Abstract
Respiratory syncytial virus (RSV) is an increasingly recognized cause of acute respiratory infection (ARI) in adults. We compared the crude in-hospital mortality of patients with RSV infection alone with that of patients with RSV–bacterial coinfection. Overall, 12 144 hospitalized patients with ARI were screened for RSV detection by polymerase chain reaction between February 2014 and April 2019. In total, 701 (5.8%) had a positive RSV result, including 85 (12.1%) with bacterial coinfection. RSV–bacterial coinfection was associated with an increase in crude in-hospital mortality in patients >65 years old (hazard ratio, 2.94; 95% CI, 1.30–6.60; P = .010). Optimized prevention and management strategies to reduce this burden are needed.
Keywords: bacterial coinfection, mortality, pneumonia, respiratory syncytial virus
Lower respiratory infections are a major cause of mortality worldwide, with an estimated 2.38 million deaths in 2016 [1]. The 4 most common causative microorganisms are, in order of frequency: Streptococcus pneumoniae, respiratory syncytial virus (RSV), Haemophilus influenza type b, and influenza viruses. Notably, this ranking is identical in children younger than 5 years and in elderly adults (>70 years). RSV is commonly responsible for bronchiolitis in children, whereas in adults it is associated with influenza-like illness (ILI) or acute respiratory infections (ARIs) including pneumonia. RSV represents an underestimated clinical burden in adults, being the causative agent of 10.6%–13.5% of community pneumonia depending on whether patients are ambulatory or hospitalized [2, 3]. Recent reports show that in hospitalized patients with RSV-ARI, intensive care unit (ICU) admission was necessary in 15%–16.8% of cases [4, 5] and mortality ranged from 8% (in-hospital mortality) [5] to 11.9% at 60 days [6], which is comparable to that associated with influenza. In addition, recent reports show that the hospital admission rate and in-hospital case fatality ratio of RSV-ARI are higher for those aged ≥65 years than for those aged 50–64 years [7].
In adult hospitalized patients with RSV-ARI, bacterial-associated pneumonia was reported in 42.3%–79.7% of cases [5, 6, 8]. In children with bronchiolitis, positive blood culture or polymerase chain reaction (PCR) on blood for the main respiratory bacterial pathogens was strongly associated with more severe RSV infection [9]. In addition, the positive association between viruses and bacteria has been widely discussed in the literature, particularly for respiratory viruses and secondary bacterial pneumonia [10].
We conducted a retrospective study over a 5-year period in the 4 university hospitals of Marseille, France. We aimed to describe the epidemiology of bacterial coinfection in patients with RSV-ARI and compare the crude in-hospital mortality of patients with RSV infection alone with that of patients with RSV–bacterial coinfection.
METHODS
Study Design
We conducted a retrospective historical cohort study of all adult patients hospitalized with RSV infection from February 1, 2014, to April 25, 2019, at the Assistance Publique–Hôpitaux de Marseille (AP-HM) in Marseille, France. Microbiological analysis was performed at the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection laboratory, which covers the 4 university hospitals of the institution.
Population
All patients aged 18 years or older who were hospitalized for ARI during the study period with an available result for RSV infection and no other respiratory virus infection during the same hospital stay were included. Hospitalization was defined as a length of stay ≥2 days. RSV requests with unavailable results and samples from outside the institution were excluded. Informed consent was not obtained from the patients, as the study design was retrospective and did not modify standard of care.
Data Collection
We screened all requests for RSV testing during the study period and used our local database to pair each RSV request with any bacteriological request made in our laboratory during the same patient’s hospital stay. The database included age, sex, date of admission, length of stay, and in-hospital death for each patient. Microbiological characteristics included nature and date of collection of the samples and name and number of bacterial agents isolated. RSV infection was considered nosocomial if the delay between admission and RSV-positive sample collection was ≥5 days (incubation period).
Virological and Microbiological Analysis
RSV positivity was defined either by a positive reverse transcriptase polymerase chain reaction (RT-PCR) specific to RSV with a cycle threshold (Ct) value ≤35 cycles or by a positive immunochromatographic test specific to RSV antigen on any of the following samples: pharyngeal swab, nasal swab, nasopharyngeal aspirate, sputum, or bronchoalveolar lavage (BAL). Syndromic and point-of-care culture and culture-independent testing including viral and bacterial detection were requested by clinicians for patients presenting ARI. Bacterial coinfection was defined as the culture of a known respiratory pathogen from blood and/or a respiratory sample (sputum, bronchial aspiration, or bronchoalveolar lavage), or a positive culture-independent test including Streptococcus pneumoniae or Legionella pneumophila antigen detection in urine, or Mycoplasma pneumoniae or Bordetella pertussis PCR, between 2 days before and 14 days after RSV-positive test. In patients with ARI, syndromic point-of-care testing was systematically performed. Patients with a blood culture or a respiratory sample positive for coagulase-negative Staphylococcus (or other bacteria without known pulmonary pathogenicity) were not considered coinfected.
Statistical Analysis
Two-group comparisons were performed with the Student test (or Mann-Whitney test when appropriate) for quantitative variables and with the Fisher exact test (or chi-square test when appropriate) for qualitative variables. Survival analyses were performed using the Kaplan-Meier curve with a log-rank test and Cox model regression (multivariate analysis). A significance threshold of .05 was adopted for all statistical analyses. Statistical analysis was performed with PASW Statistics, version 17.0.
RESULTS
Population
During the study period, we recorded 50 921 requests for RSV testing; 2 080 requests (4.1%) had unavailable RSV results and were excluded. A total of 10 155 requests (19.9%) were made hospital outpatients and were excluded. The remaining 38 686 requests represented 26 811 in-hospital patients, and among them 12 144 (45.2%) were included (Supplementary Figure 1). We excluded patients positive for influenza A or B viruses, rhinovirus, adenovirus, or metapneumovirus during the same hospital stay. In total, 11 443 (94.3%) patients were RSV-negative and 701 (5.7%) were RSV-positive; 291 (45.1%) were male. The mean age of RSV-positive patients (range) was 69.9 ± 19.3 (60–85) years, and 483 patients (68.9%) were older than 65 years. The global in-hospital mortality rate was 1.4% during the studied period. The mean age of deceased patients (range) was 80.6 ± 8.8 (73.5–87.5) years. A bacterial coinfection was found in 85 (12.1%) patients with RSV-ARI. Age, sex, length of hospital stay, and rates of RSV nosocomial acquisition did not differ according to the presence of bacterial coinfection. Crude in-hospital mortality was significantly higher in patients with RSV and bacterial coinfection when compared with RSV-ARI alone (P = .01) (Table 1).
Table 1.
Comparison of Epidemiological and Clinical Features Among RSV-Infected Patients in Absence or Presence of Bacterial Coinfection
| RSV Alone (n = 616) | Bacterial Coinfection (n = 85) | P | |
|---|---|---|---|
| Age, mean ± SD, y | 70.4 ± 19.4 | 66.6 ± 18.6 | .083 |
| Women, No. (%) | 367 (59.6) | 43 (50.6) | .098 |
| Length of stay, mean ± SD, d | 9.2 ± 7.1 | 9.6 ± 6.2 | .58 |
| Hospital-acquired RSV, No. (%) | 101 (16.4) | 15 (17.6) | .75 |
| In-hospital mortality, No. (%) | 30 (4.9) | 11 (12.9) | .01 |
Abbreviation: RSV, respiratory syncytial virus.
Microbiological Analysis
Among the 95 bacteria associated with RSV-ARI, 64 (67.4%) were identified by culture (blood culture: 22 [34.3%]; respiratory samples: 42 [65.7%]), 26 (27.4%) by culture-independent methods, and 5 (5.2%) by both techniques.
Streptococcus pneumoniae was the most frequently identified bacteria (23.5%), followed by Mycoplasma pneumoniae (14.1%), Pseudomonas aeruginosa (11.8%), and Staphylococcus aureus (10.6%). The bacteria isolated in at least 3 patients are shown in Supplementary Figure 2.
Mortality and Bacterial Coinfection in Adult Patients With RSV Infection
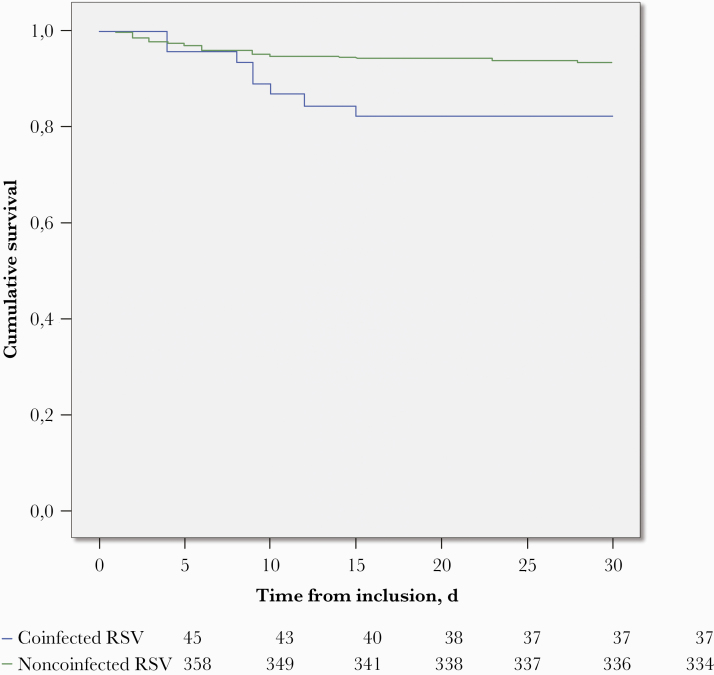
The majority of deaths (97.5%) occurred in patients >65 years old (Supplementary Figure 3). We therefore performed a survival analysis in this specific age class. In patients aged ≥65 years, survival univariate analysis showed that RSV–bacterial coinfection was associated with higher mortality (18.8% vs 7.0%; hazard ratio [HR], 2.7; 95% CI, 1.2–6.1; log-rank test, P = .010). This finding remained unchanged after adjustment for age and length of stay in multivariate analyses (Cox model regression, HR, 2.94; 95% CI, 1.30–6.60; P = .010) (Figure 1).
Figure 1.
Effect of bacterial coinfection on crude in-hospital mortality in RSV-positive hospitalized patients aged >65 years. Green line: RSV infection alone; blue line: RSV infection with bacterial coinfection. Kaplan-Meier curve, log-rank test, P = .010. Abbreviation: RSV, respiratory syncytial virus.
DISCUSSION
In this retrospective study over a 5-year period, we found that 12.1% of hospitalized patients with RSV-ARI had bacterial coinfection. This is lower than the rate reported by Jung et al. (19.7%) [11] but higher than that reported by Jeannoël et al. (9.3%) [12]. The latter study analyzed exclusively RSV-positive pneumonia, whereas we analyzed all RSV-positive ARIs. This may have contributed to differences in the frequency of associated bacteria across studies, which reported, in descending order, Streptococcus pneumonia, Haemophilus influenza, Staphylococcus aureus, Pseudomonas aeruginosa, and Moraxella catarrhalis. These ecological differences may also be partly explained by the microbiological tests used. Indeed, the systematic testing in our syndromic point-of-care testing included PCR specific to M. pneumoniae in patients with ARI.
Although the bacterial coinfection burden and outcome have been widely described in patients with viral respiratory infections [13], few studies have analyzed it in patients with RSV-ARI. Recently, Jeannnoël et al. observed a more severe outcome for RSV plus bacteria-associated pneumonia compared with only RSV-associated pneumonia. In particular, the length of hospital stay was significantly longer, and ICU hospitalization more frequent. Although difference in hospitalization characteristics was not investigated in the present study, we found that bacterial coinfection was associated with higher mortality rates in patients >65 years old with RSV-ARI. This finding remained unchanged after adjustment for age and length of stay in multivariate analysis. As RSV-ARI and bacterial coinfection may result in greater morbidity and mortality among older hospitalized adults, vaccination and early antibiotic treatment strategies need to be further evaluated and implemented in this population [3, 14]. Moreover, clinicians should perform exhaustive and systematic detection of RSV and bacterial coinfections in hospitalized adult patients with ARI, particularly in elderly subjects.
Our findings suffer from some limitations. First, the retrospective design is vulnerable to biases. Second, the lack of clinical data prevented accurate diagnosis of ARI subtype and analysis of factors such as illness severity, comorbidities, and timing of appropriate antibiotic administration. Third, outcome was only available during in-hospitality stay. Finally, the causal role of RSV infections in the clinical presentation and outcome would be better assessed in a case–control study. Nonetheless, asymptomatic RSV infection is uncommon in the elderly, and a recent meta-analysis suggests that there is strong evidence of an association between RSV and ARI [15]. Notably, among individuals with RSV-ARI, a causative role for ARI was attributable to RSV in about 88% of cases.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. No relevant financial support was provided.
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study does not include factors necessitating patient consent. The design of the work was approved by our local ethical committee.
References
- 1. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali A, Lopardo G, Scarpellini B, et al. Systematic review on respiratory syncytial virus epidemiology in adults and the elderly in Latin America. Int J Infect Dis 2020; 90:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 4. Gamiño-Arroyo AE, Moreno-Espinosa S, Llamosas-Gallardo B, et al. ; Mexico Emerging Infectious Diseases Clinical Research Network (La Red) Epidemiology and clinical characteristics of respiratory syncytial virus infections among children and adults in Mexico. Influenza Other Respir Viruses 2017; 11:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loubet P, Lenzi N, Valette M, et al. ; FLUVAC Study Group Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin Microbiol Infect 2017; 23:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 2013; 57:1069–77. [DOI] [PubMed] [Google Scholar]
- 7. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222 Supplement_(7):S577–83. [DOI] [PubMed] [Google Scholar]
- 8. Chuaychoo B, Ngamwongwan S, Kaewnaphan B, et al. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J Clin Virol 2019; 117:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cebey-López M, Pardo-Seco J, Gómez-Carballa A, et al. ; GENDRES Network Bacteremia in children hospitalized with respiratory syncytial virus infection. PLoS One 2016; 11:e0146599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung HS, Kang BJ, Ra SW, et al. Elucidation of bacterial pneumonia-causing pathogens in patients with respiratory viral infection. Tuberc Respir Dis (Seoul) 2017; 80:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeannoël M, Lina G, Rasigade JP, et al. Microorganisms associated with respiratory syncytial virus pneumonia in the adult population. Eur J Clin Microbiol Infect Dis 2019; 38:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013; 309:275–82. [DOI] [PubMed] [Google Scholar]
- 14. Levet P, Raoult D, Parola P. Treating influenza with antibiotics. Int J Antimicrob Agents 2017; 50:505–6. [DOI] [PubMed] [Google Scholar]
- 15. Shi T, Arnott A, Semogas I, et al. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta-analysis. J Infect Dis 2020; 222(Supplement_(7):S563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.