Abstract
BACKGROUND:
The majority of acute myeloid leukemia (AML) patients are older than 65 years at diagnosis and is not actively treated. We aimed to determine the prevalence, temporal trends, and factors associated with no active treatment (NAT) among older AML patients in the United States (US).
METHODS:
Retrospective analysis of Surveillance, Epidemiology and End Results (SEER)-Medicare data of 14,089 AML patients in the US who were diagnosed at the age of ≥66 years during 2001–2013. NAT was defined as not receiving any chemotherapy including HMAs. Multivariable logistic regression models were utilized to analyze sociodemographic, clinical and provider characteristics associated with NAT.
RESULTS:
The proportion of patients with NAT decreased over time, from 59.7% among patients diagnosed in 2001 to 42.8% among those diagnosed in 2013. Median OS for the entire cohort was 82 days from diagnosis. Patients with NAT had worse survival than those receiving active treatment. Variables associated with higher odds of NAT included older age, certain sociodemographic characteristics (household income in the lowest quartile, residence outside Northeast Region, being unmarried), and clinical factors (≥3 comorbidities, mental disorders, recent hospitalization, disability).
CONCLUSION:
Over half of older AML patients in the US do not receive any active leukemia-directed therapy despite the availability of lower intensity therapies such as HMAs. Lack of active therapy receipt is associated with inferior survival. Identifying predictors of NAT might improve quality of care and survival in this patient population, especially as novel therapeutic options with lower toxicity are becoming available.
Keywords: acute myeloid leukemia, AML, elderly, no active treatment, outcome, SEER/Medicare
Summary statement:
Although decreasing over time, the majority (52.7%) of older patients did not receive active treatment raising concern for potential undertreatment. Compared with actively treated patients, patients without active treatment tended to be older, had more comorbidities, and potentially worse access to specialist care.
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia with 19,520 predicted new cases and 10,670 deaths in the United States (US) in 2018.1 With a median age at diagnosis of 67 years, a considerable proportion of patients with AML fall into the “older” category.2 Treatment modalities with highest cure rates, namely intensive chemotherapy and allogeneic hematopoietic stem cell transplant (alloHSCT) are mainly reserved for younger patients. 2,3 Additionally, AML in older patients is associated with adverse cytogenetics and lower response rates, leading to a poor median overall survival of 3–6 months. 2,4–6
While high intensity treatment with curative intent might not be feasible for older patients with AML, several treatment options to alleviate symptoms, improve quality of life, reduce transfusion needs, and possibly prolong survival are available.2–4 These include hypomethylating agents (HMAs) such as azacitidine and decitabine, low dose cytarabine, and more recently, targeted therapies such as the hedgehog signaling pathway inhibitor glasdegib and the BCL-2 inhibitor venetoclax which have been approved in the US for older, unfit AML patients.7–11
While HMAs are not specifically labeled for use in AML in the US, they are the de facto standard of care among older unfit AML patients since their approval for the management of the closely related myelodysplastic syndromes (MDS) in 2004 (azacitidine) and 2006 (decitabine). Large registry and real-life data in the US show that a significant proportion of older AML patients are managed with no active therapy (NAT) which includes transfusions of blood products, growth factor support and antibiotics. 12
The underlying factors for potential undertreatment of older patients with AML include a high burden of comorbidity, poor performance status, and the adverse genetic profile of the disease. 5,12 Given the heterogeneity of both patients with AML and the disease biology as well as the greater availability of targeted and less toxic therapies, it is widely recommended that age alone should not be used as the sole criterion to make treatment decisions. 5,13,14 One of the major concerns leading to limiting active treatment in elderly AML patients is the concern for treatment-associated toxicity and impaired quality of life (QoL). 15,16 However, a previous study of azacitidine in elderly AML patients showed that even while receiving treatment QoL improved - although at a marginal level, and QoL increased over time in patients responding to treatment. 17,18
Previous population-based studies have identified various demographic and socioeconomic factors associated with a higher risk of undertreatment of cancer patients. 19–21 Now that more effective treatments for older adults with AML are available, 8,9,22 an important step in improving outcomes is to identify and overcome barriers to delivery of active treatment for older AML patients. Prior studies addressing this issue covered time periods before the wider availability of and increasing experience with HMAs and did not evaluate important variables such as access to the healthcare system or relevant characteristics of healthcare providers. We therefore conducted a retrospective cohort study utilizing Surveillance, Epidemiology and End Results (SEER)-Medicare data to identify factors associated with forgoing active therapy.
Methods
Data Sources and Study Population
The SEER-Medicare linked database, which is developed by the National Cancer Institute and the Centers for Medicare and Medicaid Services, links patient-level information on incident cancer diagnoses from SEER registries to a master file of Medicare enrollment and claims for inpatient, outpatient, and physician services. The SEER registries are nationally representative and account for approximately 30% of the US population, whereas Medicare covers health services for 97% of people aged 65 years and older. About 55% of cancer patients reported to SEER are diagnosed at ≥ 65 years of age, and approximately 94% have been successfully linked with their Medicare claims. 23,24 The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
We assembled a retrospective cohort of patients who were diagnosed with incident AML in 2001–2013. All patients fulfilled the following eligibility criteria: 1) aged 66–99 years at diagnosis, 2) known month of diagnosis, 3) diagnosis was not reported from autopsy or death certificate only, and 4) continuous Medicare fee-for-service coverage (Parts A and B) from 12 months before diagnosis through death or end of study (12/31/2014), whichever was earlier. Patients with acute promyelocytic leukemia (n=432) or who underwent alloHSCT (n=475) were excluded.
Identification of NAT
We defined NAT as not receiving any active treatment, i.e. chemotherapy, including HMAs, after AML diagnosis. Chemotherapy information was obtained via the chemotherapy procedure and administration claims (Medicare Provider Analysis and Review, National Claims History, and Outpatient Statistical Analysis File, and Durable Medical Equipment). Time between AML diagnosis and first chemotherapy was calculated and grouped (<30, 31–60, 61–90, and >90 days).
Variables of Interest
Patients were classified by age at diagnosis (66–69, 70–74, 75–79, 80–84, ≥85 years), sex, race (white, other), marital status, residence in urban/rural area (big metro, metro, and other), SEER region (Northeast, Midwest, South, and West), median income by zip code (by quartile, as a proxy for neighborhood socioeconomic status [SES]) and whether they received any state buy-in within 12 months before diagnosis (as a proxy for individual SES). We used information from SEER to identify previous history of hematologic and solid malignancies. We identified chronic conditions and mental disorders (including depression, anxiety, dementia, and psychosis) by searching inpatient, outpatient, and physician encounter claims for each patient within 12 months before diagnosis. To enhance specificity, we only included diagnosis codes that appeared at least twice on outpatient or physician claims or that had a corresponding hospital claim. For other comorbidities, a Elixhauser score25 excluding mental disorders was calculated for each patient. Since performance status is an import factor in clinical decision making, we used a method developed by Davidoff et al.26 to evaluate each patient’s disability status as a proxy of performance status before diagnosis.
To capture factors related to AML severity, we assessed whether a patient had transfusions, hospitalization due to infection or bleeding within the three months before diagnosis. To understand patients’ interaction with hematologist/oncologists before diagnosis, we identified patients’ outpatient visits with hematologist/oncologists within 1–12 months before diagnosis. We further assessed whether the first hospitalization within the month before diagnosis and the month of diagnosis was urgent or emergent. We linked with Dartmouth Health Atlas to assess hematologist/oncologist density at each HRR level. Receipt of influenza vaccination in the 12 months prior to AML diagnosis was included as an indicator for access to the healthcare system.
Statistical Analysis
Categorical variables were presented using frequencies and percentages, and continuous variables were summarized by median and interquartile range (IQR). Baseline characteristics of the patients by type of treatment were compared using χ2 test for categorical variables and t-test for continuous variables. Multivariable logistic regression models were utilized to assess potential associations between sociodemographic, clinical and provider characteristics and NAT. In addition to the overall study cohort, we also conducted stratified analyses for two age groups (66–74 years and ≥75 years) separately.
As a sensitivity analysis, we conducted analyses by adding additional time frames to define active treatment, such as receiving chemotherapy within 30 days, 60 days and 90 days after diagnosis, respectively. As findings were similar, we only presented results for NAT at any time after AML diagnosis. Additional sensitivity analyses limited to patients who survived at least 30 days and 60 days, respectively, yielded similar findings to what we observed from the overall study cohort and are therefore not included in the manuscript.
All analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC) with two-sided tests and a type I error of 5% as the threshold for statistical significance.
Results
Patient Characteristics
This study included 14,089 incident AML patients diagnosed between 2001 and 2013. Most patients were white (88.6%), male (54.5%) and married (52.5%). Median age at diagnosis was 78 (interquartile range: 73–84) years. Patients with NAT were more likely to be older (Table 1). Only 387 patients (2.7%) were alive at the end of follow-up (12/31/2014), and the median survival was 82 (95% confidence interval [CI]: 80–87) days. Patients with NAT had worse survival than those who were actively treated, with median survival of 46 (95% CI: 44–47) and 186 (95% CI: 178–193) days, respectively (p for log-rank test <0.01).
Table 1.
Characteristics of 14089 older patients with AML by treatment choice, 2001–2013
| NAT | Active Treatment | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 7425 | 6664 | |||
| Age at diagnosis (in years) | |||||
| 66–69 | 510 | 6.9 | 1188 | 17.8 | <.01 |
| 70–74 | 989 | 13.3 | 1898 | 28.5 | |
| 75–79 | 1595 | 21.5 | 1757 | 26.4 | |
| 80–84 | 1989 | 26.8 | 1211 | 18.2 | |
| ≥85 | 2342 | 31.5 | 610 | 9.2 | |
| Race | |||||
| White | 6605 | 89.0 | 5949 | 89.3 | 0.55 |
| Other | 820 | 11.0 | 715 | 10.7 | |
| Sex | |||||
| Male | 3850 | 51.9 | 3807 | 57.1 | <.01 |
| Female | 3575 | 48.1 | 2857 | 42.9 | |
| Marital status | |||||
| Unmarried | 3590 | 48.4 | 2233 | 33.5 | <.01 |
| Married | 3434 | 46.2 | 4079 | 61.2 | |
| Unknown | 401 | 5.4 | 352 | 5.3 | |
| Urban/rural | |||||
| Big metro | 3903 | 52.6 | 3706 | 55.6 | <.01 |
| Metro | 2248 | 30.3 | 1861 | 27.9 | |
| Other | 1274 | 17.2 | 1097 | 16.5 | |
| SEER region | |||||
| Northeast | 1408 | 19.0 | 1495 | 22.4 | <.01 |
| Midwest | 1088 | 14.7 | 900 | 13.5 | |
| South | 1764 | 23.8 | 1556 | 23.3 | |
| West | 3165 | 42.6 | 2713 | 40.7 | |
| Median household income at zip code level | |||||
| 1st quartile(low) | 1944 | 26.2 | 1515 | 22.7 | <.01 |
| 2nd quartile | 1899 | 25.6 | 1562 | 23.4 | |
| 3rd quartile | 1810 | 24.4 | 1648 | 24.7 | |
| 4th quartile(high) | 1644 | 22.1 | 1812 | 27.2 | |
| Unknown | 128 | 1.7 | 127 | 1.9 | |
| State buy-in before diagnosis | |||||
| No | 6271 | 84.5 | 5934 | 89.0 | <.01 |
| Yes | 1154 | 15.5 | 730 | 11.0 | |
| Previous history of hematologic malignancies | |||||
| No | 6934 | 93.4 | 5872 | 88.1 | <.01 |
| Yes | 491 | 6.6 | 792 | 11.9 | |
| Previous history of solid tumors | |||||
| No | 5421 | 73.0 | 4665 | 70.0 | <.01 |
| Yes | 2004 | 27.0 | 1999 | 30.0 | |
| Previous mental disorders | |||||
| No | 6396 | 86.1 | 6143 | 92.2 | <.01 |
| Yes | 1029 | 13.9 | 521 | 7.8 | |
| Elixhuaser score (exclude mental disorders) | |||||
| 0 | 2519 | 33.9 | 2645 | 39.7 | <.01 |
| 1–2 | 2626 | 35.4 | 2607 | 39.1 | |
| ≥3 | 2280 | 30.7 | 1412 | 21.2 | |
| Disabled | |||||
| No | 6188 | 83.3 | 6319 | 94.8 | <.01 |
| Yes | 1237 | 16.7 | 345 | 5.2 | |
| Transfusion within 3 months before diagnosis | |||||
| No | 6628 | 89.3 | 6138 | 92.1 | <.01 |
| Yes | 797 | 10.7 | 526 | 7.9 | |
| Infection related hospitalization within 3 months before diagnosis | |||||
| No | 6983 | 94.0 | 6430 | 96.5 | <.01 |
| Yes | 442 | 6.0 | 234 | 3.5 | |
| Bleeding related hospitalization within 3 months before diagnosis | |||||
| No | 7276 | 98.0 | 6580 | 98.7 | <.01 |
| Yes | 149 | 2.0 | 84 | 1.3 | |
| Hematologist/oncologist outpatient visit before diagnosis | |||||
| No | 5512 | 74.2 | 4391 | 65.9 | |
| Yes | 1913 | 25.8 | 2273 | 34.1 | <.01 |
| First hospitalization around diagnosis | |||||
| Elective/other | 2556 | 34.4 | 2825 | 42.4 | |
| Emergent/urgent | 4869 | 65.6 | 3839 | 57.6 | <.01 |
| Density of hematologist/oncologist at hospital referral region | |||||
| 1st tertile (low) | 2635 | 35.5 | 2313 | 34.7 | 0.59 |
| 2nd tertile | 2307 | 31.1 | 2079 | 31.2 | |
| 3rd tertile (high) | 2483 | 33.4 | 2272 | 34.1 | |
| Influenzas vaccine before diagnosis | |||||
| No | 4201 | 56.6 | 3648 | 54.7 | 0.03 |
| Yes | 3224 | 43.4 | 3016 | 45.3 | |
Treatment Patterns
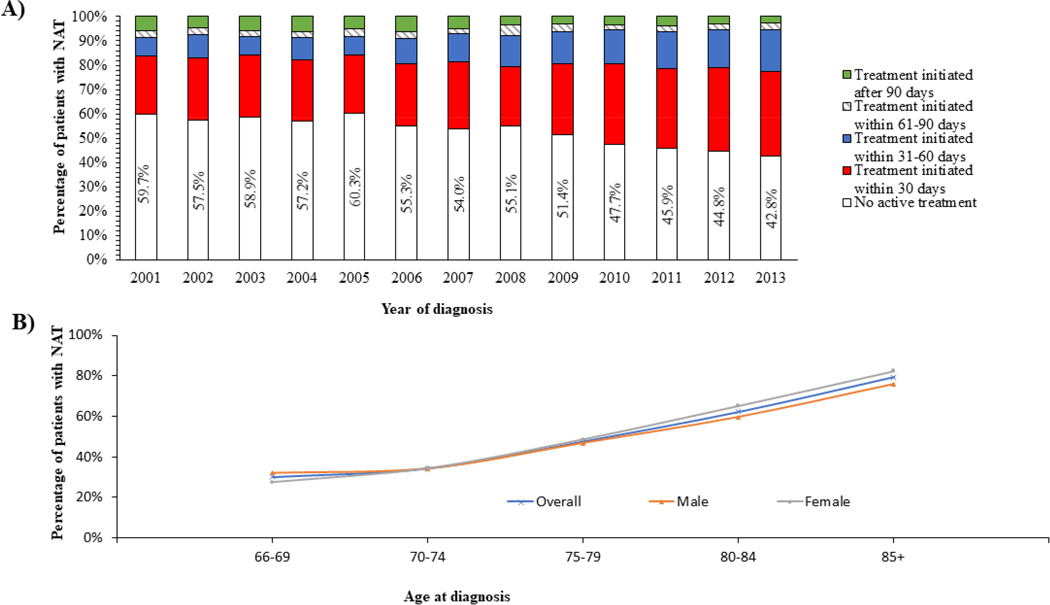
A total of 7,425 (52.7%) patients received NAT. As shown in Figure 1, the proportion of patients with NAT decreased over time, from 59.7% (635 out of 1,063 patients) of those diagnosed in 2001 to 42.8% (523 out of 1,220 patients) of those diagnosed in 2013 (Figure 1). Among 6,664 patients who received active treatment, 84.9% received their first therapy within 60 days after diagnosis. The proportion of patients whose treatment was initiated within 60 days increased over time, from 78.3% among those diagnosed in 2001 to 90.5% among those diagnosed in 2013.
Figure 1: Temporal trends of treatment patterns in elderly patients with AML.
(A) illustrates the temporal trends of treatment patterns during the study period. The proportion of patients with NAT decreased from 59.7% (635 out of 1,063 patients) of those diagnosed in 2001 to 42.8% (523 out of 1,220 patients) of those diagnosed in 2013. Among the 6,664 patients who received active treatment, 84.9% received their first therapy course within 60 days after diagnosis. The proportion of patients whose treatment was initiated within 60 days increased over time, from 78.3% among those diagnosed in 2001 to 90.5% among those diagnosed in 2013. (B) overall females were more likely than man to receive NAT (55.6% of female patients vs. 50.3% of male patients). This overall trend was also present in all age subgroups except for the age group 66–69 years in which men (31.9%) were more likely than women to receive NAT (27.5%).
As expected, the proportion of patients with NAT increased with advancing age of AML diagnosis. Only 30.0% of patients diagnosed at age 66–69 years had NAT; among those diagnosed at ≥85 years, the percentage was as high as 82.4%. Overall, compared with their male counterparts, female patients were more likely to have NAT (55.6% vs. 50.3%). This finding was present among each age group except for the age cohort of 66–69 years in which more males (31.9%) had NAT than females (27.5%).
Factors associated with NAT
In addition to older patients, those who were unmarried (odds ratio [OR]=1.36, 95% CI: 1.26– 1.47), had ≥ 3 comorbid conditions (OR = 1.30, 95% CI: 1.17– 1.44) or mental disorders (OR=1.43, 95% CI: 1.26– 1.63), or were disabled (OR = 2.31, 95% CI: 2.01– 2.66) were more likely to receive NAT (Table 2). Patients who had been hospitalized due to infections within 3 months before diagnosis (OR = 1.39, 95% CI: 1.15–1.67) or if the first hospitalization around diagnosis was emergent/urgent (OR = 1.21, 95% CI: 1.12–1.31) were more likely to receive NAT. Compared with patients residing in big metro areas, those residing in metro areas were 16% more likely to have NAT (95% CI: 1.06–1.26). Interestingly, patients with a previous history of hematologic (OR=0.59, 95% CI: 0.51–0.68) or solid malignancies (OR=0.85, 95% CI: 0.78–0.93) were less likely to have NAT. In addition, patients who received influenza vaccination (OR=0.87, 95% CI: 0.81– 0.94) or had an outpatient visit with hematologist/oncologist (OR = 0.77, 0.71–0.85) within one year of diagnosis were less likely to receive NAT than those who did not have such encounters with the healthcare system. Compared with those residing in neighborhoods with the lowest median household income, patients from the highest-income neighborhoods (OR=0.75, 95% CI: 0.67–0.85) were less likely to receive NAT. We also stratified the analysis by patient’s age at AML diagnosis (Table 3). The findings were similar to the overall study cohort.
Table 2.
Factors associated with NAT among 14089 older patients with AML, 2001–2013
| Odds ratio* | 95% Confidence interval* | p | |
|---|---|---|---|
| Age at diagnosis (in years) | |||
| 66–69 | 1.00 | ||
| 70–74 | 1.26 | 1.10– 1.44 | <.01 |
| 75–79 | 2.19 | 1.93– 2.49 | <.01 |
| 80–84 | 3.95 | 3.46– 4.50 | <.01 |
| ≥85 | 8.19 | 7.10– 9.44 | <.01 |
| Marital status | |||
| Unmarried | 1.36 | 1.26– 1.47 | <.01 |
| Married | 1.00 | ||
| Unknown | 1.14 | 0.96– 1.34 | 0.12 |
| Urban/rural | |||
| Big metro | 1.00 | ||
| Metro | 1.16 | 1.06– 1.26 | <.01 |
| Other | 1.08 | 0.96– 1.21 | 0.21 |
| SEER region | |||
| Northeast | 1.00 | ||
| Midwest | 1.27 | 1.11– 1.45 | <.01 |
| South | 1.27 | 1.12– 1.43 | <.01 |
| West | 1.31 | 1.19– 1.45 | <.01 |
| Median household income at zip code level | |||
| 1st quartile(low) | 1.00 | ||
| 2nd quartile | 0.97 | 0.87– 1.08 | 0.57 |
| 3rd quartile | 0.90 | 0.81– 1.01 | 0.08 |
| 4th quartile(high) | 0.75 | 0.67– 0.85 | <.01 |
| Unknown | 0.92 | 0.70– 1.22 | 0.56 |
| Previous history of hematologic malignancies | |||
| No | 1.00 | ||
| Yes | 0.59 | 0.51– 0.68 | <.01 |
| Previous history of solid tumors | |||
| No | 1.00 | ||
| Yes | 0.85 | 0.78–0.93 | <.01 |
| Mental disorders | |||
| No | 1.00 | ||
| Yes | 1.43 | 1.26–1.63 | <.01 |
| Elixhuaser score (exclude mental disorders) | |||
| 0 | 1.00 | ||
| 1–2 | 0.96 | 0.88– 1.05 | 0.34 |
| ≥3 | 1.30 | 1.17– 1.44 | <.01 |
| Disabled | |||
| No | 1.00 | ||
| Yes | 2.31 | 2.01– 2.66 | <.01 |
| Infection related hospitalization within 3 months before diagnosis | |||
| No | 1.00 | ||
| Yes | 1.39 | 1.15– 1.67 | <.01 |
| First hospitalization around diagnosis | |||
| Elective/other | 1.00 | ||
| Emergent/urgent | 1.21 | 1.12– 1.31 | <.01 |
| Hematologist/oncologist outpatient visit before diagnosis | |||
| No | 1.00 | ||
| Yes | 0.77 | 0.71– 0.85 | <.01 |
| Influenzas vaccine before diagnosis | |||
| No | 1.00 | ||
| Yes | 0.87 | 0.81– 0.94 | <.01 |
All variables in the table were included in a multivariable logistic regression model simultaneously.
Table 3.
Factors associated with no active treatment (NAT) among older patients with AML by age at diagnosis, 2001–2013
| NAT n (%) | 66–74 years Active treatment n (%) | OR (95% CI) | p | NAT n (%) | 75–99 years Active treatment n (%) | OR (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (in years) | ||||||||
| 66–69 | 510(34.0) | 1188(38.5) | 1.00 | |||||
| 70–74 | 989(66.0) | 1898(61.5) | 1.25(1.09–1.43) | <.01 | ||||
| 75–79 | 1595(26.9) | 1757(49.1) | 1.00 | |||||
| 80–84 | 1989(33.6) | 1211(33.8) | 1.82(1.65–2.02) | <.01 | ||||
| ≥85 | 2342(39.5) | 610(17.0) | 3.79(3.37–4.26) | <.01 | ||||
| Sex | ||||||||
| Male | 878(58.6) | 1755(56.9) | 1.00 | |||||
| Female | 621(41.4) | 1331(43.1) | 0.81(0.71–0.93) | <.01 | ||||
| Marital status | ||||||||
| Unmarried | 595(39.7) | 904(29.3) | 1.53(1.33–1.77) | <.01 | 2995(50.5) | 1329(37.1) | 1.31(1.19–1.44) | <.01 |
| Married | 831(55.4) | 2034(65.9) | 1.00 | 2603(43.9) | 2045(57.2) | 1.00 | ||
| Unknown | 73(4.9) | 148(4.8) | 1.19(0.88–1.60) | 0.27 | 328(5.5) | 204(5.7) | 1.10(0.91–1.35) | 0.33 |
| Urban/rural | ||||||||
| Big metro | 3147(53.1) | 2093(58.5) | 1.00 | |||||
| Metro | 1781(30.1) | 966(27.0) | 1.22(1.10–1.36) | <.01 | ||||
| Other | 998(16.8) | 519(14.5) | 1.20(1.04–1.38) | 0.01 | ||||
| SEER region | ||||||||
| Northeast | 220(14.7) | 615(19.9) | 1.00 | 1188(20.0) | 880(24.6) | 1.00 | ||
| Midwest | 189(12.6) | 431(14.0) | 1.23(0.95–1.59) | 0.11 | 899(15.2) | 469(13.1) | 1.34(1.14–1.57) | <.01 |
| South | 418(27.9) | 851(27.6) | 1.38(1.08–1.78) | 0.01 | 1346(22.7) | 705(19.7) | 1.33(1.14–1.54) | <.01 |
| West | 672(44.8) | 1189(38.5) | 1.71(1.38–2.11) | <.01 | 2493(42.1) | 1524(42.6) | 1.23(1.09–1.39) | <.01 |
| Median household income at zip code level | ||||||||
| 1st quartile(low) | 422(28.2) | 757(24.5) | 1.00 | 1522(25.7) | 758(21.2) | 1.00 | ||
| 2nd quartile | 414(27.6) | 745(24.1) | 1.05(0.88–1.26) | 0.57 | 1485(25.1) | 817(22.8) | 0.94(0.83–1.08) | 0.39 |
| 3rd quartile | 348(23.2) | 764(24.8) | 0.86(0.71–1.03) | 0.11 | 1462(24.7) | 884(24.7) | 0.94(0.82–1.08) | 0.39 |
| 4th quartile(high) | 276(18.4) | 749(24.3) | 0.75(0.61–0.92) | <.01 | 1368(23.1) | 1063(29.7) | 0.77(0.66–0.89) | <.01 |
| Unknown | 39(2.6) | 71(2.3) | 1.00(0.66–1.53) | 0.99 | 89(1.5) | 56(1.6) | 0.85(0.59–1.23) | 0.40 |
| Previous history of hematologic malignancies | ||||||||
| No | 1370(91.4) | 2714(87.9) | 1.00 | 5564(93.9) | 3158(88.3) | 1.00 | ||
| Yes | 129(8.6) | 372(12.1) | 0.56(0.45–0.71) | <.01 | 362(6.1) | 420(11.7) | 0.57(0.48–0.67) | <.01 |
| Previous history of solid tumors | ||||||||
| No | 1141(76.1) | 2259(73.2) | 4280(72.2) | 2406(67.2) | 1.00 | |||
| Yes | 358(23.9) | 827(26.8) | 0.84(0.72–0.98) | 0.02 | 1646(27.8) | 1172(32.8) | 0.85(0.77–0.93) | <.01 |
| Mental disorders | ||||||||
| No | 1298(86.6) | 2836(91.9) | 1.00 | 5098(86.0) | 3307(92.4) | 1.00 | ||
| Yes | 201(13.4) | 250(8.1) | 1.36(1.09–1.69) | <.01 | 828(14.0) | 271(7.6) | 1.46(1.24–1.71) | <.01 |
| Elixhuaser score (exclude mental disorders) | ||||||||
| 0 | 595(39.7) | 1359(44.0) | 1.00 | 1924(32.5) | 1286(35.9) | 1.00 | ||
| 1–2 | 473(31.6) | 1189(38.5) | 0.89(0.77–1.03) | 0.12 | 2153(36.3) | 1418(39.6) | 0.98(0.89–1.09) | 0.78 |
| ≥3 | 431(28.8) | 538(17.4) | 1.57(1.32–1.88) | <.01 | 1849(31.2) | 874(24.4) | 1.19(1.05–1.35) | <.01 |
| Disabled | ||||||||
| No | 1320(88.1) | 2955(95.8) | 1.00 | 4868(82.1) | 3364(94.0) | 1.00 | ||
| Yes | 179(11.9) | 131(4.2) | 2.14(1.66–2.75) | <.01 | 1058(17.9) | 214(6.0) | 2.43(2.06–2.88) | <.01 |
| Infection related hospitalization within 3 months before diagnosis | ||||||||
| No | 1402(93.5) | 2985(96.7) | 1.00 | 5581(94.2) | 3445(96.3) | 1.00 | ||
| Yes | 97(6.5) | 101(3.3) | 1.45(1.06–1.98) | 0.02 | 345(5.8) | 133(3.7) | 1.33(1.06–1.66) | 0.01 |
| Hematologist/oncologist outpatient visit before diagnosis | ||||||||
| No | 4437(74.9) | 2309(64.5) | 1.00 | |||||
| Yes | 1489(25.1) | 1269(35.5) | 0.73(0.66–0.82) | <.01 | ||||
| First hospitalization around diagnosis | ||||||||
| Elective/other | 1996(33.7) | 1551(43.3) | 1.00 | |||||
| Emergent/urgent | 3930(66.3) | 2027(56.7) | 1.31(1.19–1.43) | <.01 | ||||
| Density of hematologist/oncologist at hospital referral region | ||||||||
| 1st tertile(low) | 581(38.8) | 1199(38.9) | 1.00 | |||||
| 2nd tertile | 439(29.3) | 926(30.0) | 0.98(0.83–1.15) | 0.77 | ||||
| 3rd tertile(high) | 479(32.0) | 961(31.1) | 1.28(1.06–1.54) | <.01 | ||||
| Influenza vaccine within 1 year before diagnosis | ||||||||
| No | 973(64.9) | 1843(59.7) | 1.00 | 3228(54.5) | 1805(50.4) | 1.00 | ||
| Yes | 526(35.1) | 1243(40.3) | 0.86(0.75–0.98) | 0.02 | 2698(45.5) | 1773(49.6) | 0.88(0.81–0.97) | <.01 |
All variables in the table mutually adjusted in the model.
Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
In this large, retrospective cohort study, we found that more than half (52.7%) of older AML patients (aged ≥66 years at diagnosis) received no active leukemia-directed therapy. Even in 2013, nine years after HMAs became available in the US, 42% of older AML patients did not receive any active therapy for their malignancy. Variables associated with higher odds of NAT included older age, certain socioeconomic characteristics (household income in the lowest quartile,residence outside the Northeast Region, not being married, state buy-in insurance coverage prior to diagnosis), and clinical factors (≥3 comorbidities, mental disorders, recent hospitalization, disability), all of which are in line with findings from other studies of patients with both solid and other hematologic malignancies. 12,19,21,27 Given the high morbidity and mortality related to intensive chemotherapy, high prevalence of comorbidities and poor organ function, and aggressive disease biology among older AML patients, it is not surprising that many such patients received NAT.28,29
A novel finding of our study is that patients with a previous diagnosis of solid or hematologic malignancy, had undergone chemotherapy or were seen by a hematologist/oncologist within the year prior to diagnosis were more likely to be actively treated for their AML. While this might seem counterintuitive initially as patients with a previous malignancy and undergoing chemotherapy might have a reduced performance status compared to other elderly patients, this finding is potentially due to a better access to specialist care and patient preference to pursue aggressive treatment. Additionally, patients who received the influenza vaccine within the last year were more likely to be actively treated for their AML which is also suggestive of better access to and more frequent contact with the healthcare system.
The retrospective and population-based nature of our study precluded assessment of the reason why individual patients received NAT. While age, burden of comorbidity, and concern about treatment-related mortality may be medically justifiable, we also identified additional predictors of NAT, including low household income, unmarried status, female sex, and residence outside the Northeast Region. Lower household income and unmarried status (suggesting a potential deficit in social support) could limit access to hematologists/oncologists as patients may prioritize other basic needs over medical treatment or have difficulties in arranging for transportation to their appointments which is especially relevant for patients receiving azacitidine as a daily injection. Potential disparity in access to AML therapy is concerning, as patients who received NAT had a significantly worse survival than those who received active treatment in our study and others. 14,30,31. However, it needs to be kept in mind that the overall survival for elderly patients with AML is poor in general even if they are receiving leukemia-directed therapy. Nonetheless, identifying and overcoming socioeconomic and health system factors that are associated with NAT may help improve the quality of care and survival of older AML patients as improved therapeutic options become available.
Encouragingly, the percentage of older AML patients managed with NAT has decreased over the last decade which mirrors a modest improvement in survival. 4,13 This trend may be due to the introduction of less-toxic regimens such as HMAs and improved supportive care measures. 32 It remains to be seen how the recent approvals of effective and generally well-tolerated novel oral agents for unfit older patients and those with comorbidities, such as venetoclax, ivosidenib, and glasdegib, will impact treatment patterns and outcomes in this patient population.8,9 Additionally, the oral administration of these agents may decrease the logistic burden for patients associated with travel to and from treatment centers and the inconvenience of HMA injections potentially leading to increased therapy adherence.
While NAT does not necessarily imply undertreatment, another approach to improving care of older AML patients is to change physicians’ perceptions of the risks and benefits associated with AML therapy. In physician surveys, often-quoted reasons for not offering systemic therapy include the poor overall outcome, concern about treatment-related morbidity and mortality, and patient preference. 27 While QoL can worsen initially with therapy, previous studies suggest that it rebounded subsequently in some patients and survival improved. 27,33,34 Despite the availability of validated tools to estimate risks of intensive therapies and risk of disease relapse based on clinical and biological factors, estimating the prognosis of an individual patient and potential benefits and risks of active treatment remains very challenging.
While the acuity of AML diagnosis can be overwhelming for patients, a majority wants to be involved in the decision-making process about treatment options. 35–38 Patients who believe their prognosis is more favorable are more likely to pursue aggressive treatment. 37 Most patients are overestimating their chance of cure for both AML and other types of cancer. 27,35,39,40
It is important to emphasize that NAT might be an appropriate treatment strategy for some AML patients, especially in case of a poor performance status and a significant burden of comorbidity. 37 For most patients, QoL is more important than length of life, and NAT may therefore not necessarily reflect undertreatment. 27,28,29 However, NAT should be part of a broader, multidisciplinary treatment concept that includes palliative care and hospice services. Previous data from our group and others showed that end-of-life care in older AML patients in this regard may be suboptimal. 28,29,41–43 Not surprisingly, the factors associated with a lower likelihood of NAT in our study match factors that were previously identified to be linked with a lower likelihood of hospice and palliative care enrollment. 28,29
Like any retrospective cohort study, our study has limitations. Our dataset only included AML patients with Medicare coverage and therefore results may not be generalizable to all patients with AML. As a claims-based study, we do not have any information regarding the preference of physicians and patients, or the medical appropriateness of NAT versus chemotherapy on an individual patient level. This limitation is especially important as individual preferences of an informed patient should be the main factor in decisions about treatment options. Additionally, we could not assess whether chemotherapy was administered in a curative or palliative intent such as limiting transfusion burden.
Despite these limitations, our study is the largest to date that examines factors associated with NAT in AML patients. Given the large number of patients as well as the population-based and longitudinal design, we were able to assess trends in treatment approaches over a 13-year study period. Our study also spans the longest study period which is an advantage over other studies as the armamentarium of AML treatments is continuously expanding. Additionally, the availability of a wide spectrum of data on medical history, treatment and healthcare access allowed us to identify several novel factors that were associated with a higher likelihood of NAT in older AML patients.
Conclusions
In conclusion, we found that more than half of older AML patients in the US received NAT and that the likelihood of NAT increased with patient age, burden of comorbidity and various sociodemographic factors. Notably, patients were more likely to receive AML-specific treatment if they were diagnosed more recently, or if they had more frequent contact with the healthcare system in general and hematologists/oncologists in particular. Identifying potential barriers to optimal treatment is important to improve outcomes and quality of life in this patient population especially as novel oral therapies are entering the US market.
Table 4:
Overview of factors associated with a higher likelihood of NAT for acute myeloid leukemia in previously published studies
| Study (Ref.) | Patients | % NATonly | Sex | Age | Comorbidity | Race | SES | Marital status | Geographic region | Provider characteristics |
|---|---|---|---|---|---|---|---|---|---|---|
| Medeiros et al. 12 | AML >65 years of age | 60% | Female | Yes | Yes | No | Low income | Widowed | Other than Midwest | Not specified |
| Doria-Rose et al. 44 | AML >60 years of age | 15% | No | Yes | No | No | No | Other than married | Not specified | No |
| Lang et al. 45 | AML >65 years of age | 66% | No | Yes | Yes | No | Not specified | Not specified | Other than South | Not specified |
| Meyers et al.30 | AML >65 years of age | 57% | No | Yes | Yes | Black | No | Not specified | Not specified | Not specified |
| Oran et al.13 | AML >65 years of age | 61% | Female | Yes | Yes | No | No | Not specified | Not specified | Not specified |
| Bhatt et al. 46 | All AML | 25% | Female | Yes | Yes | Black | Low income, insurance status | Not specified | Not specified | Lower hospital volume, non-academic, shorter travel |
| Patel et al.47 | All AML | Not specified | Female | Yes | Yes | Black | Not specified | Not specified | Not specified | Not specified |
| Current study | AML >65 years of age | 53% | Female | Yes | Yes | No | Low income | Non-married | Other than Northeast | Not specified |
Abbreviations: BSC best supportive care, SES socioeconomic status
Acknowledgement/funding:
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute (NCI)’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
This research was funded by an investigator-initiated grant from Celgene Corp (PI: XM). AMZ was partly supported by the Dennis Cooper Hematology Young Investigator Award. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
AMZ received research funding (institutional) from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, ADC Therapeutics. AMZ had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Ariad, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Ariad, and Takeda. AMZ received honoraria from and was a speaker for Takeda (past). NAP received research funding (institutional) from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Celator, Pfizer, Astex Pharmaceuticals, CTI BioPharma, Genentech, LAM Therapeutics and Samus Therapeutics. NAP received research funding from Celgene. NAP had a consultancy with and received honoraria from Agios, Alexion and Pfizer. SFH received research funding (institutional) from Celgene, TG Therapuetics, DTRM, Genentech. SFH reports personal fees from Celgene, personal fees from Pharmacyclics, personal fees from Genentech, personal fees from Bayer, outside the submitted work; S.D.G. has consulted for and receives research funding from Celgene. AJD reports grants from Celgene during the conduct of the study; personal fees and other from Abbvie, grants from Boehringer-Ingelheim, grants from Pharmaceutical Research and Manufacturers of America Foundation outside of the submitted work. XM and RW received research funding from Celgene Corp, which supported the development of this manuscript, and consulted for Celgene and Incyte.
Footnotes
Declaration of conflicts of interest: The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin 68:7–30, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Podoltsev NA, Stahl M, Zeidan AM, et al. : Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev 31:43–62, 2017 [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MR, Tallman MS, Abboud CN, et al. : Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:926–957, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Lancet JE: Is the overall survival for older adults with AML finally improving? Best Pract Res Clin Haematol 31:387–390, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, Weber D, Fromm E, et al. : Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol 96:1993–2003, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juliusson G, Antunovic P, Derolf A, et al. : Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113:4179–87, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Lancet JE, Uy GL, Cortes JE, et al. : Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. Journal of Clinical Oncology 34:7000–7000, 2016 [Google Scholar]
- 8.DiNardo CD, Pratz KW, Letai A, et al. : Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 19:216–228, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Cortes JE, Douglas Smith B, Wang ES, et al. : Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. Am J Hematol 93:1301–1310, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. : Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28:562–9, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Dombret H, Seymour JF, Butrym A, et al. : International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros BC, Satram-Hoang S, Hurst D, et al. : Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 94:1127–38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oran B, Weisdorf DJ: Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 97:1916–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah BK, Ghimire KB: Improved survival among older acute myeloid leukemia patients - a population-based study. Acta Oncol 53:935–8, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Forsythe A, Kwon CS, Bell T, et al. : Health-related quality of life in acute myeloid leukemia patients not eligible for intensive chemotherapy: results of a systematic literature review. ClinicoEconomics and outcomes research : CEOR 11:87–98, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter RB, Estey EH: Management of older or unfit patients with acute myeloid leukemia. Leukemia 29:770–775, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korol EE, Wang S, Johnston K, et al. : Health-Related Quality of Life of Patients with Acute Myeloid Leukemia: A Systematic Literature Review. Oncology and therapy 5:1–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minden MD, Dombret H, Seymour JF, et al. : The effect of azacitidine on health-related quality of life (HRQL) in older patients with newly diagnosed acute myeloid leukemia (AML): results from the AZA-AML-001 trial. Haematologica. 2015;22(100):40–41., 2015 [Google Scholar]
- 19.Fakhri B, Fiala MA, Tuchman SA, et al. : Undertreatment of Older Patients With Newly Diagnosed Multiple Myeloma in the Era of Novel Therapies. Clin Lymphoma Myeloma Leuk 18:219–224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shavers VL, Brown ML: Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 94:334–57, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Aizer AA, Chen MH, McCarthy EP, et al. : Marital status and survival in patients with cancer. J Clin Oncol 31:3869–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bewersdorf JP, Stahl M, Zeidan AM: Are we witnessing the start of a therapeutic revolution in acute myeloid leukemia? Leuk Lymphoma:1–16, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3–18, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Noone AM, Howlader N, Krapcho M, et al. : SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018., 2018 [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 36:8–27, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Davidoff AJ, Gardner LD, Zuckerman IH, et al. : Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care 52:500–10, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekeres MA, Stone RM, Zahrieh D, et al. : Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia 18:809–16, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Zeidan AM, Halene S, et al. : Health Care Use by Older Adults With Acute Myeloid Leukemia at the End of Life. Journal of Clinical Oncology 35:3417–3424, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Jawahri AR, Abel GA, Steensma DP, et al. : Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer 121:2840–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyers J, Yu Y, Kaye JA, et al. : Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy 11:275–86, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Percival ME, Tao L, Medeiros BC, et al. : Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer 121:2004–12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl M, DeVeaux M, Montesinos P, et al. : Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv 2:923–932, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alibhai SM, Breunis H, Timilshina N, et al. : Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol 6:262–71, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Alibhai SM, Leach M, Kermalli H, et al. : The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol 64:19–30, 2007 [DOI] [PubMed] [Google Scholar]
- 35.El-Jawahri A, Nelson-Lowe M, VanDusen H, et al. : Patient-Clinician Discordance in Perceptions of Treatment Risks and Benefits in Older Patients with Acute Myeloid Leukemia. Oncologist, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagerty RG, Butow PN, Ellis PM, et al. : Communicating with realism and hope: incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol 23:1278–88, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Matsuyama R, Reddy S, Smith TJ: Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24:3490–6, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Gurmankin AD, Baron J, Hershey JC, et al. : The role of physicians’ recommendations in medical treatment decisions. Med Decis Making 22:262–71, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Mackillop WJ, Stewart WE, Ginsburg AD, et al. : Cancer patients’ perceptions of their disease and its treatment. Br J Cancer 58:355–8, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Jawahri A, Nelson-Lowe M, VanDusen H, et al. : Patient-Clinician Discordance in Perceptions of Treatment Risks and Benefits in Older Patients with Acute Myeloid Leukemia. Oncologist 24:247–254, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Jawahri A, LeBlanc TW, Burns LJ, et al. : What Do Transplant Physicians Think About Palliative Care? A National Survey Study. Cancer, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui D, Park M, Liu D, et al. : Attitudes and Beliefs Toward Supportive and Palliative Care Referral Among Hematologic and Solid Tumor Oncology Specialists. Oncologist 20:1326–32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui D, Didwaniya N, Vidal M, et al. : Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer 120:1572–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria-Rose VP, Harlan LC, Stevens J, et al. : Treatment of de novo acute myeloid leukemia in the United States: a report from the Patterns of Care program. Leuk Lymphoma 55:2549–55, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Lang K, Earle CC, Foster T, et al. : Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs Aging 22:943–55, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Bhatt VR, Shostrom V, Gundabolu K, et al. : Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood Adv 2:1277–1282, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel MI, Ma Y, Mitchell B, et al. : How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev 24:344–9, 2015 [DOI] [PubMed] [Google Scholar]