Abstract
Background:
Amdoxovir acts synergistically with zidovudine in vitro and the combination prevents or delays the selection of thymidine analogue and K65R mutations. In silico studies have shown that a reduced dose of zidovudine (200 mg) results in decreased zidovudine-monophosphate levels, associated with toxicity, while maintaining zidovudine-triphosphate levels, which are associated with antiviral effects. Here, we aimed to assess the short-term tolerability and antiviral activity of amdoxovir in combination with reduced and standard doses of zidovudine.
Methods:
The study was a double-blind, placebo-controlled study in HIV-1-infected patients not receiving antiretroviral therapy and with plasma HIV-1 RNA ≥5,000 copies/ml. Patients were randomized to 10 days of twice-daily treatment with 200 mg zidovudine, 300 mg zidovudine, 500 mg amdoxovir, 500 mg amdoxovir plus 200 mg zidovudine or 500 mg amdoxovir plus 300 mg zidovudine. The mean change in viral load (VL) log10 and area under the virus depletion curve (AUCVL) from baseline to day 10 were determined. Laboratory and clinical safety monitoring were performed.
Results:
Twenty-four patients were enrolled. The mean VL log10 change was 0.10 with placebo, −0.69 with zidovudine 200 mg, −0.55 with zidovudine 300 mg, −1.09 with amdoxovir, −2.00 with amdoxovir plus zidovudine (200 mg) and −1.69 with amdoxovir plus zidovudine (300 mg). Amdoxovir plus zidovudine (200 mg) was significantly more potent than amdoxovir monotherapy in AUCVL and mean VL decline (P=0.019 and P=0.021, respectively), suggesting synergy. There was markedly decreased VL variability with the combination compared with amdoxovir alone. All adverse events were mild to moderate.
Conclusion:
The combination of amdoxovir plus zidovudine appeared synergistic with reduced VL variability. This combined therapy, including the use of a lower zidovudine dosage, warrants further development for the therapy of HIV infection.
Introduction
Nucleoside reverse transcriptase inhibitors (NRTIs) form the backbone of currently used first-line regimens for the treatment of HIV-1 infections and are the preferred drug class of treatment in combination with other classes of drugs [1]. Suboptimal adherence mainly due to poor tolerability will lead to treatment failure in all regimens because of the subsequent development of drug resistance. Such failure would lead to limited future therapeutic options [2].
Amdoxovir (AMDX, DAPD, (−)-β-d-2,6-diaminopurine dioxolane) is an NRTI currently in advanced Phase II clinical trials that has been safely administered to nearly 200 patients. This prodrug of DXG (β-d-[2R,4R]-1,3-dioxolane guanosine) is the only guanosine nucleoside analogue in clinical development for the treatment of HIV-1 infections. DXG has shown good activity in vitro against HIV-1 strains resistant to lamivudine or emtricitabine (M184V/I) and strains containing zidovudine/stavudine-resistant thymidine analogue mutations (TAMs), including the 69SS double insert [3–5]. In vitro, DXG demonstrated synergistic antiviral activity in combination with other NRTIs, including zidovudine and lamivudine [3]. Selection studies showed a delayed emergence of HIV-1-resistant viruses to DXG, which was associated with K65R or L74V mutations in MT-2 cells [6].
Previous reports suggest that the K65R mutation, which confers cross-resistance to DXG and amdoxovir, can revert zidovudine-resistant virus to zidovudine-sensitive virus [3,5–7]. As observed for the K65R variant, virus containing the L74V mutation remained sensitive to zidovudine [6]. In vitro, the combination of amdoxovir plus zidovudine resulted in the absence of drug-resistant mutations through week 28 [8]. The bidirectional phenotypic antagonism between the K65R and TAM pathways has been elucidated previously, demonstrating that K65R reduces the enzymatic excision of chain-terminating analogues, such as zidovudine, and that TAMs counteract K65R-mediated resistance by reducing the selectivity of the virus to incorporate natural substrate over the nucleoside analogue [7,9–11]. Retrospective analyses of clinical databases of patient genotypes also demonstrate the negative association of K65R with specific TAMs [7,9,12,13].
Zidovudine is an attractive drug for use in combination with amdoxovir, as these drugs are activated by different phosphorylation pathways (thymidine kinase and 5′-nucleotidase, respectively) and the combination does not affect the nucleoside triphosphate (NTP) levels of each drug in vitro [14–16]. On the basis of the favourable mutational resistance mechanisms, counter-selection of K65R and TAMs, and synergistic antiviral activity, it is expected that coadministration of zidovudine with amdoxovir could delay the emergence of NRTI resistance and prolong treatment response [17–20].
The major toxicity of amdoxovir in extensive animal toxicology studies was obstructive nephropathy at high doses, secondary to the precipitation of amdoxovir/DXG in the renal tubules. This was probably due to the poor aqueous solubility of DXG, similar to that observed for the related nucleoside analogues acyclovir and cidofovir. In a 52-week study, five cynomolgus monkeys, receiving amdoxovir at 800 or 1,200 mg/kg per day, developed obstructive nephropathy and associated uraemia after 26 weeks of treatment, which was reversible after early identification and discontinuation of medication. In addition, islet cell atrophy and hyperglycaemia occurred. Lens opacities were observed, which was thought to be secondary to hyperglycaemia and not a direct effect of amdoxovir. No patients enrolled in clinical trials of amdoxovir have developed any renal abnormalities attributable to the drug [21].
Amdoxovir has been well-tolerated in six human Phase I/II studies [21–24]. In one study involving 18 individuals on amdoxovir therapy (300 or 500 mg twice daily), non-gradable lens opacities were observed in four patients between weeks 6.8 and 15, and mild posterior subcapsular cataract was identified at week 3.6 in one patient; all five patients were withdrawn from the study. As ophthalmologic examinations were added only mid-study, it is not known whether these findings were present at baseline. Lens abnormalities were visually insignificant in all five patients and there were no renal or glucose toxicities [21]. There were no lens abnormalities noted in the other 13 patients in this study, who were administered amdoxovir for up to 96 weeks (n=4) or received amdoxovir at a dose of 300 mg twice daily (n=9) for up to 48 weeks in the AIDS Clinical Trials Group (ACTG) study A5118; likewise, no abnormalities were seen in patients receiving amdoxovir at a dose of 500 mg twice daily (n=49) for up to 96 weeks (n=5) in ACTG study A5165 [23,24].
A recent in silico study demonstrated that reduced dose zidovudine at 200 mg twice daily produces decreased zidovudine-monophosphate levels without reducing zidovudine-triphosphate levels, which are necessary for the antiviral effect [25]. Therefore, a proof-of-concept study was conducted in HIV-infected patients to evaluate the short-term tolerability and antiviral activity of amdoxovir (500 mg twice daily) in combination with reduced and standard doses of zidovudine.
Methods
Approval
The protocol was approved by the local ethics committees (Facultad de Medicina-Universidad de Buenos Aires, Comité Independiente de Etica en Investigación [CIEI-FM-UBA]) and the Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT) of Argentina.
Study design
This was a Phase Ib/IIa randomized, double-blind, proof-of-concept study to evaluate antiviral activity and short-term tolerability of amdoxovir in combination with two different doses of zidovudine. Patients were randomly assigned (1:1:1) to one of three treatment groups and within each treatment group patients were randomly assigned (3:1) to amdoxovir or placebo. Treatment A was 500 mg amdoxovir or placebo twice daily, treatment B was 500 mg amdoxovir or placebo plus 300 mg zidovudine twice daily and treatment C was 500 mg amdoxovir or placebo plus 200 mg zidovudine twice daily. Computer-generated randomization codes were used. Zidovudine administration was open-label; amdoxovir administration was blinded. Patients, investigators and laboratory personnel were blinded to amdoxovir treatment assignment. Twenty-four patients were required to obtain eight evaluable patients per treatment group. Within a group, six patients were randomized to amdoxovir and two to placebo. The protocol allowed for replacement of a patient who withdrew consent or discontinued the study prior to day 10 for any reason.
Patients
Patients were HIV-1 infected men and women ≥18 years of age who were either antiretroviral therapy naive or had not received any antiretroviral therapy in the previous 90 days. All patients included had a plasma HIV-1 RNA (viral load, VL) ≥5,000 copies/ml and a CD4+ T-cell count ≥200 cells/mm3. Females of child-bearing potential were required to have a negative pregnancy test and agree to adequate contraception.
Exclusionary criteria were any active AIDS-defining illness [26], active acute hepatitis A and/or chronic hepatitis B or C with detectable viraemia, abnormal clinical laboratory tests, any active clinically significant disease or findings that in the investigator’s opinion would compromise the outcome of the study, concomitant use of antiviral drugs, prophylactic drugs for opportunistic infections, nephrotoxic drugs, competitors of renal excretion or immunosuppressive drugs within 30 days of study entry, visual abnormalities (for example, cataracts or macular degeneration) other than non-organic decreased visual acuity, diabetes mellitus type 1 or 2, active alcohol or drug use and pregnancy or lactation.
The patients received the assigned study medication twice daily from days 1 to 9, but only one dose on day 10. The morning dose was administered at the study site, whereas the evening dose was taken by patients at home 12 h later. Those patients assigned to the amdoxovir plus zidovudine combination arms took both drugs at the same time. The time of the evening dose taken at home was reported in a diary from days 2 to 9. The patients returned for follow-up visits on days 11, 12, 15 and 20.
Plasma HIV-1 RNA was measured at baseline and on days 1–10, day 12 and day 20 using the standard PCR assay Amplicor HIV Monitor Assay, version 1.5 (Roche Diagnostics, Indianapolis, IN, USA). Viral genotyping at baseline and day 10 was carried out using Trugene (Bayer Health Care LLC, Tarrytown, NY, USA).
Statistical analysis
All patients who completed 10 days of treatment were evaluable for antiviral activity analyses. All patients who received at least one dose of study medication and had at least one post-baseline safety evaluation were included in the safety evaluation. Missing data were not imputed.
Descriptive statistics were calculated for antiviral activity, and immunological and safety parameters. The area under the virus depletion curve (AUCVL) and the change in viral load from baseline were analysed using analysis of covariance (ANCOVA), including amdoxovir/placebo and zidovudine dose (none, 200 mg or 300 mg) as factors, the amdoxovir plus zidovudine interaction arm, and baseline log10 HIV-1 RNA as a covariate. The difference between least squares means and the 95% confidence interval on the difference was calculated for pairwise comparisons. The pairwise comparisons examined the effect of amdoxovir separately with each dose of zidovudine (none, 200 mg or 300 mg) and evaluated the effect of the three doses of zidovudine (20 0 mg versus none, 300 mg versus none and 300 mg versus 200 mg) in combination with amdoxovir. Unadjusted P-values for the pairwise comparisons between least squares means were obtained from the ANCOVA; the Bonferroni adjustment was applied to protect the type I error rate for the pairwise comparison of treatments.
Several antiviral activity endpoints were assessed: AUCVL from day 1 to day 10; change in viral load from baseline through day 12 and on day 20; the lowest viral load value through day 12 and day 20; the maximum reduction in viral load from baseline through day 12 and day 20; the proportion of patients with a decrease of at least 1.0 and 1.5 in log10 viral load on each day; the proportion of patients with a plasma HIV-1 RNA value <400 copies/ml on each day; and the proportion of patients with a plasma HIV-1 RNA value <50 copies/ml on each day.
Immunological changes were determined by changes in CD4+ and CD8+ T-cell counts from baseline at day 10 by treatment arm. The incidence of adverse events and laboratory toxicities were summarized by treatment group. The data were analysed using SAS version 9.1 (SAS Institute Inc, Cary, NC, USA). The adverse events were coded in MedDRA (MSSO, Chantilly, VA, USA).
Results
Baseline characteristics
All 24 patients who were dosed completed the study and complied with the protocol. The primary baseline characteristics of the patients according to treatment groups are summarized in Table 1. At baseline, the mean age was 34 years, log10 viral load was 4.5 copies/ml and CD4+ and CD8+ T-cell counts were 417 cells/mm3 and 975 cells/mm3 respectively. Of the total number of patients, 54% were treatment-naive and 46% had previous antiretroviral therapy. Prior resistance testing was not available for the treatment-experienced patients as routine resistance testing is not necessarily part of the clinical management of HIV-1 infection in Argentina. The patients, by choice or per discretion of treating physicians, were off treatment and not monitored for resistance. Of significance, no patients at baseline had NRTI mutations, but two patients in the amdoxovir plus zidovudine 300 mg arm had NNRTI mutations. Protease inhibitor (PI) mutations were observed in all study participants; however, the PI mutations probably represent polymorphisms and not primary mutations. In 96% of patients, no baseline resistance to PI or boosted PI regimens was detected. One patient in the amdoxovir plus zidovudine 200 mg group had possible resistance to atazanavir and tipranavir/ritonavir at baseline. No new mutations were detectable at day 10.
Table 1.
Baseline patient characteristics according to treatment group
| Characteristic | Placebo (n=2) | ZDV 200 mg (n=2) | ZDV 300 mg (n=2) | AMDX 500 mg (n=6) | AMDX 500 mg + ZDV 200 mg (n=6) | AMDX 500 mg + ZDV 300 mg (n=6) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male, n (%) | 2 (100) | 0 | 1 (50) | 5 (83) | 4 (67) | 0 |
| Female, n (%) | 0 | 2 (100) | 1 (50) | 1 (17) | 2 (33) | 6 (100) |
| Mean age ± sd, years | 27.0 ± 4.2 | 47.5 ± 3.5 | 24.0 ± 2.8 | 36.7 ± 10.4 | 37.3 ± 9.7 | 30.0 ± 5.2 |
| (min-max)a | (24.0–30.0) | (45.0–50.0) | (22.0–26.0) | (21.0–49.0) | (23.0–52.0) | (21.0–36.0) |
| Mean log10 viral load, copies/ml | 4.1 | 3.8 | 5.0 | 4.7 | 4.5 | 4.5 |
| (min-max)a | (3.9–4.2) | (3.6–3.9) | (4.9–5.1) | (4.3–5.0) | (3.7–6.0) | (4.0–5.1) |
| Patients with viral load >5 log10, n | 0 | 0 | 1 | 0 | 1 | 1 |
| Mean CD4+ T-cell count, cells/mm3 | 588 | 366 | 336 | 378 | 472 | 388 |
| (min-max)a | (570–606) | (270–461) | (227–445) | (234–614) | (237–1071) | (201–580) |
| Any previous ART, n (%) | 1 (50) | 0 | 1 (50) | 2 (33) | 2 (33) | 5 (83) |
| ATC class | ||||||
| None, % | 50 | 100 | 50 | 67 | 67 | 17 |
| PI, % | 50 | 0 | 0 | 33 | 0 | 0 |
| NRTI, % | 50 | 0 | 50 | 33 | 67 | 83 |
| NNRTI, % | 0 | 0 | 50 | 17 | 33 | 33 |
| ART combination | ||||||
| None, % | 50 | 100 | 50 | 67 | 67 | 17 |
| NRTI, % | 0 | 0 | 0 | 0 | 17 | 50 |
| PI+NRTI, % | 50 | 0 | 0 | 17 | 0 | 0 |
| NRTI+NNRTI, % | 0 | 0 | 50 | 0 | 17 | 33 |
| PI+NRTI+NNRTI, % | 0 | 0 | 0 | 17 | 0 | 0 |
| Patients with NRTI mutations, n | 0 | 0 | 0 | 0 | 0 | 0 |
| Patients with NNRTI mutations, n | 0 | 0 | 0 | 0 | 0 | 2 |
| Patients with PI mutations, nb | 2 | 2 | 2 | 6 | 6 | 6 |
min–max, minimum and maximum values.
Mutations probably represent polymorphisms and not primary protease inhibitor mutations. AMDX, amdoxovir; ART, antiretroviral therapy; ATC, anatomical, therapeutic and chemical; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; ZDV, zidovudine.
Antiviral activity
The virus depletion AUCVL of plasma HIV-1 RNA over the 10 day treatment period according to treatment group is shown in Figure 1. The mean ± sd for AUCVL was 0.41 ± 1.17 with placebo, −4.50 ± 0.19 with zidovudine 200 mg, −2.42 ± 1.80 with zidovudine 300 mg, −6.69 ± 4.57 with amdoxovir, −11.98 ± 1.86 with amdoxovir plus zidovudine 200 mg and −11.32 ± 1.44 with amdoxovir plus zidovudine 300 mg. The two amdoxovir plus zidovudine combination groups had the greatest mean AUCs, which were comparable. The ANCOVA results showed amdoxovir/placebo (P<0.0001) and zidovudine (P=0.013) to be significant cofactors with no significant amdoxovir–zidovudine interaction effect (P=0.597). Three significant pairwise comparisons were noted: amdoxovir plus zidovudine 300 mg versus zidovudine 300 mg (P=0.005), amdoxovir plus zidovudine 200 mg versus amdoxovir (P=0.019) and amdoxovir plus zidovudine 300 mg versus amdoxovir (P=0.045).
Figure 1.
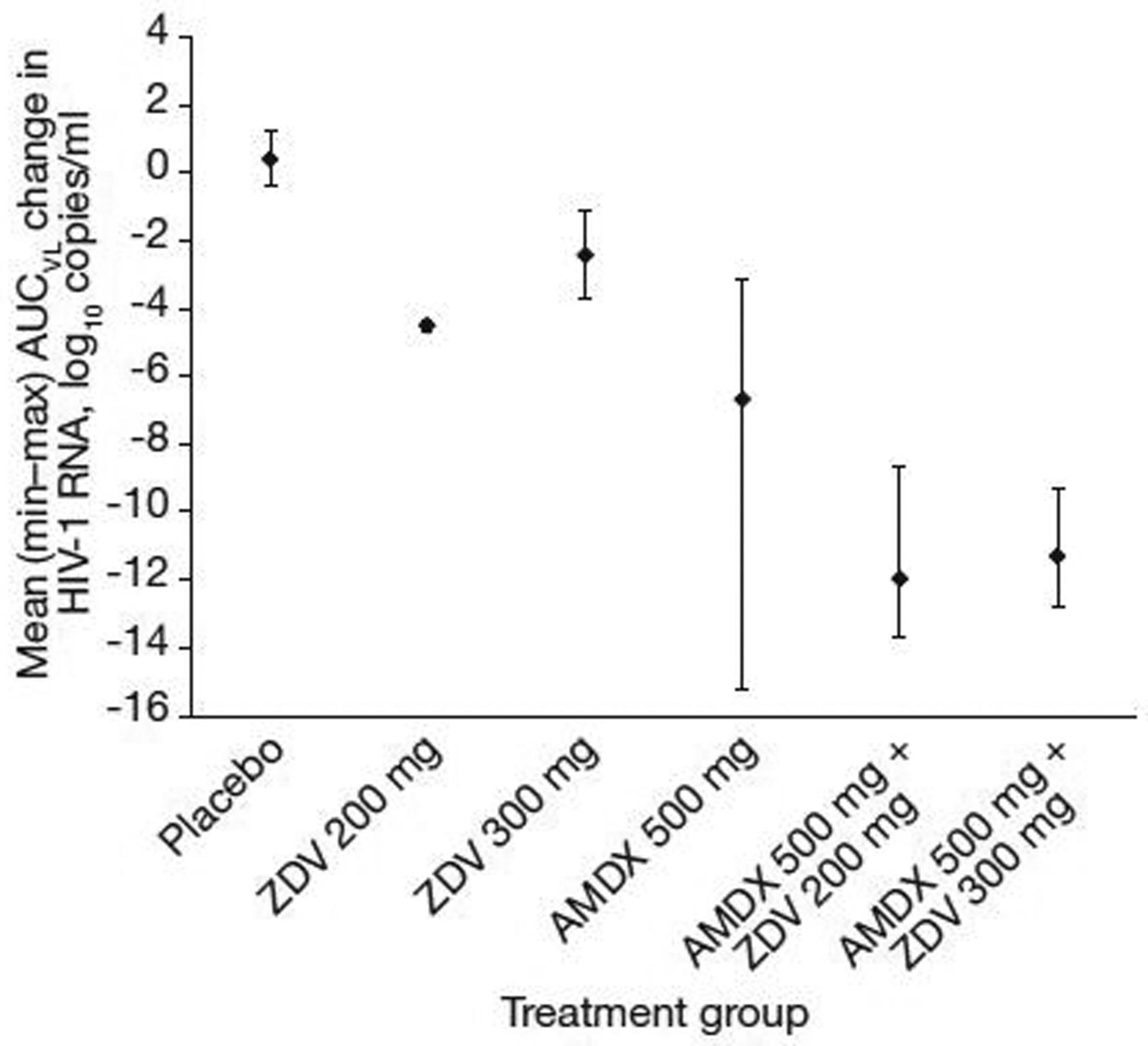
Area under the virus depletion curve by treatment
The mean is represented by a black diamond; bars represent minimum and maximum values. AMDX, amdoxovir; AUC, area under the curve; HIV-1, HIV type-1; VL, viral load; ZDV, zidovudine.
The mean change in viral load (log10 copies/ml) by treatment group over time is shown in Figure 2. The log10 mean change in viral load from baseline at the end of treatment was as follows: 0.10 (placebo), −0.69 (zidovudine 200 mg), −0.55 (zidovudine 300 mg), −1.1 (amdoxovir 500 mg), −2.0 (amdoxovir plus zidovudine 200 mg) and −1.7 (amdoxovir plus zidovudine 300 mg). Viral load reductions for amdoxovir plus zidovudine 200 mg were significantly greater than for amdoxovir monotherapy at days 8–12 (P=0.003, 0.008, 0.021, 0.005 and 0.016, respectively), suggesting synergy (as calculated by adding log10 viral load declines for the individual drugs of the combination) and persisted 2 days post-treatment. Viral load decline was significantly improved with amdoxovir plus zidovudine 200 mg and 300 mg combinations, compared with zidovudine monotherapy at days 11 (P=0.036 and P=0.015, respectively) and 12 (P=0.027 and P=0.024, respectively). There was no significant difference between amdoxovir plus zidovudine 200 mg and amdoxovir plus zidovudine 300 mg combination treatments. Furthermore, there was markedly decreased viral load variability with both amdoxovir plus zidovudine combinations compared with amdoxovir alone. Viral load returned to pre-treatment levels following treatment cessation, but there was a continued post-treatment antiviral effect with amdoxovir in combination with zidovudine 200 or 300 mg groups.
Figure 2.
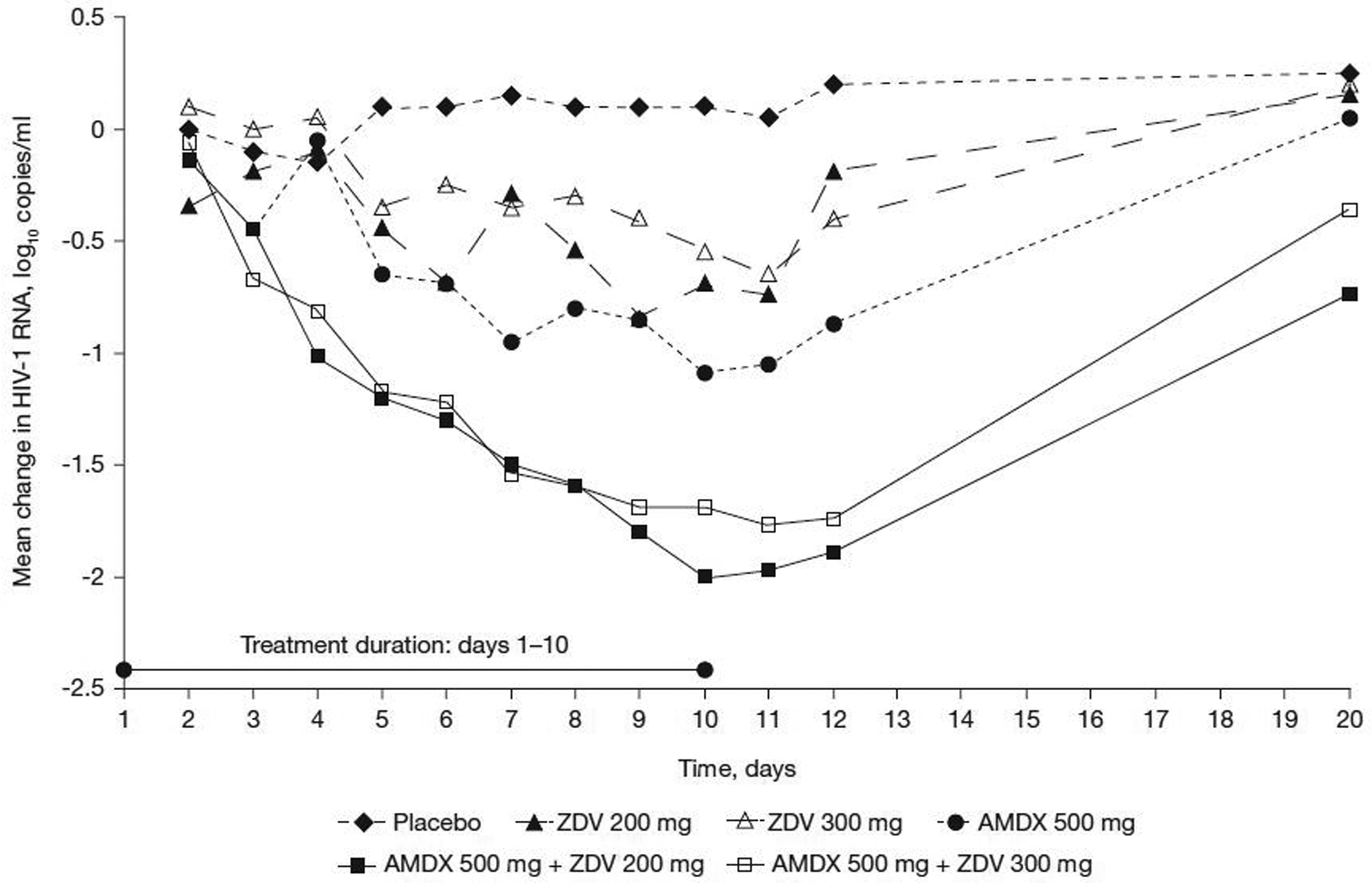
Mean change in plasma viral load levels by treatment and day
AMDX, amdoxovir; HIV-1, HIV type-1; ZDV, zidovudine.
The proportion of patients with at least 1.0, 1.5 log10 copies/ml decline in viral load or who had HIV-1 RNA<400 copies/ml on the last day of treatment is shown in Figure 3. As anticipated, none of the patients in the placebo or zidovudine 200 mg or 300 mg monotherapy groups exhibited a ≥1.0 log10 decrease or HIV-1 RNA<400 copies/ml at the end of treatment, whereas all of the patients in the two amdoxovir plus zidovudine groups had a >1.0 log10 decrease with 50% achieving HIV-1 RNA<400 copies/ml. All patients in the amdoxovir plus zidovudine 200 mg group and five out of six patients in the amdoxovir plus zidovudine 300 mg group exhibited a 1.5 log10 drop in viral load. One patient in the amdoxovir plus zidovudine 200 mg group reached HIV-1 RNA<50 copies/ml at days 11–12. The greatest mean maximum reduction in viral load value from baseline through day 12 was seen in the amdoxovir plus zidovudine 200 mg group (‒2.07 ± 0.16 log10 copies/ml) followed by the amdoxovir plus zidovudine 300 mg group (−1.84 ± 0.28 log10 copies/ml) and the amdoxovir monotherapy group (−1.22 ± 0.30 log10 copies/ml). This coincided with the lowest viral load value observed through day 12: amdoxovir plus zidovudine 200 mg group (2.50 ± 0.72 log10 copies/ml) followed by the amdoxovir plus zidovudine 300 mg group (2.70 ± 0.37 log10 copies/ml) and the amdoxovir monotherapy group (3.48 ± 0.67 log10 copies/ml). The ANCOVA results indicated that amdoxovir/placebo and zidovudine had significant effects on the maximum reduction in viral load and produced the lowest viral load values. Significant pairwise comparisons for both outcomes were observed for amdoxovir plus zidovudine 300 mg versus zidovudine 300 mg monotherapy (P=0.008; P=0.008) and amdoxovir plus zidovudine 200 mg versus amdoxovir monotherapy (P=0.015; P=0.015).
Figure 3.
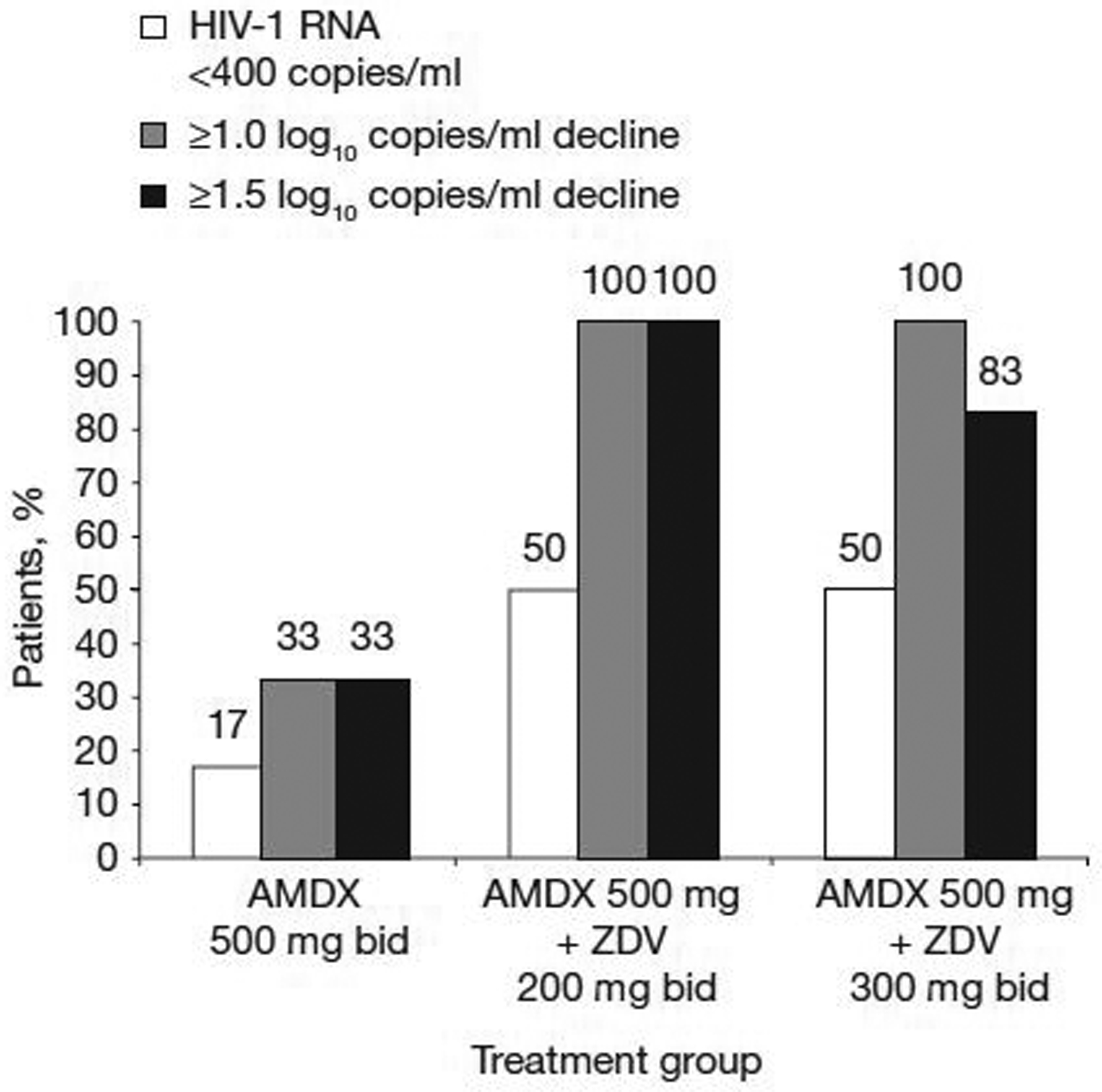
Proportion of individuals (n=6 per group) with at least 1.0, 1.5 log10 copies/ml viral load decline or HIV-1 RNA<400 copies/ml at day 10
AMDX, amdoxovir; bid, twice daily; HIV-1, HIV type-1; ZDV, zidovudine.
During the short treatment period, there were no clinically significant changes in the absolute CD4+ or CD8+ T-cell counts in all treatment groups by day 10. Changes in the absolute CD4+ and CD8+ T-cell counts ranged from −315 to 161 cells/mm3 and −402 to 817 cells/mm3, respectively.
Tolerability
The 10-day treatment with amdoxovir/placebo with or without zidovudine was well-tolerated; there were no early withdrawals, discontinuations or serious adverse events reported in the study. The adverse events assessed by the principal investigator as at least remotely related to the study medication are presented in Table 2.
Table 2.
Treatment-emergent and treatment-related adverse events according to treatment group
| Adverse event | Placebo (n=2) | ZDV 200 mg (n=2) | ZDV 300 mg (n=2) | AMDX 500 mg (n=6) | AMDX 500 mg plus ZDV 200 mg (n=6) | AMDX 500 mg plus ZDV 300 mg (n=6) | Total (n=24) |
|---|---|---|---|---|---|---|---|
| At least one adverse event, n (%) | 2 (100) | 2 (100) | 0 | 4 (67) | 5 (83) | 6 (100) | 19 (79) |
| Headache, n (%) | 1 (50) | 1 (50) | 0 | 1 (17) | 3 (50) | 3 (50) | 9 (37) |
| Nausea, n (%) | 0 | 0 | 0 | 0 | 3 (50) | 3 (50) | 6 (25) |
| Dyspepsia, n (%) | 2 (100) | 1 (50) | 0 | 1 (17) | 0 | 1 (17) | 5 (21) |
| Abdominal pain upper, n (%) | 0 | 1 (50) | 0 | 0 | 1 (17) | 0 | 2 (8) |
| Back pain, n (%) | 1 (50) | 0 | 0 | 0 | 1 (17) | 0 | 2 (8) |
| Constipation, n (%) | 0 | 0 | 0 | 1 (17) | 0 | 1 (17) | 2 (8) |
| Stomach discomfort, n (%) | 0 | 0 | 0 | 0 | 0 | 2 (33) | 2 (8) |
| Abdominal pain, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (4) |
| Anxiety, n (%) | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Chest pain, n (%) | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Diarrhoea, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (4) |
| Fatigue, n (%) | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Mucosal inflammation, n (%) | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Muscle contracture, n (%) | 0 | 1 (50) | 0 | 0 | 0 | 0 | 1 (4) |
| Rhinitis seasonal, n (%) | 0 | 0 | 0 | 1 (17) | 0 | 0 | 1 (4) |
| Vomiting, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (4) |
AMDX, amdoxovir; ZDV, zidovudine.
Discussion
This 10-day monotherapy and dual-therapy design provided a direct and rapid assessment of the potential activity and tolerability of amdoxovir in combination with zidovudine and the short duration of the study limited the potential for the development of drug resistance. A direct characterization of efficacy, safety and tolerability without the confounding effects of other antiretroviral agents is possible with short-term monotherapy trials involving antiretroviral-naive patients, although this study evaluated treatment-naive and treatment-experienced patients who had not been treated for at least 90 days [27].
The significant antiviral activity observed for the two combination groups compared with the amdoxovir or zidovudine monotherapy groups after 10 days of treatment suggests a synergistic interaction between the two NRTIs, as noted by the greater than additive log10 reduction in viral load. Among all the actively treated patients, those randomized to amdoxovir plus zidovudine 200 mg exhibited the greatest reduction in viral load; 50% of the patients achieved at least a 2 log10 drop in viral load at day 10. As anticipated from the in silico studies [25], the antiviral activity observed in the amdoxovir plus zidovudine 300 mg group was not significantly different from amdoxovir plus zidovudine 200 mg, although the magnitude of change was numerically higher in the latter group at day 10. Likewise, the magnitude of the overall incidence rate of treatment-related adverse events in the amdoxovir plus zidovudine 300 mg group was comparable, but numerically higher than for the amdoxovir plus zidovudine 200 mg group. The results support the hypothesis that lowering the dose of zidovudine when administered in combination with amdoxovir does not affect antiviral activity and is synergistic. These data were in agreement with the in silico finding that a lower dose of zidovudine retains the antiviral effect of the standard dose while decreasing its toxicity [25].
The mean viral load decline from baseline of −1.1 log10 at day 10 observed with amdoxovir monotherapy was consistent with that reported previously. In a short-term study involving treatment-naive patients, the median viral load reduction was −1.3 log10 at day 15 [22]. Clinically significant improvements in CD4+ T-cell count would not be expected following 10 days of treatment and was not observed in this study. The active metabolite of amdoxovir (DXG) produces reasonably high levels of DXG-triphosphate, which has a long intracellular half-life of 16 h in activated peripheral blood mononuclear cells [16]. Thus, in combination with zidovudine, it is reasonable to observe a gradual decline in antiviral effect following cessation of therapy. There were no serious adverse events and no treatment discontinuations. Likewise, the pharmacokinetic data from this study did not show any drug–drug interactions [28]. This apparently benign tolerability profile and the potent antiviral activity observed in this study makes amdoxovir plus zidovudine a promising NRTI combination product. However, the generalization of the results is limited by the small sample size and the short treatment duration.
In conclusion, amdoxovir in combination with reduced or approved dose of zidovudine showed promising antiviral activity, good short-term tolerability and a low propensity for resistance selection in patients treated for 10 days. These results are significant and provide initial clinical data to support the viability of a combination of amdoxovir plus the approved or reduced dose of zidovudine for the treatment of HIV-1 infection. It should be noted that in certain countries like Thailand, the National Guidelines already recommend zidovudine at the reduced dose of 200 mg twice daily in persons <60 kg in weight [29]. In persons failing current first-line therapies, there is an ongoing need for new antiretroviral agents of existing classes with improved efficacy, tolerability and a high genetic barrier to resistance that could prevent or delay the emergence of resistance. On the basis of the clinical study presented herein and previously published studies [21], the combination of amdoxovir plus zidovudine has the potential to provide benefit as an important nucleoside treatment option, particularly in delaying the emergence of NRTI resistance. This combination also has the potential to provide a bridging regimen in failing persons with the option to postpone the introduction of new drug classes. Additional long-term studies in patients failing therapy is warranted to further evaluate the long-term efficacy and safety of this combination.
Acknowledgements
The authors acknowledge the assistance of the following individuals: Jose Luis Ippolito MD in managing this study (ACLIRES-Argentina, Buenos Aires, Argentina); Elizabeth Belsey PhD for statistical analysis and programming (SigmaClinical, Mas de Cause, France); Marina Machera PhD for clinical site monitoring (ACLIRES-Argentina, Buenos Aires, Argentina) and all the patients who participated in this study. The work presented herein was supported by RFS Pharma, LLC. RFS is supported by the National Institutes of Health sponsored Center for AIDS (2P30-AI-0504) and the Department of Veterans Affairs. ClinicalTrials.gov number: NCT00432016
Footnotes
Disclosure statement
RFS, as an inventor of the amdoxovir technology and founder of RFS Pharma, has a financial interest that has been reviewed and approved by Emory University in compliance with its conflict of interest policies. RLM is a consultant to RFS Pharma. The remaining authors declare no competing interests.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services (Updated 3 November 2008; Accessed 12 December 2008). Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 2.Pereira CF, Paridaen JT. Anti-HIV drug development – an overview. Curr Pharm Des 2004; 10:4005–4037. [DOI] [PubMed] [Google Scholar]
- 3.Gu Z, Wainberg MA, Nguyen-Ba N, et al. Mechanism of action and in vitro activity of 1′,3′-dioxolanylpurine nucleoside analogues against sensitive and drug-resistant human immunodeficiency virus type 1 variants. Antimicrob Agents Chemother 1999; 43:2376–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Z, Wainberg MA, Nguyen-Ba P, L’Heureux L, de Muys JM, Rando RF. Anti-HIV-1 activities of 1,3-dioxolane guanine and 2,6-diaminopurine dioxolane. Nucleosides Nucleotides 1999; 18:891–892. [DOI] [PubMed] [Google Scholar]
- 5.Mewshaw JP, Myrick FT, Wakefield DACS, et al. Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails. J Acquir Immune Defic Syndr 2002; 29:11–20. [DOI] [PubMed] [Google Scholar]
- 6.Bazmi HZ, Hammond JL, Cavalcanti SCH, Chu CK, Schinazi RF, Mellors JW. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (−)-beta-D-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother 2000; 44:1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh UM, Bacheler L, Koontz DL, Mellors JW. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J Virol 2006; 80:4971–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapp KL, Ruckstuhl M, Schinazi RF. The combination of zidovudine and amdoxovir prevents the selection of thymidine analogue mutations in primary human lymphocytes. 16th International HIV Drug Resistance Workshop 12–16 June 2007, Barbados Abstract 117. [Google Scholar]
- 9.Parikh UM, Barnas DC, Faruki H, Mellors JW. Antagonism between the HIV-1 reverse transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J Infect Dis 2006; 194:651–660. [DOI] [PubMed] [Google Scholar]
- 10.Parikh UM, Sluis-Cremer N, Mellors JW. Kinetic mechanism by which thymidine analogue mutations antagonize K65R in HIV-1 reverse transcriptase. Antivir Ther 2005; 10:S95. [Google Scholar]
- 11.Parikh UM, Zalina S, Sluis-Cramer N, Mellors JW. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS 2007; 21:1405–1407. [DOI] [PubMed] [Google Scholar]
- 12.Segondy M, Montes B. Prevalence and conditions of selection of the K65R mutation in the reverse transcriptase gene of HIV-1. J Acquir Immune Defic Syndr 2005; 38:110–111. [DOI] [PubMed] [Google Scholar]
- 13.Valer L, Martin-Carbonero L, de Mendoza C, Corral A, Soriano V. Predictors of selection of K65R: tenofovir use and lack of thymidine analogue mutations. AIDS 2004; 18:2094–2096. [DOI] [PubMed] [Google Scholar]
- 14.Stein DS, Moore KHP. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy 2001; 21:11–34. [DOI] [PubMed] [Google Scholar]
- 15.Feng JY, Parker WB, Krajewski ML, et al. Anabolism of amdoxovir: phosphorylation of dioxolane guanosine and its 5′-phosphates by mammalian phosphotransferases. Biochem Pharmacol 2004; 68:1879–1888. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Santiago BI, Obikhod A, Fromentin E, Hurwitz SJ, Schinazi RF. Cellular pharmacology of 9-(beta-D-1,3-dioxolan-4-yl)guanine and its lack of drug interactions with zidovudine in primary human lymphocytes. Antivir Chem Chemother 2007; 18:343–346. [DOI] [PubMed] [Google Scholar]
- 17.Gilliam BL, Sajadi MM, Amoroso A, Davis CE, Cleghorn FR, Redfield RR. Tenofovir and abacavir combination therapy: lessons learned from an urban clinic population. AIDS Patient Care STDS 2007; 21:240–246. [DOI] [PubMed] [Google Scholar]
- 18.Lanier ER, Givens N, Stone C, et al. Effect of concurrent zidovudine use on the resistance pathway selected by abacavir containing regimens. HIV Med 2004; 5:394–399. [DOI] [PubMed] [Google Scholar]
- 19.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004; 350:1850–1861. [DOI] [PubMed] [Google Scholar]
- 20.Elion R, Cohen C, DeJesus E, et al. COL40263: resistance and efficacy of once-daily trizivir and tenofovir DFin antiretroviral naive subjects. 11th Conference on Retroviruses and Opportunistic Infections 8–11 February 2004, San Francisco, CA, USA Abstract 53. [Google Scholar]
- 21.Won SY, Kessler HA. Amdoxovir The use of antibiotics: a clinical review of antibacterial, antifungal and antiviral drugs. Sixth edition The Bath Press, Avon, UK; in press 2009. [Google Scholar]
- 22.Thompson MA, Kessler HA, Eron JJ Jr, et al. Short-term safety and pharmacodynamics of amdoxovir in HIV-infected patients. AIDS 2005; 19:1607–1615. [DOI] [PubMed] [Google Scholar]
- 23.Gripshover BM, Ribaudo H, Santana J, et al. (A5118 Team). Amdoxovir versus placebo with enfuvirtide plus optimized background therapy for HIV-1-infected subjects failing current therapy (ACTG A5118). Antivir Ther 2006; 11:619–623. [PubMed] [Google Scholar]
- 24.Margolis DM, Mukherjee AL, Fletcher CV, et al. W for the ACTG 5165 team. The use of β-D-2,6-diaminopurine dioxolane with or without mycophenolate mofetil in drug-resistant HIV infection. AIDS 2007; 21:2025–2032. [DOI] [PubMed] [Google Scholar]
- 25.Hurwitz SJ, Asif G, Kivel NM, Schinazi RF. Development of an optimized dose for coformulation of zidovudine with drugs that select for the K65R mutation using a population pharmacokinetic and enzyme kinetic simulation model. Antimicrob Agents Chemother 2008; 52:4241–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers of Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993; 269:729–730. [PubMed] [Google Scholar]
- 27.Wainberg MA, Sawyer JP, Montaner JS, et al. Challenges for the clinical development of new nucleoside reverse transcriptase inhibitors for HIV infection. Antivir Ther 2005; 10:13–28. [PubMed] [Google Scholar]
- 28.Hurwitz SJ, Asif G, Fromentin E, Tharnish PM,Schinazi RF. Lack of pharmacokinetic interaction between amdoxovir and reduced and standard close zidovudine in HIV-1 infected individuals. Antimicrob Agents Chemother 2009; 54:1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thai Ministry of Public Health. National Guidelines for The Clinical Management of HIV Infection in Children and Adults. 7th ed. Nonthaburi, Thailand: Department of Disease Control, Ministry of Public Health; 2002. [Google Scholar]


