Abstract
Purpose
Knowledge regarding the systemic inflammatory response syndrome (SIRS) associated with emergent large vessel occlusion (ELVO) is still insufficient. We aimed to investigate the occurrence rate, predictors, and clinical outcomes of SIRS in patients with ELVO after endovascular treatment (EVT).
Patients and Methods
We retrospectively collected EVT data of patients with ELVO from July 2015 to August 2019 in our center. SIRS in the absence of infection was recorded in detail. A favorable outcome was defined as obtaining a 90-day modified Rankin Scale (mRS) score ≤2.
Results
Among the 256 patients who received EVT, 91 (35.5%) developed SIRS. The patients who developed SIRS had a reduced favorable outcome (OR 4.112 [95% CI 1.705 to 9.920]; p=0.002) and higher mortality (OR 25.336 [95% CI 8.578 to 74.835]; p<0.001) at 90 days. A greater SIRS burden was positively correlated with the NIHSS scores at discharge and mRS scores at 90 days (r=0.265, p=0.011; r=0.245, p=0.019). The development of SIRS was associated with neutrophilic leukocytosis, hyperglycemia, higher NIHSS scores at admission, and worse collateral circulation.
Conclusion
The patients with SIRS had higher odds of poor functional outcomes and higher mortality at 90 days in the EVT-treatment setting. The severity of the inflammatory response was positively correlated with the clinical outcomes of the patients. Clinically, SIRS was associated with neutrophilic leukocytosis, hyperglycemia, baseline stroke severity, and worse collateral circulation.
Keywords: endovascular treatment, outcome, stroke, inflammation
Introduction
The inflammatory response is an important pathophysiological process in stroke.1 Previous studies have shown that inflammation plays a dual role in particular stages of the stroke process and has both detrimental and beneficial effects.2–4 On the one hand, the strong inflammatory reaction characterized by the activation of resident glial cells, upregulation of pro-inflammatory cytokines, infiltration of leukocytes and monocytes, and breakdown of the blood-brain barrier (BBB) may potentiate secondary brain injury during the acute phase.3 On the other hand, inflammation can contribute to propagating brain regeneration and recovery during the late postischemic phase.4 Therefore, regulating the inflammatory process in stroke may be a potential target for neuroprotective treatment.5
Systemic inflammatory response syndrome (SIRS) is a continuous uncontrolled generalized inflammatory state. Clinical studies have shown that patients with more severe strokes have a higher risk of suffering from SIRS6,7 and that the inflammatory response can be significantly attenuated by successful thrombolysis.6 Additionally, experimental studies have suggested that middle cerebral artery (MCA) occlusion stroke generates cytokine-driven acute-phase inflammation,8,9 which may lead to secondary brain damage and systemic inflammatory response, and the results vary depending on the presence of permanent or transient patterns.9
Recently, endovascular treatment (EVT) has been validated to be safe and effective in patients with stroke caused by emergent large vessel occlusion (ELVO) in the anterior circulation.10 However, even if timely and technically successful therapy is administered, nearly half of patients are unable to obtain functional independence.11 Since leukocytes12 and body temperature,13 which are important components of SIRS, have been confirmed to contribute to poor functional outcomes in EVT-treated patients, we hypothesize that SIRS may affect the clinical outcomes of patients with ELVO after EVT.
Therefore, in the present study, we aimed to investigate the incidence of SIRS and related factors influencing the development of SIRS and determine whether the occurrence of SIRS is associated with functional outcomes in EVT-treated patients.
Patients and Methods
Patient Selection
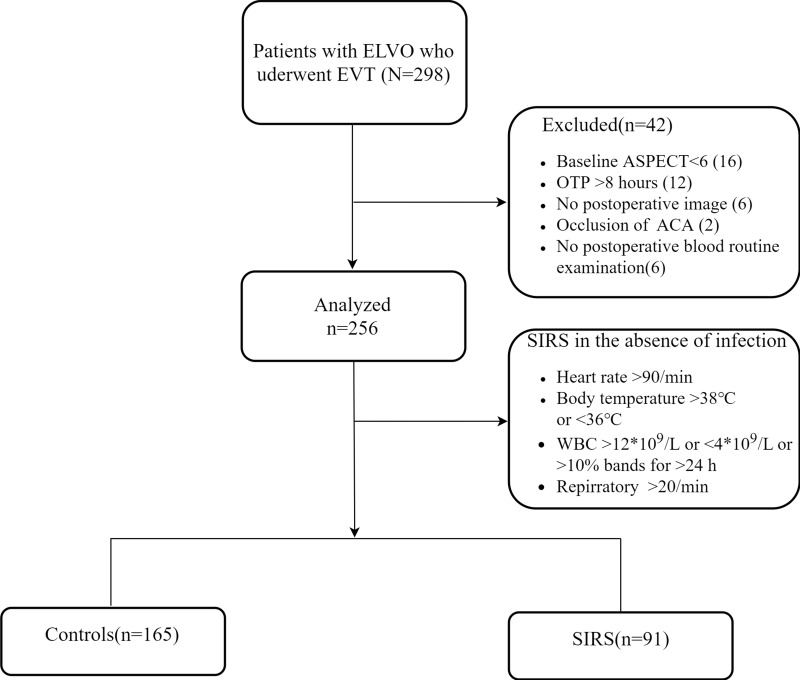
We retrospectively reviewed all consecutive patients with ELVO who underwent EVT at a comprehensive stroke center The First Affiliated Hospital of Wannan Medical College) between July 2015 and August 2019. The endovascular procedure was performed under local anesthesia. For very few patients with obvious irritability, diazepam was administered during surgery. The study was approved by the Ethics Committee of the First Affiliated Hospital of Wannan Medical College (201900039). All private data of the participants were anonymized and maintained with confidentiality. The inclusion criteria were as follows: (1) time from onset to groin puncture (OTP) ≤8 h; (2) baseline National Institutes of Health Stroke Scale (NIHSS) score ≥6; (3) pretreatment Alberta Stroke Program Early Computerized Tomography (ASPECT) score ≥6 and pre-stroke modified Rankin Scale (mRS) score <2; (4) occlusion of the internal carotid artery (ICA) or MCA was confirmed by computed tomographic angiography/magnetic resonance angiography/digital subtraction angiography (DSA); and (5) patients undergoing EVT. A flow chart of the inclusion of the study population is displayed in Figure 1.
Figure 1.
Inclusion flow chart.
For all enrolled patients, we recorded the demographic data, personal medical history (atrial fibrillation, mellitus, and hypertension), and stroke cause according to the Trial of ORG 10,172 in the Acute Stroke Treatment (TOAST) classification,14 baseline ASPECT score, baseline NIHSS score, and thrombolytic therapy before EVT. Perioperative variables including the occlusion site, collateral circulation, and modified Thrombolysis in Cerebral Infarction (mTICI) grading were recorded in detail by the operators. In addition, postoperative imaging was assessed by two interventionalists (Ke Yang and Qian Yang) who were blinded to the clinical information. Blood samples were collected within 24 h after EVT. The results of at least one imaging technique (computerized tomography or magnetic resonance imaging) were available during follow-up.
Variable Definitions
Bridging therapy was defined as combined treatment with endovascular thrombectomy preceded by intravenous alteplase in patients who had ELVO in the anterior circulation.
Collateral circulation was evaluated based on retrograde contrast opacification of the vessels within the occluded territory on delayed DSA images and was graded from absent to good (0–2) by the following criteria: grade 0 (no filling or<1/3 filling of the occluded territory), grade 1 (≥1/3 but<2/3 filling of the occluded territory), and grade 2 (≥2/3 filling of the occluded territory).15 Vessel recanalization after EVT was assessed by the modified TICI scale with scores ranging from 0 to 2a defined as unsuccessful and scores ranging from 2b to 3 defined as successful reperfusion. The functional outcome was assessed by the mRS at 90 days. A favorable outcome was defined as obtaining an mRS score ≤2, indicating functional independence.
SIRS in the absence of infection was defined as the presence of ≥2 of the following: (1) heart rate >90, (2) body temperature >38°C or <36°C, (3) white blood cells >12,000/mm or <4000/mm or >10% bands for >24 h, and (4) respiratory rate >20.16 Patients with systemic inflammatory response caused by validated infection were excluded and placed in the control group. Regarding the patients with concurrent infection and a systemic inflammatory response, we consulted two infectious disease experts to determine whether to include these patients in the case group. In addition, a scoring system was created to indicate the SIRS severity. Each diagnostic indicator received 1 point, and the score was accumulated successively to calculate the SIRS score. Notably, if a patient suffering from tachycardia atrial fibrillation (AF), the heart rate was not scored.
Statistical Analysis
We categorized the patients according to the presence of SIRS or favorable and unfavorable outcomes. The normally distributed continuous variables are summarized as the mean ± SD, and the nonnormally distributed continuous variables are expressed as the median and interquartile range (IQR). The categorical variables are presented as percentages. The differences between the groups were analyzed using Χ2 tests, t-tests, and Fisher’s exact tests as appropriate. We employed a Spearman correlation analysis to investigate the association between the SIRS burden and the clinical outcomes (NIHSS score at discharge and 90-day mRS score). A univariate regression analysis was performed to evaluate the relationship between SIRS and the clinical characteristics. Multivariate models were constructed to assess the impact of the variables contributing to SIRS, including the variables with a p<0.10 in the univariate analysis. Similar models were applied to assess the prognostic impact of different outcome definitions.
A propensity score-matched (PSM) analysis was used to compare the patients with direct EVT and bridging therapy with a caliper of 0.15 at a 1:1 ratio. The matched variables in the PSM in this study included age, sex, hypertension, AF, diabetes mellitus, baseline NIHSS score, baseline ASPECT score, OTP, occlusion site, and collateral status. IBM SPSS Statistics for Windows (version 25.0; IBM Corp, Armonk, NY) software and R version 3.4.3 (R Development Core Team, Vienna, Austria) were used for all statistical analyses.
Results
Patient Baseline Characteristics
In total, 298 patients with ELVO who received EVT under local anesthesia were enrolled in the study, and 42 patients were excluded. Of the 256 included patients, the mean age was 68.1±11.6 years, the median baseline ASPECT score was 8 (IQR 8–10), and the median baseline NIHSS score was 16 (IQR 13–19). Additionally, 32 (12.5%) received bridging therapy, and 187 (73.0%) achieved successful recanalization. In total, 113 (44.1%) patients reached 90-day functional independence, and the overall mortality at 90 days after EVT was 25.0% (64). The baseline characteristics and outcomes of all enrolled patients are shown in Table 1.
Table 1.
Demographics and Baseline and Outcome Characteristics Stratified by SIRS
| All Patients (n=256) |
Non-SIRS (n=165) |
SIRS (n=91) |
P-value | Odds Ratio (95% CI) |
P-value | |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 68.1 (11.6) | 67.9 (11.7) | 68.4 (11.7) | 0.787 | ||
| Male, n (%) | 139 (54.3) | 86 (52.1) | 53 (58.2) | 0.347 | ||
| Hypertension | 180 (70.3) | 106 (64.2) | 74 (81.3) | 0.004 | ||
| Diabetes mellitus | 43 (16.8) | 23 (13.9) | 20 (22.0) | 0.100 | ||
| Clinical characteristics, median (IQR) | ||||||
| Baseline NIHSS score | 16 (13–19) | 14 (12–18) | 18 (15–20) | <0.001 | 1.115 (1.022–1.217) | 0.014 |
| Baseline ASPECT score | 8 (8–10) | 9 (8–10) | 8 (8–9) | 0.006 | ||
| Stroke cause, n (%) | ||||||
| LAA | 84 (32.8) | 59 (35.8) | 25 (27.5) | 0.195 | ||
| Cardioembolic | 141 (55.1) | 84 (50.9) | 57 (62.6) | |||
| Undetermined or other | 31 (12.1) | 22 (13.3) | 9 (9.9) | |||
| Occlusion site, n (%) | ||||||
| ICA | 107 (41.8) | 62 (37.6) | 45 (49.5) | 0.065 | ||
| MCA | 149 (58.2) | 103 (62.4) | 46 (50.5) | |||
| OTP, median (IQR) | 260 (210–300) | 270 (212–300) | 255 (210–300) | 0.411 | ||
| Bridging therapy, n (%) | 32 (12.5) | 23 (13.9) | 9 (9.9) | 0.348 | ||
| Collateral score, n (%) | ||||||
| Grade 0 | 58 (22.7) | 21 (12.7) | 37 (40.7) | <0.001 | Reference | |
| Grade 1 | 99 (38.7) | 63 (38.2) | 36 (39.6) | 0.346 (0.153–0.783) | 0.011 | |
| Grade 2 | 99 (38.7) | 81 (49.1) | 18 (19.8) | 0.225 (0.093–0.545) | 0.001 | |
| mTICI, 2b/3, n (%) | 187 (73.0) | 125 (75.8) | 62 (68.1) | 0.188 | ||
| Independence at 90 days, n (%) | 113 (44.1) | 100 (60.6) | 13 (14.3) | <0.001 | ||
| Mortality at 90 days, n (%) | 64 (25.0) | 10 (6.1) | 54 (59.3) | <0.001 | ||
| Laboratory examination | ||||||
| FBG*(mmol/L), mean (SD) | 7.4 (3.5) | 6.5 (2.6) | 8.9 (4.4) | <0.001 | 1.140 (1.036–1.256) | 0.007 |
| Leucocytes, 109/L | 10.2 (8.3–13.3) | 9.4 (7.9–11.3) | 13.7 (10.8–17.2) | <0.001 | ||
| Neutrophils,109/L | 8.7 (6.8–11.6) | 7.7 (6.5–9.5) | 12.0 (9.4–15.2) | <0.001 | 1.367 (1.219–1.534) | <0.001 |
| Lymphocytes, 109/L | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) | 0.8 (0.6–1.2) | 0.011 |
Note: *Missing data in six patients.
Abbreviations: SIRS, systemic inflammatory response syndrome; ASPECT, Alberta Stroke Program Early CT; ICA, internal carotid artery; LAA, large-artery atherosclerosis; mTICI, modified thrombolysis in cerebral infarction; ICA, internal carotid artery; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; OTP, symptom onset to groin puncture time; FBG, fasting blood glucose.
Baseline Characteristics of All Patients with or without SIRS Who Received EVT
Of all enrolled patients, 91 (35.5%) patients developed SIRS. In total, 24 met all SIRS criteria, 43 met 3 of the SIRS criteria, and the remaining 24 met 2 of the SIRS criteria. In the univariate analysis, the proportion of hypertension in the patients with SIRS was higher than that in the patients without SIRS (81.3% versus 64.2%; p=0.004). The patients with SIRS had higher baseline NIHSS scores (median, 18 versus 14; p<0.001) and lower pretreatment ASPECT scores (median, 8 versus 9; p=0.006). Poor collateral scores (grade 0.40.7% versus 12.7%; grade 1, 39.6% versus 38.2%; grade 2, 19.8% versus 49.1%; p<0.001) were more common in the patients with SIRS than in those without SIRS (Table 1).
In addition, higher neutrophil counts (IQR 9.4–15.2) but lower lymphocyte counts (IQR 0.6–1.2) were observed in the patients who developed SIRS. Furthermore, the patients with SIRS had significantly higher levels of fasting blood glucose (FBG: 8.9±4.4 mmol/L versus 6.5±2.6 mmol/L; p<0.001) than the non-SIRS patients.
As expected, there were significant differences in both the entire range of the mRS estimates and 90-day mortality between the SIRS patients and non-SIRS patients. Compared with the non-SIRS patients, in the patients diagnosed with SIRS, favorable outcomes were less common (60.6% versus 14.3%; p<0.001). Unsurprisingly, the 90-day mortality in the patients with SIRS was proportionally higher than that in the patients without SIRS (59.3% versus 6.1%; p<0.001). However, we did not find a difference in the rate of successful reperfusion between the two groups (68.1% versus 75.8%; p=0.188).
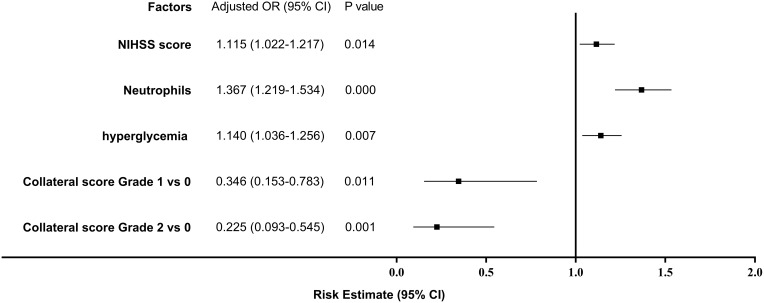
In the multivariate logistic analysis, increased neutrophils (odds ratio OR 1.367 [95% CI 1.219 to 1.534]; p<0.001), elevated FBG (OR 1.140 [95% CI 1.036 to 1.256]; p=0.007), and the baseline NIHSS (OR 1.115 [95% CI 1.022 to 1.217]; p=0.014) were associated with SIRS development after endovascular treatment. However, good collaterals were associated with a reduced likelihood of suffering from SIRS (grade 1 versus grade 0: OR 0.346 [95% CI 0.153 to 0.783]; p=0.011; grade 2 versus grade 0: OR 0.225 [95% CI 0.093 to 0.545]; p=0.001; Figure 2).
Figure 2.
Odds ratios (OR) of the development of systemic inflammatory response syndrome (SIRS) in all enrolled patients.
Systemic Inflammatory Response Syndrome and Outcomes
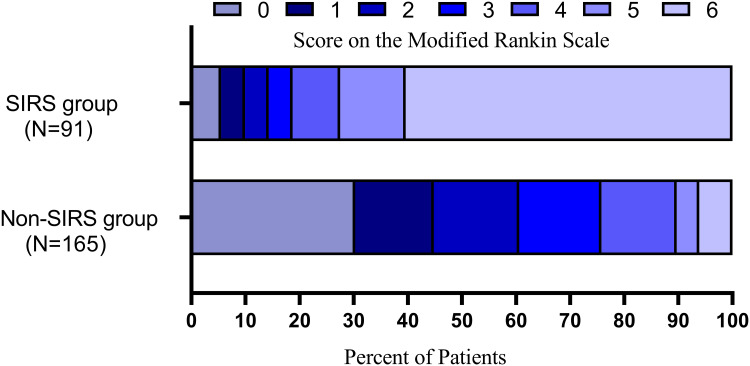
After adjusting for confounding factors, including age, sex, stroke etiology, baseline NIHSS score and lymphocytes, the multivariate logistic analysis showed that the presence of SIRS (OR 4.112 [95% CI 1.705 to 9.920]; p=0.002; Table 2) was inversely associated with favorable functional outcomes at 90 days. The overall distribution of the mRS scores at 90 days is shown in Figure 3. Furthermore, there was a significant correlation between the presence of SIRS (OR 25.336 [95% CI 8.578 to 74.835]; p<0.001; see online Supplementary Table S1) and 90-day mortality. Given the collateral circulation, the baseline ASPECT score and admission NHISS score significantly differed between the Non-SIRS group and SIRS group; to reduce interference from these factors in the prognostic analysis, a verified PSM analysis was adopted to compare the Non-SIRS group with the SIRS group. After the propensity score-matched analysis, 67 patients (mean age 67.8 ± 11.5 years, median NIHSS score 16 [IQR 14–20]) in the Non-SIRS group and 67 patients in the SIRS group (mean age 68.5 ± 11.7 years, median NIHSS score 17 [IQR 14–19]) were further compared. The results showed that the patients with SIRS had poorer functional outcomes (17.9% vs 44.8%) and higher mortality (56.7% vs 7.5%) at 90 days (see online Supplementary Table S2). Nevertheless, there was no difference in recanalization between the two groups. In addition, a Spearman correlation analysis was adopted to explore the correlation between the severity of SIRS and the clinical prognosis, and the results suggested that the SIRS severity was positively correlated with the NIHSS scores at discharge and mRS scores at 90 days (r=0.265, p=0.011; r=0.245, p=0.019).
Table 2.
Comparison of the Variables Stratified by Outcome in the Overall Cohort
| Favorable Outcome (n=113) |
Poor Outcome (n=143) |
P-value | Odds Ratio (95% CI) |
P-value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 65.7 (11.2) | 69.9 (11.8) | 0.004 | ||
| Male, n (%) | 70 (61.9) | 69 (48.3) | 0.029 | 2.043 (0.984–4.242) | 0.055 |
| Medical history, n (%) | |||||
| Hypertension | 75 (66.4) | 105 (73.4) | 0.220 | ||
| Diabetes mellitus | 12 (10.6) | 31 (21.7) | 0.019 | ||
| Clinical characteristics, median (IQR) | |||||
| Baseline NIHSS score | 14 (12–18) | 17 (14–20) | <0.001 | ||
| Baseline ASPECT score | 9 (8–10) | 8 (8–9) | <0.001 | 0.680 (0.491–0.942) | 0.020 |
| Stroke cause, n (%) | |||||
| LAA | 50 (44.2) | 34 (23.8) | <0.001 | ||
| Cardioembolic | 47 (41.6) | 94 (65.7) | |||
| Undetermined or other | 16 (14.2) | 15 (10.5) | |||
| Occlusion site, n (%) | |||||
| ICA | 33 (29.2) | 74 (51.7) | <0.001 | 2.663 (1.287–5.514) | 0.008 |
| MCA | 80 (70.8) | 69 (48.3) | Reference | ||
| OTP, median (IQR) | 270 (224–303) | 250 (210–300) | 0.208 | ||
| Bridging therapy, n (%) | 16 (14.2) | 16 (11.2) | 0.475 | ||
| Collateral score, n (%) | |||||
| Grade 0 | 8 (7.1) | 50 (35.0) | <0.001 | Reference | |
| Grade 1 | 38 (33.6) | 61 (42.7) | 0.344 (0.119–0.994) | 0.049 | |
| Grade 2 | 57 (59.3) | 32 (22.4) | 0.115 (0.038–0.343) | <0.001 | |
| mTICI, 2b/3, n (%) | 98 (86.7) | 89 (62.2) | <0.001 | 0.223 (0.095–0.525) | 0.001 |
| SIRS, n (%) | 13 (11.5) | 78 (54.5) | <0.001 | 4.112 (1.705–9.920) | 0.002 |
| Laboratory examination | |||||
| FBG* (mmol/L), mean (SD) | 6.1 (2.2) | 8.4 (4.0) | <0.001 | ||
| Leucocytes, 109/L | 9.3 (7.9–11.3) | 11.8 (8.9–14.8) | <0.001 | ||
| Neutrophils,109/L | 7.7 (6.4–9.5) | 10.1 (7.4–12.7) | <0.001 | 1.171 (1.032–1.328) | 0.015 |
| Lymphocytes, 109/L | 1.1 (0.7–1.4) | 0.8 (0.6–1.2) | 0.005 |
Note: *Missing data in six patients.
Abbreviations: SIRS, systemic inflammatory response syndrome; ASPECT, Alberta Stroke Program Early CT; ICA, internal carotid artery; LAA, large-artery atherosclerosis; mTICI, modified thrombolysis in cerebral infarction; ICA, internal carotid artery; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; OTP, symptom onset to groin puncture time; FBG, fasting blood glucose.
Figure 3.
Distribution of mRS scores at 90 days according to the presence of systemic inflammatory response syndrome (SIRS).
Systemic Inflammatory Response Syndrome and Bridging Therapy
The baseline demographics and outcomes of the direct EVT and bridging therapy in the unmatched subgroups of patients are shown in online Supplementary Table S3. After the propensity score-matched analysis, 30 patients (mean age 68.4 ± 9.9 years, median NIHSS score 16 [IQR 14–19]) in the direct EVT group and 30 patients in the bridging therapy group (mean age 68.8 ± 11.5 years, median NIHSS score 16 [IQR 12–19]) were further compared. The neutrophil and leukomonocyte counts were very similar between these two groups (p>0.05). The rate of SIRS did not differ between the two groups (36.7% versus 26.7%, p=0.405). Furthermore, the rate of the 90-day mortality (20% versus 20%, p=1.000) and functional independence (50.0% versus 50.0%, p=1.000) at 90 days did not differ between the groups (see online Supplementary Table S3).
Discussion
Our results showed summarized findings of this study, first, we observed that the prevalence of SIRS (35.5%) was high after EVT regardless of recanalization. Second, factors such as neutrophil activation, elevated FBG, severity at admission and worse collateral circulation was associated with SIRS. Third, developing SIRS was detected to be independently associated with poorer functional outcomes in terms of the overall distribution of the mRS scores and mortality. Additionally, bridging therapy seemed to have no additional function on alleviating SIRS.
Previous studies aiming to elucidate the prevalence of SIRS have shown that approximately 18–53% of ischemic stroke patients experience SIRS in hospitals.6,7,17 Boehme et al7 found that approximately 20% of stroke patients treated with rt-PA developed SIRS and that the presence of SIRS was positively related to the initial stroke severity and poor short-term functional outcomes. However, the outcomes of SIRS in patients after EVT remain unclear. To the best of our knowledge, this study is the first to assess the SIRS characteristics and clinical impact in EVT-treated patients.
In our study, we observed a high prevalence of SIRS (35.5%) in this setting. The occurrence rate of SIRS in our study was higher than that in Boehme’s study (18%) but lower than that in Alicia’s (53%) study. This discrepancy can be explained by the fact that our baseline NIHSS scores were higher than those in Boehme’s study (Non-SIRS group median: 14 versus 7; SIRS group median: 18 versus 9). In Alicia’s study, 69.2% of the patients had posterior circulation stroke located in heat-regulating centers. Although the incidence of SIRS after EVT was unclear, we considered that SIRS may be a common condition in patients with ELVO who receive EVT regardless of recanalization.
In our research, the development of SIRS in the patients with ELVO who underwent EVT was associated with neutrophil activation, elevated FBG and higher NIHSS scores upon hospital admission. During cerebral ischemia and reperfusion, leukocytes can adhere to vascular endothelial cells and block microvessels, leading to microvascular occlusion and the phenomenon of “no reflow”. Infiltrating neutrophils massively accumulate in ischemic cerebral tissue, where they abundantly release enzymes, including myeloperoxidase and elastase, and reactive oxygen species (ROS), which are known to cause local and systemic inflammation.18,19 Moreover, patients with high blood glucose levels are more likely to develop SIRS, which is consistent with a previous study by Yoshimoto, who indicated that plasma glucose was highly related to SIRS.20 A prior study indicated that hyperglycemia may trigger a massive accumulation of neutrophils, lead to endothelial damage and BBB disruption, and exacerbate downstream microvascular thromboinflammation, which precipitates neurovascular damage.21 Consistent with previous observations, we observed that patients with more severe stroke, as indicated by higher NIHSS scores at admission, had higher odds of developing SIRS.6,7 Audebert et al6 showed that SIRS depends on the initial stroke severity, implying that SIRS reflects the extent of tissue damage caused by stroke.
The quality of the collateral circulation may affect the risk of SIRS after EVT. In the adjusted analysis, we observed that favorable collateral circulation protected the patients from developing SIRS. In fact, the collateral circulation provides leukocytes access to the infarct core and penumbra.22 However, interestingly, SIRS was significantly alleviated in the group with good collateral circulation. A generally accepted view is that the amount of collateral-dependent blood flow is a dominating determinant of the infarct volume after ELVO.23 Therefore, rarefaction of the preexisting collateral circulation results in more severe tissue injury and augmented neuronal necrosis following arterial occlusion; then, endogenous molecules are released from damaged cells, amplifying inflammatory mediator expression and tissue damage by triggering and inducing the activation of Toll-like receptors (TLRs)24 and some other damage-associated molecular patterns (DAMPs).25 Furthermore, collateral remodeling involves endothelial cell activation, leukocyte recruitment, matrix changes and cell proliferation; in patients with poor collateral circulation, the proinflammatory process involving angiogenesis will be more obvious.26
Unexpectedly, we did not find that recanalization could reduce the incidence SIRS. We speculated that several etiologies may explain this phenomenon. On the one hand, inflammatory cell infiltration readily occurs following cerebral ischemia throughout the infarcted tissue and may not depend on blood perfusion being restored.18 On the other hand, the inflammatory process is initiated prior to recanalization.19 This phenomenon was demonstrated in the postischemic mouse brain, and animal studies have shown that a profound infiltration of inflammatory cells occurs 3 hours after focal ischemia, even in permanent middle cerebral artery occlusion.27 Additionally, reperfusion injury frequently leads to an exacerbation of tissue damage and a profound inflammatory response.28
Previous studies have shown that SIRS following ischemic stroke is associated with poorer short-term neurological outcomes and a prolonged length of stay,7 but its long-term consequences have not been completely determined. Our study confirmed that the presence of SIRS was inversely associated with favorable functional outcomes at 90 days. Additionally, the multivariate analysis showed that SIRS predicted an increased 90-day mortality. Furthermore, the SIRS burden was positively correlated with the NIHSS scores at discharge and mRS scores at 90 days. Our findings might encourage future research to investigate whether regulating the inflammatory process during the acute phase can improve outcomes in EVT-treated patients. In fact, novel immunotherapeutic approaches have shown promising prospects in regulating inflammation and improving outcomes.29
Notably, we did not find differences in the occurrence of SIRS between the patients with bridging therapy and the patients with direct EVT, which contradicts a prior study. The study showed that stroke-induced SIRS could be alleviated by successful thrombolysis.6 We speculate that this discrepancy may be related to the lower rate of intravenous thrombolysis in our center. The low percentage of intravenous thrombolysis insufficiently detect the effect of intravenous thrombolysis on SIRS. Additionally, all enrolled patients had large vessel occlusions requiring thrombectomy; thus, rt-PA did not result in recanalization.
Several shortcomings should be noted. Our study was limited by its retrospective nature and monocentric design. Furthermore, many patients were transferred from the primary stroke center, and we did not review the routine blood tests at admission in our hospital. Routine blood tests and other tests were not incomplete at admission in our center. Finally, inflammatory markers such as highly sensitive C-reactive protein or various interleukin levels were not available.
Conclusions
In conclusion, SIRS is frequently present in patients with ELVO who receive EVT and is an independent predictor of the outcome. Future efforts aiming to understand the pathogenesis underlying the occurrence of SIRS may facilitate modulation of this inflammation by combining reperfusion therapies as a potential therapeutic strategy for acute large vessel occlusion stroke.
Funding Statement
This work was supported by the Scientific Research Project for Middle-aged and Young People of Wannan Medical College (No. WK2020F24).
Abbreviations
SIRS, systemic inflammatory response syndrome; ELVO, emergent large vessel occlusion; EVT, endovascular treatment; mRS, modified Rankin Scale; BBB, blood-brain barrier; MCA, middle cerebral artery; ASPECT, Alberta Stroke Program Early CT; ICA, internal carotid artery; LAA, large-artery atherosclerosis; mTICI, modified thrombolysis in cerebral infarction; TOAST, Trial of ORG 10,172 in Acute Stroke Treatment; ICA, internal carotid artery; NIHSS, National Institutes of Health Stroke Scale; OTP, symptom onset to groin puncture time; DSA, digital subtraction angiography; FBG, fasting blood glucose; AF, atrial fibrillation; IQR, interquartile range; PSM, propensity score-matched; ROS, reactive oxygen species, TLRs, Toll-like receptors; DAMPs, damage-associated molecular patterns.
Data Sharing Statement
The data are available upon reasonable request.
Ethical Approval
This study was reviewed by the Ethics Committee of the First Affiliated Hospital of Wannan Medical College (201900039). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. This study got an exemption notice from Independent Ethics Committee approval as it was a retrospective data analysis. We confirmed that all private data of the participants were anonymized and maintained with confidentiality.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Zhu T, Li H, et al. Plasma interleukin-37 is elevated in acute ischemic stroke patients and probably associated with 3-month functional prognosis. Clin Interv Aging. 2020;15:1285–1294. doi: 10.2147/CIA.S230186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dénes A, Ferenczi S, Kovács KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. doi: 10.1186/1742-2094-8-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15(8):946–950. doi: 10.1038/nm.1999 [DOI] [PubMed] [Google Scholar]
- 5.Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi: 10.1016/S1474-4422(19)30078-X [DOI] [PubMed] [Google Scholar]
- 6.Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35(9):2128–2133. doi: 10.1161/01.STR.0000137607.61697.77 [DOI] [PubMed] [Google Scholar]
- 7.Boehme AK, Kapoor N, Albright Karen C, et al. Systemic inflammatory response syndrome in tissue-type plasminogen activator–treated patients is associated with worse short-term functional outcome. Stroke. 2013;44(8):2321–2323. doi: 10.1161/STROKEAHA.113.001371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–1698. doi: 10.1038/jcbfm.2012.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129(2):239–257. doi: 10.1007/s00401-014-1381-0 [DOI] [PubMed] [Google Scholar]
- 10.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–e418. [DOI] [PubMed] [Google Scholar]
- 11.Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. 2019;14(1):23–31. doi: 10.1177/1747493018799979 [DOI] [PubMed] [Google Scholar]
- 12.Boisseau W, Desilles JP, Fahed R, et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology. 2019;93(5):e467–e475. doi: 10.1212/WNL.0000000000007859 [DOI] [PubMed] [Google Scholar]
- 13.Diprose WK, Liem B, Wang MTM, et al. Impact of body temperature before and after endovascular thrombectomy for large vessel occlusion stroke. Stroke. 2020;51(4):1218–1225. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 15.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26(7):1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 17.Zha A, Vahidy F, Randhawa J, et al. Association between splenic contraction and the systemic inflammatory response after acute ischemic stroke varies with age and race. Transl Stroke Res. 2018;9(5):484–492. doi: 10.1007/s12975-017-0596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129(2):259–277. doi: 10.1007/s00401-014-1355-2 [DOI] [PubMed] [Google Scholar]
- 19.Kollikowski AM, Schuhmann MK, Nieswandt B, Müllges W, Stoll G, Pham M. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol. 2020;87(3):466–479. doi: 10.1002/ana.25665 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32(9):1989–1993. doi: 10.1161/hs0901.095646 [DOI] [PubMed] [Google Scholar]
- 21.Desilles JP, Syvannarath V, Ollivier V, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke. 2017;48(7):1932–1940. doi: 10.1161/STROKEAHA.117.017080 [DOI] [PubMed] [Google Scholar]
- 22.Semerano A, Laredo C, Zhao Y, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. 2019;50(12):3456–3464. [DOI] [PubMed] [Google Scholar]
- 23.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69(6):963–974. doi: 10.1002/ana.22354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Culebras A, Durán-Laforet V, Peña-Martínez C, et al. Role of TLR4 (toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke. 2019;50(10):2922–2932. [DOI] [PubMed] [Google Scholar]
- 25.Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–598. doi: 10.1523/JNEUROSCI.2439-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. 2012;18(8):494–501. doi: 10.1016/j.molmed.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Chu HX, Kim HA, Lee S, et al. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Met. 2013;34:450–459. doi: 10.1038/jcbfm.2013.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holger K, Eltzschig TE. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16(3):217–226. [DOI] [PubMed] [Google Scholar]