Abstract
Objectives:
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues is a promising treatment for patients with inoperable, well to moderately-differentiated metastatic neuroendocrine tumors (NETs). In continuation of our novel study with the radionuclide Lutetium 177 (Lu-177), we now present further results of Lu-177 DOTATATE therapy in managing NETs and other somatostatin receptor (SSTR) expressing tumors in a larger and more diverse patient group.
Methods:
144 consecutive patients (84 men and 50 women; age range 11- 87 year; mean age 58.5) with histologically confirmed NET were enrolled. 143 patients received at least 1 cycle of treatment. Among them 132 were deemed evaluable by having at least 1 cycle of treatment and a post-treatment MRI or CT scan for assessment based on modified Response Evaluation Criteria in Solid Tumors (RECIST) .Response to therapy was evaluated in terms of progression free survival (PFS), overall survival (OS) as well as radiologic, biochemical and clinical responses. Further, analysis of symptoms was reviewed during therapy and also in subsequent follow-ups for safety evaluation. Renal, gastrointestinal, hepatic, and hematological adverse events were evaluated using National Cancer Institute (NCI) common toxicities criteria V4.03, through full blood panels, as well as consultation with patients for any symptoms and/or adverse events.
Results:
As of July, 2016, out of 28 patients who have completed 177Lu-DOTATATE therapy (completion of 4 or more cycles of treatment and all designated follow-ups), no patient showed complete response (CR), 8 patients (28.57%) showed partial response (PR), 16 patients (57.14%) showed stable disease (SD) and progressive disease (PD) was observed in 4 patients (14.28%). The Objective Response Rate (CR+PR) of this group was 28.57% (n= 8) with a Cumulative Disease Control (CR+PR+SD) of 85.71% (n= 24).
Among 132 evaluable patients, assessment of treatment response using modified RECIST criteria revealed complete response (CR) in none of the patients, partial response (PR) in 12 patients (9.09%), stable disease (SD) in 66 patients (50%) while progressive disease (PD), which included patients who passed away, was observed in 54 patients (40.90%) yielding an Objective Response Rate of 9.09% (n=12) and a Cumulative Disease Control Rate of 59.09% (n=78).
Symptoms including abdominal pain, diarrhea, flushing, and fatigue improved in over 50% of the patients while weight loss improved in 28.26% of the patients. No grade III or grade IV renal toxicities were found, though eleven grade III and five grade IV hematological as well as five grade III hepatotoxicities were reported. Grade III hematotoxicity lasted an average of 2.7 months and grade IV lasted for only 0.9 months while grade III hepatotoxicity lasted an average of 3.1 months.
Conclusion:
Lu-177 Octreotate PRRT has shown promising potential as a safe and effective targeted therapy in inoperable, well to moderately differentiated metastatic neuroendocrine cancers. The results of the multicenter randomized clinical trial conducted in U.S. and Europe are concordant with current study.
Keywords: Lu-177 Octreotate Peptide Receptor Radionuclide Therapy (PRRT), NET, Neuroendocrine tumors, Somatostatin Receptor, Progression Free Survival
Introduction and Background
Neuroendocrine Tumors (NETs) are relatively rare and slow growing hormone secreting neoplasms originating from neuroendocrine tissues with considerable variability in clinical presentation 1, 2. NETs can result in a number of diverse symptoms secondary to hyper-secretion of affected hormones as well as neurotransmitter serotonin. Together, this can lead to the most common symptoms like diarrhea, flushing, and in some cases right-sided heart disease, often referred to as carcinoid syndrome. Additionally, associated conditions such as insulinoma, glucagonoma, VIPoma and gastrinoma can also arise 3. Although these tumors are considered rare, there has been a steady rise in the incidence of NETs over the last few decades. A study in 2008 using Surveillance, Epidemiology, and End Results (SEER) program registries, found that between the years of 1973 and 2005, the age-adjusted incidence rate increased from 1.09/100,000 to 5.25/100,000. It also showed that patients with well-differentiated stage G1 tumors had a 21% rate of multiple distant metastases, G2 with a 30% rate, and poorly/well differentiated G3/G4 stage tumors had a 50% rate of multiple distant metastases 3. Surgical resection is the therapy of choice for patients with operable and localized disease, though the nonspecific symptoms and high rate of metastases by the time of diagnosis makes successful and curative tumor resection difficult or often impossible 4. Current approved systemic therapies for NETs in the U.S. include streptozocin, everolimus, and sunitinib, and lanreotide. Multiple European studies have shown that systemic cytotoxic chemotherapy (cisplatin or etoposide) has a minimal effect on low (grade 1-2) NETs, but is effective in treating high-grade (grade 3) NETs 5, 6.
Treatment using somatostatin analogues can elicit a significant symptomatic relief in patients with functional NETs as the hormonal hyperactivity slows or shuts down. Based on the data from the PROMID (placebo-controlled, randomized study of octreotide long acting release in metastatic neuroendocrine midgut tumors) study 7, 8, this is indicated by showing prolonged disease free survival of midgut carcinoid and reduction in the level of biochemical markers such as chromogranin A, neurokinin A, serotonin in blood and its metabolite (5-HIAA) in the urine.
Radiolabeled somatostatin analogues called Peptide Receptor Radionuclide Therapy (PRRT) for treatment of patients with NET have been available since the 1990’s 9. Since then several radionuclides have been used for treatment including Indium-111 followed by Yttrium-90, however, since 2000’s Lutetium-177 has been in use as the radionuclide agent of choice for most therapy trials moving forward.
Lu-177-Octreotate (DOTATATE) PRRT uses the Lu-177 radionuclide, which is generated from Ytterbium (Yb), has an approximate half-life of 6.7 days and is capable of delivering precise small value doses of Beta energies between 0.149–0.479 MeV with ranges of tissue penetration between 0.5mm to 2.0mm allowing for localized radiation with limited collateral damage to the normal tissues compared to Y-90. Additionally, Lu-177 also has two main gamma emission energies, 0.113 MeV (relative abundance 6%) and 0.208 MeV (11%), thereby providing the needed radiotracer for during and post-therapy scintigraphic imaging, bio-distribution and dosimetry studies 10.
Previous studies including our own study have shown the safety and tumor burden reduction effects of repeated treatments with 100–200 mCi (3.7-7.4 GBq) of Lu-177 DOTATATE 11. One such study by Kwekkeboom et al, showed that Objective tumor response rate (combination of complete response (CR), minimal response (MR), and partial response (PR) was as high as 46% and stable disease (SD) was reported in 35% of the 310 patients treated for NET 12. Other reports have also attested to the anti-tumor capabilities as well as limited toxicity potential in patients treated with Lu-177 13, 14.
The use of somatostatin analogue octreotate, which is attached to radiotracer Lu-177 through a chelating molecule DOTA is in itself a step forward in treatment delivery options. [DOTA0, Tyr3] Octreotate, has shown up to nine times the affinity for SSTR2 (most abundantly found somatostatin receptor subtype on the surface of NETs) as compared with [DOTA0, Tye3] Octreotide, and shows significant impact on tumor regression due to concentrated delivery of the β and γ emitting radiation produced by the Lu-177 radionuclide 15. The chemical composition difference between the somatostatin analogues [DTPA0, Tyr3] Octreotate and [DTPA0, Tyr3] Octreotide is minor, only in that the C-terminal threoninol of Octreotide is replaced with a C-terminal threonine in Octreotate 16. Yet this small difference increases the analogues’ ability to deliver more radioactive dose directly to the tumor, given same amount of injected octreotide and octreotate.
Though PRRT is proving to be a more manageable and safer option over other treatments such as chemotherapy, toxicity remains a safety concern especially in terms of renal damage. Kidney protection is, however, established to a certain extent with the simultaneous infusion of positively charged amino acids (AA). Several studies have shown that prolonged infusion of Lys-Arg was effective in reducing renal reuptake during and following completion of the PRRT injection, via reduced tubular reabsorption of the radionuclide thereby also allowing for higher absorbed doses by the tumor cells. This protection was enhanced with continued AA infusion for up to 10 hours, however, even 4 hours infusion showed a significant improvement 17. There is no established protocol measure for renal protection against Lu-177 Octreotate PRRT to date, but in our study we used 15% Clinisol® infusion for 4 hours to mitigate the toxicity risk.
Lu-177 Octreotate PRRT has shown promise, as a potential standard of care method in treatment of NETs and other SSTR expressing tumors. This study was to further establish phase II data on efficacy and safety of tumor management and symptom control in treatment of NETs and other SSTR expressing tumors and to provide this treatment to eligible patients under FDA-approved expanded access trial.
Materials and Methods:
Patient Profile
144 consecutive patients were enrolled into the trial program for repeated cycles of Lu-177 DOTATATE therapy to further evaluate the safety and efficacy of this treatment. One patient signed the consent form but before starting the PRRT experienced sever adverse event (SAE) which excluded him from the study, leaving 143 patients (84 men and 59 women, ages ranged from 11-87 years old, mean age 58.5 years ) who had undergone at least one cycle of treatment. Racial breakdown of patients: Caucasian (132 patients, 92.3% of total); Hispanic (5, 3.5%); African American (4, 2.8%); Asian (2, 1.4%). All patients had histopathologically confirmed diagnosis of somatostatin receptor expressing NET with a RECIST profile of progressive disease on diagnostic CT/MRI before the therapy. Patients had inoperable primary and /or multiple distant metastases and had responded poorly to previous standard of care treatments, including surgery, chemotherapy, radiotherapy, chemo and/or radio-embolization, or cold-somatostatin analogue therapy. Patients had following types of NETs: Mid-gut neuroendocrine tumor (NET) (59 patients; 41.2% of total); Pancreatic (48, 33.5%); Pulmonary (14, 9.8%); Unknown primary (10, 6.9%); Paraganglioma (4, 2.8%), Thymic (2, 1.4%); Merkel cell (2, 1.4%); Pheochromocytoma (1, 0.7%); Neuroblastoma (1, 0.7%); Prostate (1, 0.7%); and Ovarian (1, 0.7%). Patient prerequisites for trial treatment included hemoglobin concentration greater than or equal to 8.9 g/dL; white blood cell count greater than or equal to 2 x 109 /L (2000/μL); platelet count greater than or equal to 100 x 109 /L (100 x 103 /μL), total bilirubin equal to or less than 3 times upper normal limit, serum albumin greater than 30 g/L (3 g/dL) (or ≤ 3 g/L with normal prothrombin time), and serum creatinine level less than or equal to 150 μmol/L or less than or equal to 1.7 mg/dL; and a measured 24-hour creatinine clearance greater than or equal to 50 mL/min. Further, a somatostatin-based scintigraphy within 6 months of the first dose of Lu-177 DOTATATE, a Karnofsky performance status of at least 60% and a life expectancy of greater than 12 weeks was required for participation in the study. Patients receiving long acting somatostatin analogue therapy were required to have a 28 day washout period before the initiation of each therapy cycle.
Preparation of 177Lu-Octreotate
Lu-177-C13 was purchased from the Missouri University Research Reactor, Columbia, MO. DOTATATE kits were manufactured by Iso-Tex, Inc. in Friendswood Texas. The radiolabeled solution was compounded at the South Texas Nuclear Pharmacy (Houston, TX) and delivered to Excel Nuclear Oncology Center in Houston, TX. Analytical and quality assurance tests were performed by IsoTherapeutics, Inc. in Angleton, TX before the dose was administered to the patients as described previously11.
Treatment Protocol
We evaluated information from the 457 treatments provided to 143 patients started on October 2010. Patient based analysis was evaluated in 132 patients who underwent at least one cycle of therapy and had two comparable scans available to evaluate response based on RECIST criteria. Patients who failed to follow-up after 1st therapy cycle and did not have a comparison scan available, were excluded from evaluation (n=11). We further evaluated treatment response in a subgroup of patients who had completed the study (4+ cycles of therapy and all 3-6-12 and 18 month follow-ups). Number of cycles completed in this study ranged from one to six. 23 (16.08%) patients completed only 1 cycle, 18 (12.58%) patients completed two, 23 (16.08%) completed three, 71 patients (49.65%) completed four cycles, three patients (2.09%) completed 5 cycles, and five patients (3.49%) completed 6 cycles.
In each treatment cycle, patients received approximately 200 mCi (7.4 GBq; +/−10%) of Lu-177 DOTATATE via intravenous (IV) infusion administered over 30 minutes. An infusion of 1000mL of 15% Clinisol® was used for kidney protection; this infusion was started 30 minutes before the administration of 177Lu-DOTATATE and was continued for a total of 4 hours (250mL/h). Thirty minutes into 15% Clinisol® infusion, the Lu-177 DOTATATE infusion was started and completed in 30 minutes. All procedures were carried out in an outpatient setting with radiation exposure at 1m at the time of discharge was between 3-6 mR/h.
All patients were evaluated for any renal, gastrointestinal(GI) , hepatic, hematologic or other adverse events beginning immediately after the start of procedure, throughout the course of procedure and continued at least until completion of last follow-up using Common Terminology Criteria for Adverse Events (CTCAE) criteria version 4.03 established by National Cancer Institute (NCI). Total duration of follow-ups ranged from 2.5 months to 49 months, with a median of 12.3 months and an average of 13.1 months. Safety monitoring included routine determination of complete blood cell count (CBC), comprehensive metabolic panel (CMP), and tumor markers including chromogranin A, serotonin, pancreastatin, gastrin, neurokinin A, pancreatic polypeptide, and 24-hour urine 5-hydroxyindole acetic acid (5-HIAA), 1 week prior to each cycle of therapy and also 4 weeks after the therapy. Patients were followed up for 3, 6, 12 and 18 months after the fourth cycle. The Lu-177 DOTATATE therapy goal for this study was 4 cycles of therapy (at 6–9 weeks interval, up to a cumulative dose of 800 mCi [29.6 GBq]). Patients who had achieved stable disease status for at least 6 months were offered to receive two additional cycles upon disease progression. Patients were also evaluated for clinical response before each cycle through a complete history and physical examination, completion of a quality of life questionnaire, and imaging studies, such as CT scan, MRI, Octreoscan™, renal scan, MUGA scan and 18F-FDG PET/CT scan. In order to prevent and manage severe nausea and vomiting associated with amino acid infusion, following regimens were employed before and during the therapy: Sixty to ninety minutes before AA infusion, a single oral dose of Aprepitant 125mg was given. Within thirty minutes before AA infusion, dexamethasone 10mg IV diluted in 50 ml N/Saline and promethazine 25 mg PO were administered along with Ondansetron 8 mg IV diluted in 50 ml N/Saline over 15 minutes. If needed ondansetron was repeated at one hour intervals. All evaluable patients were categorized by their RECIST criteria to be further evaluated for Disease Control, Objective Response Rate, Kaplan- Meier estimates of Overall Survival and Progression free survival (PFS).
The study was conducted under FDA IND 78,256. Informed consent obtained and all the guidelines for experimental investigation with human subjects required by the formal institutional review board (IRB), and Health Insurance Portability and Accountability Act (HIPAA) compliance have been followed.
Statistical Analysis
The main objective of this study was to determine overall survival via the surrogate primary end point of progression free survival (PFS). The distributions of duration of PFS were estimated using the Kaplan-Meier method. Additional secondary end points of this trial were to determine safety of Lu-177 PRRT and also efficacy of this therapeutic modality evaluated through radiological response, clinical response, and biochemical response rates.
Results
143 patients (84 men and 59 women) with somatostatin receptor-positive NETs were treated with high activity Lu-177 DOTATATE (200 mCi [7.4GBq]; ±10% per cycle) between October 2010 and November 2015. The mean age was 58.5 years ranged from 11-87 years old. Demographic data for all patients is summarized in Table 1.
TABLE 1.
Demographic Data for all patients
| Type | n=143 |
|---|---|
| Mid-Gut | 59 |
| Pancreas | 48 |
| Pulmonary | 14 |
| Others | |
| Paraganglioma | 4 |
| Thymic | 2 |
| Merkel Cell | 2 |
| Pheochromocytoma | 1 |
| Neuroblastoma | 1 |
| Prostate | 1 |
| Ovarian | 1 |
| Unknown | 10 |
Response to Therapy:
As of July 2016, 23 patients were treated with 1 cycle of Lu-177 DOTATATE, with an average dose of 197.92 mCi. 18 patients were treated with 2 cycles with an average dose of 192.83 mCi. 23 Patients were treated with 3 cycles with an average dose of 196.91 mCi. 71 Patients were treated with 4 cycles with an average dose of 198.19 mCi. Eight patients were deemed able to undergo further cycles after their fourth cycle; three patients received 5 cycles of treatment with average dose of 198.27 mCi and five patients received 6 cycles of treatment with an average dose of 198.36 mCi (Table 2)
Table 2.
Average dose for number of cycles completed
| Number of Cycles Completed | Number of Patients | Percentage of Patients | Average Dose mCi |
|---|---|---|---|
| 1 | 23 | 16% | 197.92 |
| 2 | 18 | 12.6 | 192.83 |
| 3 | 23 | 16% | 196.91 |
| 4 | 71 | 49.6% | 198.19 |
| 5 | 3 | 2.1% | 198.27 |
| 6 | 5 | 3.5% | 198.36 |
As is evident in the following table (Table 3) we have evaluated RECIST criteria for all 143 patients who received at least one cycle of treatment as of July 2016. RECIST for patients who had only one cycle of therapy and did not come back for second cycle (either due to voluntarily withdrawal or death) were rendered not evaluable. Among a total of 143 patients, 132 patients received a follow-up and were able to be further evaluated for RECIST criteria, overall survival, and any toxicity. Eleven patients who did not return for follow-up after first cycle and therefore did not have a comparable scan for RECIST evaluation were excluded from analysis:
TABLE 3:
RECIST Criteria Evaluation for All Patients
| Organ | Number of cases (%) |
PD (%) |
SD (%) |
PR (%) |
CR (%) |
Not Evaluable (%) |
|---|---|---|---|---|---|---|
| MID-GUT | 59 (41.2%) |
19 (32.2%) |
32 (50.1%) |
2 (6.8%) |
--- | 6 (10.2%) |
| PANCREAS | 48 (33.53%) |
23 (47.9%) |
18 (37.5%) |
6 (12.5%) |
--- | 1 (2.08%) |
| PULMONARY | 14 (9.8%) |
5 (37.5%) |
6 (42.9%) |
2 (14.2%) |
--- | 1 (7.1%) |
| THYMUS | 2 (1.4%) |
2 (100%) |
--- | --- | --- | --- |
| PROSTATE | 1 (0.7%) |
--- | --- | --- | --- | 1 (100%) |
| PHEOCROMOCYTOMA | 1 (0.7%) |
--- | 1 (100%) |
--- | --- | --- |
| PARAGANGLIOMA | 4 (2.8%) |
1 (25%) |
3 (75%) |
--- | --- | --- |
| NEUROBLASTOMA | 1 (0.7%) |
1 (100%) |
--- | --- | --- | --- |
| OVARY | 1 (0.7%) |
--- | --- | 1 (100%) |
--- | --- |
| MERCKEL CELL | 2 (1.4%) |
1 (50%) |
--- | --- | --- | 1 (50%) |
| UNKNOWN | 10 (7%) |
2 (20%) |
6 (60%) |
1 (10%) |
--- | 1 (10%) |
| TOTAL | 143 | 54 (37.7%) | 66 (46.1%) | 12 (8.39%) | 0 (0%) | 11 (7%) |
Among 132 patients who had undergone at least one therapy cycle and returned for a follow-up scan, complete response (CR) wasn’t observed in any patient, partial response (PR) was seen in 12 patients (9.09%), and stable disease (SD) was observed in 66 patients (50%). Progressive disease (PD), which included those patients who passed away, was observed in 54 patients (40.90%) yielding an Objective Response Rate (ORR=CR+PR) of 9.09% (n=12) and a Cumulative Disease Control Rate (CR + PR+ SD) of 59.09% (n=78). Among the total 143 patients, 51 deaths were reported as of July 2016. Cause of 41 deaths was attributed to the tumor burden, whereas the cause of remaining deaths were documented as secondary to respiratory failure, portal vein thrombosis, hemorrhagic stroke, cardiac arrest, sepsis, pulmonary embolism and Cushing disease. None of the death events confirmed to be related to Lu-177 PRRT.
As of July 2016, among the 132 patients evaluated, 28 patients who completed the Lu-177 DOTATATE treatment (defined as having completed four cycles of treatment and all designated follow-ups) had RECIST criteria assessed. None of the patients were observed with complete response, 8 with partial response (28.57%), 16 with stable disease (57.14%), and progressive disease was observed in 4 patients (14.28%). The Objective Response Rate of this group was approximately 28.57% with a Cumulative Disease Control of 85.71%.
Overall survival was calculated for all patients and a Kaplan-Meir survival curve was generated (Figure 1) for all evaluable patients in this study. Additionally, progression free survival was also calculated (including deaths) (Figure 2). 11 patients were excluded due to lack of follow-up and a cut-off of July, 2016 was set for patients that had no progression to date and had a follow-up visit recorded.
FIGURE 1:
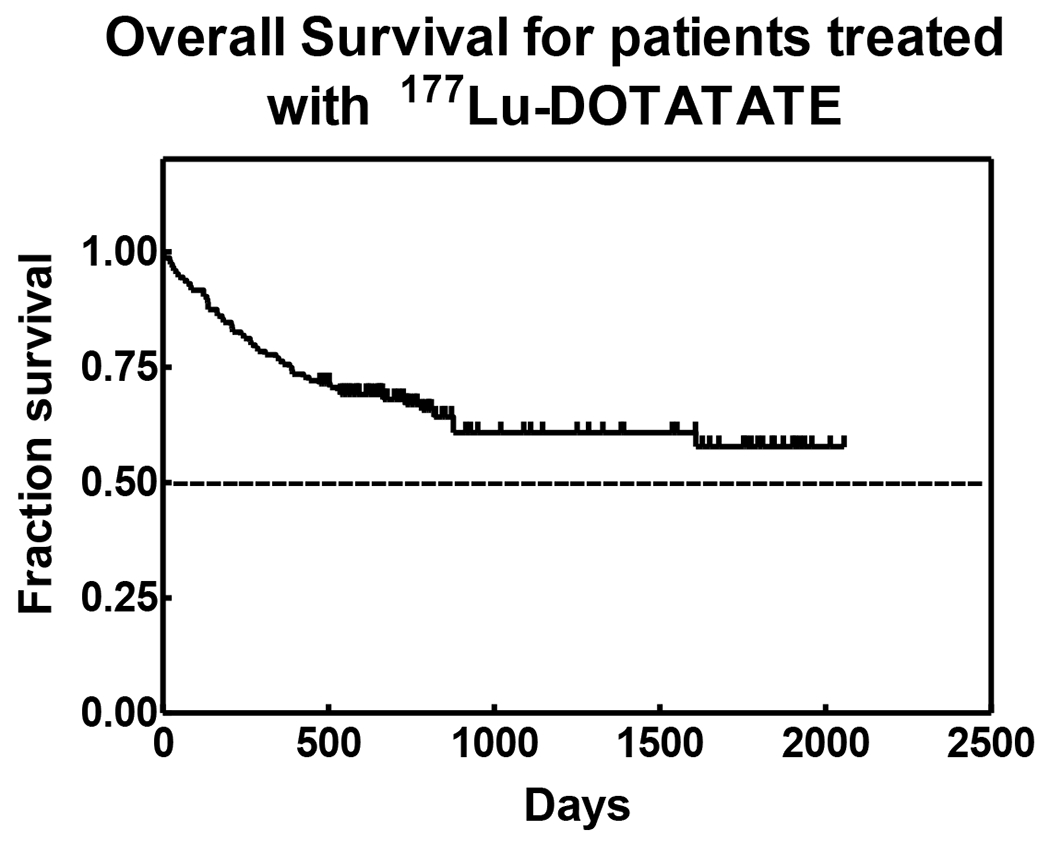
Overall survival for patients treated with 177Lu DOTATATE.
FIGURE 2:
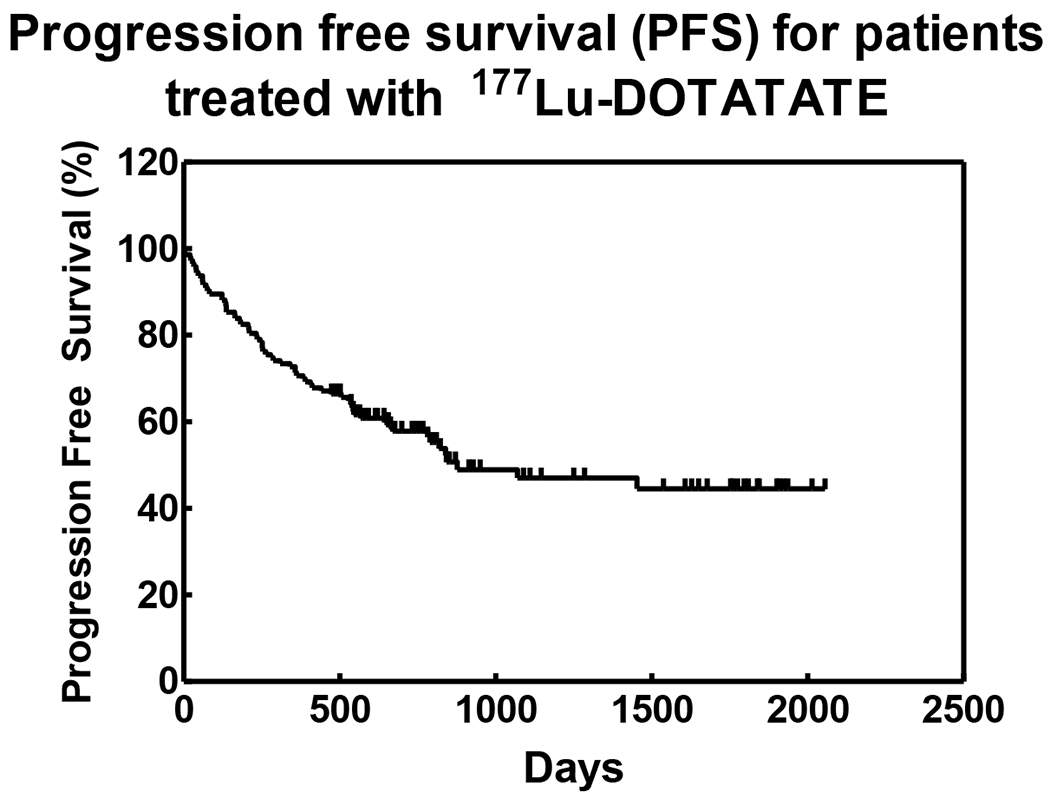
Progression-free survival for patients treated with 177Lu DOTATATE.
Average PFS for all patients regardless of the type of response was 11.2 months (337.4 days; range 1–1070 days). As of July 2016, PFS of 21 patients who completed Lu-177 DOTATATE PRRT and still experiencing favorable response (SD+PR) was 50.94 months (range 29.10 – 69.2 months). PFS of 69 evaluable patients (52.27%) who are alive as of July 2016 and have demonstrated and maintained favorable response (SD+PR) was found to be an average 31.9 months (range 15.8 – 68.6 months). These results indicate the survival benefit of repeated cycles of treatment. Kaplan-Meier Curves for overall survival and progression free survivals have been depicted in Figures 1 and 2.
Clinical response was evaluated based on symptomatology questionnaire filled out by the patient after each therapy cycle. Evaluation of common symptoms of NET patients to PRRT therapy showed that diarrhea which was reported as the most debilitated symptom improved in about 70% of patients (p <0.0001) followed by abdominal pain 63% (p <0.0001), flushing 64% (p 0.0012), fatigue 53% (p 0.0008) and finally weight loss with 28% (p 0.6855) improvement as the least responsive symptom to PRRT.
Summary of the symptom analysis is presented in Table 4 and Figure 3:
Table 4:
Change in symptoms following PRRT
| Symptoms before Therapy | Number of patients | Improved (%) | Progressed(%) | Unchanged (%) |
|---|---|---|---|---|
| Abdominal Pain | 57 | 63.15% | 17.54% | 19.29% |
| Diarrhea | 41 | 70.73% | 7.31% | 21.95% |
| Flushing | 42 | 64.28%% | 16.66% | 19.04% |
| Fatigue | 62 | 53.22% | 29.03% | 17.74% |
| Weight loss | 92 | 28.26%% | 22.82% | 48.19% |
Figure 3:
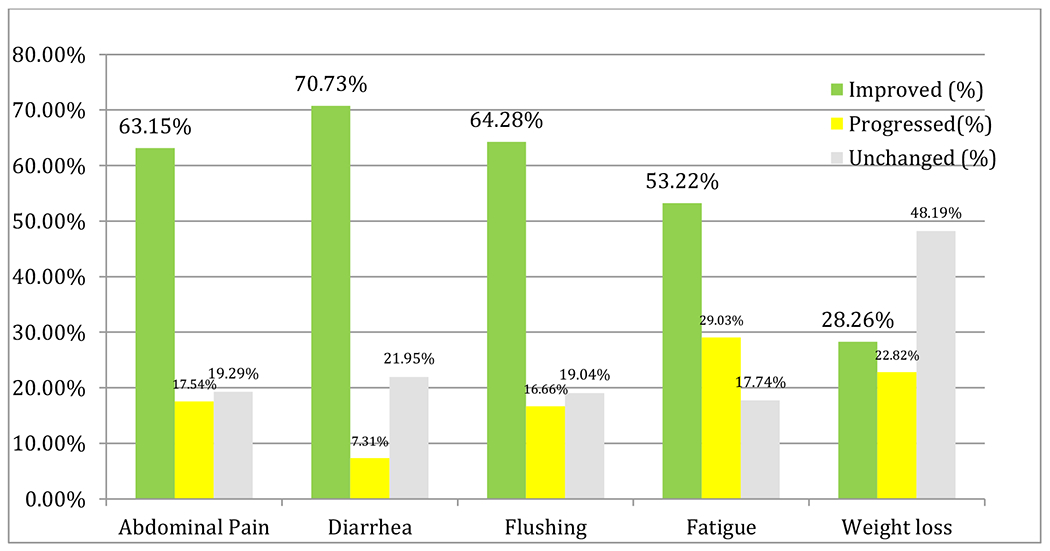
Change in symptoms following PRRT
Quality of life (QOL) of patients which is the most important aspect of any cancer therapy was evaluated using the European Organization for Research and Treatment of Cancer (EORTC QLQ-C30). We performed both quantitative and qualitative assessments of changes in QOL following PRRT. Out of 97 evaluable EORTC-QLQs, 46 (47.4%) patients reported improvement, 47(48.4%) patients reported worsening, while 4 (4.1%) patients reported no change in their quality of life (p 0.59) (Table 5; Figure 4)
TABLE 5:
Qualitative Assessments of Changes in QOL After PRRT
| Condition | Number of Patients | Percentage | |
|---|---|---|---|
| Total | Improvement | 46 | 47.40% |
| Worsening | 47 | 48.40% | |
| No Change | 4 | 4.10% |
figure 4.
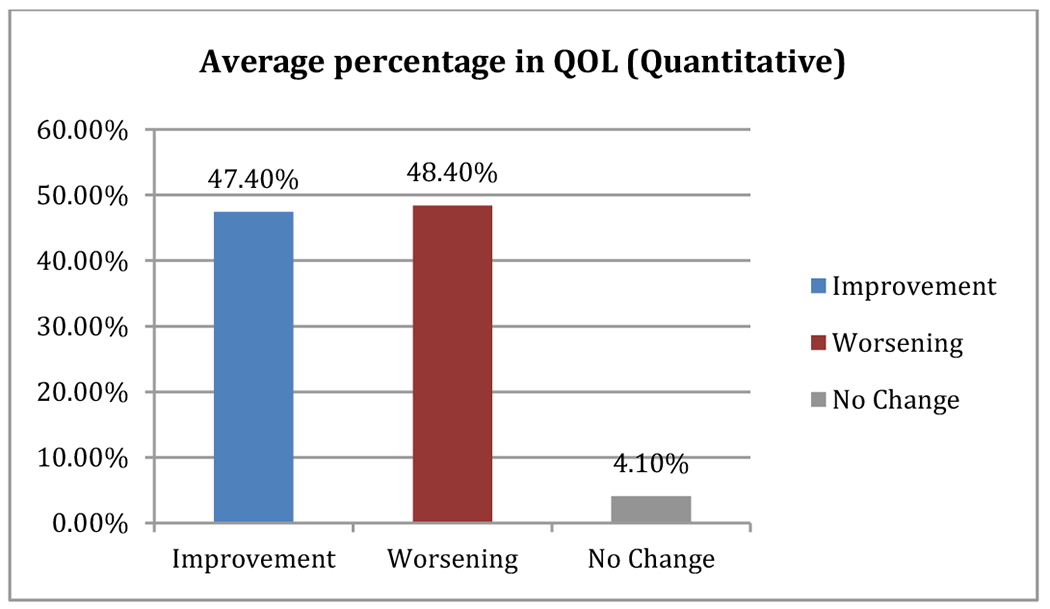
Average percentage in QOL (quantitative)
Among patients who experienced improvement in their QOL the average improvement from the baseline was 12% (range: 1 to 42) while patients who experienced worsening in QOL, the average of worsening from the baseline was 6.7% (range: −1 to −28) (Figure 5).
Figure 5.
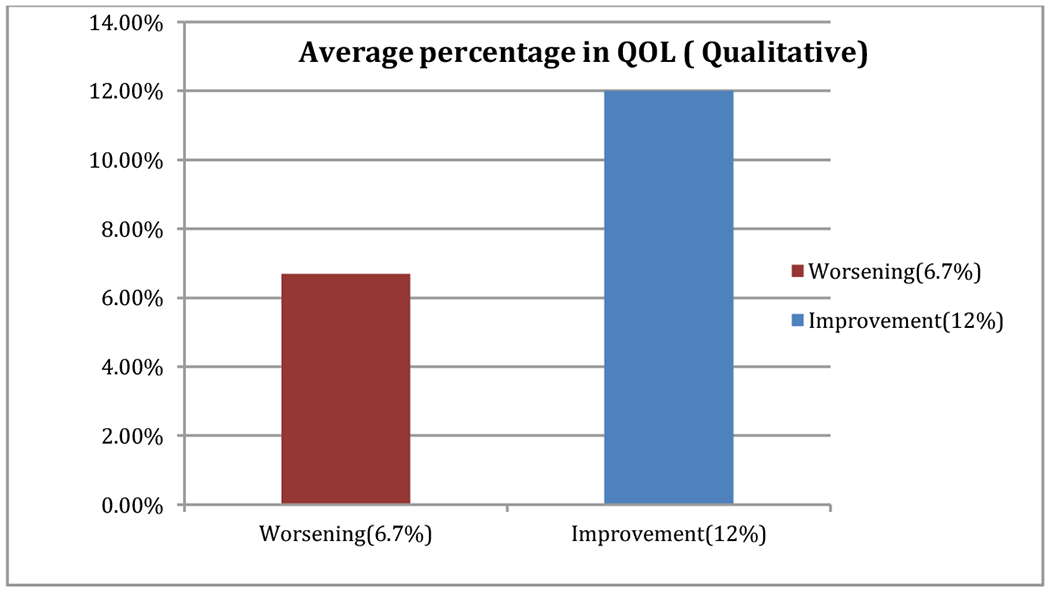
Average percentage in QOL (qualitative).
This data emphasizes on the fact that the degree of improvement in QOL in the particular fraction of patients that showed improvement was much higher in magnitude than the degree of worsening in patients with diminished QOL.
We evaluated biochemical response through pre therapy and post last respective therapy cycle levels of CgA, 5-HIAA and serotonin.
31 patients (25.2%) showed >50% decrease in the CgA level whereas 36 patients (29.2%) showed >50% increase in CgA level. For 5-HIAA 18 patients (15.6%) showed >50% decrease in the 5-HIAA level whereas 26 patients (22.6%) showed >50% increase in 5-HIAA level, and finally for Serotonin 55 patients (50%) showed >50% decrease in the Serotonin level where as 11 patients (10%) showed >50% increase in Serotonin level. For the rest of percentage changes in the tumor markers refer to table 6.
Table 6.
Percentage change in tumor markers following PRRT
| Tumor Markers (Evaluable) | |||
|---|---|---|---|
| CgA | 5-HIAA | Serotonin | |
| Number of evaluable cases | 123 | 115 | 110 |
| % change Decrease | |||
| >50% | 31 (25.2%) | 18 (15.65%) | 55 (50%) |
| 25-50% | 15 (12.19%) | 23 (20%) | 20 (18.18%) |
| <25% | 17 (13.82%) | 20 (17.39%) | 13 (11.81%) |
| % change Increase | |||
| >50% | 36 (29.26%) | 26 (22.60%) | 11 (10%) |
| 25-50% | 12 (9.75%) | 14 (12.17%) | 2 (1.81%) |
| <25% | 11 (8.94%) | 14 (12.17%) | 8 (7.27%) |
| No Change | 1 (0.81%) | 1 (0.90%) | |
There was no significant acute toxicity observed among the patients during or immediately following the treatment. Common clinical complaints occurred either as solitary symptoms or a combination of symptoms, most notably nausea (198 events), vomiting (54 events), abdominal pain (12 events), back pain (10 events), headache (5 events), pain at the site of injection (18 events), and flushing (62 events).
Patients were further evaluated for GI, hematological, hepatic, or renal toxicity using the National Cancer Institute common toxicity criteria and followed up for an average of 13 months. To date, no significant G3, G4 or other clinically significant renal toxicity has been observed. There were ten G3 and four G4 instances of hematological toxicities post- treatment. It is of note that all four patients with grade 4 hematologic toxicity had already undergone significant cytotoxic chemotherapy prior to PRRT. In addition, four instances of G3 hepatic toxicity were also observed. The G3 toxicities lasted an average of 2.7 months (range 1.9 to 4.3 months) and the G4 toxicities lasted an average of 0.9 months (range 0.2 to 2.3 months). A complete breakdown of post-treatment toxicity profile is summarized in Table 7 and Table 8:
Table 7.
Toxicity profile following PRRT
| Hematotoxicity | Hepatotoxicity | Kidney Toxicity | ||||
|---|---|---|---|---|---|---|
| Grade | Duration/M | Range/M | Duration/M | Range/M | Duration/M | Range/M |
| G1 | 4.3 | 0.3-35.7 | 2.8 | 0.4-8.0 | 4.3 | 0.7-12.3 |
| G2 | 5 | 0.4-8 | 2.4 | 0.4-6.4 | 1.5 | 1.5 |
| G3 | 2.7 | 1.9-4.3 | 3.1 | 2.2-4.9 | N/A | N/A |
| G4 | 0.9 | 0.2-2.3 | N/A | N/A | N/A | N/A |
Table 8.
Most frequent and the most serious adverse experiences by body system
| Body System | Side effect | Frequency |
|---|---|---|
| Hematologic | Thrombocymiddleenia | |
| GI | 37 (30.8%) | |
| GII | 13 (10.82%) | |
| GIII | 6 (4.9%) | |
| GIV | 5 (4.16%) | |
| Low Hb | ||
| GI | 78 (65%) | |
| GII | 20 (16.6%) | |
| GIII | 4 (3.33%) | |
| Low WBC | ||
| GI | 19 (15.8%) | |
| GII | 26 (21.6%) | |
| GIII | 1(0.83%) | |
| Hepatic | Bilirubin Elevation | |
| GI | 8 (6.6%) | |
| GII | 7 (5.83%) | |
| GIII | 3 (2.5%) | |
| Low Albumin | ||
| GI | 20 (16.6%) | |
| GII | 11 (9.16%) | |
| Renal | Serum Cr Elevation | |
| GI | 24 (20%) | |
| GII | 2 (1.66%) | |
| Gastrointestinal | Nausea | |
| G I | 144 (35.6%) | |
| G II | 54 (13.3%) | |
| Vomiting | ||
| G I | 40 (9.9%) | |
| G II | 14 (4.2%) | |
| Abdominal pain | 12 (2.9%) | |
| Skin | ||
| Flushing | 62 (15.3%) | |
| Others | ||
| Back pain | 10 (2.4%) | |
| Headache | 5 (1.23%) | |
| Pain in the site of injection | 3 (0.74%) |
Discussion:
The results of our study exhibited the strength and potential of the radionuclide based approach to neuroendocrine cancer treatment by demonstrating significant improvements in patient survival and progression free survival based on RECIST criteria and by considerably reducing associated symptoms. It is encouraging to see that with renal protection protocols in place, no clinically significant nephrotoxicity has been observed in this study with long term follow up.
Safety data from previous studies as well as data from our own previous studies has indicated that 4 cycles of Lu-177 DOTATATE administered at 6 to 9 week intervals at the dose of around 200 mCi (5.55-7.4 GBq)/cycle is a safe and effective method of tumor burden reduction 14, 18–22. Hematological toxicities of grade G3 were seen in 10 and G4 on 4 occasions respectively. Circulating levels of radioactivity in the blood being absorbed into the bone marrow is probably the cause of hematotoxicities and therefore protection options are very limited 23. Although no lasting severe hematological toxicities were found in this study, as the G3 toxicities lasted an average of 2.7 months and G4 an average of 0.9 months, other severe instances of hematological toxicities have been observed including a few cases of myelodysplastic syndrome (MDS) in some phase I trials with radionuclide therapies 24. However, in toxicity analysis of 504 patients treated with [Lu-177-DOTA0, Tyr3] Octreotate in doses between 27.8-29.6 GBq, usually in four treatment cycles (similar to the parameters of this study) has identified that MDS and liver toxicities were very rare, occurring only in approximately 1% of patients and study investigators concluded that MDS occurrences indicated radiation is due to direct absorption through the bone marrow during treatment or variation existing between patients in stem cell susceptibility to treatment 12.
Symptom management was also promising in this study and achieved through pre-therapy combination of anti-nausea regimen including, ondansetron IV, aprepitant PO, dexamethasone IV (non-diabetic patients) and promethazine PO. Cold somatostatin analogues have also been noted to improve symptoms for the last couple of decades, showing reduction in urinary 5-HIAA metabolites, the objective disease indicator, and improvement to most carcinoid syndrome symptoms including flushing and diarrhea 7.
Overall response to treatment can be considered encouraging. A disease control (CR, PR, SD) rate of 59.09% in patients with 1 or more cycles of Lu-177 DOTATATE indicates favorable tumor burden reduction results. Average PFS for all patients was 10.12 months or 337.4 days (range: 1-1070) and for patients who completed Lu-177 DOTATATE PRRT (4+ cycles) was 50.94 months (range 29.10 – 69.2 months). A study by Kwekkeboom et al showed patients treated with [Lu-177 DOTA0, Tyr3] Octreotate and a cumulative dose of 600 to 800 mCi (22.2 to 29.6 GBq) of Lu-177 Octreotate had a median time to progression* of over 36 months, while others reported progression free survival of 26 months with overall survival lasting upwards of 55 months indicating long-term outcomes for patients with pancreatic NET and non-pancreatic GEP NET. 25, 26.
In order to correctly evaluate the response to therapy, it is our view that it would be imprudent to ignore patients who achieved “Stable Disease” (SD) in this patient population. Achieving symptom control, improved quality of life, and stability of previously progressive cancer by RECIST criteria are meaningful improvements and are of utmost importance for individual patients who have cancer. The patients enrolled in this protocol all had progressive disease and had exhausted all other therapeutic options. An analysis to compare RECIST between July 2015 and July 2016 interestingly revealed fraction of mid-gut type tumors showing no change over the year but patients with Non mid-gut neuroendocrine carcinoma who experienced stable disease maintained their stability more than the patients who experienced partial response, and this is another attestation to the fact that stable disease is a very important and favorable response in Lu-177 PRRT (Table 9).
Table 9.
Effectiveness of Lu-177 DOTA- Octreotate with breakdown according to “mid-gut type” and “Non mid-gut type” neuroendocrine tumors from July 2015 to July 2016
| Number of cases (%) |
PD (%) |
SD (%) |
PR (%) |
CR (%) |
Not Evaluable (%) |
|
|---|---|---|---|---|---|---|
| MID-GUT 2015 & 2016 | 59 (41.2%) |
19 (32.2%) |
32 (54.23%) |
2 (3.38%) |
--- | 6 (10.2%) |
| Non Mid-Gut 2015 |
84 (58.8%) |
25 (29.7%) |
33 (39.2%) |
21 (25%) |
--- | 5 (5.9%) |
| Non Mid-Gut 2016 |
84 (58.8%) |
34 (40%) |
33 (39.2%) |
12 (14.3%) |
--- | 5 (5.9%) |
Recent publications on this topic conclude that patients with PD as a treatment outcome had significantly shorter PFS and overall survival (OS) than patients with an objective response (OR) or stable disease with all 4 scoring systems 27. PFS and OS were comparable for patients with tumor regression and stable disease.
In comparing Lu-177 PRRT with chemotherapy regimens for NET, most of the literature indicates minimal activity of chemotherapy in tumor reduction accompanied by higher reported toxicities. Phase II data on early studies of dimethyltriazenoimidazole carboxamide (DTIC) reported moderate toxicities and survival time of all patients as 20 months 28. Sunitinib, approved for treatment of renal cell carcinoma and gastrointestinal tumors as well as pancreatic neuroendocrine tumors, reported median progression free survival of 11.4 months and objective response rate of 9.3% while everolimus combined with octreotide LAR produced a PFS of 60 weeks in NET patients 29, 30. Current direction of research in this space is toward identification of more potent SSTR ligands such as antagonists or radionuclides such as alpha-emitters. Finding more effective kidney protective agents with less side effects such as severe nausea and vomiting is another unmet need for having more effective and safe PRRT.
Conclusions
In this study, we continued to evaluate the safety and efficacy of Lu-177 DOTATATE therapy in patients with a broad range of progressive somatostatin expressing neuroendocrine tumors. Treatment with Lu-177 DOTATATE has yielded few toxicity events that have since resolved spontaneously and the therapy has proven to manage tumors while reducing symptoms, improving quality of life as well as overall survival compared to cytotoxic chemotherapeutic agents. The most promising results in this study were with patients who received 4 or more cycles indicating that Lu-177 DOTATATE could prove to be a viable treatment option for patients in conjunction with conventional standard of care treatment options. Further studies need to be conducted in future in relation to complementing the effects of radionuclide therapy in combination with radio sensitizing agents such as oxygen therapies and radiosensitizing chemotherapeutic agents such as capecitabine and 5-FU. However the toxicity profile needs to be carefully evaluated when designing studies for combination therapies.
References
- 1.Raut CP, Kulke MH, Glickman JN, et al. Carcinoid tumors. Curr Probl Surg. 2006;43:383–450. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 3.Chan JA, Kulke MH. Progress in the treatment of neuroendocrine tumors. Curr Oncol Rep. 2009;11:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80 Suppl 1:3–7. [DOI] [PubMed] [Google Scholar]
- 5.Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowa-Staszczak A, Pach D, Chrzan R, et al. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur J Nucl Med Mol Imaging. 38:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kvols LK, Moertel CG, O’Connell MJ, et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. [DOI] [PubMed] [Google Scholar]
- 8.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 9.Krenning EP, Kooij PP, Bakker WH, et al. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci. 1994;733:496–506. [DOI] [PubMed] [Google Scholar]
- 10.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 40:800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 43:518–525. [DOI] [PubMed] [Google Scholar]
- 12.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen C, Faraggi M, Giraudet AL, et al. Long-term efficacy of radionuclide therapy in patients with disseminated neuroendocrine tumors uncontrolled by conventional therapy. J Nucl Med. 2004;45:1660–1668. [PubMed] [Google Scholar]
- 14.Thapa P, Ranade R, Ostwal V, et al. Performance of 177Lu-DOTATATE-based peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumor: a multiparametric response evaluation correlating with primary tumor site, tumor proliferation index, and dual tracer imaging characteristics. Nucl Med Commun. 37:1030–1037. [DOI] [PubMed] [Google Scholar]
- 15.Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001;28:1319–1325. [DOI] [PubMed] [Google Scholar]
- 16.Kam BL, Teunissen JJ, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 39 Suppl 1:S103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamar F, Barone R, Mathieu I, et al. 86Y-DOTA0)-D-Phe1-Tyr3-octreotide (SMT487)--a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30:510–518. [DOI] [PubMed] [Google Scholar]
- 18.Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Clin Oncol. 2004;22:2724–2729. [DOI] [PubMed] [Google Scholar]
- 19.Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 17:R53–73. [DOI] [PubMed] [Google Scholar]
- 20.Claringbold PG, Brayshaw PA, Price RA, et al. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 38:302–311. [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Krenning EP, van Essen M, et al. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med. 52:1361–1368. [DOI] [PubMed] [Google Scholar]
- 22.van Vliet EI, Teunissen JJ, Kam BL, et al. Treatment of gastroenteropancreatic neuroendocrine tumors with peptide receptor radionuclide therapy. Neuroendocrinology. 97:74–85. [DOI] [PubMed] [Google Scholar]
- 23.Cremonesi M, Ferrari M, Zoboli S, et al. Biokinetics and dosimetry in patients administered with (111)In-DOTA-Tyr(3)-octreotide: implications for internal radiotherapy with (90)Y-DOTATOC. Eur J Nucl Med. 1999;26:877–886. [DOI] [PubMed] [Google Scholar]
- 24.Forrer F, Valkema R, Kwekkeboom DJ, et al. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. 2007;21:111–129. [DOI] [PubMed] [Google Scholar]
- 25.Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–2762. [DOI] [PubMed] [Google Scholar]
- 26.Ezziddin S, Attassi M, Yong-Hing CJ, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 55:183–190. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet EI, Krenning EP, Teunissen JJ, et al. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med. 54:1689–1696. [DOI] [PubMed] [Google Scholar]
- 28.Bukowski RM, Tangen CM, Peterson RF, et al. Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer. 1994;73:1505–1508. [DOI] [PubMed] [Google Scholar]
- 29.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 364:501–513. [DOI] [PubMed] [Google Scholar]


