Abstract
Puberty is a developmental period characterized by a broad range of physiologic changes necessary for the acquisition of adult sexual and reproductive maturity. These changes mirror complex modifications within the central nervous system, including within the hypothalamus. These modifications result in the maturation of a fully active hypothalamic–pituitary–gonadal (HPG) axis, the neuroendocrine cascade ensuring gonadal activation, sex steroid secretion, and gametogenesis. A complex and finely regulated neural network overseeing the HPG axis, particularly the pubertal reactivation of gonadotropin-releasing hormone (GnRH) secretion, has been progressively unveiled in the last 3 decades. This network includes kisspeptin, neurokinin B, GABAergic, and glutamatergic neurons as well as glial cells. In addition to substantial modifications in the expression of key targets, several changes in neuronal morphology, neural connections, and synapse organization occur to establish mature and coordinated neurohormonal secretion, leading to puberty initiation. The aim of this review is to outline the current knowledge of the major changes that neurons secreting GnRH and their neuronal and glial partners undergo before and after puberty. Emerging mediators upstream of GnRH, uncovered in recent years, are also addressed herein. In addition, the effects of sex steroids, particularly estradiol, on changes in hypothalamic neurodevelopment and plasticity are discussed.
Keywords: puberty, neurodevelopment, plasticity, hypothalamus, GnRH neuron
Puberty is a critical period of development characterized by the acquisition of sexual maturity. In humans, the age of puberty onset varies according to multiple factors, such as genetic background, ethnicity, and gender. The first pubertal signs are generally observed between the ages of 8 and 12 years in girls, with the appearance of mammary buds (thelarche) and between 9 and 14 years in boys, with the onset of testicular enlargement (1). A number of hormonal, neural, and behavioral changes are observed during this period, highlighting the important contribution of neurodevelopment and brain plasticity (2). The hypothalamic–pituitary–gonadal (HPG) axis is the physiologic cascade ensuring gonadal activation and triggering the onset of puberty. The HPG axis, already active during the final months of prenatal life and shortly after birth, remains silent throughout childhood and resumes activity just prior to pubertal changes (3). At the level of the hypothalamus, this reactivation results from the awakening of a complex neuronal network that culminates in the release of the gonadotropin-releasing hormone (GnRH) neuropeptide (Fig. 1) (4, 5). An extensive body of knowledge has identified GnRH as the master regulator of the HPG axis (6, 7). GnRH neurons originate from the olfactory placode and follow a migratory route through the forebrain to settle in discrete hypothalamic regions. Once in place, these neurons project their axons to the median eminence, from which they release the decapeptide GnRH in a pulsatile manner. GnRH enters the hypophyseal portal circulation to stimulate the secretion of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone, that stimulate the gonads to synthesize and secrete sex steroids and produce gametes. Circulating gonadal sex steroids, responsible for the development of external genitalia along with other physical changes, in turn exert either negative or positive feedback at the level of the hypothalamus to regulate the release of GnRH. Over the past 3 decades, a complex and finely regulated neural network overseeing GnRH activity has been progressively unveiled. Neural elements producing kisspeptin, neurokinin B, glutamate, GABA, and other neuropeptides have been identified as major regulators of pulsatile GnRH secretion. These neuronal systems, together with glial and other non-neuronal partners, undergo a host of morphological, synaptic, and molecular changes during development, particularly as puberty approaches (2, 8). While many aspects of pubertal maturation of the brain are not yet fully understood, particularly within the hypothalamus, this review aims to present the current knowledge of pubertal hypothalamic development and plasticity and to discuss future challenges.
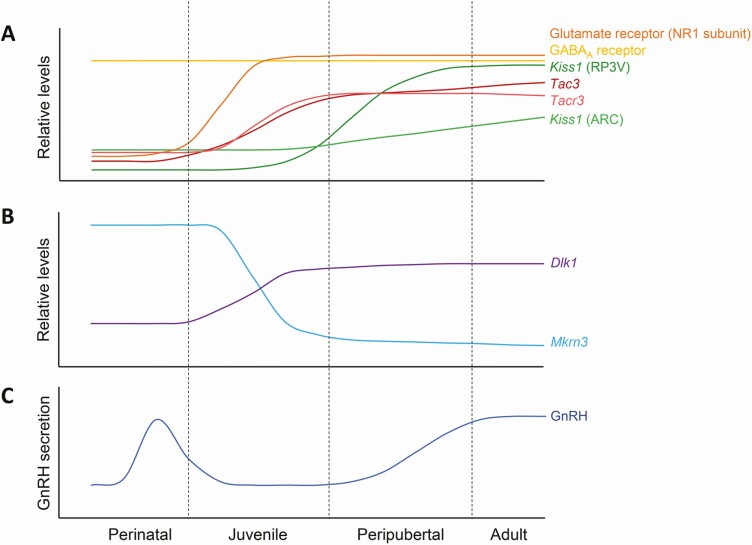
Figure 1.
Schematic depiction of levels of hypothalamic factors involved in the regulation of the GnRH network according to developmental stage. The upper panel (A) shows changes in protein levels of GABA and glutamate receptor components and mRNA levels of neuropeptides involved in regulation of the GnRH network. The central panel (B) shows changes in mRNA levels of Mkrn3 and Dlk1, 2 genes in which loss of function mutations in humans result in central precocious puberty. The lower panel (C) shows GnRH secretion over time during postnatal development.
Human Brain Development and Plasticity During Puberty
In humans, the brain develops from the third week of prenatal life until adolescence. Neurogenesis, gliogenesis, and cell migration are the first embryonic developmental processes. Under the effect of locally produced guidance cues, neurons migrate to their final destination, where they differentiate, grow dendrites and axons, and begin to form synapses (8). This neuronal maturation begins prenatally and continues throughout adolescence. It is well demonstrated, especially in the cortex, that there is developmental overproduction of neurons and synapses. A high level of neuronal cell death occurs prenatally, which is critical for the establishment of effective and functional neural circuits (8). In addition, there is a peak of synapse formation between the first and the second year of life, which is later eliminated in the course of postnatal and pubertal development. This process of refinement is referred to as synaptic pruning and has proven to be essential for efficient information processing in adulthood (9, 10). In parallel, glial cell differentiation and maturation (astrocytes, microglia, oligodendrocytes) take place primarily postnatally, lasting through adolescence (8). In adult brains, astrocytes outnumber neurons and provide several functions, going far beyond trophic support. Oligodendrocytes are particularly essential for neuronal maturation, as they form the myelin sheaths surrounding axons, referred to as myelination, which is a prerequisite for effective neurotransmission (11, 12). Finally, in addition to the intrinsic properties of both neurons and glial cells and neuron–glia interaction, extracellular matrix and cell adhesion molecules have been shown to play prominent roles in brain development by guiding cell migration, neurite outgrowth, and synaptogenesis (13, 14).
Throughout the lifespan, changes in the brain can occur in each of these processes, driven by both genetic and environmental influences. Brain plasticity (or neuroplasticity) refers to the ability of the brain to change its activity in response to intrinsic or extrinsic stimuli by reorganizing its structures, functions, or connections. Interestingly, structural and functional changes are particularly enhanced at the time of puberty (15). Magnetic resonance imaging (MRI) technologies have allowed the assessment of human brain changes across development and puberty. Apart from conventional T1- and T2-weighted images, the most common radiofrequency pulse sequences that highlight fat tissue (T1) or fat and water (T2), a variety of additional magnetic resonance–based techniques have helped to refine our understanding of brain structure and provided additional data relevant to plasticity. For instance, water diffusion, assessed by diffusion tensor imaging, has permitted indirect investigation of the microstructural components of white matter and quantitative myelination (16). Cortical gray matter density, indirectly measuring glia, vasculature, and neurons (including dendrites and synapses), can be described by an inverted U-shaped curve, peaking in late childhood and then declining throughout adolescence (17, 18). This decline temporally correlates with postmortem findings of increased synaptic pruning during adolescence (9). By contrast, cortical white matter, constituted essentially by myelinated axons, increases progressively from birth to adolescence across most brain regions (19, 20). This observation is consistent with postmortem histological evidence of ongoing myelination during this period (21).
While initial studies analyzed brain development in relation to chronological age, more recent studies have shown that the development of several cortical and subcortical regions correlates more specifically with pubertal stages than with chronological age (22, 23). Indeed, independently of age, larger white matter volumes and increased maturation have been observed in postpubertal girls compared with prepubertal girls (16, 24). Likewise, at equivalent ages, girls showing signs of pubertal maturation develop central nervous system (CNS) changes, such as smaller gray matter volumes in several regions of the brain, including the frontal cortex, the amygdala, and hippocampus, compared with girls who had not yet entered puberty (22). These findings strengthen the hypothesis of a direct influence of pubertal timing on brain development and plasticity (2).
Hypothalamic Development and Plasticity in the Regulation of Pubertal Timing
Human studies of neuroplasticity have focused primarily on cortical and subcortical regions, particularly the cortex, hippocampus, and amygdala. To our knowledge, no structural or functional studies have been reported on the human hypothalamus at puberty. The absence of data about hypothalamic structural changes during puberty could in part be explained by the lack of sufficient fine spatial resolution of current neuroimaging techniques, which do not allow precise delimitation of hypothalamic areas and nuclei. New advances in neuroimaging should permit a more detailed exploration of the human hypothalamus across puberty. In this regard, an atlas of the adult human hypothalamus has been described using MRI and optimized protocols using high-resolution functional MRI (fMRI) have been developed (25-28). Diffusion tensor imaging (DTI) followed by fiber tractography has also been used recently in adults to identify fibers passing through specific hypothalamic nuclei (29).
While human studies regarding the mechanisms underlying pubertal hypothalamic neuroplasticity are limited, the use of animal models has allowed a better understanding of the pubertal changes that occur at the level of the GnRH neuron and its neural network. Studies in sheep and primates have considerably enhanced our knowledge of the physiological regulation of pulsatile GnRH secretion and have been thoroughly reviewed elsewhere (30, 31). This review will focus on the regulation of the timing of puberty in rodents in which the majority of studies regarding hypothalamic development and plasticity have been conducted.
GnRH neurons
GnRH neurons have a unique characteristic within the CNS of being born outside the brain in the olfactory placode and migrating through the forebrain to the hypothalamus during embryonic development. After migration, GnRH cell bodies are diffusely scattered within the medial septum, the organum vasculosum of the lamina terminalis, and the rostral preoptic area of the hypothalamus. The number of GnRH cell bodies is relatively low, with approximately 1000 neurons in rodent and 1500 to 2000 in human hypothalami (32, 33). Remarkably, using whole-mount immunolabeling and 3DISCO technology, a recent study identified around 10 000 GnRH cells in the human brain during fetal development, with around 2000 GnRH neurons located in the hypothalamus and 8000 GnRH neurons in extrahypothalamic regions (34). These data corroborate previous observations using in situ hybridization (35). The role of this extrahypothalamic GnRH neuronal population is still unknown and needs further investigation.
Once discretely settled in the hypothalamus, GnRH neurons send extensive projections to the median eminence (5, 36). These projections may extend for long distances (up to 1000 μm) and feature both axonal and dendritic properties (37, 38). Indeed, GnRH projections have spike initiation sites and actively conduct action potentials, and at the same time receive and integrate synaptic inputs along their entire length, thereby regulating the excitability of the neuron. These unique projections have been termed “dendrons” (38).
Although this organization is established before birth, morphological analyses in rodents have revealed remodeling of the dendritic structure and spine density of GnRH neurons across postnatal and prepubertal development. Immature multipolar GnRH neurons located in the rostral preoptic area are submitted to synaptic pruning and a complex dendritic reorganization to achieve unipolar or bipolar arrangements typical of mature GnRH neurons (Fig. 2) (39, 40). Concomitantly, a postnatal increase in the number of spines has been observed at the level of both somal and proximal dendrites (39, 41). Electrophysiological analyses show that GnRH neurons of juvenile mice, although displaying the same passive membrane properties as adult neurons, exhibit an enhanced heterogeneity in their firing properties (42). Interestingly, a recent study evaluating the firing activity of GnRH neurons by extracellular recording of GnRH-green fluorescent protein (GFP) neurons revealed that GnRH neurons are more active during the prepubertal period than in adulthood. Indeed, there is an increase in GnRH neuronal activity during the first 3 weeks of development, followed by a decline to adult levels (43). It is worth mentioning that, if experimentally isolated from the rest of the brain, prepubertal GnRH neurons are capable of generating pulses (44). This suggests that a strong network upstream of the GnRH neuron controls the juvenile restraint of GnRH neuronal activity, which is subsequently counterbalanced by an increase in excitatory influences. How these inhibitory and stimulatory networks develop and mature during the postnatal/prepubertal period and the underlying neuroplasticity of these networks remain unclear. The current knowledge of the major GnRH regulators and their roles in neurodevelopment and neuroplasticity are presented in the following sections.
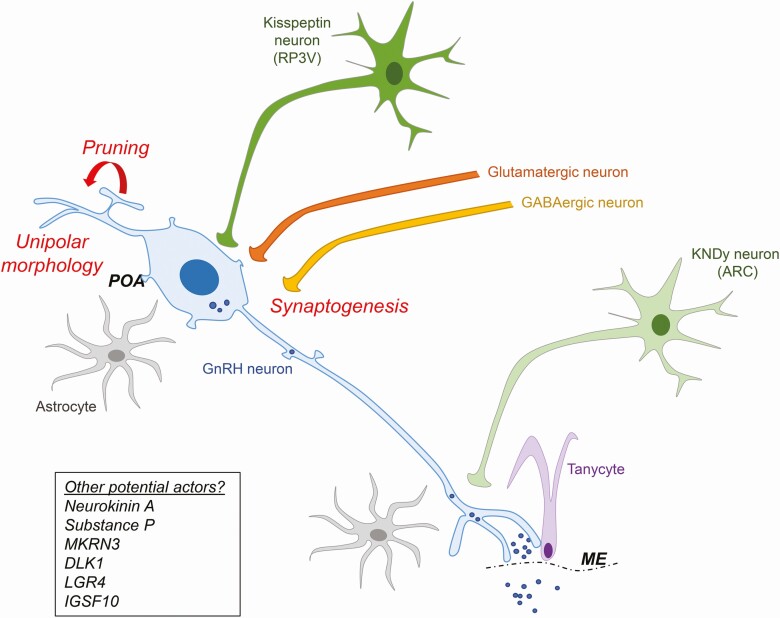
Figure 2.
Schematic representation of pubertal morphological changes in GnRH neurons and the upstream neural network. During pubertal development, the GnRH neuron undergoes synaptic pruning, dendritic reorganization and increased synaptogenesis. There is an increase in GABAergic and glutamatergic projections on GnRH neurons, as well as an increase in kisspeptin neuron projections. GnRH secretion is also regulated by glial cells including astrocytes and tanycytes. POA, preoptic area; ME, median eminence. RP3V, rostral periventricular area.
Kisspeptin neurons
The large postnatal/prepubertal increase in spine density of GnRH neurons suggests an important increase in excitatory inputs directly at the level of the GnRH neuron in order to activate the HPG axis. A large body of knowledge based on clinical, molecular, and pharmacological studies has clearly demonstrated that kisspeptin is 1 of the most robust activators of GnRH neurons. Kisspeptin neurons are indispensable in the initiation of puberty and in the maintenance of a fully functional HPG axis throughout adult life (45-48). Mutations in genes encoding kisspeptin (KISS1) or its receptor (KISS1R) in humans, as well as deletion of these genes in mice, led to severe GnRH deficiency and failure to enter puberty (49-51). In rodents, kisspeptin neurons are prevalent in 2 distinct hypothalamic regions, the rostral periventricular area (RP3V) and the arcuate nucleus (ARC), respectively involved in the generation of the female LH surge and the activation of the GnRH pulse generator in both sexes. The mutual role of these 2 distinct kisspeptin populations in the control of puberty onset is, however, still debated. Although variable according to the species, a postnatal increase in Kiss1 mRNA and kisspeptin protein expression has been detected in both regions, with a more marked increase observed in the female RP3V (Fig. 1) (52). The pubertal regulation of Kiss1 expression in the ARC is tightly regulated by both repressive and stimulatory epigenetic pathways, particularly involving the Polycomb group (PcG-repressor) and the Trithorax group (TrxG-facilitator) complexes (53). It is important to note that a third population of kisspeptin neurons has more recently been identified in the medial amygdala, and appears to contribute to the timing of puberty (54, 55). A pubertal increase of Kiss1 expression has also been reported in this region (56).
GnRH neurons receive kisspeptin projections and express Kiss1r mRNA and kisspeptin receptor protein as early as embryonic day (E)13.5 in mice (57, 58). The percentage of GnRH neurons expressing Kiss1r increases markedly across postnatal development, from ~40% at birth to ~70% by postnatal day 20 (PND20) (59). Kisspeptin projections to GnRH neurons also increase between PND25 and adulthood (Fig. 2) (52). In addition, electrophysiological analyses reveal a significant increase in the percentage of GnRH neurons responsive to kisspeptin stimulation with pubertal maturation, from ~25% in juvenile to >90% in adult mice (60). Together, these findings reveal the presence of important morphological and functional neuronal changes within the kisspeptin/GnRH system across pubertal maturation.
KNDy neurons
In the rodent ARC, kisspeptin, neurokinin B (NKB) and dynorphin are expressed in the same neurons, referred to as KNDy neurons. The KNDy neuron population is essential in order to coordinate GnRH pulses (61, 62). In adult mice, this process appears to be regulated through autocrine and paracrine regulatory loops involving either stimulatory (kisspeptin and NKB) or inhibitory (dynorphin) actions (61). Beyond kisspeptin, NKB has been shown to be another major stimulator of GnRH and the HPG axis. Its role is supported by evidence of GnRH deficiency and pubertal failure in patients with loss-of-function mutations in NKB (encoded by TAC3 gene) and its receptor (TAC3R). In contrast, Tac3 knockout in female mice results in pubertal delay but maintenance of fertility (63, 64). In rodents, Tac3 and Tacr3 expression increases in the ARC during pubertal maturation (Fig. 1) (65, 66). The pubertal control of Tac3 expression is regulated, like Kiss1, by an epigenetic switch from transcriptional repression to activation (53). Several studies have also revealed the involvement of neurokinin A and substance P, belonging to the family of tachykinins, in the modulation of pubertal timing in rodents (67, 68). How this tachykinin neuronal system develops during the postnatal/prepubertal period and its underlying synaptic plasticity remains to be elucidated.
GABAergic neurons
Apart from kisspeptin and KNDy neurons, GnRH neurons also receive significant synaptic inputs from both GABAergic and glutamatergic neurons, the principal inhibitory and excitatory systems in the adult brain (Fig. 2). These 2 systems have been shown to undergo significant modifications across postnatal and pubertal brain maturation (11, 69, 70). GnRH neurons express functional GABA (GABAA and GABAB) and glutamate (NMDA and AMPA) receptors (71-73). Little is known about the locations of glutamatergic and GABAergic cell bodies that project to GnRH neurons, but the RP3V seems to represent a primary site (74). In addition, kisspeptin neurons in the ARC appear to be mostly glutamatergic, while those in the RP3V are predominantly GABAergic and thereby account for some of the projections (75, 76).
GnRH neurons express functional GABAA receptors from the earliest stages of embryonic development (Fig. 1) (77). GABAA receptors are pentameric chloride channels composed of specific α, β, γ, and δ subunits. A postnatal increase in GABAA receptor sensitivity is observed in conjunction with the maturation of the GnRH neuron. Indeed, electrophysiological analyses showed that juvenile animals required up to 10-fold higher GABA concentrations than adults to establish a concentration–response curve and displayed a reduction in the level of membrane depolarization in response to GABA compared with adults (78). This increase is accompanied by a prepubertal reorganization of GABAA receptor signaling, with a change in receptor subunit composition and consequently a change in cell signaling properties (78, 79). A recent electrophysiological study revealed an increase in GABAergic transmission frequency in GnRH neurons during the first 4 weeks of postnatal/prepubertal development (80). Although heavily debated, the prevailing view supports an inhibitory action of GABA, through GABAA receptors, on adult LH secretion. Nevertheless, surprisingly both excitatory and inhibitory actions of GABAA receptor activation on adult GnRH neurons have been reported. This appears to be dependent on a variable concentration of chloride (Clˉ) in the intracellular milieu of the GnRH neuron (81). It is important to note that in most brain regions, GABA signaling typically shifts from being depolarizing (with excitatory effects) in early postnatal stages to hyperpolarizing (inhibitory) in adulthood. This effect is seemingly mediated by GABAA receptors and is in part explained by a decline in intracellular Clˉ levels during postnatal life (82). Differential expression of the sodium potassium chloride cotransporter 1 (NKCC1) and the potassium chloride cotransporter 2 (KCC2) contribute to this decline. The excitatory property of early GABAergic projections seems to play an important role in neuronal differentiation and dendritic arborization (83). Within the hypothalamus, it is currently recognized that GABAA receptor activation exerts a predominantly depolarizing and excitatory response on embryonic and prepubertal GnRH neurons (84, 85). However, the depolarizing/hyperpolarizing switch of GABA action, observed in most brain regions, still needs to be demonstrated in GnRH populations (86, 87).
Glutamatergic neurons
Glutamatergic stimulation of GnRH neurons is considered another essential factor in the reactivation of GnRH secretion at puberty. An increase in glutamatergic transmission is observed during postnatal development in many brain regions, including the hippocampus and the hypothalamus (11, 69, 70). In rodents, GnRH neurons express the three types of ionotropic glutamate receptors, the α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, the N-methyl-D-aspartic acid (NMDA) receptor, and the kainate receptor. Neuroanatomical studies showed that GnRH neurons express all 4 AMPA receptor subunits (GluR1-4), the NR1 and NR2 NMDA subunits, as well as both GluK1 and GluK5 kainate subunits (71). The specific combination of subunits determines the biophysical and pharmacological receptor properties. GnRH neurons appear to express NMDA and AMPA receptors at a later stage of postnatal development than GABAergic receptors, which are expressed since embryonic development. In GnRH neurons, a progressive increase in expression of the NMDA receptor NR1 subunit is observed during postnatal/prepubertal development in mice and rats (Fig. 1) (70, 88). One study in rat also showed a pubertal increase in expression of the kainate receptor GluK5 subunit (89). In addition, electrophysiological analyses revealed an increase in the percentage of GnRH neurons responding to AMPA in adult compared to prepubertal (PND18-25) mice. In contrast, the number of GnRH neurons responding to NMDA appeared relatively stable between these 2 groups (90). Altogether, these findings suggest that, similarly to other regions of the brain, a delicate interplay of GABAergic/glutamatergic transmission occurs at the hypothalamic level, and more specifically at the level of the GnRH network. During the prenatal period, there is formation of excitatory GABAergic synapses, followed by progressive postnatal development of glutamatergic synapses (5, 70). The progressive increase in excitatory transmission from these 2 neurotransmitters on GnRH neurons is likely to play a critical role in remodeling the synaptic network required for the proper control of the onset of puberty.
Glial cells
Beyond neuronal regulation of GnRH secretion, accumulating evidence exists for a critical role of glial cells in the regulation of brain plasticity during puberty. Within the hypothalamus, astrocytes and tanycytes have been shown to play a role in regulating not only dendritic plasticity but also cell adhesion, cell–cell communication, and axon guidance (91). Glial regulation has been demonstrated in both the preoptic area, where GnRH cell bodies reside, and the median eminence, where GnRH projections and terminals are located (Fig. 2) (91).
Several astrocyte signaling molecules have been identified to contribute to puberty initiation, including prostaglandin E2 (PGE2), tyrosine kinase receptors belonging to the erythroblastosis B (ERBB) family (ERBBs), and synaptic cell adhesion molecule 1 (SynCAM1) (91). In rodents, an estradiol-induced increase in hypothalamic PGE2 synthesis occurs during prepubertal development and triggers the first preovulatory GnRH surge (92). The PGE2 release requires the activation of ERBBs, known to be expressed in hypothalamic astrocytes that physically interact with GnRH cell bodies. The expression of ERBB increases during pubertal development (91, 93, 94). In astrocytes, selective genetic disruption of targets involved in ERBB1 and ERBB4 expression or in PGE2 synthesis is able to delay puberty in mice (95, 96). PGE2 has been shown to mediate the postnatal, estradiol-induced increase in dendritic spine plasticity in the preoptic area (97). Whether these changes specifically affect GnRH neurons remains to be determined.
Selective disruption of SynCAM1 in astrocytes has also been associated with delayed puberty (98). SynCAM1 is a synaptic cell adhesion molecule known for promoting synapse formation and enhancing excitatory synaptic transmission. Interestingly, SynCAM1 expression increases in the brain over the first 3 weeks of postnatal development, the major period of synaptogenesis in rodents (99). Within the hypothalamus, SynCAM1 is expressed by both astrocytes and GnRH neurons and has been shown to drive the adhesiveness of astrocytes to GnRH neuron cell bodies (100). These results highlight the importance of cell–cell adhesion and communication between astrocytes and GnRH neurons for proper GnRH neuron maturation.
In the median eminence, GnRH axon terminals are in close apposition to the endfeet of tanycytes, specialized unciliated cells lining the floor of the third ventricle (91, 101). In adult female mice, tanycytes demonstrate a high degree of structural plasticity across the estrous cycle. The retraction of tanycyte endfeet before ovulation allows direct access of GnRH neurons to the pericapillary space, thereby facilitating the release of GnRH into the pituitary portal circulation (102). This process involves PGE2 and ERBB signaling (103). Interestingly, recent studies revealed that this mechanism also involves semaphorins, a family of secreted and membrane proteins first identified as axon guidance cues. This class of molecules, together with their receptors neuropilins and plexins, regulate cell migration by exerting chemoattracting/repulsing properties. GnRH neuron development and migration have been shown to be regulated by semaphorin signaling during embryogenesis (104-107). Their contribution to activation of the HPG axis is underscored by the occurrence of GnRH deficiency in patients harboring deleterious mutations in SEMA3A, SEMA3E, and SEMA7A genes, as well as in their receptors PLXNA1, NRP1, and NRP2 (108-111). Together with PGE2 and ERBBs, endothelium-secreted SEMA3A and tanycyte-derived SEMA7A have been demonstrated to participate to tanycyte/GnRH terminal plasticity in the median eminence at adulthood (112, 113). In effect, in addition to cell attraction/repulsion processes, semaphorin signaling appears to be involved in neuroplasticity, axonal pruning, and synapse formation (114). Surprisingly, this morphological plasticity of GnRH projections at the level of the median eminence has only been studied in adulthood. Whether median eminence plasticity would promote the reactivation of GnRH secretion to contribute to the onset of puberty remains to be elucidated.
Additional partners: multigenic control of puberty initiation
Puberty has been demonstrated to be regulated by hundreds to thousands of genes (115). In humans, a recent genome-wide association study identified 389 independent regions associated with age at menarche (116). Interestingly, a substantial number of these regions encompassed genes linked to neuron development (116). Moreover, whole-exome sequencing studies of families with disorders of puberty such as central precocious puberty and delayed puberty have identified several new genes associated with pubertal timing with putative roles in neurodevelopment (117-120). These data underscore the importance of the early embryonic and prepubertal development periods, in complement to the pubertal period, in controlling the timing of puberty.
Makorin ring finger protein 3 (MKRN3) and Delta-like homolog 1 (DLK1) were the first genes to be identified as GnRH inhibitors. Loss-of-function mutations in both MKRN3 and DLK1 are associated with central precocious puberty (117, 118). MKRN3 belongs to the makorin family of E3 ubiquitin ligases (121, 122). In the rodent hypothalamus, Mkrn3 expression is high from E10.5 to the second week of postnatal development, then decreases sharply before puberty initiation (117, 123-125). Mkrn3 expression has been found in the mediobasal hypothalamus (containing the ARC) and in the preoptic area. Interestingly, Mkrn3 is expressed in kisspeptin neurons in the mediobasal hypothalamus. Moreover, in vitro analyses demonstrated that MKRN3 selectively inhibits KISS1 and TAC3 promoter activity, suggesting an action of MKRN3 upstream of GnRH secretion via regulation of kisspeptin and NKB (125). Increasing evidence shows that E3 ubiquitin ligases, and particularly the makorin protein family, play important roles in neurodevelopment and synaptic plasticity from embryonic stages through adolescence (126-129).
DLK1 is a transmembrane protein and member of the epidermal growth factor-like family, which is involved in cell differentiation and cell fate determination (130). In the mouse hypothalamus, Dlk1 expression has been shown as early as E10.5 and increases progressively during pubertal development (123, 131). Dlk1 acts as a negative regulator of the Notch signaling pathway (132). Interestingly, while the mechanism of action of Dlk1 in the regulation of puberty initiation is still unknown, the Notch signaling pathway has been shown to be critical for neurogenesis and development of the hypothalamus, specifically for the formation of kisspeptin neurons (133). Taken together, these observations suggest potential roles for MKRN3 and DLK1 in the neural development of the GnRH network before puberty initiation.
Whole-exome sequencing studies of families with delayed puberty have also identified additional targets involved in GnRH neuron development, such as immunoglobulin superfamily member 10 (IGSF10) and leucine-rich repeat containing G protein–coupled receptor 4 (LGR4) (119, 120). IGSF10 is a secreted protein involved in the control of early migration of GnRH neurons. Although the presence of specific receptors for IGSF10 on GnRH bodies or dendrites has not been formally demonstrated, lack of functional IGSF10 in zebrafish prevents GnRH neurons from extending projections to the hypothalamus (119). LGR4 has been shown to mediate Wnt/β-catenin signaling, which is critical to the development of GnRH neurons (120). These findings highlight the importance of early developmental processes in the regulation of pubertal timing.
Regulation of Pubertal Neuroplasticity by Sex Steroids
Within the HPG axis, gonadal sex steroids participate in the dynamic control of GnRH secretion through negative and positive feedback regulatory loops that are organized during postnatal and pubertal development. Indeed, sex steroid levels increase in both males and females during postnatal/prepubertal development and, in parallel with steroid-independent mechanisms, have been shown to contribute to the timing of puberty (134-136). Sex steroids have been associated with brain development and plasticity during puberty. Human MRI studies have shown changes in cortical gray and white matter volumes, thickness of the cortex, and hippocampus and amygdala morphology in association with rising levels of testosterone and estradiol (137, 138). There is also considerable evidence that gonadal hormones impact synaptogenesis, dendritic branching, synaptic pruning, and glial function (135).
In the rodent hypothalamus, GnRH neurons do not appear to express the androgen receptor (encoded by AR) or the estrogen receptor (ER) α (encoded by Esr1), the main receptors mediating the reproductive actions of testosterone and estradiol, respectively (139, 140). Nevertheless, while the mechanisms are still unclear, ERβ (encoding by Esr2) has been found in GnRH neurons, and both neural and GnRH neuron-specific ablation of Esr2 result in delayed puberty (141, 142). Remarkably, the dendritic morphological changes observed during postnatal development of GnRH neurons occur independently of gonadal steroid feedback (40). Thus, it would seem that sex steroid regulation of hypothalamic neuroplasticity occurs upstream of the GnRH neuron.
Unlike GnRH neurons, kisspeptin neurons strongly express Esr1 and AR in rodent RP3V and ARC. Moreover, Kiss1 expression in these regions is differentially regulated by sex steroids. Estradiol inhibits Kiss1 expression in the ARC neuronal population involved in negative feedback regulation of GnRH secretion. In contrast, estradiol stimulates Kiss1 expression in the RP3V, which controls the positive feedback regulation of GnRH secretion in females (143). Esr1 deletion in murine kisspeptin neurons produces early signs of pubertal progression, indicating that estradiol exerts a prepubertal restraint on GnRH secretion (144, 145). However, these mice fail to ovulate, probably because of defective kisspeptin neuron maturation in the RP3V. Similarly, ablation of Esr1 from Tac2-expressing cells advanced puberty, consistent with ERα restraint of GnRH activation via the ARC KNDy neurons (146). These findings have contributed to the development of a model, at least in rodents, in which interplay between the ARC and RP3V populations of kisspeptin neurons determines the timing of puberty and overall HPG integrity (147). It has been shown that ERβ also participates in the increase of postnatal Kiss1 mRNA and kisspeptin protein expression in the female RP3V (141). Taken together, these data show that estradiol controls postnatal/prepubertal expression of Kiss1 in the hypothalamus; however, its impact on the formation and plasticity of kisspeptin projections to GnRH neurons remains to be investigated.
While the involvement of glutamate and GABA in mediating estradiol feedback in adulthood has been known for decades, few studies have been devoted to understanding the impact on prepubertal development of GnRH neurons (148-150). A subset of GABAergic and glutamatergic cells in the RP3V and ARC have been shown to express Esr1 (151). Recent findings in mice showed that the specific deletion of Esr1 from Vgat-expressing (GABAergic) neurons did not alter puberty initiation. In contrast, Esr1 ablation in Vglut2-expressing (glutamatergic) neurons caused advanced puberty. Thus, Esr1-modulated glutamate transmission appears to be a critical component of the estradiol-induced prepubertal restraint on GnRH neuron activity (151). Further studies are needed to better understand the contribution of estradiol to the postnatal increase in glutamatergic synaptic transmission to GnRH neurons and to the GABAergic synaptic changes occurring postnatally.
In addition to neuronal cells, estradiol also regulates the function of glial cells, which also express estrogen receptors (152). Within the hypothalamus, an increase in the synthesis of astrocytic PGE2 correlates with the pubertal increase in estradiol levels (92). Similarly, the morphological plasticity of the tanycytes observed across the estrous cycle in female is estrogen dependent, and both SEMA3A and SEMA7A expression are regulated by ovarian steroids (91, 112, 113).
Additional studies are needed to understand whether gonadal steroids might also regulate other partners of the HPG axis. While no studies have yet been conducted on the newly identified regulators of puberty, DLK1, IGSF10, and LGR4, recent evidence shows that hypothalamic Mkrn3 expression is not regulated by sex steroids. Indeed, Mkrn3 mRNA levels in the ARC and RP3V were unchanged in the GnRH deficient hypogonadal hpg mouse, which carry a genomic rearrangement deleting the Gnrh1 locus. Moreover, treatment of PND11 female mice with estradiol did not modify hypothalamic Mkrn3 expression (125). Further investigation is needed to better understand the effects of sex steroids on hypothalamic neurodevelopment and neuroplasticity.
Conclusion
Puberty is a critical transitional developmental period during which extensive physical, hormonal, neural, and behavioral changes take place. These changes are essential for entering adulthood with reproductive maturity and with cognitive and emotional independence. It is a period of intense cerebral neuroplasticity, finely tuned by numerous factors working in concert to enable proper brain maturation. While our understanding of the neuroendocrine and physiological bases of puberty has improved tremendously in recent decades, the underlying processes impacting neuroplasticity are largely unknown. Both neurons and glial cells are known to contribute to neuroplasticity and regulation of GnRH neuron plasticity is no exception. In comparison with other CNS neurons, GnRH neurons have some distinct characteristics, such as their origin outside the brain, their diffuse distribution throughout the anterior hypothalamus, and their small population. Synchronization of GnRH neurons is critical for correct temporal pacing and for generating coordinated secretory pulses. These observations highlight the importance of high synaptic morphological and adaptive plasticity, to drive the pattern of GnRH secretion and efficiently stimulate pituitary gonadotropes. Interestingly, our current understanding underscores the remarkable morphological changes and neural plasticity that occur during the first few postnatal weeks in rodents, well before the onset of puberty. These and other findings suggest that pubertal development is not simply the result of a neural network awakening at a prespecified time from a previously period of quiescence. Rather, it appears that a broad set of plastic changes occur dynamically and continuously throughout a spectrum of developmental stages, beginning well before birth, to ensure the proper development and maturation of neural circuits to enable the juvenile–adult transition. Across these developmental stages, the fetal, perinatal, and prepubertal periods have been defined as critical windows of plasticity during which neural circuits are highly receptive to genetic, epigenetic and environmental factors. These periods are essential for the organization of hypothalamic circuits that underlie the regulation of the HPG. Thus, the development and maturation of the hypothalamus occurs in conjunction with developmental regulation of the activity of the HPG axis via both internal and external factors. For instance, the activation of GnRH secretion during the perinatal period demonstrates the ability of immature GnRH neurons to be activated. Hence, alongside the maturation of the GnRH neuron itself, there exists a fine regulation of the afferent network that controls the secretion of GnRH. In contrast, the postnatal/prepubertal period of development is a period of high level of plasticity within the hypothalamus, while the HPG axis is maintained in quiescence. These paradoxes highlight the existence of strong inhibitory influences of internal and external factors concomitantly with the development of the hypothalamus. While the mechanisms underlying this inhibitory effect are still unclear, the recent identification of the inhibitory factors Mkrn3 and Dlk1 open a new area of research. Furthermore, we can propose an important role of plasticity events in the modulation of this inhibitory action. In addition, external factors, particularly gonadal steroids, regulate GnRH secretion. The gonads are silent during early postnatal development, thereby participating in the quiescence of the HPG axis until puberty initiation. It is likely that a combination of both steroid-dependent and steroid-independent mechanisms govern puberty initiation. It is worth noting that the model proposing these changes in plasticity apply primarily to mice and rats, as most studies have focused on rodents. It is possible that these mechanisms may differ across species. For instance, while prepubertal negative feedback by sex steroids on GnRH neurons is prominent in rodents, steroid-independent mechanisms seem more important in primates (30). Studies of hypothalamic neuroplasticity during puberty are lacking in humans. Further investigations are needed to evaluate whether neurodevelopment and neuroplasticity processes may account in part for the sex-based differences in puberty initiation and more generally for the regulation of the HPG axis.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health R01HD082314 (to UK), by Brigham and Women’s Hospital Women’s Brain Initiative awards (to LN and UK) and by a French Society of Endocrinology Research Award (to LM).
Glossary
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid
- ARC
arcuate nucleus
- CNS
central nervous system
- DTI
diffusion tensor imaging
- ER
estrogen receptor
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic–pituitary–gonadal
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- NKB
neurokinin B
- NMDA
N-methyl-D-aspartic acid
- PGE2
prostaglandin E2
- PND
postnatal day
- RP3V
rostral periventricular area
- SynCAM1
synaptic cell adhesion molecule 1
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84-88. [DOI] [PubMed] [Google Scholar]
- 2. Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017;44:122-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001;86(6):2364-2368. [DOI] [PubMed] [Google Scholar]
- 4. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452-466. [DOI] [PubMed] [Google Scholar]
- 6. de Roux N, Young J, Misrahi M, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337(22):1597-1602. [DOI] [PubMed] [Google Scholar]
- 7. Mason AJ, Hayflick JS, Zoeller RT, et al. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234(4782):1366-1371. [DOI] [PubMed] [Google Scholar]
- 8. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195-205. [DOI] [PubMed] [Google Scholar]
- 10. Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66(6):578-590. [DOI] [PubMed] [Google Scholar]
- 11. Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244-252. [DOI] [PubMed] [Google Scholar]
- 13. Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136:101-108. [DOI] [PubMed] [Google Scholar]
- 14. Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuhrmann D, Knoll LJ, Blakemore S-J. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19(10):558- 566. [DOI] [PubMed] [Google Scholar]
- 16. Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20(9):2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31(19):7174-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859-861. [DOI] [PubMed] [Google Scholar]
- 19. Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863. [DOI] [PubMed] [Google Scholar]
- 20. Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309-315. [DOI] [PubMed] [Google Scholar]
- 21. Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, ed. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific; 1967:3-70. [Google Scholar]
- 22. Bramen JE, Hranilovich JA, Dahl RE, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perrin JS, Leonard G, Perron M, et al. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055-1066. [DOI] [PubMed] [Google Scholar]
- 25. Baroncini M, Jissendi P, Balland E, et al. MRI atlas of the human hypothalamus. Neuroimage. 2012;59(1):168-180. [DOI] [PubMed] [Google Scholar]
- 26. Simon JJ, Stopyra MA, Mönning E, et al. Neuroimaging of hypothalamic mechanisms related to glucose metabolism in anorexia nervosa and obesity. J Clin Invest. 2020;130(8):4094-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jastreboff AM, Sinha R, Arora J, et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes. 2016;65(7):1929-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemaire JJ, De Salles A, Coll G, et al. MRI atlas of the human deep brain. Front Neurol. 2019;10:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Florent V, Baroncini M, Jissendi-Tchofo P, et al. Hypothalamic structural and functional imbalances in anorexia nervosa. Neuroendocrinology. 2020;110(6):552-562. [DOI] [PubMed] [Google Scholar]
- 30. Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nestor CC, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL. Regulation of GnRH pulsatility in ewes. Reproduction. 2018;156(3):R83-R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161-164. [DOI] [PubMed] [Google Scholar]
- 33. Crowley WF Jr, Pitteloud N, Seminara S. New genes controlling human reproduction and how you find them. Trans Am Clin Climatol Assoc. 2008;119:29-37; discussion 37. [PMC free article] [PubMed] [Google Scholar]
- 34. Casoni F, Malone SA, Belle M, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969-3981. [DOI] [PubMed] [Google Scholar]
- 35. Rance NE, Young WS 3rd, McMullen NT. Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1994;339(4):573-586. [DOI] [PubMed] [Google Scholar]
- 36. Forni PE, Wray S. GnRH, anosmia and hypogonadotropic hypogonadism–where are we? Front Neuroendocrinol. 2015;36:165-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146(3):1163-1169. [DOI] [PubMed] [Google Scholar]
- 38. Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689-12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(8):3652-3661. [DOI] [PubMed] [Google Scholar]
- 40. Ybarra N, Hemond PJ, O’Boyle MP, Suter KJ. Spatially selective, testosterone-independent remodeling of dendrites in gonadotropin-releasing hormone (GnRH) neurons prepubertally in male rats. Endocrinology. 2011;152(5):2011-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wray S, Hoffman G. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology. 1986;43(2):93-97. [DOI] [PubMed] [Google Scholar]
- 42. Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21(3):1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dulka EA, Moenter SM. Prepubertal development of gonadotropin-releasing hormone neuron activity is altered by sex, age, and prenatal androgen exposure. Endocrinology. 2017;158(11):3943-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060-15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barroso A, Roa J, Tena-Sempere M. Neuropeptide control of puberty: beyond kisspeptins. Semin Reprod Med. 2019;37(4):155-165. [DOI] [PubMed] [Google Scholar]
- 46. Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324(1-2):45-50. [DOI] [PubMed] [Google Scholar]
- 47. Terasawa E, Garcia JP, Seminara SB, Keen KL. Role of kisspeptin and neurokinin B in puberty in female non-human primates. Front Endocrinol (Lausanne). 2018;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trevisan CM, Montagna E, de Oliveira R, et al. Kisspeptin/GPR54 system: what do we know about its role in human reproduction? Cell Physiol Biochem. 2018;49(4):1259-1276. [DOI] [PubMed] [Google Scholar]
- 49. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-1627. [DOI] [PubMed] [Google Scholar]
- 51. d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714-10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toro CA, Wright H, Aylwin CF, Ojeda SR, Lomniczi A. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat Commun. 2018;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li XF, Hu MH, Hanley BP, et al. The posterodorsal medial amygdala regulates the timing of puberty onset in female rats. Endocrinology. 2015;156(10):3725-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology. 2016;157(10):4021-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J Neurosci. 2014;34(10):3756-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150(3):1400-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herbison AE, de Tassigny XD, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):312-321. [DOI] [PubMed] [Google Scholar]
- 60. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349-11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32(7):2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gill JC, Navarro VM, Kwong C, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153(10):4883-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simavli S, Thompson IR, Maguire CA, et al. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. León S, Fergani C, Talbi R, et al. Characterization of the role of NKA in the control of puberty onset and gonadotropin release in the female mouse. Endocrinology. 2019;160(10):2453-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990;15(1):41-70. [DOI] [PubMed] [Google Scholar]
- 70. Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol. 2006;254-255:32-38. [DOI] [PubMed] [Google Scholar]
- 71. Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010;1364:35-43. [DOI] [PubMed] [Google Scholar]
- 72. Moore AM, Abbott G, Mair J, Prescott M, Campbell RE. Mapping GABA and glutamate inputs to gonadotrophin-releasing hormone neurones in male and female mice. J Neuroendocrinol. 2018;30(12):e12657. [DOI] [PubMed] [Google Scholar]
- 73. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu X, Porteous R, d’Anglemont de Tassigny X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31(7):2421-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A. 1995;92(9):3918-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12(10):3497-3504. [DOI] [PubMed] [Google Scholar]
- 79. Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol. 2005;17(9):591-599. [DOI] [PubMed] [Google Scholar]
- 80. Berg T, Silveira MA, Moenter SM. Prepubertal development of GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J Neurosci. 2018;38(9):2283-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rivera C, Voipio J, Payne JA, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251-255. [DOI] [PubMed] [Google Scholar]
- 83. Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014;279:187-219. [DOI] [PubMed] [Google Scholar]
- 84. Hales TG, Sanderson MJ, Charles AC. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology. 1994;59(3):297-308. [DOI] [PubMed] [Google Scholar]
- 85. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412-419. [DOI] [PubMed] [Google Scholar]
- 86. Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143(4):1459-1466. [DOI] [PubMed] [Google Scholar]
- 87. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872-2891. [DOI] [PubMed] [Google Scholar]
- 88. Simonian SX, Herbison AE. Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of GnRH neurons during embryogenesis. J Neurosci. 2001;21(3):934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eyigor O, Jennes L. Expression of glutamate receptor subunit mRNAs in gonadotropin-releasing hormone neurons during the sexual maturation of the female rat. Neuroendocrinology. 1997;66(2):122-129. [DOI] [PubMed] [Google Scholar]
- 90. Constantin S, Jasoni CL, Wadas B, Herbison AE. Gamma-aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):262-270. [DOI] [PubMed] [Google Scholar]
- 91. Clasadonte J, Prevot V. The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol. 2018;14(1):25-44. [DOI] [PubMed] [Google Scholar]
- 92. Ojeda SR, Campbell WB. An increase in hypothalamic capacity to synthesize prostaglandin E2 precedes the first preovulatory surge of gonadotropins. Endocrinology. 1982;111(4):1031-1037. [DOI] [PubMed] [Google Scholar]
- 93. Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci U S A. 1990;87(24):9698-9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Clasadonte J, Sharif A, Baroncini M, Prevot V. Gliotransmission by prostaglandin e(2): a prerequisite for GnRH neuronal function? Front Endocrinol (Lausanne). 2011;2:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Prevot V, Rio C, Cho GJ, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23(1):230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prevot V, Lomniczi A, Corfas G, Ojeda SR. erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology. 2005;146(3):1465-1472. [DOI] [PubMed] [Google Scholar]
- 97. Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22(19):8586-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sandau US, Mungenast AE, Alderman Z, et al. SynCAM1, a synaptic adhesion molecule, is expressed in astrocytes and contributes to erbB4 receptor-mediated control of female sexual development. Endocrinology. 2011;152(6):2364-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Biederer T, Sara Y, Mozhayeva M, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297(5586):1525-1531. [DOI] [PubMed] [Google Scholar]
- 100. Sandau US, Mungenast AE, McCarthy J, Biederer T, Corfas G, Ojeda SR. The synaptic cell adhesion molecule, SynCAM1, mediates astrocyte-to-astrocyte and astrocyte-to-GnRH neuron adhesiveness in the mouse hypothalamus. Endocrinology. 2011;152(6):2353-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518(7):943-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Prevot V, Croix D, Bouret S, et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94(3):809-819. [DOI] [PubMed] [Google Scholar]
- 103. Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity. J Neurosci. 2003;23(33):10622-10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Oleari R, Lettieri A, Paganoni A, Zanieri L, Cariboni A. Semaphorin signaling in GnRH neurons: from development to disease. Neuroendocrinology. 2019;109(3):193-199. [DOI] [PubMed] [Google Scholar]
- 105. Vanacker C, Trova S, Shruti S, et al. Neuropilin-1 expression in GnRH neurons regulates prepubertal weight gain and sexual attraction. EMBO J. 2020;39(19):e104633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cariboni A, Hickok J, Rakic S, et al. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci. 2007;27(9):2387-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Marcos S, Monnier C, Rovira X, et al. Defective signaling through plexin-A1 compromises the development of the peripheral olfactory system and neuroendocrine reproductive axis in mice. Hum Mol Genet. 2017;26(11):2006-2017. [DOI] [PubMed] [Google Scholar]
- 108. Young J, Metay C, Bouligand J, et al. SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development. Hum Reprod. 2012;27(5):1460-1465. [DOI] [PubMed] [Google Scholar]
- 109. Cariboni A, André V, Chauvet S, et al. Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J Clin Invest. 2015;125(6):2413-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Känsäkoski J, Fagerholm R, Laitinen EM, et al. Mutation screening of SEMA3A and SEMA7A in patients with congenital hypogonadotropic hypogonadism. Pediatr Res. 2014;75(5):641-644. [DOI] [PubMed] [Google Scholar]
- 111. Hanchate NK, Giacobini P, Lhuillier P, et al. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS Genet. 2012;8(8):e1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Giacobini P, Parkash J, Campagne C, et al. Brain endothelial cells control fertility through ovarian-steroid-dependent release of semaphorin 3A. PLoS Biol. 2014;12(3):e1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Parkash J, Messina A, Langlet F, et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat Commun. 2015;6:6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13(9):605-618. [DOI] [PubMed] [Google Scholar]
- 115. Lomniczi A, Wright H, Castellano JM, Sonmez K, Ojeda SR. A system biology approach to identify regulatory pathways underlying the neuroendocrine control of female puberty in rats and nonhuman primates. Horm Behav. 2013;64(2):175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Day FR, Thompson DJ, Helgason H, et al. ; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium . Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dauber A, Cunha-Silva M, Macedo DB, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102(5):1557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Howard SR, Guasti L, Ruiz-Babot G, et al. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016;8(6):626-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mancini A, Howard SR, Marelli F, et al. LGR4 deficiency results in delayed puberty through impaired Wnt/β-catenin signaling. JCI Insight. 2020;5(11):e133434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54(3):R131-R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Naulé L, Kaiser UB. Evolutionary conservation of MKRN3 and other makorins and their roles in puberty initiation and endocrine functions. Semin Reprod Med. 2019;37(4):166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shimogori T, Lee DA, Miranda-Angulo A, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13(6):767-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019;17(11):e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Upadhyay A, Joshi V, Amanullah A, et al. E3 ubiquitin ligases neurobiological mechanisms: development to degeneration. Front Mol Neurosci. 2017;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gray TA, Hernandez L, Carey AH, et al. The ancient source of a distinct gene family encoding proteins featuring RING and C(3)H zinc-finger motifs with abundant expression in developing brain and nervous system. Genomics. 2000;66(1):76-86. [DOI] [PubMed] [Google Scholar]
- 128. Yang PH, Cheung WK, Peng Y, et al. Makorin-2 is a neurogenesis inhibitor downstream of phosphatidylinositol 3-kinase/Akt (PI3K/Akt) signal. J Biol Chem. 2008;283(13):8486-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu H, Kong X, Chen F. Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget. 2017;8(49):85102-85109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Macedo DB, Kaiser UB. DLK1, Notch signaling and the timing of puberty. Semin Reprod Med. 2019;37(4):174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Villanueva C, Jacquier S, de Roux N. DLK1 is a somato-dendritic protein expressed in hypothalamic arginine-vasopressin and oxytocin neurons. PLoS One. 2012;7(4):e36134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sánchez-Solana B, Nueda ML, Ruvira MD, et al. The EGF-like proteins DLK1 and DLK2 function as inhibitory non-canonical ligands of NOTCH1 receptor that modulate each other’s activities. Biochim Biophys Acta. 2011;1813(6):1153-1164. [DOI] [PubMed] [Google Scholar]
- 133. Biehl MJ, Raetzman LT. Rbpj-κ mediated Notch signaling plays a critical role in development of hypothalamic Kisspeptin neurons. Dev Biol. 2015;406(2):235-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rehbein E, Hornung J, Sundström Poromaa I, Derntl B. Shaping of the female human brain by sex hormones – a review [published online ahead of print March 11, 2020]. Neuroendocrinology. 2020. Doi: 10.1159/000507083. [DOI] [PubMed] [Google Scholar]
- 135. Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040-1047. [DOI] [PubMed] [Google Scholar]
- 136. Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64(2):203-210. [DOI] [PubMed] [Google Scholar]
- 137. Neufang S, Specht K, Hausmann M, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464-473. [DOI] [PubMed] [Google Scholar]
- 138. Peper JS, Brouwer RM, Schnack HG, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332-342. [DOI] [PubMed] [Google Scholar]
- 139. Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22(4):292-308. [DOI] [PubMed] [Google Scholar]
- 140. Hrabovszky E, Shughrue PJ, Merchenthaler I, et al. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141(9):3506-3509. [DOI] [PubMed] [Google Scholar]
- 141. Naulé L, Robert V, Parmentier C, et al. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum Mol Genet. 2015;24(25):7326-7338. [DOI] [PubMed] [Google Scholar]
- 142. Novaira HJ, Negron AL, Graceli JB, et al. Impairments in the reproductive axis of female mice lacking estrogen receptor β in GnRH neurons. Am J Physiol Endocrinol Metab. 2018;315(5):E1019-E1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 144. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107(52):22693-22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Estradiol restrains prepubertal gonadotropin secretion in female mice via activation of ERα in Kisspeptin neurons. Endocrinology. 2016;157(4):1546-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Greenwald-Yarnell ML, Marsh C, Allison MB, et al. ERα in Tac2 neurons regulates puberty onset in female mice. Endocrinology. 2016;157(4):1555-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017;23(6):737-763. [DOI] [PubMed] [Google Scholar]
- 148. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302-330. [DOI] [PubMed] [Google Scholar]
- 149. Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69(6):1771-1778. [DOI] [PubMed] [Google Scholar]
- 150. Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;21(4):327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cheong RY, Czieselsky K, Porteous R, Herbison AE. Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice. J Neurosci. 2015;35(43):14533-14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta. 2010;1800(10):1106-1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.