timed breathing, which aims to normalise respiratory rate. Individual breathing patterns and pathophysiology should be considered in their selection.26
A review of 16 trials in COPD (n=1233) found breathing retraining over 4–15 weeks improved functional exercise capacity, though effects on breathlessness and health-related QoL were inconsistent.25 Widespread use as a stand-alone intervention was not recommended. A large trial (n=655) in patients with incompletely controlled asthma of a self-guided breathing retraining intervention, delivered either face-to-face or as a digital audio–visual programme plus printed booklet, improved QoL over 12 months.27 A single acute trial in patients (n=63) with lung cancer (n=32), COPD and asthma, who were breathless at rest, found 20 min mindful breathing control reduced breathlessness within 5 min. A follow-on effect was not studied.28
Mobility aids
Mobility aids help to improve both breathlessness and mobility through an increased ventilatory capacity and/or reduced metabolic cost.29 30 Despite the absence of studies in patients with cancer, several randomised crossover studies in breathless patients with COPD demonstrate that use of a rollator improves self-paced walking distance in both indoor31 32 and outdoor environments,33 especially in patients severely limited by breathlessness including those using ambulatory oxygen.34 Using a modern ‘draisine’ (bicycle without pedals) improved indoor walking distance further still, with the same metabolic requirements and breathlessness scores,35 but in outdoor environments with obstacles it had a detrimental effect on mobility compared with unaided walking.33 All the above studies have been conducted over a short time period and prolonged satisfaction and benefit should not be assumed. If mobility aids are prescribed, a subsequent review is recommended.
Education and self-management
For patients receiving curative treatment, education is often framed around enhancing recovery and returning to a healthy lifestyle. In advanced cancer, where deterioration is expected, it can be framed around making the most of the present and staying independent for as long as possible. Patients should be assured that being breathless is itself not dangerous, and that breathlessness is a normal exertional response that settles with rest. Even following incremental exercise to a symptom-limited maximum, breathlessness in people with lung cancer typically recovers within a few minutes.36 Advice on positions to aid recovery following exertion, or during an episode of breathlessness, can be useful.37 A ‘forward-lean’ position may reduce accessory muscle work, improving diaphragm function and ventilatory capacity.38 Relaxed sitting (with hands or elbows rested on thighs) or standing (using a wall as support) and high side lying (supporting head and chest) are frequently recommended within breathlessness services.20
Activity pacing (balancing rest and activity) education teaches patients to moderate activity behaviour and to avoid extremes of rest or activity for optimising the use of available energy. A change in routine is often required to avoid ‘boom or bust’ situations where patients push themselves too much and then need a prolonged period of recovery. Study of activity pacing as a standalone intervention for breathlessness is lacking, but evidence from its use in cancer-related fatigue39 and/or pain40 is generally applicable. Distraction using music or visualisation has been tested in small studies. An uncontrolled study (n=53) found guided imagery with theta music reduced breathlessness in patients with advanced cancer,41 and a retrospective analysis reported positive responses to music therapy sessions in a palliative care setting.42 In COPD, auditory distraction with music improved the effect of exercise training on functional exercise performance, but was inconsistent in its short-term effect on exercise testing and in reducing breathlessness at rest.43 Bredin et al found a nurse-led clinic combining these educational strategies with psychological support improved breathlessness, performance status and emotional state over 8 weeks in people with lung cancer.44
Pulmonary rehabilitation
A rehabilitation approach incorporating exercise should be considered for most patients with chronic breathlessness to reduce the impact of the symptom on physical function. Breathlessness tends to lead towards reduced physical activity, which in turn precipitates a downward spiral of deconditioning. Rehabilitation helps to counter this downward spiral by improving the physical condition of patients, by exposing them to being breathless while staying in control.
As the spiral of deconditioning involves physical inactivity, an approach that targets lower limb function and mobility is generally helpful. Breathlessness can also limit upper limb activities, for example, cooking and shopping, as the muscles around the shoulder have a dual role for breathing and stability. By improving physical capacity, the relative level of ventilatory demand for a fixed activity becomes lower, and perceived exertion is reduced. Following exercise training, the anxiety related to breathlessness is also reduced allowing patients to improve functional performance.45
Both pulmonary and cardiac rehabilitation have a strong evidence of effectiveness in improving breathlessness, functional exercise capacity and health-related QoL.46 Though most evidence for pulmonary rehabilitation relates to COPD, consistent effects are found across other respiratory diseases, including lung cancer.47 However, the efficacy of pulmonary rehabilitation in patients with advanced cancer is unclear, especially when these patients may be too weak to participate in exercise sessions. Referral and uptake to these programmes should be encouraged. The availability of cancer rehabilitation programmes is relatively low, but with accumulating evidence of the effect on symptom burden, fitness and QoL48 and exercise recommendations in national treatment guidelines,49 50 this should improve.
Programmes typically incorporate a combination of aerobic and resistance training, plus tailored education. In general, supervised training leads to a greater exercise workload and response47; however, home-based unsupervised training is also beneficial if completed regularly. Mind–body movement therapies (eg, yoga, tai chi and qigong) may be preferred by some patients, and when compared with conventional training modalities, outcomes related to breathlessness are similar.51 Low-intensity resistance training using novel approaches, for example, neuromuscular muscle stimulation52 or partitioned training, may be suited to patients where exertional breathlessness is severely limiting.53 The low ventilatory demand of these modalities allows the severely breathless patient to exercise to help counteract the deconditioning associated with physical inactivity.
Complementary therapies
A few, mostly small RCTs have examined the role of acupressure or acupuncture in patients with cancer and reported short-term benefit on breathlessness.54–56 One open-label trial involving 152 patients with lung cancer compared prolonged acupuncture (with indwelling studs) with morphine or a combination of both and found similar reduction on breathlessness but fewer side-effects in the acupuncture group.55 A recent systematic review of 12 clinical trials involving 190 patients with cancer and 347 patients with COPD found overall benefit from acupuncture on breathlessness severity.57 Additional therapies, including aromatherapy massage, hypnotherapy, meditation, reflexology and reiki, may provide limited short-term benefit.58 Further high-quality studies are needed to examine the risks and benefits of acupuncture and other complementary therapies in the long term, especially in patients with cancer.
Pharmacological symptomatic interventions
Recommendations on the pharmacological management were informed by searches in Medline using the terms (dyspnea/dyspnoea/breathlessness) and cancer and, in turn, the treatment of interest: (opioid/morphine/fentanyl), benzodiazepine, steroid, oxygen, high-flow oxygen, or (non-invasive/non invasive/NIV), antidepressant and cannabinoid. In addition, reference lists of major papers and systematic reviews were hand searched.
Opioids
Among the various pharmacological options for breathlessness, opioids have been studied the most. However, existing randomised trials were generally small and only a handful had specifically focused on patients with cancer. Although opioids appear to have a pharmacological effect on breathlessness, the overall evidence is low and there is much uncertainty how they should be used in clinical practice.59–62
Efficacy of systemic opioids on chronic breathlessness was shown in the latest Cochrane report from 2016 of 12 RCTs (198 participants of whom 41 (21%) had cancer).59 61 There is no clear evidence to support use of nebulised opioids.61 Compared with placebo, systemic opioids decreased breathlessness intensity by a pooled mean of 0.8 points on a 0–10 NRS,59 which represents between a small and a moderate effect size.63 64 Assuming a moderate effect size represents clinically meaningful improvement, this value is just below this level and suggests that only a proportion of patients derived an improvement. Several methodological issues may have impacted the study findings. For instance, the trials were mostly small, cross-over trials (11 out of 12) with short follow-up.59 61 When the cross-over design was taken into account in the statistical analysis, the precision around the findings improved.59
Opioid-naïve patients
A multicentre parallel group RCT (<20% with cancer) reported that neither 20 mg/day of sustained-release morphine nor 5 mg every 8 hours of extended-release (ER) oxycodone improved the primary outcome of intensity of 'breathless now', compared with placebo over 7 days.65 66 However, several methodological limitations complicate its interpretation: ‘rescue’ immediate-release morphine (MIR) was available for all patients with higher usage in the placebo groups; early termination of the oxycodone group; difficulties with enrolment; opioid dosing; and not all patients were opioid-naïve. While the best evidence for opioids is in people with COPD, a pooled data study showed no predictive association of underlying disease,61 although younger patients and patients with more severe breathlessness were more likely to gain benefit.67 Given methodological limitations in existing studies, further research is needed to examine the efficacy of opioids and optimal administration in opioid-naïve patients.
There are no head-to-head comparisons of immediate-release (IR) and ER formulations for breathlessness. The Therapeutic Goods Administration has extended the licence for one ER formulation of oral morphine (morphine sulfate pentahydrate) to include chronic breathlessness in Australia. A dose range of 10–30 mg per 24 hours is licensed, with an explicit statement that additional morphine sulfate IR doses should not be given for breathlessness but may be needed for concurrent pain.68
Opioid-tolerant patients
There are few RCTs to inform use of opioids for treatment of breathlessness in patients with cancer taking regular opioids for pain. A cross-over, placebo-controlled trial found that a single dose of subcutaneous morphine given at 50% higher than the scheduled 4-hourly dose resulted in a significant reduction of breathlessness consistently over the first hour compared with placebo among patients with cancer.69 In a second dose-comparison study, patients with persistent breathlessness were given a dose of MIR at 25% or 50% more than their usual 4-hourly dose.70 There was no difference in the improvement of breathlessness between the two groups. Preliminary data from a small randomised trial comparing fentanyl buccal tablet with morphine sulfate for treatment of episodic breathlessness suggested that fentanyl had a faster onset and provided greater relief.71 Several small placebo-controlled trials have examined the use of prophylactic fentanyl given prior to exertion in opioid-tolerant patients with cancer and reported a benefit (discussed below).72–74 Taken together, the available evidence signals that opioids have a positive effect on breathlessness among opioid-tolerant individuals, giving justification for further studies to examine these findings in confirmatory trials and to determine the optimal dose and timing of opioids for breathlessness.
Prophylactic use of opioids prior to exertion among opioid-tolerant patients
Because breathlessness predictably increases with exertion, the use of IR opioids prior to activity (or indeed, the use of sustained-release opioid) may potentially reduce this distressing symptom and thereby maximise activity level.75 Rapid-onset fentanyl formulations may be appealing for this purpose given their fast onset of action and ease of administration. To date, only a handful of small RCTs have examined the use of opioids in patients with cancer for prophylaxis. Small double-blind, placebo-controlled studies of subcutaneous fentanyl,72 fentanyl pectin nasal spray73 and fentanyl buccal tablet74 showed within-arm improved breathlessness and/or exercise capacity; however, these studies were not powered for between-arm comparisons. Recently, a dose-finding study suggested the use of higher proportional dose of fentanyl sublingual spray (35%–45% of morphine equivalent daily dose (MEDD)) conferred a clinical benefit in contrast to a lower dose (15%–25% of MEDD) in the prophylactic setting.76 Outside of the cancer setting, two randomised trials also suggested that nebulised fentanyl may improve dyspnoea during exercise.77 78 Importantly, the long-term use of rapid-onset fentanyl has not been examined systematically and the risk of addiction with these agents needs to be further investigated. Limited use of prophylactic opioids should only be considered among patients with severe functional impairment and/or distress related to specific activities causing breathlessness and when the benefits likely outweigh potential risks. Larger pragmatic studies are needed to examine how opioids could be used on a prophylactic basis to improve episodic breathlessness.
Choice of opioid
The panel would like to emphasise that there are significant limitations among existing clinical trials on opioids, particularly for people with cancer, which contribute to variable interpretation of these studies. When formulating treatment recommendations, the panel carefully weighed the risks and benefits of opioid therapy based on all available literature, while recognising significant variations in practices across regions. In a patient with severe, debilitating chronic breathlessness at rest or provoked by minimal exertion despite non-pharmacological management, treatment with ER morphine provides the smoothest steady-state where the effect size appears to be greatest, at least in COPD studies. A starting dose in an opioid-naïve patient could be between 5 and 15 mg of ER morphine two times a day,79 although the doses may need to be lower for selected populations (eg, renal failure). Titration should be tailored to the individual starting with the lowest possible dose. A placebo-controlled dose titration RCT in COPD is expected in 2021.80 Similar to pain,81 a rescue dose or IR morphine of about 1/6 of the patient’s total daily morphine dose may be considered, if needed. Most research evidence pertains to doses equivalent to 10–30 mg oral morphine per day,59 61 82 83 and it is unknown if higher doses give further benefit; data are awaited.
Most evidence, including the adequately powered trials, relates to morphine and to people with non-malignant disease. There is emerging evidence for fentanyl and adequately powered trials would be useful to inform use in people with poor renal function, or where different pharmacokinetic profiles may be helpful. Theoretically, opioids given at equianalgesic doses are expected to provide similar levels of benefits on breathlessness; however, some patients may respond better to selected opioids. More research is needed to investigate this. All patients starting opioids should be offered prophylaxis for constipation with laxatives and, as needed, antiemetics. For further discussion of opioid-related adverse effects and principles of safe opioid use see supplemental material available at ESMO Open online.
Benzodiazepines
Benzodiazepines are frequently used for the relief of breathlessness in clinical practice, especially when breathlessness is associated with symptoms of anxiety or panic. However, there is currently insufficient evidence from clinical trials to support the use of benzodiazepines for the relief of breathlessness, either in malignant or non-malignant disease.84 The Cochrane systematic review of Simon et al included eight small RCTs, most of them with a unclear risk of bias, that tested the effect of benzodiazepines for the relief of breathlessness in adult patients with advanced disease, out of which 66 had COPD and 148 patients had cancer.84 Only one trial showed a significant reduction of breathlessness intensity in patients receiving midazolam in comparison with the morphine group, which is conflicting with the result of a second study by the same research team.85 86 The other seven studies included in the review did not demonstrate any significant difference for benzodiazepines compared with placebo or morphine. Two additional RCTs have been identified both without any significant effect of benzodiazepines for the relief of breathlessness intensity.87 88
The most common side-effects of benzodiazepines were drowsiness and somnolence, which were significantly more frequent compared with placebo but less frequent compared with morphine.84 Patients with cancer having breathlessness are at high risk of delirium, and the use of benzodiazepines may compound this concern.
It has been hypothesised that benzodiazepines may improve the coping capacity or reduce the unpleasantness related to breathlessness by a better coping of anxiety. However, the studies cited above measured intensity of breathlessness alone. There is a need for well-conducted and adequately powered studies measuring predictors and symptoms associated with breathlessness that may be modulated by benzodiazepines.
In the last days of life, some patients may continue to experience severe breathlessness despite maximising all other palliative measures within the limited time frame. In these unique situations, palliative sedation may be considered to alleviate suffering. Benzodiazepines such as midazolam infusion and/or lorazepam may be titrated carefully to reduce consciousness as little as possible while maximising comfort.89 90 This should only be considered a treatment of last resort and only after careful discussion with patients/families. When given to patients with only days of life expectancy, palliative sedation has not been associated with shorter survival.91 92
Corticosteroids
The efficacy of systematic steroids for the relief of cancer-related breathlessness was investigated in a systematic review of two RCTs.93 The first RCT, a placebo-controlled, double-blind pilot trial, tested the effect of dexamethasone among 41 patients with cancer on the reduction of breathlessness intensity 'now' as the primary outcome (NRS 0–10, subscale of the Edmonton Symptom Assessment System (ESAS), Modified Dyspnoea Borg Scale).94 Between-group differences were not statistically significant, although there was a significant within-arm reduction of breathlessness intensity at day 4 in the dexamethasone group of 1.9 on ESAS (95% CI 3.3 to 0.5; p=0.01), whereas the placebo group showed a non-significant reduction of 0.7 (95% CI 2.1 to 0.6; p=0.38). The authors concluded that dexamethasone may provide rapid relief from breathlessness. A larger confirmatory randomised trial is currently under way to examine the effect of dexamethasone on breathlessness in patients with cancer (clinicaltrials.gov NCT03367156).
Several randomised trials have examined breathlessness as a secondary outcome and reported some improvement.95 96
There are no published data regarding inhaled corticosteroids in patients with cancer, and although commonly used in people with asthma and COPD, the very different pathophysiological characteristics of the underlying disease would make application of indirect evidence questionable. From clinical experience and biological rationale, the use of steroids for breathlessness due to lymphangitis carcinomatosis or tumour-induced respiratory obstruction may have positive effects, although evidence is insufficient.97
Supplemental oxygen
The benefit of supplemental oxygen for relief of breathlessness (palliative oxygen therapy) and for improving physical capacity and activity in daily life has not been established.98–100 While supplemental oxygen is indicated in patients with chronic severe hypoxaemia (partial pressure of oxygen (PaO2) <7.4 kPa or peripheral oxygen saturation (SpO2) <89% at rest) to prolong life,101 oxygen has not been consistently shown to relieve breathlessness in the palliative setting in patients with mild or no hypoxaemia in advanced disease98 99 including cancer.100 Recent guidelines from the British Thoracic Society and the National Guideline for Palliative Care in patients with non-curative cancer in Germany state that palliative oxygen therapy is not indicated in patients with SpO2 ≥92%, and that other treatments should be considered first, such as treatment of underlying causes, exercise-based rehabilitation, a hand-held fan and opioids.101 In the largest double-blind RCT to date by Abernethy et al of patients with advanced disease (n=239; 16% with cancer) with no or mild hypoxaemia (PaO2 >7.3 kPa, corresponding to a SpO2 >88%), there was no difference in breathlessness between supplemental oxygen and room air over 7 days.98
However, in patients with chronic breathlessness despite other evidence-based treatments, a trial of supplemental oxygen could be justified,101 based on indirect data that supplemental oxygen can improve exercise capacity and breathlessness observed during exercise testing in the laboratory for patients with COPD and interstitial lung disease.99 102 Individualised information and shared decision-making with patients and caregivers are important.101 103 If tried, supplemental oxygen should be re-evaluated within a few days and discontinued if the patient perceives no benefit.60 98 Based on RCT data98 and clinical experience, a cut-off for defining clinically relevant hypoxaemia as SpO2 <90% is recommended.
High-flow oxygen therapy can deliver up to 60 L/min of humidified and heated oxygen via nasal canulae. To date, only one cross-over RCT study has specifically evaluated its effect on breathlessness in hospitalised patients with cancer in the palliative care setting.104 This randomised trial found that high-flow oxygen and bilevel positive airway pressure (BiPAP) were both similarly effective in reducing breathlessness. More research is needed to examine its impact on breathlessness, particularly in the home setting.
Non-invasive ventilation
Non-invasive ventilation (NIV) may improve both oxygenation and hypoventilation, while supporting chest wall muscles. In a randomised trial of 200 patients with advanced solid tumours, NIV decreased breathlessness compared with supplemental oxygen, especially in patients with hypercapnia. NIV was associated with lower use of rescue opioid and had an acceptable tolerance and safety profile.105 Another RCT reported that BiPAP reduced breathlessness by 3.2 (95% CI 1.3 to 5.1) points on a 0–10 NRS.104 However, some patients could not tolerate the positive pressure and there are multiple contraindications to NIV.
Antidepressants
Depression and anxiety are associated with increased breathlessness.106 107 Five RCTs (n=336) and three case studies (n=19) investigated antidepressants (nortriptyline, paroxetine, citalopram, sertraline, protriptyline) to relieve breathlessness mostly in patients with COPD.108–114 The largest randomised trial (n=223) tested sertraline against placebo in patients with chronic breathlessness, of whom around 20% had cancer.109 None of the RCTs reported any significant improvement in breathlessness with antidepressants. The three case studies reported breathlessness relief by citalopram, sertraline or mirtazapine.112 113 115 A preliminary trial of mirtazapine shows promise, although further investigation is needed.116
Cannabinoids
Few studies of cannabinoids for the relief of breathlessness have been published, evaluating small samples of healthy participants or patients with COPD, with no effect observed.117 118 A broader clinical experience and adequately powered clinical trials are missing.
Multimodal interventions
Recognition that breathlessness is a multidimensional construct leads to development of multimodal interventions that include both non-pharmacological and pharmacological treatments. Specialist palliative care teams, because of their interdisciplinary nature and symptom management expertise, may be particularly suited to manage breathlessness.119 120
A systematic review of holistic breathlessness services for patients with advanced cancer and non-cancer diagnoses identified 37 articles across 18 different services. Most comprised 4–6 contacts over 4–6 weeks. Commonly used interventions included the hand-held fan, breathing techniques, psychological support and relaxation techniques, although there was significant variation in the structure and processes for these services. Meta-analyses of randomised trials demonstrated reductions in NRS distress due to breathlessness (n=324; NRS mean difference (MD) −2.30, 95% CI −4.43 to −0.16, p=0.03) and Hospital Anxiety and Depression Scale (HADS) depression scores (n=408, MD −1.67, 95% CI −2.52 to −0.81, p<0.001) compared with usual care. Statistically non-significant effects were observed for Chronic Respiratory Questionnaire mastery (n=259, MD 0.23, 95% CI −0.10 to 0.55, p=0.17) and HADS anxiety scores (n=552, MD −1.59, 95% CI −3.22 to 0.05, p=0.06).20 An analysis of pooled individual datasets (n=259) found outcomes of reduced mastery and distress were influenced by baseline scores for these variables, but not by patient diagnosis, lung function or health status. Therefore stratifying patients by levels of mastery and/or distress due to breathlessness appears appropriate for research and clinical services.121 In a meta-synthesis of qualitative interviews (n=216), patients and carers accessing breathlessness services valued tailored education, self-management interventions and expert staff providing person-centred, dignified care.20 These components should be integrated into clinical practice.
Taken together, there is good evidence to support the use of multimodal breathlessness services. However, the availability of these services remains limited. In the absence of such dedicated services, there is good evidence to support that palliative care teams can improve patient and caregiver outcomes.122–125 Thus, a timely referral is recommended.
The impact of breathlessness on caregivers
There is now a substantial body of data demonstrating that carers of someone suffering from chronic breathlessness experience profound anxiety, isolation, exhaustion and poor sleep. This is heightened when they witness their loved ones having an episode of breathlessness126 127 and feel powerless to help them. It is also clear that they can become exhausted from the extra physical work and psychological support they need to give to their loved one. There are no comparable controlled data to guide clinical teams on the most effective interventions for carers’ stress—although recognition and acknowledgement seem to be useful first steps.119
The onset of breathlessness in cancer tends to be over a much shorter period than in other illnesses, giving less time for the patient and carer to adjust to a new reality. It also progresses more quickly (in spite of recent advances in treatment). There are some data to suggest that unscheduled use of clinical services may often be related to carer as well as patient anxiety about seemingly inexplicable breathlessness.
The psychological and physical effects of the stress of caring for a family member with a serious illness like cancer are becoming recognised.128 Given the significant impact on caregivers, patient and carer may benefit from a joint assessment to examine the impact of breathlessness on their lives. A structured needs-assessment tool such as the Carer Needs Assessment Tool may be helpful.
Psychoeducational interventions may help them manage episodes of breathlessness, exploring what happens at these times with development of a ‘ritual’ for crises. Where possible these interventions should be delivered as part of a complex interdisciplinary intervention. The best evidence for impact is when they are part of a specialist breathlessness service.119 120 The limited research to date suggests that these interventions may help reduce anxiety and distress in both patient and carer and mitigate the stress and health impact of long-term caring.129
Recommendations
Treat the underlying cause
Clinicians should identify and treat any potentially reversible condition(s) contributing to breathlessness (II, A*).
Non-pharmacological symptomatic interventions
Consider use of a hand-held fan directed to the face and inform patients of the potential mechanisms and benefit. This may be useful alone in people without hypoxaemia, or as an adjunct to those requiring oxygen supplementation for hypoxaemia (II, B).
Advise patients on relevant breathing retraining techniques and/or refer to specialist services such as a physiotherapist (II, B).
Consider a trial of a mobility aid to assess possible impact on breathing during ambulation and functional activities (II, B).
Educate and inform patients on strategies including activity pacing, relieving positions and distraction techniques to encourage self-management (II, B).
Refer patients to available exercise-based rehabilitation programmes, including pulmonary or cardiac rehabilitation for patients with comorbid chronic lung or heart disease (I, A).
Provide individualised advice on aerobic and resistance exercises, suitable to the patient’s functional status and degree of limiting breathlessness (II, B).
Consider a therapeutic trial of acupressure or acupuncture according to patient preference (II, C).
Pharmacological symptomatic interventions
Regular, oral, low-dose morphine is the first-line pharmacological treatment for severe chronic breathlessness, which persists despite non-pharmacological measures (II, B).
In opioid-naïve patients, a starting daily dose of scheduled morphine 10–30 mg over 24 hours can be used, with individual titration depending on the patient’s symptoms (II, B).
In opioid-tolerant patients, an increase in the baseline dose of opioid by 25%–50% may be considered (V, C).
In opioid-tolerant patients with severe exertional breathlessness associated with defined triggering situations leading to significant functional impairment and/or distress despite standard treatments, consider prophylactic use of opioids prior to the episodes. Patients should use prophylactic doses sparingly (e.g. ≤2×/day) and only with close monitoring given that the long-term safety risk is not known (II, C).
All patients starting opioids should be offered prophylaxis for constipation with laxatives and, as needed, antiemetics (I, A).
Patients on opioids for breathlessness should be educated on safe opioid use and monitored longitudinally with various risk mitigation strategies (III, A*).
Because of significant risk of sedation and delirium, benzodiazepines should not be used for breathlessness as first-line pharmacological therapy (III, D).
Benzodiazepines may be used with caution in patients with cancer for the relief of breathlessness with associated anxiety if opioids are not effective (V, C).
In the last days of life, benzodiazepines may be considered for palliative sedation in patients with refractory breathlessness despite other treatments (IV, C).
Corticosteroids may be considered for palliation of cancer-related breathlessness refractory to other treatments (II, C).
Palliative oxygen is not recommended in patients with resting SpO2 ≥90% (II, D).
High-flow oxygen therapy may be considered in selected patients for treatment of breathlessness, especially if they have hypoxaemic respiratory failure (II, B).
A therapeutic trial for NIV can be considered in patients with cancer with severe chronic breathlessness, especially in patients with acute hypercapnic respiratory failure (II, B).
Sertraline is not recommended for chronic breathlessness (II, D).
The use of other antidepressants for breathlessness should only be limited to the clinical trials context at this time (V, C).
The use of cannabinoids for chronic breathlessness is not recommended given the insufficient evidence and potential adverse event profile (IV, D).
Multimodal interventions
Patients with cancer with chronic breathlessness should be referred to specialist multimodal breathlessness services if available (I, A).
Timely referral to palliative care services should be considered in centres in which holistic breathlessness services are not available (II, B).
The impact of breathlessness on caregivers
The oncology team should routinely assess the psychological status, information needs, and support network for carer(s) of breathless individuals with cancer (III, B*).
A properly implemented caregiver needs-assessment tool may be helpful (II, B).
Consider making referrals to specialist breathlessness services for patients suffering from breathlessness and their carers (I, A).
Methodology
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development http://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology. The relevant literature has been selected by the expert authors. Levels of evidence and grades of recommendation have been applied using the system shown in online supplemental table S1), available at ESMO Open online.130 The * notation is assigned to the grade of recommendation for statements on topics for which clinical trials are not available because they are inherently difficult to design or not justified due to ethical reasons while these statements are considered justified by standard clinical practice by the experts and the ESMO faculty.
Statements without grading were considered justified standard clinical practice by the experts and the ESMO faculty.
Acknowledgments
The ESMO Guidelines Committee would like to thank the ESMO Faculty who provided critical reviews of these ESMO Clinical Practice Guidelines.
Footnotes
Presented at: †Approved by the ESMO Guidelines Committee: July 2015. Last update October 2020. This publication supersedes the previously published version – Ann Oncol 2015; 26 (suppl 5): v169–v173.
Funding: No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Competing interests: DH has reported research grants from National Cancer Institute (US), National Institute of Nursing Research (US), American Cancer Society, Helsinn, Insys, Teva and Depomed. MM has reported research grants from National Institute for Health Research and consultancy fees from Helsinn and Fresenius Kabi. MJ has received institutional payments for clinical consultancy to Mayne Pharma and honoraria from Novartis. ME has reported honoraria from AstraZeneca, Boehringer Ingelheim, Novartis and Roche. SS has reported research grants from Federal Ministry of Education and Research in Germany and from the European Commission. CR has received funding from InPharm, Kyowa Kirin, Amgen Europe and Molteni SpA. ACO and SB have declared no potential conflicts of interest.
Patient consent for publication: Not required.
Provenance and peer review: ESMO-commissioned faculty review completed; externally peer reviewed.
Data availability statement: All published ESMO Clinical Practice Guidelines can be found on https://www.esmo.org/guidelines.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Anon Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med 1999;159:321–40. 10.1164/ajrccm.159.1.ats898 [DOI] [PubMed] [Google Scholar]
- 2.Parshall MB, Schwartzstein RM, Adams L, et al. . An official American thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435–52. 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahler DA, Selecky PA, Harrod CG, et al. . American College of chest physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137:674–91. 10.1378/chest.09-1543 [DOI] [PubMed] [Google Scholar]
- 4.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. . Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J 2017;49:1602277 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 5.Tishelman C, Petersson L-M, Degner LF, et al. . Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. Journal of Clinical Oncology 2007;25:5381–9. 10.1200/JCO.2006.08.7874 [DOI] [PubMed] [Google Scholar]
- 6.Lovell N, Etkind SN, Bajwah S, et al. . Control and context are central for people with advanced illness experiencing breathlessness: a systematic review and thematic synthesis. J Pain Symptom Manage 2019;57:140–55. e142 10.1016/j.jpainsymman.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 7.Cuervo Pinna MA, Mota Vargas R, Redondo Moralo MJ, et al. . Dyspnea--a bad prognosis symptom at the end of life. Am J Hosp Palliat Care 2009;26:89–97. 10.1177/1049909108327588 [DOI] [PubMed] [Google Scholar]
- 8.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58–69. 10.1016/j.jpainsymman.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Neumann C, Brenneis C, et al. . Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care 2000;16:16–21. 10.1177/082585970001600304 [DOI] [PubMed] [Google Scholar]
- 10.Mercadante S, Aielli F, Adile C, et al. . Epidemiology and characteristics of episodic breathlessness in advanced cancer patients: an observational study. J Pain Symptom Manage 2016;51:17–24. 10.1016/j.jpainsymman.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 11.Dudgeon DJ, Kristjanson L, Sloan JA, et al. . Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage 2001;21:95–102. 10.1016/s0885-3924(00)00258-x [DOI] [PubMed] [Google Scholar]
- 12.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage 2008;36:451–60. 10.1016/j.jpainsymman.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Hui D, Morgado M, Vidal M, et al. . Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med 2013;16:274–80. 10.1089/jpm.2012.0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell ML, Templin T, Walch J. A respiratory distress observation scale for patients unable to self-report dyspnea. J Palliat Med 2010;13:285–90. 10.1089/jpm.2009.0229 [DOI] [PubMed] [Google Scholar]
- 15.Bausewein C, Booth S, Higginson IJ. Measurement of dyspnoea in the clinical rather than the research setting. Curr Opin Support Palliat Care 2008;2:95–9. 10.1097/SPC.0b013e3282ffafe8 [DOI] [PubMed] [Google Scholar]
- 16.Bausewein C, Farquhar M, Booth S, et al. . Measurement of breathlessness in advanced disease: a systematic review. Respir Med 2007;101:399–410. 10.1016/j.rmed.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med 2007;21:177–91. 10.1177/0269216307076398 [DOI] [PubMed] [Google Scholar]
- 18.Dorman S, Jolley C, Abernethy A, et al. . Researching breathlessness in palliative care: consensus statement of the National cancer research Institute palliative care breathlessness subgroup. Palliat Med 2009;23:213–27. 10.1177/0269216309102520 [DOI] [PubMed] [Google Scholar]
- 19.Simoff MJ, Lally B, Slade MG, et al. . Symptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e455S–97. 10.1378/chest.12-2366 [DOI] [PubMed] [Google Scholar]
- 20.Brighton LJ, Miller S, Farquhar M, et al. . Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019;74:270–81. 10.1136/thoraxjnl-2018-211589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luckett T, Phillips J, Johnson MJ, et al. . Contributions of a hand-held FAN to self-management of chronic breathlessness. Eur Respir J 2017;50:1700262. 10.1183/13993003.00262-2017 [DOI] [PubMed] [Google Scholar]
- 22.Spathis A, Booth S, Moffat C, et al. . The breathing, thinking, functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. npj Prim Care Resp Med 2017;27:27 10.1038/s41533-017-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Wu Y, Rozman de Moraes A, et al. . Fan Therapy for the Treatment of Dyspnea in Adults: A Systematic Review. J Pain Symptom Manage 2019;58:481–6. 10.1016/j.jpainsymman.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 24.Swan F, Newey A, Bland M, et al. . Airflow relieves chronic breathlessness in people with advanced disease: an exploratory systematic review and meta-analyses. Palliat Med 2019;33:618–33. 10.1177/0269216319835393 [DOI] [PubMed] [Google Scholar]
- 25.Holland AE, Hill CJ, Jones AY, et al. . Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;10:CD008250. 10.1002/14651858.CD008250.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bott J, Blumenthal S, Buxton M, et al. . Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax 2009;64:i1–52. 10.1136/thx.2008.110726 [DOI] [PubMed] [Google Scholar]
- 27.Bruton A, Lee A, Yardley L, et al. . Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018;6:19–28. 10.1016/S2213-2600(17)30474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S-B, Liam C-K, Pang Y-K, et al. . The effect of 20-Minute mindful breathing on the rapid reduction of dyspnea at rest in patients with lung diseases: a randomized controlled trial. J Pain Symptom Manage 2019;57:802–8. 10.1016/j.jpainsymman.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Probst VS, Troosters T, Coosemans I, et al. . Mechanisms of improvement in exercise capacity using a rollator in patients with COPD. Chest 2004;126:1102–7. 10.1378/chest.126.4.1102 [DOI] [PubMed] [Google Scholar]
- 30.Hill K, Dolmage TE, Woon LJ, et al. . Rollator use does not consistently change the metabolic cost of walking in people with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2012;93:1077–80. 10.1016/j.apmr.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 31.Solway S, Brooks D, Lau L, et al. . The short-term effect of a rollator on functional exercise capacity among individuals with severe COPD. Chest 2002;122:56–65. 10.1378/chest.122.1.56 [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Goldstein R, Brooks D. The acute effects of a rollator in individuals with COPD. J Cardiopulm Rehabil 2006;26:107–11. 10.1097/00008483-200603000-00011 [DOI] [PubMed] [Google Scholar]
- 33.Vaes AW, Meijer K, Delbressine JM, et al. . Efficacy of walking aids on self-paced outdoor walking in individuals with COPD: a randomized cross-over trial. Respirology 2015;20:932–9. 10.1111/resp.12570 [DOI] [PubMed] [Google Scholar]
- 34.Crisafulli E, Costi S, De Blasio F, et al. . Effects of a walking aid in COPD patients receiving oxygen therapy. Chest 2007;131:1068–74. 10.1378/chest.06-2108 [DOI] [PubMed] [Google Scholar]
- 35.Vaes AW, Annegarn J, Meijer K, et al. . The effects of a "new" walking aid on exercise performance in patients with COPD: a randomized crossover trial. Chest 2012;141:1224–32. 10.1378/chest.11-1076 [DOI] [PubMed] [Google Scholar]
- 36.Maddocks M, Taylor V, Klezlova R, et al. . When will I get my breath back? recovery time of exercise-induced breathlessness in patients with thoracic cancer. Lung Cancer 2012;76:128–9. 10.1016/j.lungcan.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 37.Simon ST, Weingärtner V, Higginson IJ, et al. . “I Can Breathe Again!” Patients’ Self-Management Strategies for Episodic Breathlessness in Advanced Disease, Derived From Qualitative Interviews. J Pain Symptom Manage 2016;52:228–34. 10.1016/j.jpainsymman.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 38.Sharp JT, Drutz WS, Moisan T, et al. . Postural relief of dyspnea in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1980;122:201–11. 10.1164/arrd.1980.122.2.201 [DOI] [PubMed] [Google Scholar]
- 39.Mustian KM, Alfano CM, Heckler C, et al. . Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue. JAMA Oncol 2017;3:961–8. 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews NE, Strong J, Meredith PJ. Activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: a systematic review and meta-analysis. Arch Phys Med Rehabil 2012;93:2109–21. 10.1016/j.apmr.2012.05.029 [DOI] [PubMed] [Google Scholar]
- 41.Lai W-S, Chao C-SC, Yang W-P, et al. . Efficacy of guided imagery with theta music for advanced cancer patients with dyspnea: a pilot study. Biol Res Nurs 2010;12:188–97. 10.1177/1099800409347556 [DOI] [PubMed] [Google Scholar]
- 42.Gallagher LM, Lagman R, Rybicki L. Outcomes of music therapy interventions on symptom management in palliative medicine patients. Am J Hosp Palliat Care 2018;35:250–7. 10.1177/1049909117696723 [DOI] [PubMed] [Google Scholar]
- 43.Lee AL, Desveaux L, Goldstein RS, et al. . Distractive auditory stimuli in the form of music in individuals with COPD. Chest 2015;148:417–29. 10.1378/chest.14-2168 [DOI] [PubMed] [Google Scholar]
- 44.Bredin M, Corner J, Krishnasamy M, et al. . Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ 1999;318:901–4. 10.1136/bmj.318.7188.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadell K, Webb KA, Preston ME, et al. . Impact of pulmonary rehabilitation on the major dimensions of dyspnea in COPD. COPD 2013;10:425–35. 10.3109/15412555.2012.758696 [DOI] [PubMed] [Google Scholar]
- 46.Richardson CR, Franklin B, Moy ML, et al. . Advances in rehabilitation for chronic diseases: improving health outcomes and function. BMJ 2019;195:l2191 10.1136/bmj.l2191 [DOI] [PubMed] [Google Scholar]
- 47.Troosters T, Blondeel A, Janssens W, et al. . The past, present and future of pulmonary rehabilitation. Respirology 2019;24:830–7. 10.1111/resp.13517 [DOI] [PubMed] [Google Scholar]
- 48.Stout NL, Baima J, Swisher AK, et al. . A systematic review of exercise systematic reviews in the cancer literature (2005-2017). Pm R 2017;9:S347–84. 10.1016/j.pmrj.2017.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buffart LM, Galvão DA, Brug J, et al. . Evidence-Based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 2014;40:327–40. 10.1016/j.ctrv.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 50.Schmitz KH, Campbell AM, Stuiver MM, et al. . Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA A Cancer J Clin 2019;69:468–84. 10.3322/caac.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gendron LM, Nyberg A, Saey D, et al. . Active Mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;10:CD012290. 10.1002/14651858.CD012290.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones S, Man WD-C, Gao W, et al. . Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 2016;10:Cd009419. 10.1002/14651858.CD009419.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanfleteren LEGW, Gloeckl R. Add-On interventions during pulmonary rehabilitation. Respirology 2019;24:899–908. 10.1111/resp.13585 [DOI] [PubMed] [Google Scholar]
- 54.Doğan N, Taşcı S. The effects of Acupressure on quality of life and dyspnea in lung cancer: a randomized, controlled trial. Altern Ther Health Med 2020;26:49–56. [PubMed] [Google Scholar]
- 55.Minchom A, Punwani R, Filshie J, et al. . A randomised study comparing the effectiveness of acupuncture or morphine versus the combination for the relief of dyspnoea in patients with advanced non-small cell lung cancer and mesothelioma. Eur J Cancer 2016;61:102–10. 10.1016/j.ejca.2016.03.078 [DOI] [PubMed] [Google Scholar]
- 56.Bauml J, Haas A, Simone CB, et al. . Acupuncture for dyspnea in lung cancer: results of a feasibility trial. Integr Cancer Ther 2016;15:326–32. 10.1177/1534735415624138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Trott P, Oei SL, Ramsenthaler C. Acupuncture for Breathlessness in Advanced Diseases: A Systematic Review and Meta-analysis. J Pain Symptom Manage 2020;59:327–38. 10.1016/j.jpainsymman.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 58.Zeng YS, Wang C, Ward KE, et al. . Complementary and alternative medicine in hospice and palliative care: a systematic review. J Pain Symptom Manage 2018;56:781–94. 10.1016/j.jpainsymman.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 59.Ekström M, Bajwah S, Bland JM, et al. . One evidence base; three stories: do opioids relieve chronic breathlessness? Thorax 2018;73:88–90. 10.1136/thoraxjnl-2016-209868 [DOI] [PubMed] [Google Scholar]
- 60.Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ 2014;349:g7617 10.1136/bmj.g7617 [DOI] [PubMed] [Google Scholar]
- 61.Barnes H, McDonald J, Smallwood N, et al. . Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016;3:CD011008. 10.1002/14651858.CD011008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ekström M, Nilsson F, Abernethy AA, et al. . Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann Am Thorac Soc 2015;12:1079–92. 10.1513/AnnalsATS.201501-034OC [DOI] [PubMed] [Google Scholar]
- 63.Ekström M, Johnson MJ, Huang C, et al. . Minimal clinically important differences in average, best, worst and current intensity and unpleasantness of chronic breathlessness. Eur Respir J 2020;56:1902202. 10.1183/13993003.02202-2019 [DOI] [PubMed] [Google Scholar]
- 64.Hui D, Shamieh O, Paiva CE, et al. . Minimal clinically important differences in the Edmonton symptom assessment scale in cancer patients: a prospective, multicenter study. Cancer 2015;121:3027–35. 10.1002/cncr.29437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Currow D, Louw S, McCloud P, et al. . Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax 2020;75:50–6. 10.1136/thoraxjnl-2019-213681 [DOI] [PubMed] [Google Scholar]
- 66.Ferreira DH, Louw S, McCloud P, et al. . Controlled-Release oxycodone vs. placebo in the treatment of chronic Breathlessness-A multisite randomized placebo controlled trial. J Pain Symptom Manage 2020;59:581–9. 10.1016/j.jpainsymman.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 67.Johnson MJ, Bland JM, Oxberry SG, et al. . Opioids for chronic refractory breathlessness: patient predictors of beneficial response. Eur Respir J 2013;42:758–66. 10.1183/09031936.00139812 [DOI] [PubMed] [Google Scholar]
- 68.Administration TG Prescription medicines: new or extended uses, or new combinations of registered medicines. in. Symonston, Australia: Australian Department of Health, 2019. [Google Scholar]
- 69.Bruera E, MacEachern T, Ripamonti C. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med 1993;119:906–7. 10.7326/0003-4819-119-9-199311010-00007 [DOI] [PubMed] [Google Scholar]
- 70.Allard P, Lamontagne C, Bernard P, et al. . How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? A randomized continuous sequential clinical trial. J Pain Symptom Manage 1999;17:256–65. 10.1016/S0885-3924(98)00157-2 [DOI] [PubMed] [Google Scholar]
- 71.Simon ST, Kloke M, Alt-Epping B, et al. . EffenDys—Fentanyl buccal tablet for the relief of episodic breathlessness in patients with advanced cancer: a multicenter, open-label, randomized, Morphine-Controlled, crossover, phase II trial. J Pain Symptom Manage 2016;52:617–25. 10.1016/j.jpainsymman.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 72.Hui D, Xu A, Frisbee-Hume S, et al. . Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage 2014;47:209–17. 10.1016/j.jpainsymman.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hui D, Kilgore K, Park M, et al. . Impact of Prophylactic Fentanyl Pectin Nasal Spray on Exercise-Induced Episodic Dyspnea in Cancer Patients: A Double-Blind, Randomized Controlled Trial. J Pain Symptom Manage 2016;52:459–68. 10.1016/j.jpainsymman.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui D, Kilgore K, Frisbee-Hume S, et al. . Effect of prophylactic fentanyl buccal tablet on episodic exertional dyspnea: a pilot double-blind randomized controlled trial. J Pain Symptom Manage 2017;54:798–805. 10.1016/j.jpainsymman.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson MJ, Hui D, Currow DC. Opioids, exertion, and dyspnea: a review of the evidence. Am J Hosp Palliat Care 2016;33:194–200. 10.1177/1049909114552692 [DOI] [PubMed] [Google Scholar]
- 76.Hui D, Hernandez F, Larsson L, et al. . Prophylactic fentanyl sublingual spray for episodic exertional dyspnea in cancer patients: a pilot double-blind randomized controlled trial. J Pain Symptom Manage 2019;58:605–13. 10.1016/j.jpainsymman.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen D, Alsuhail A, Viola R, et al. . Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manage 2012;43:706–19. 10.1016/j.jpainsymman.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 78.Kotrach HG, Bourbeau J, Jensen D. Does nebulized fentanyl relieve dyspnea during exercise in healthy man? J Appl Physiol 2015;118:1406–14. 10.1152/japplphysiol.01091.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Currow DC, McDonald C, Oaten S, et al. . Once-Daily Opioids for Chronic Dyspnea: A Dose Increment and Pharmacovigilance Study. J Pain Symptom Manage 2011;42:388–99. 10.1016/j.jpainsymman.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 80.Currow D, Watts GJ, Johnson M, et al. . A pragmatic, phase III, multisite, double-blind, placebo-controlled, parallel-arm, dose increment randomised trial of regular, low-dose extended-release morphine for chronic breathlessness: breathlessness, exertion and morphine sulfate (beams) study protocol. BMJ Open 2017;7:e018100 10.1136/bmjopen-2017-018100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fallon M, Giusti R, Aielli F, et al. . Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol 2018;29:iv166–91. 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 82.Currow D, Ekström M, Fazekas B, et al. . A phase III, multi-site, randomised, double blind, placebo controlled parallel arm study of daily extended release (ER) morphine for chronic breathlessness. Eur Respir J 2016;48:OA4808 10.1136/bmjopen-2017-018100 [DOI] [Google Scholar]
- 83.Currow DC, McDonald C, Oaten S, et al. . Once-Daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage 2011;42:388–99. 10.1016/j.jpainsymman.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 84.Simon ST, Higginson IJ, Booth S, et al. . Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2016;10:CD007354. 10.1002/14651858.CD007354.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navigante AH, Castro MA, Cerchietti LC. Morphine Versus Midazolam as Upfront Therapy to Control Dyspnea Perception in Cancer Patients While Its Underlying Cause Is Sought or Treated. J Pain Symptom Manage 2010;39:820–30. 10.1016/j.jpainsymman.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 86.Navigante AH, Cerchietti LCA, Castro MA, et al. . Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage 2006;31:38–47. 10.1016/j.jpainsymman.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 87.Hart DE, Corna NE, Horwood F, et al. . Randomised control trial of intranasal midazolam or oral lorazepam for the relief of dyspnoea in severe respiratory disease. Am J Respir Crit Care Med 2012;185:A2959 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A2953 [DOI] [Google Scholar]
- 88.Hardy J, Randall C, Pinkerton E, et al. . A randomised, double-blind controlled trial of intranasal midazolam for the palliation of dyspnoea in patients with life-limiting disease. Support Care Cancer 2016;5:3069–76. 10.1007/s00520-016-3125-2 [DOI] [PubMed] [Google Scholar]
- 89.Cherny NI, ESMO Guidelines Working Group . ESMO clinical practice guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann Oncol 2014;25 Suppl 3:iii143–52. 10.1093/annonc/mdu238 [DOI] [PubMed] [Google Scholar]
- 90.Hui D, Frisbee-Hume S, Wilson A, et al. . Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care. JAMA 2017;318:1047–56. 10.1001/jama.2017.11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maeda I, Morita T, Yamaguchi T, et al. . Effect of continuous deep sedation on survival in patients with advanced cancer (J-Proval): a propensity score-weighted analysis of a prospective cohort study. Lancet Oncol 2016;17:115–22. 10.1016/S1470-2045(15)00401-5 [DOI] [PubMed] [Google Scholar]
- 92.Maltoni M, Scarpi E, Rosati M, et al. . Palliative sedation in end-of-life care and survival: a systematic review. JCO 2012;30:1378–83. 10.1200/JCO.2011.37.3795 [DOI] [PubMed] [Google Scholar]
- 93.Haywood A, Duc J, Good P, et al. . Systemic corticosteroids for the management of cancer-related breathlessness (dyspnoea) in adults. Cochrane Database Syst Rev 2019;2:CD012704. 10.1002/14651858.CD012704.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hui D, Kilgore K, Frisbee-Hume S, et al. . Dexamethasone for dyspnea in cancer patients: a pilot double-blind, randomized, controlled trial. J Pain Symptom Manage 2016;52:8–16. 10.1016/j.jpainsymman.2015.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. . Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. JCO 2013;31:3076–82. 10.1200/JCO.2012.44.4661 [DOI] [PubMed] [Google Scholar]
- 96.Chow E, Meyer RM, Ding K, et al. . Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463–72. 10.1016/S1470-2045(15)00199-0 [DOI] [PubMed] [Google Scholar]
- 97.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft - Deutsche Krebshilfe - AWMF) S3-Leitlinie Palliativmedizin für Patienten MIT einer nicht heilbaren Krebserkrankung, Langversion 1.1, AWMF-Registernummer: 128/001OL, 2015. http://www.dgpalliativmedizin.de/images/stories/LL_Palliativmedizin_Langversion_1_1.pdf, Accessed 22 Nov 2020. [Google Scholar]
- 98.Abernethy AP, McDonald CF, Frith PA, et al. . Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010;376:784–93. 10.1016/S0140-6736(10)61115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ekström M, Ahmadi Z, Bornefalk-Hermansson A, et al. . Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev 2016;11:CD006429. 10.1002/14651858.CD006429.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben-Aharon I, Gafter-Gvili A, Leibovici L, et al. . Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol 2012;51:996–1008. 10.3109/0284186X.2012.709638 [DOI] [PubMed] [Google Scholar]
- 101.Hardinge M, Annandale J, Bourne S, et al. . British thoracic Society guidelines for home oxygen use in adults: accredited by NICE. Thorax 2015;70:i1–43. 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 102.Schaeffer MR, Ryerson CJ, Ramsook AH, et al. . Effects of hyperoxia on dyspnoea and exercise endurance in fibrotic interstitial lung disease. Eur Respir J 2017;49:1602494. 10.1183/13993003.02494-2016 [DOI] [PubMed] [Google Scholar]
- 103.Collier A, Breaden K, Phillips JL, et al. . Caregivers' Perspectives on the Use of Long-Term Oxygen Therapy for the Treatment of Refractory Breathlessness: A Qualitative Study. J Pain Symptom Manage 2017;53:33–9. 10.1016/j.jpainsymman.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 104.Hui D, Morgado M, Chisholm G, et al. . High-Flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage 2013;46:463–73. 10.1016/j.jpainsymman.2012.10.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nava S, Ferrer M, Esquinas A, et al. . Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol 2013;14:219–27. 10.1016/S1470-2045(13)70009-3 [DOI] [PubMed] [Google Scholar]
- 106.Lovell N, Wilcock A, Bajwah S, et al. . Mirtazapine for chronic breathlessness? A review of mechanistic insights and therapeutic potential. Expert Rev Respir Med 2019;13:173–80. 10.1080/17476348.2019.1563486 [DOI] [PubMed] [Google Scholar]
- 107.von Leupoldt A, Taube K, Lehmann K, et al. . The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest 2011;140:730–6. 10.1378/chest.10-2917 [DOI] [PubMed] [Google Scholar]
- 108.Borson S, McDonald GJ, Gayle T, et al. . Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics 1992;33:190–201. 10.1016/S0033-3182(92)71995-1 [DOI] [PubMed] [Google Scholar]
- 109.Currow DC, Ekström M, Louw S, et al. . Sertraline in symptomatic chronic breathlessness: a double blind, randomised trial. Eur Respir J 2019;53. 10.1183/13993003.01270-2018. [Epub ahead of print: 17 01 2019]. [DOI] [PubMed] [Google Scholar]
- 110.Eiser N, Harte R, Spiros K, et al. . Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD 2005;2:233–41. 10.1081/COPD-57596 [DOI] [PubMed] [Google Scholar]
- 111.Lacasse Y, Beaudoin L, Rousseau L, et al. . Randomized trial of paroxetine in end-stage COPD. Monaldi Arch Chest Dis 2004;61:140–7. 10.4081/monaldi.2004.692 [DOI] [PubMed] [Google Scholar]
- 112.Perna G, Cogo R, Bellodi L. Selective serotonin re-uptake inhibitors beyond psychiatry: are they useful in the treatment of severe, chronic, obstructive pulmonary disease? Depress Anxiety 2004;20:203–4. 10.1002/da.20041 [DOI] [PubMed] [Google Scholar]
- 113.Smoller JW, Pollack MH, Systrom D, et al. . Sertraline effects on dyspnea in patients with obstructive airways disease. Psychosomatics 1998;39:24–9. 10.1016/S0033-3182(98)71377-5 [DOI] [PubMed] [Google Scholar]
- 114.Ström K, Boman G, Pehrsson K, et al. . Effect of protriptyline, 10 Mg daily, on chronic hypoxaemia in chronic obstructive pulmonary disease. Eur Respir J 1995;8:425–9. 10.1183/09031936.95.08030425 [DOI] [PubMed] [Google Scholar]
- 115.Lovell N, Bajwah S, Maddocks M, et al. . Use of mirtazapine in patients with chronic breathlessness: a case series. Palliat Med 2018;32:1518–21. 10.1177/0269216318787450 [DOI] [PubMed] [Google Scholar]
- 116.Higginson IJ, Wilcock A, Johnson MJ, et al. . Randomised, double-blind, multicentre, mixed-methods, dose-escalation feasibility trial of mirtazapine for better treatment of severe breathlessness in advanced lung disease (BETTER-B feasibility). Thorax 2020;75:176–9. 10.1136/thoraxjnl-2019-213879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdallah SJ, Smith BM, Ware MA, et al. . Effect of Vaporized cannabis on exertional breathlessness and exercise endurance in advanced chronic obstructive pulmonary disease. A randomized controlled trial. Ann Am Thorac Soc 2018;15:1146–58. 10.1513/AnnalsATS.201803-198OC [DOI] [PubMed] [Google Scholar]
- 118.Pickering EE, Semple SJ, Nazir MS, et al. . Cannabinoid effects on ventilation and breathlessness: a pilot study of efficacy and safety. Chron Respir Dis 2011;8:109–18. 10.1177/1479972310391283 [DOI] [PubMed] [Google Scholar]
- 119.Farquhar MC, Prevost AT, McCrone P, et al. . Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? findings of a mixed-method randomised controlled trial. BMC Med 2014;12:194. 10.1186/s12916-014-0194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Higginson IJ, Bausewein C, Reilly CC, et al. . An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979–87. 10.1016/S2213-2600(14)70226-7 [DOI] [PubMed] [Google Scholar]
- 121.Brighton LJ, Gao W, Farquhar M, et al. . Predicting outcomes following holistic breathlessness services: a pooled analysis of individual patient data. Palliat Med 2019;33:462–6. 10.1177/0269216319830299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hui D, Hannon BL, Zimmermann C, et al. . Improving patient and caregiver outcomes in oncology: team-based, timely, and targeted palliative care. CA Cancer J Clin 2018;68:356–76. 10.3322/caac.21490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kavalieratos D, Corbelli J, Zhang D, et al. . Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316:2104–14. 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gaertner J, Siemens W, Meerpohl JJ, et al. . Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357:j2925. 10.1136/bmj.j2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haun MW, Estel S, Rücker G, et al. . Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017;6:Cd011129. 10.1002/14651858.CD011129.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care 2003;1:337–44. 10.1017/S1478951503030499 [DOI] [PubMed] [Google Scholar]
- 127.Hutchinson A, Barclay-Klingle N, Galvin K, et al. . Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J 2018;51:1701477. 10.1183/13993003.01477-2017 [DOI] [PubMed] [Google Scholar]
- 128.Grunfeld E, Coyle D, Whelan T, et al. . Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ 2004;170:1795–801. 10.1503/cmaj.1031205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA 2012;307:398–403. 10.1001/jama.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dykewicz CA, Centers for Disease Control and Prevention (U.S.), Infectious Diseases Society of America . Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33:139–44. 10.1086/321805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001038supp001.pdf (146.1KB, pdf)