Abstract
The COVID-19 outbreak caused by SARS-CoV-2 challenges the medical system by interfering with routine therapies for many patients with chronic diseases. In patients with cancer receiving immune checkpoint inhibitors (ICIs), difficulties also arise from the incomplete understanding of the intricate interplay between their routine treatment and pathogenesis of the novel virus. By referring to previous ICI-based investigations, we speculate that ICIs themselves are not linked to high-infection risks of respiratory diseases or inflammation-related adverse effects in patients with cancer. Moreover, ICI treatment may even enhance coronavirus clearance in some patients with malignant tumor by boosting antiviral T-cell responsiveness. However, the ‘explosive’ inflammation during COVID-19 in some ICI-treated patients with cancer was illustrated as exuberant immunopathological damage or even death. In case of the COVID-19 immunopathogenesis fueled by ICIs, we propose a regular monitor of pathogenic T-cell subsets and their exhaustion marker expression (eg, Th17 and interleukin (IL)-6-producing Th1 subsets with surface programmed death 1 expression) to guide the usage of ICI. Here we aimed to address these considerations, based on available literature and experience from our practice, that may assist with the decision-making of ICI administration during the pandemic.
Keywords: immunotherapy, inflammation, biomarkers, tumor
Since the outbreak of COVID-19, patients with cancer have been associated with a higher incidence of infection and poorer clinical outcomes.1 Questions about the infection risk of the novel coronavirus and disease severity in patients with cancer associated with the treatment of immune checkpoint inhibitors (ICIs), including blockers to cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1), in different scenarios are under discussion. Theoretically, these therapeutic modalities enable the restoration of T-cell cytotoxicity, thus potentiating enhanced antiviral effects. However, immunopathological findings of COVID-19 are characterized by significant inflammatory damage, especially during the late period of disease, which may be worsened by ICI treatment. Regrettably, data from ICI-treated patients infected with COVID-19 are scant. Therefore, the management of ICIs in patients with cancer during the outbreak is a crucial and urgent knowledge gap facing clinicians.
To achieve a rational approach to decision-making, we first aimed to assess the risks of ICI-related pneumonia (infection-related) and pneumonitis (inflammation damage-related) in patients with cancer by searching clinical trials and published articles from clinicaltrials.gov and PubMed through 10 October 2020 since COVID-19 largely affects the lung and respiratory tissues. Although no specific conclusion regarding SARS-CoV-2 infection in ICI-treated patients has been made, previous clinical investigations indicate that the overall infection rate of ICI-related pneumonia in patients with a wide variety of cancers (including melanoma, non-small cell lung carcinoma, head and neck squamous cell carcinoma, malignant mesothelioma, urothelial carcinoma, gastric cancer and prostate cancer) was 0.3%–6.0% for monotherapy, compared with 1%-2.6% for combination therapy, with the death rate around 0.3% (table 1). Factors that positively correlate to the pulmonary infection rate in patients with cancer include (1) underlying lung diseases (asthma, interstitial lung disease and chronic obstructive pulmonary disease) and (2) combined immunosuppressive therapies controlling the immune-related adverse effects (IrAEs), while ICIs themselves are not supposed to cause higher infection risk of pneumonia.2 3 The fact that only a limited number of gathered cases in the many cohorts4–10 reported concurrent SARS-CoV-2 infection among the ICI-treated patients with cancer also confirms its relative safety with regard to infection rate. Moreover, given that ICI-induced pneumonitis might predispose patients to the development of more progressive COVID-19, we also cautiously reviewed the incidence of ICI-related pneumonitis in different patients with cancer. The rates of immune-mediated pneumonitis of all grades and pneumonitis-associated mortality caused by ICIs were about 0.4%–10% and 0.2%–1%, respectively (table 1). Among them, patients bearing (1) decreased baseline pulmonary function and (2) certain types of tumors (especially lung cancer and renal cell cancer) are associated with higher incidence of adverse effects.11 More importantly, evidence from some relevant investigations revealed that ICIs will not promote COVID-19 severity or poor prognosis in patients with cancer.7–9 Taken together, ICIs themselves are not likely linked to much higher risk of SARS-CoV-2 infection or associated immunopathological damage of the lung in patients without the aforementioned high-risk factors.
Table 1.
Incidence and mortality rate of ICI-related pneumonia and pneumonitis (including ARDS) for patients with cancer
| Agent (target) | Tumor type | Treated patients(n) | Patients, n (%) | Reference | ||||
| Pneumonia | Pneumonia-associated death | All-grade pneumonitis | Pneumonitis-associated death | ARDS | ||||
| Nivolumab (PD-1) | Melanoma | 313 | 2 (0.6%) | NA | 5 (1.6%) | NA | NA | Hodi FS, et al. Lancet Oncol 2018;19:1480–92. |
| 206 | NA | NA | 3 (1.5%) | NA | NA | Robert C, et al. N Engl J Med 2015;372:320–30. | ||
| 268 | NA | NA | 5 (1.9%) | NA | NA | Weber JS, et al. Lancet Oncol 2015;16:375–84. | ||
| NSCLC | 287 | 17 (5.9%) | NA | 8 (2.8%) | NA | NA | Borghaei H, et al. N Engl J Med 2015; 373:1627–39. | |
| 391 | NA | NA | 9 (2.3%) | 1 (0.3%) | NA | Hellmann MD, et al. N Engl J Med 2018;378:2093–104. | ||
| 131 | NA | NA | 6 (4.6%) | NA | NA | Brahmer J, et al. N Engl J Med 2015;373:123–35. | ||
| HNSCC | 236 | 10 (4.2%) | NA | 5 (2.1%) | 1 (0.4%) | NA | Ferris RL, et al. N Engl J Med 2016;375:1856–67. | |
| Pembrolizumab (PD-1) | Melanoma | 277 | NA | NA | 5 (1.8%) | NA | NA | Robert C, et al. N Engl J Med 2015;372:2521–32. |
| 357 | NA | NA | 3 (0.8%) | NA | NA | Ribas A, et al. Lancet Oncol 2015;16:908–18. | ||
| 277 | NA | NA | 3 (1.1%) | NA | NA | Schachter J, et al. Lancet 2017;390:1853–62. | ||
| NSCLC | 339 | 5 (1.5%) | 1 (0.3%) | 16 (4.7%) | 2 (0.6%) | NA | Herbst RS, et al. Lancet 2016;387:1540–50. | |
| 636 | NA | NA | 43 (6.8%) | 1 (0.2%) | NA | Mok TSK, et al. Lancet 2019;393:1819–30. | ||
| 550 | NA | NA | 10 (1.8%) | NA | NA | Leigh NB, et al. Lancet Respir Med 2019;7:347–57. | ||
| 154 | NA | NA | 12 (7.8%) | 1 (0.6%) | NA | Reck M, et al. J Clin Oncol 2019;37:537–46. | ||
| 154 | NA | NA | 9 (5.8%) | NA | NA | Reck M, et al. N Engl J Med 2016;375:1823–33. | ||
| Urothelial Cancer | 370 | 1 (0.3%) | 1 (0.3%) | 5 (1.4%) | NA | NA | Balar AV, et al. Lancet Oncol 2017;18:1483–92. | |
| Urothelial carcinoma | 266 | NA | NA | 11 (4.1%) | 1 (0.4%) | NA | Bellmunt J, et al. N Engl J Med 2017;376:1015–26. | |
| Gastric cancer | 294 | NA | NA | 8 (2.7%) | NA | NA | Shitara K, et al. Lancet 2018;392:123–33. | |
| Atezolizumab (PD-L1) | NSCLC | 142 | 4 (3%) | NA | 4 (3%) | NA | NA | Fehrenbacher L, et al. Lancet 2016;387:1837–46. |
| 609 | NA | NA | 6 (1%) | NA | NA | Rittmeyer A, et al. Lancet 2017;389:255–65. | ||
| Ipilimumab (CTLA-4) | Melanoma | 311 | NA | NA | 5 (1.6%) | NA | NA | Hodi FS, et al. Lancet Oncol 2018;19:1480–92. |
| 256 | NA | NA | 1 (0.4%) | NA | NA | Robert C, et al. N Engl J Med 2015;372:2521–32. | ||
| Prostate cancer | 393 | 24 (6%) | 1 (0.3%) | 5 (1.3%) | NA | NA | Kwon ED, et al. Lancet Oncol 2014;15:700–12. | |
| Tremelimumab (CTLA-4) | Malignant mesothelioma | 380 | 18 (4.7%) | 1 (0.3%) | 3 (0.8%) | NA | 1 (0.3%) | Maio M, et al. Lancet Oncol 2017;18:1261–73. |
| Melanoma | 325 | 1 (0.3%) | 1 (0.3%) | NA | NA | NA | Ribas A, et al. J Clin Oncol 2013;31:616–22. | |
| Ipilimumab+nivolumab (CTLA-4+PD-1) | Melanoma | 313 | NA | NA | 23 (7.3%) | NA | NA | Hodi FS, et al. Lancet Oncol 2018;19:1480–92 |
| 313 | NA | NA | 22 (7%) | NA | NA | Wolchok JD, et al. N Engl J Med 2017;377:1345–56. | ||
| 94 | 1 (1%) | NA | 9 (10%) | 1 (1%) | NA | Hodi FS, et al. Lancet Oncol 2016;17:1558–68. | ||
| NSCLC | 39 | 1 (2.6%) | NA | 2 (5.1%) | NA | NA | Hellmann MD, et al. Lancet Oncol 2017;18:31–41. | |
| 576 | NA | NA | 22 (3.8%) | 3 (0.5%) | NA | Hellmann MD, et al. N Engl J Med 2018;378:2093–104. | ||
| SCLC | 61 | NA | NA | 2 (3.2%) | NA | NA | Antonia SJ, et al. Lancet Oncol 2016;17:883–95. | |
ARDS, acute respiratory distress syndrome; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; NA, not applicable; NSCLC, non-small cell lung cancer; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; SCLC, small cell lung cancer.
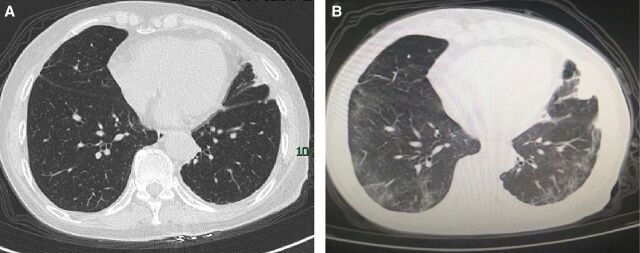
On infection, studies have shown that patients with COVID-19 develop lymphopenia, which becomes exacerbated during disease progression.12 Alongside this overall reduction in lymphocytes, patients with COVID-19 with disease progression were shown to have a marked loss of multifunctional interferon (IFN)-γ+tumor necrosis factor-α+IL-2+CD4+ T cells and excessive exhaustion of PD-1+CTLA-4+TIGIT+CD8+ T cells.13 Importantly, studies from previous coronavirus infections, including SARS and Middle East respiratory syndrome coronavirus, similarly demonstrated a major pathological characterization with sensitized T-cell apoptosis and delayed IFN production.14 Functional impairment of CD4+ T lymphocytes and exhaustion of CD8+ cytotoxic T lymphocytes may lead to compromised humoral and cellular immunity against viral infections during COVID-19 progression.15 In our experience, one 70-year-old female non-smoker with non-small cell lung cancer (NSCLC) at stage IV, who received PD-1 inhibitor (from October 2019 to January 2020) plus taxanes chemotherapy, was infected with the SARS-CoV-2 in February in Wuhan, the peak and epicenter of COVID-19, which came along with generally high mortality. Notably, she experienced only mild symptoms of COVID-19 with no need for supplemental oxygen before her self-recovery in a mobile cabin hospital. The CT scans of the chest before (figure 1A) and amid (figure 1B) COVID-19 infection are shown as follows. Her immunotherapy was resumed with scrutiny in July. In her latest admission check on 12 October, the anti-SARS-CoV-2 IgG antibody was present, while no inflammatory signs were detected from lab tests or radiographs, which indicates a smooth recovery from the virus infection. Taken together, it is possible that ICI treatment in some SARS-CoV-2-infected cases may be beneficial due to the boosting of antiviral T-cell function. Recently, a clinical trial to investigate the anti-infection efficacy of PD-1 blocking antibody in COVID-19-induced sepsis has been launched (NCT04268537).
Figure 1.
Chest CT images from the patient with cancer (A) before the COVID-19 pneumonia on 16 January, which was clear in both lung fields; and (B) amid the infection on 28 February, showing mild bilateral ground-glass opacity and multiple consolidation (with permission from the patient).
Notably, there are concerns that certain T-cell subsets would promote the immunopathological damage of COVID-19. In the postmortem biopsy of a patient with COVID-19, the inflammatory infiltrates in both lungs were dominated by a large portion of lymphocytes, with increased ratios of both proinflammatory CCR6+ Th17 and cytotoxic CD8+ T cells.16 Another mechanistic study of COVID-19 further identified a pathogenic IFN-γ+ Th1 subset that could secrete high amounts of GM-CSF and IL-6 to activate inflammatory CD14+CD16+IL-6+ monocytes, possessing a critical role in the generation of cytokine storm syndrome during pulmonary damage.17 Correspondingly, there are also a series of cohort studies4–6 10 and case reports18–20 indicating that ICI treatment stands as a risk factor for COVID-19 exacerbation in patients with cancer. Strikingly, Lovly et al reported in a case report that the coronavirus infection prior to ICI treatment in a patient with lung cancer resulted in fulminant inflammatory damage in both lung alveolar and death.20
So far, lab tests are usually not prompt or specific enough to detect T-cell subsets and functional changes in patients with cancer infected with COVID-19, which calls for the identification of useful clinicopathological factors to evaluate the severity of COVID-19-induced immunopathogenesis and to guide the usage of ICI and other T cell-targeted therapies in patients with cancer during the pandemic. Based on the aforementioned findings, pathogenic T-cell subsets with great clinical significance, including IL-6-producing Th1 subset17 and Th17/IL-17 pathway,21–23 could be applied as important clinicopathological indicators and should also be regularly screened in ICI-treated patients when combined with COVID-19. Notably, the expression levels of PD-1 and CTLA-4 molecules on these pathological T subsets are also of great importance, since the overactivation of these pathogenic T subsets induced by ICIs could elicit adverse outcomes in some patients with cancer in the form of an ‘explosive’ lung or systemic inflammation. Thus, routine lab examinations of pathogenic T-cell numbers and phenotypes will be extremely useful to guide ICI usage in patients with cancer or those who recovered from COVID-19 infection.
In conclusion, previous studies generally found no additional severe risks of respiratory infection or IrAEs from ICI treatment in the vast majority of patients with cancer with COVID-19. Given all the evidence, it is postulated that for patients with cancer, ICI application prior to the onset of COVID-19 infection or even at the early stage of COVID-19 infection enables the activation of effector T-cell functions that could be beneficial to both antitumoral and anti-COVID-19 immune responses. However, exuberant inflammatory damage during ICI treatments could also occur in certain patients with cancer with COVID-19 infection, perhaps due to the activation of pathogenic T subsets. The conflicting results imply a time-dependent shift of T-cell differentiation and their activation states along the COVID-19 course in patients with cancer. As a result, we propose a regular screening and tracking of not only the total lymphocyte count but also the pathogenic T-cell subsets and their surface exhaustion markers, which would guide clinical ICI strategies in patients with cancer amid the COVID-19 pandemic.
Acknowledgments
We thank Professor Adam J Adler and Dr Upendra P Hegde for critically reading the manuscript.
Footnotes
Contributors: Drafting of the manuscript: CS, investigation and data collection: QL, patient data collection: YW and YL, revision and supervision: JL and JT.
Funding: This work was supported by National Natural Science Foundation of China (81902795 and 81874135).
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cadranel J, Canellas A, Matton L, et al. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev 2019;28. 10.1183/16000617.0058-2019. [Epub ahead of print: 30 Sep 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Del Castillo M, Romero FA, Argüello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016;63:1490–3. 10.1093/cid/ciw539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a new York hospital system. Cancer Discov 2020;10:935–41. 10.1158/2159-8290.CD-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov 2020;10:1121–8. 10.1158/2159-8290.CD-20-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 2020;21:914–22. 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020;6:1108–10. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H, Guo X, Zhou J, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer 2020;11:191–7. 10.1111/1759-7714.13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng H-Y, Zhang M, Yang C-X, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 2020;17:541–3. 10.1038/s41423-020-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017;39:529–39. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020;217:e20200678. 10.1084/jem.20200678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Fu B, Zheng X, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv 2020. 10.1093/nsr/nwaa041 [DOI] [Google Scholar]
- 18. Bonomi L, Ghilardi L, Arnoldi E, et al. A rapid fatal evolution of coronavirus disease-19 in a patient with advanced lung cancer with a long-time response to nivolumab. J Thorac Oncol 2020;15:e83–5. 10.1016/j.jtho.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Noia V, D'Aveni A, Squadroni M, et al. Immune checkpoint inhibitors in SARS-CoV-2 infected cancer patients: the spark that ignites the fire? Lung Cancer 2020;145:208–10. 10.1016/j.lungcan.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lovly CM, Boyd KL, Gonzalez-Ericsson PI, et al. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv 2020. 10.1101/2020.04.29.20085738. [Epub ahead of print: 01 May 2020]. [DOI] [Google Scholar]
- 21. De Biasi S, Meschiari M, Gibellini L, et al. Marked T cell activation, senescence, exhaustion and skewing towards Th17 in patients with COVID-19 pneumonia. Nat Commun 2020;11:3434. 10.1038/s41467-020-17292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol 2020;20:271–2. 10.1038/s41577-020-0312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 2020;20:269–70. 10.1038/s41577-020-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]