Abstract
We assessed fungal diversity present in air and freshly deposited snow samples obtained from Livingston Island, Antarctica, using DNA metabarcoding through high throughput sequencing (HTS). A total of 740 m3 of air were pumped through a 0.22 µm membrane. Snow obtained shortly after deposition was kept at room temperature and yielded 3.760 L of water, which was filtered using Sterivex membranes of 0.22 µm mesh size. The total DNA present was extracted and sequenced. We detected 171 fungal amplicon sequence variants (ASVs), 70 from the air and 142 from the snow. They were dominated by the phyla Ascomycota, Basidiomycota, Mortierellomycota and Mucoromycota. Pseudogymnoascus, Cladosporium, Mortierella and Penicillium sp. were the most dominant ASVs detected in the air in rank order. In snow, Cladosporium, Pseudogymnoascus, Penicillium, Meyerozyma, Lecidea, Malassezia, Hanseniaspora, Austroplaca, Mortierella, Rhodotorula, Penicillium, Thelebolus, Aspergillus, Poaceicola, Glarea and Lecanora were the dominant ASVs present. In general, the two fungal assemblages displayed high diversity, richness, and dominance indices, with the assemblage found in snow having the highest diversity indices. Of the total fungal ASVs detected, 29 were only present in the air sample and 101 in the snow sample, with only 41 present in both samples; however, when only the dominant taxa from both samples were compared none occurred only in the air and, among the rare portion, 26 taxa occurred in both air and snow. Application of HTS revealed the presence of a more diverse fungal community in the air and snow of Livingston Island in comparison with studies using traditional isolation methods. The assemblages were dominated by cold-adapted and cosmopolitan fungal taxa, including members of the genera Pseudogymnoascus, Malassezia and Rhodotorula, which include some taxa reported as opportunistic. Our results support the hypothesis that the presence of microbiota in the airspora indicates the possibility of dispersal around Antarctica in the air column. However, further aeromycology studies are required to understand the dynamics of fungal dispersal within and beyond Antarctica.
Subject terms: Environmental microbiology, Fungi, Fungal ecology
Introduction
Antarctica represents one of the most pristine regions of the planet and, despite the multiple extreme conditions that characterize it, harbours a considerable terrestrial biodiversity, mainly of microorganisms, that are able to survive and colonize its different environments. Due the continent’s isolation from lower latitudes by the oceanic Antarctic Circumpolar Current and atmospheric circulation, the lack of trophic complexity, and the vulnerability of its endemic biodiversity to environmental changes and anthropogenic influences, Antarctica provides a unique opportunity for microbial aerobiology studies seeking to understand how airspora are transported to and within Antarctica1,2. The extent to which Antarctic environments receive microbial propagules, potentially including globally cosmopolitan species from outside Antarctica, remains largely unstudied, although they have been detected in the air column and after deposition, for instance in snow and ice3–7. According to Archer et al.2, microbial communities present in ecosystems of isolated regions of Antarctica, such as the Victoria Land Dry Valleys, display limited connectivity to the global microbial pool due the strong selection that occurs during atmospheric transport, resulting in regionally isolated airborne inputs and highly specialized soil communities, with fungi also displaying greater isolation from non-polar sources than bacteria. However, detailed information about the aerial routes by which microorganisms arrive and circulate in Antarctica is lacking8,9.
Biological dispersal by aerial means can be an important factor shaping patterns of biodiversity9,10. Viable organisms or their propagules present in the air column may be in dormant and cryptobiotic states, where they are metabolically inactive due the harsh dry, cold, low nutrient and high irradiance conditions. Diverse groups of microorganisms have been recorded in the few Antarctic aerobiological studies completed to date (reviewed by Pearce et al.9), including viruses, bacteria, microalgae and fungi.
Mycological studies in Antarctica have shown that much of the Antarctic fungal community is represented by cold tolerant (psychrophilic or psychrotolerant) species, many of which have wide and even globally cosmopolitan distributions, with presence in polar, temperate, and tropical environments11. de Menezes et al.12 suggested that the high densities of cosmopolitan fungi present in snow are consistent with them being present in air masses arriving at the Antarctic Peninsula from beyond Antarctica, which are then entrained in snow precipitation, and become concentrated in the snow. Snow and ice can provide an indirect record of the presence and deposition of fungal propagules (e.g. spores or hyphal fragments) from the air column over time12. In snow samples obtained from six different regions of the Antarctic Peninsula, de Menezes et al.13 reported a rich fungal diversity assigned to 51 species in 26 genera and dominated by cold tolerant cosmopolitan fungi. However, in ice from continental Antarctica and the Antarctic Peninsula, Rogers et al.14 and de Menezes et al.15, respectively, reported much lower fungal diversity. In the present study, we assessed fungal diversity present in air and freshly deposited snow samples obtained from Livingston Island, Antarctica, using DNA metabarcoding through high-throughput sequencing (HTS).
Material and methods
Snow and air sampling
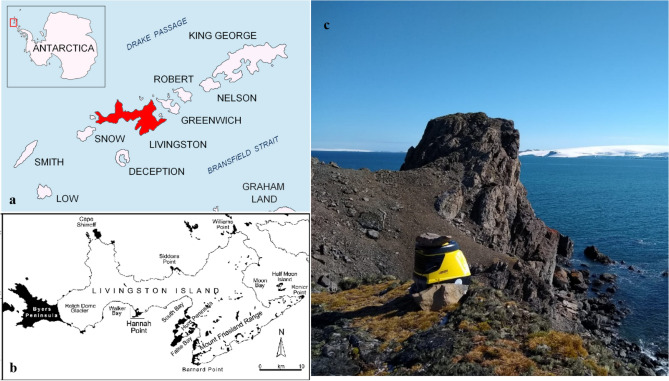
Air and snow samples were collected at Punta Polaca (62°40′16″ S; 60°22′43″ W), Hurd Peninsula, Livingston Island, South Shetland Islands, near to the Spanish station Juan Carlos I (Fig. 1). Two air samples were collected with a high flow glass impinger following Šantl-Temkiv et al.16,17. The chamber was filled with 2 L of sampling liquid (ddH2O) and the sampler was run for 5 min, so that the liquid came in contact with the entire chamber, after which 0.5 L of the sampling liquid was removed, stored as a control, and analyzed along with the samples. The control represented a field blank to certify that the samples were not contaminated by external organisms. The resulting solution was filtered directly on the Sterivex filter units for the air, as described by Lever et al.18. Air was collected over c. 5 h on March 11th 2019. In addition, the two separate air DNA extractions were combined together in order to increase DNA yield. Two freshly deposited snow samples were collected on March 20th 2019 at the same site using a sterilized shovel. Both pairs of samples were separately combined in order to increase DNA yield. Snow was melted at room temperature, under strictly sterile conditions, for 24 h in the laboratory at Juan Carlos I Station and then filtered using Sterivex filters18.
Figure 1.
Location of soil sample collections. (a) Antarctic Peninsula, (b) Livingston Island and (c) Punta Polaca at Hurd Peninsula, where the air and snow were sampled [62°40′16″ S; 60°22′43″ W]. Photo (c) by T Šantl-Temkiv.
DNA extraction and data analysis
Total DNA was extracted from environmental samples using the Qiagen Power Soil Kit (Qiagen, USA) following the manufacturer’s instructions. Extracted DNA was used as template for generating PCR amplicons. The internal transcribed spacer 2 (ITS2) of the nuclear ribosomal DNA was used as a DNA barcode for molecular species identification19,20. PCR amplicons were generated using the universal primers ITS3 and ITS421 and were sequenced by high-throughput sequencing at Macrogen Inc. (South Korea) on an Illumina MiSeq sequencer, using the MiSeq Reagent Kit v3 (600-cycle) following the manufacturer’s protocol.
Raw fastq files were filtered using BBDuk version 38.34 (BBMap—Bushnell B.—sourceforge.net/projects/bbmap/) to remove Illumina adapters, known Illumina artifacts, and PhiX Control v3 Library. Quality read filtering was carried out using Sickle version 1.33-q 30-l 5022, to trim ends 3′ or 5′ with low Phred quality score. Sequences shorter than 50 bases were discarded. These sequences were imported to QIIME2 version 2019.10 (https://qiime2.org/) for bioinformatics analyses23. The qiime2-dada2 plugin is a complete pipeline that was used for filtering, dereplication, turning paired-end fastq files into merged reads, and removal of chimeras24. Taxonomic assignment was carried out for the amplicon sequence variants (ASVs) using qiime2-feature-classifier25 classify-sklearn against the UNITE fungal ITS database version 7.226 and trained with Naive Bayes classifier. A confidence threshold of 98.5% was used. All raw sequences have been deposited in the NCBI database under the codes SRR12830238, SRR12830240 and SRR12830239.
Many factors, including extraction, PCR, and primer bias, can affect the number of reads27, and thus lead to misinterpretation of abundance28. However, Giner et al.29 concluded that such biases did not affect the proportionality between reads and cell abundance, implying that more reads are linked with higher abundance29,30. Therefore, for comparative purposes we used the number of reads as a proxy for relative abundance.
All sequences obtained from air and snow samples were matched with sequences present in the list of the top 50 ‘most wanted’ fungi according to Nilsson et al.31. The sequences were merged, filtered, dereplicated, and clustered into > 97% identity ASVs using USEARCH version 1032. Nucleotide-Nucleotide BLAST 2.6.0 + was used to compare these ASVs against the top50_release_04.02.2020.fasta33, considering just subject matches with aligned length longer than 250 bp and > 98% identity.
Fungal diversity and distribution
To quantify species diversity, richness, and dominance, we used the following indices: (i) Fisher’s α, (ii) Margalef’s, and (iii) Simpson’s, respectively. The numbers of DNA reads of the amplicon sequence variants (ASVs) were used to quantify the fungal taxa present in the air sampled, where fungal ASVs with more than 1,000 reads were considered dominant and < 1,000 minor components (rare) of the fungal community. All of the results were obtained with 95% confidence, and bootstrap values were calculated from 1,000 iterations. Taxon species accumulation curves were obtained using the Mao Tao index. All diversity indices and species accumulation curves calculations were performed using PAST v. 1.9034. Venn diagrams were prepared according to Bardou et al.35 to compare the fungal assemblages present in both air and snow samples. The functional assignments of fungal ASVs at species and genera levels are shown using FunGuild36.
Results
Fungal taxonomy
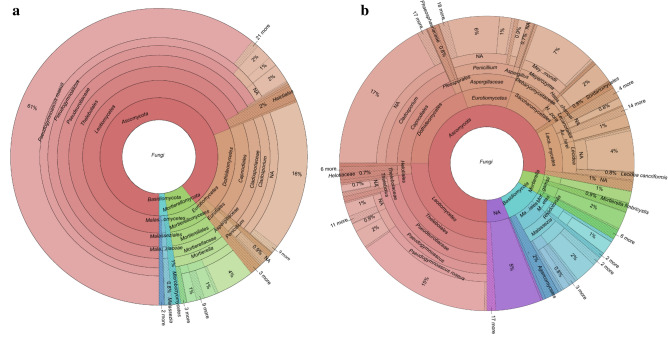
The number of reads in the air sample was 162,038 and that in snow 268,710. From these, we detected 171 fungal amplicon sequence variants (ASVs), 70 in 740 m3 of air and 142 in 3.76 L of snow from Livingston Island, Antarctica (Table 1; Fig. 2). The ASVs were dominated by the phyla Ascomycota, Basidiomycota and Mortierellomycota. In the air sample, ASVs identified as Pseudogymnoascus roseus, Cladosporium sp., Mortierella sp. 1, Pseudogymnoascus sp. 3, Pseudogymnoascus sp. 2, Mortierella fimbricystis, Mortierella gamsii and Penicillium sp. were the most dominant taxa (all with > 1,000 reads), in rank order. In contrast, 27 fungal ASVs (Cladosporium sp., Pseudogymnoascus roseus, Penicillium sp., Meyerozyma guilliermondii, Lecidea sp., Malassezia restricta, Pseudogymnoascus sp. 3, Hanseniaspora lachancei, Pseudogymnoascus sp. 2, Austroplaca darbishirei, Mortierella gamsii, Malassezia globosa, Rhodotorula diobovata, Mortierella sp. 1, Ascomycota sp., Mortierella fimbricystis, Penicillium polonicum, Lecanorales sp., Thelebolus sp., Lecidea cancriformis, Aspergillus sp., Poaceicola agrostina, Glarea sp., Pseudogymnoascus sp. 1, Mortierella sp. 2, Thelebolus globosus and Lecanora physciella) were present as dominant fungi in snow. A further 177 ASVs (62 in air and 115 in snow) were detected less frequently (< 1,000 reads) and may represent the rare portion of the fungal assemblages. In addition, 78 ASVs could only be assigned to higher taxonomic levels (phylum, class, order or family). A total of 29,069 sequences from the air and 6,223 from the snow samples were matched with the sequences of 11 unidentified species hypotheses in the list of the top 50 most wanted fungi31 with the alignment length longer than 250 bp and > 98% identity (Suppl. Table 1).
Table 1.
Numbers of sequence reads of fungal amplicon sequence variants (ASVs) detected in air and snow samples from Livingston Island, South Shetlands, Antarctica.
| Hierarchical level | Fungal taxa (ASVs)* | Reference sequences | Reads by Samples | Total | |
|---|---|---|---|---|---|
| Air | Snow | ||||
| Fungi | Fungi sp. | 39** | 20,958 | 20,997 | |
| Ascomycota | Pseudogymnoascus roseus | SH1557165.08FU | 61,935 | 0 | 61,935 |
| Cladosporium sp. | SH1521536.08FU | 20,801 | 0 | 20,801 | |
| Pseudogymnoascus sp. | SH1557215.08FU | 2,035 | 1,5650 | 17,685 | |
| Meyerozyma sp. | SH1516625.08FU | 0 | 1,5735 | 15,735 | |
| Penicillium sp. | SH1530043.08FU | 431 | 9,385 | 9,816 | |
| Lecidea cancriformis | SH2711223.08FU | 0 | 6,781 | 6,781 | |
| Hanseniaspora sp. | SH1547214.08FU | 0 | 4,708 | 4,708 | |
| Austroplaca darbishirei | SH1633428.08FU | 0 | 3,165 | 3,165 | |
| Thelebolus globosus | SH1647628.08FU | 271 | 1,614 | 1,885 | |
| Helotiales sp. | SH1648813.08FU | 1,075 | 404 | 1,479 | |
| Penicillium polonicum | SH1529888.08FU | 0 | 1,233 | 1,233 | |
| Pseudogymnoascus appendiculatus | SH1939321.08FU | 1138 | 0 | 1,138 | |
| Septoriella sp. | SH1525156.08FU | 0 | 902 | 902 | |
| Lecanora physciella | SH1636780.08FU | 0 | 738 | 738 | |
| Cyberlindnera sp. | SH1648567.08FU | 571 | 0 | 571 | |
| Mitrulinia sp. | SH1574181.08FU | 0 | 482 | 482 | |
| Cleistothelebolus nipigonensis | SH1630064.08FU | 0 | 433 | 433 | |
| Chalara pseudoaffinis | SH1522386.08FU | 368 | 0 | 368 | |
| Pestalotiopsis sp. | SH1562655.08FU | 0 | 364 | 364 | |
| Neoascochyta paspali | SH1547057.08FU | 329 | 4 | 333 | |
| Paraconiothyrium africanum | SH1525457.08FU | 0 | 331 | 331 | |
| Debaryomyces sp. | SH1516581.08FU | 62 | 251 | 313 | |
| Phaeoacremonium hungaricum | SH1644597.08FU | 0 | 287 | 287 | |
| Lecidea sp. | SH1524770.08FU | 0 | 277 | 277 | |
| Colletotrichum sp. | SH1636843.08FU | 186 | 90 | 276 | |
| Rhizoscyphus sp. | SH1543082.08FU | 169 | 103 | 272 | |
| Aspergillus sp. | SH1536361.08FU | 0 | 249 | 249 | |
| Schwanniomyces polymorphus | SH1649127.08FU | 0 | 244 | 244 | |
| Septoriella hirta | SH2714710.08FU | 0 | 225 | 225 | |
| Ascomycota sp. | SH1574206.08FU | 123 | 82 | 205 | |
| Penicillium fluviserpens | SH1536160.08FU | 0 | 199 | 199 | |
| Saccharomyces cerevisiae | SH1583301.08FU | 0 | 193 | 193 | |
| Aspergillus niger | SH3322875.08FU | 0 | 183 | 183 | |
| Volucrispora graminea | SH1605412.08FU | 0 | 154 | 154 | |
| Aspergillus sydowii | SH1550060.08FU | 38 | 113 | 151 | |
| Penicillium steckii | SH1692788.08FU | 0 | 150 | 150 | |
| Leptosphaeria sclerotioides | SH1624038.08FU | 147 | 0 | 147 | |
| Leotiomycetes sp. | SH1647738.08FU | 136 | 0 | 136 | |
| Pseudallescheria sp. | SH2328594.08FU | 0 | 132 | 132 | |
| Buellia russa | SH1551132.08FU | 0 | 130 | 130 | |
| Chaetothyriales sp. | SH1545109.08FU | 0 | 129 | 129 | |
| Penicillium brasilianum | SH1692798.08FU | 0 | 123 | 123 | |
| Phaeosphaeria dennisiana | SH1530704.08FU | 120 | 0 | 120 | |
| Pseudallescheria ellipsoidea | SH2328455.08FU | 0 | 112 | 112 | |
| Lodderomyces elongisporus | SH1507873.08FU | 103 | 0 | 103 | |
| Candida tropicalis | SH1542296.08FU | 101 | 0 | 101 | |
| Yamadazyma sp. | SH1539910.08FU | 101 | 0 | 101 | |
| Trichoderma sp. | SH1542292.08FU | 0 | 91 | 91 | |
| Didymellaceae sp. | SH1547074.08FU | 82 | 0 | 82 | |
| Penicillium paxilli | SH1530009.08FU | 8 | 73 | 81 | |
| Parmeliaceae sp. | SH1541255.08FU | 71 | 0 | 71 | |
| Paraphoma fimeti | SH1616190.08FU | 0 | 70 | 70 | |
| Colletotrichum annellatum | SH2219599.08FU | 0 | 67 | 67 | |
| Polysporina subfuscescens | SH1596449.08FU | 0 | 67 | 67 | |
| Pseudeurotium sp. | SH3332798.08FU | 67 | 0 | 67 | |
| Dermateaceae sp. | SH1522957.08FU | 66 | 0 | 66 | |
| Penicillium astrolabium | SH1530010.08FU | 0 | 66 | 66 | |
| Cladosporium halotolerans | SH1525346.08FU | 37 | 27 | 64 | |
| Diaporthales sp. | SH1657193.08FU | 64 | 0 | 64 | |
| Lecanoromycetes sp. | SH1517968.08FU | 0 | 60 | 60 | |
| Lecanora contractula | SH1527996.08FU | 0 | 55 | 55 | |
| Ramalinaceae sp. | SH1522446.08FU | 0 | 51 | 51 | |
| Cystodendron sp. | SH1524864.08FU | 50 | 0 | 50 | |
| Penicillium cairnsense | SH2190109.08FU | 0 | 50 | 50 | |
| Cladonia rei | SH3326345.08FU | 49 | 0 | 49 | |
| Neodevriesia capensis | SH3331962.08FU | 0 | 49 | 49 | |
| Neopestalotiopsis sp. | SH3324784.08FU | 49 | 0 | 49 | |
| Penicillium sumatraense | SH1585145.08FU | 9 | 37 | 46 | |
| Mycosphaerella tassiana | SH1607937.08FU | 0 | 44 | 44 | |
| Pseudeurotiaceae sp. | SH1556184.08FU | 44 | 0 | 44 | |
| Fusarium solani | SH2721166.08FU | 43 | 0 | 43 | |
| Placopsis contortuplicata | SH1521544.08FU | 0 | 40 | 40 | |
| Schwanniomyces sp. | SH2154634.08FU | 38 | 0 | 38 | |
| Bacidina arnoldiana | SH3321741.08FU | 0 | 28 | 28 | |
| Penicillium citrinum | SH1539276.08FU | 15 | 13 | 28 | |
| Zymoseptoria verkleyi | SH1544001.08FU | 21 | 0 | 21 | |
| Sarocladium sp. | SH1542060.08FU | 17 | 0 | 17 | |
| Aspergillus penicillioides | SH1537266.08FU | 16 | 0 | 16 | |
| Pichia kluyveri | SH1527730.08FU | 16 | 0 | 16 | |
| Botryosphaeriaceae sp. | SH3317647.08FU | 0 | 6 | 6 | |
| Fusarium asiaticum | SH2456121.08FU | 0 | 4 | 4 | |
| Usnea sp. | SH1550545.08FU | 0 | 3 | 3 | |
| Basidiomycota | Malassezia restricta | SH2734004.08FU | 401 | 4,740 | 5,141 |
| Malassezia globosa | SH1565779.08FU | 165 | 2,946 | 3,111 | |
| Rhodotorula diobovata | SH1585138.08FU | 0 | 3,060 | 3,060 | |
| Agaricomycetes sp. | SH1575746.08FU | 0 | 2,581 | 2,581 | |
| Malassezia sp. | SH1546915.08FU | 22 | 1,548 | 1,570 | |
| Marasmius sp. | SH1514868.08FU | 912 | 0 | 912 | |
| Rhodotorula mucilaginosa | SH1558606.08FU | 750 | 120 | 870 | |
| Leucosporidiella creatinivora | SH1651377.08FU | 404 | 0 | 404 | |
| Heterochaete shearii | SH1561152.08FU | 75 | 259 | 334 | |
| Malasseziales sp. | SH1547455.08FU | 46 | 266 | 312 | |
| Calyptella capula | SH1635872.08FU | 0 | 170 | 170 | |
| Pluteus ephebeus | SH2724840.08FU | 158 | 0 | 158 | |
| Malassezia equina | SH2723257.08FU | 0 | 95 | 95 | |
| Vishniacozyma victoriae | SH1572254.08FU | 94 | 0 | 94 | |
| Phanerochaete sordida | SH1573517.08FU | 83 | 0 | 83 | |
| Hyphodontia microspora | SH1651385.08FU | 82 | 0 | 82 | |
| Peniophora laxitexta | SH1646415.08FU | 56 | 0 | 56 | |
| Gymnopus sp. | SH1560298.08FU | 50 | 0 | 50 | |
| Vishniacozyma tephrensis | SH1691243.08FU | 48 | 0 | 48 | |
| Microbotryomycetes sp. | SH2750674.08FU | 40 | 0 | 40 | |
| Vanrija humicola | SH1514178.08FU | 30 | 0 | 30 | |
| Basidiomycota sp. | SH1514435.08FU | 0 | 19 | 19 | |
| Polyporales sp. | SH1651381.08FU | 15 | 0 | 15 | |
| Malassezia sympodialis | SH3313592.08FU | 0 | 12 | 12 | |
| Mortierellomycota | Mortierella sp. | SH1557435.08FU | 5,878 | 744 | 6,622 |
| Mortierella fimbricystis | SH2452854.08FU | 2,260 | 0 | 2,260 | |
| Mortierella gamsii | SH1556972.08FU | 1,416 | 155 | 1,571 | |
| Mortierella parvispora | SH1629873.08FU | 396 | 0 | 396 | |
| Mortierella alpina | SH1503809.08FU | 158 | 0 | 158 | |
| Mortierella elongatula | SH1574597.08FU | 0 | 74 | 74 | |
| Mortierella turficola | SH3338068.08FU | 0 | 56 | 56 | |
| Mucoromycota | Densospora sp. | SH3319965.08FU | 0 | 145 | 145 |
*ASVs = amplicon sequence variants; **number of the reads.
Figure 2.
Krona chart of (a) fungal assemblages detected in the air and (b) in snow from Livingston Island, South Shetland Islands, Antarctica.
Fungal diversity
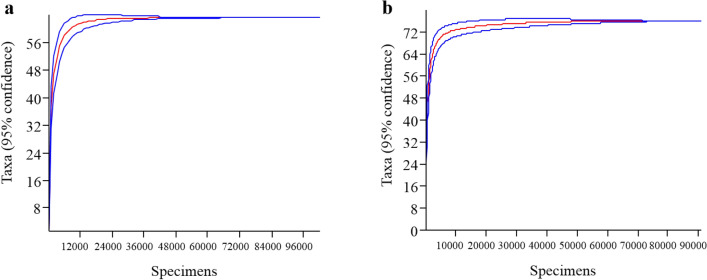
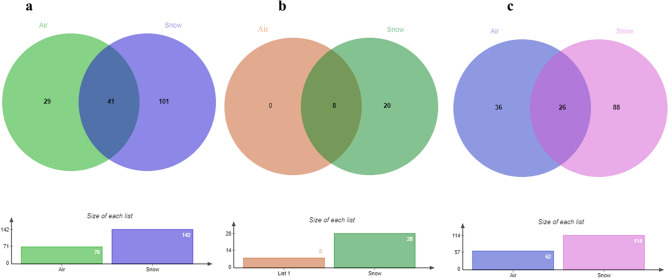
The Mao Tao rarefaction curves of the fungal assemblages present in air and snow reached asymptote for both fungal assemblages (Fig. 3), indicating that the data provided a good description of the diversity present. In general, both fungal assemblages displayed high diversity, richness, and dominance indices (Table 2). The assemblage present in the snow was more diverse, rich, and included a wider range of dominant fungi when compared with that from the air sample. Of the total fungal ASVs detected, 29 were only present in the air sample and 101 in the snow sample, with 41 present in both samples (Fig. 4a). However, when only the dominant ASVs (> 1,000 reads) from both samples were compared, none occurred only in the air (Fig. 4b) and, among the rare portion, 26 occurred in both air and snow (Fig. 4c). In addition, the ecological functional assignments of fungal ASVs in species and genera levels were showed in Suppl. Table 2 and Suppl. Table 3, respectively.
Figure 3.
Rarefaction curves for samples from fungal assemblages present in the (a) air and (b) snow on Livingston Island, South Shetlands, Antarctica. Blue lines represent confidence limits inferred using bootstrap values calculated from 1,000 iterations using PAST, version 1.9034.
Table 2.
Sample data and ecological indices of the fungal DNA recovered from air and snow samples from Livingston Island, South Shetlands, Antarctica.
| Ecological indices | Sample | ||
|---|---|---|---|
| Air | Snow | Total | |
| Number of reads | 162,038 | 268,710 | 430,748 |
| Number of taxa | 70 | 142 | 171 |
| Fisher α | 6.96 | 14.44 | 16.85 |
| Margalef | 5.75 | 11.3 | 13 |
| Simpson | 0.6 | 0.92 | 0.85 |
Figure 4.
(a) Venn diagram showing the (a) total, (b) dominant (those with > 1,000 reads) and (c) rare fungal taxa distribution detected in air and snow of Livingston Island, South Shetlands, Antarctica.
Discussion
Fungal taxonomy and diversity
Despite an increase in mycological studies, fungal diversity in Antarctica remains poorly known11. According to Bridge and Spooner37, around 1,000 fungal species have been described from Antarctica, identified using a range of approaches including traditional methods for cultivable fungi such as macro- and/or micromorphology of colonies and fruiting bodies as well as DNA sequencing of mycelia of cultivable fungi. Most airborne mycological studies in Antarctica have relied on traditional morphological methods. Marshall4 monitored airborne fungal spores over 13.6 months at three sites on Signy Island (South Orkney Islands) in the maritime Antarctic, reporting that Epicoccum spp. and Cladosporium spp. dominated the diversity present. Duncan et al.38 sampled air inside the historic wooden huts on Ross Island, finding Cladosporium cladosporioides, Pseudeurotium desertorum, Pseudogymnoascus sp. and Antarctomyces psychrotrophicus as dominant viable fungal propagules and Cadophora sp. and Thebolus sp. as minor components of the outdoor airborne fungal assemblage. Archer et al.2 compared microbial diversity in near-ground and high-altitude air above the Victoria Land Dry Valleys as well as that of underlying soil microbial communities, finding basidiomycete yeasts to be dominant in the air and unclassified fungi to be common in soils. However, the more recent fungal inventories using metabarcoding approaches have demonstrated that fungal diversity in Antarctica is greater than previously recognised39–41.
As air and snow are typically ultra-oligotrophic microhabitats, few viable fungal taxa are expected to be present, as reported by de Menezes et al.12 who, using cultivation techniques, reported only 14 fungal taxa in snow samples from several different Antarctic islands. However, despite analysing only a small a small absolute sample size of air and snow collected in the Livingston Island, use of the HTS approach in the current study revealed the presence of much greater fungal diversity in both air and snow, many of which display mechanisms that render them well-adapted to survive atmospheric transport, such as the production of resistant spores and UV protective compounds42,43.
The dominant taxa detected in the air included representatives of Pseudogymnoascus, Cladosporium, Mortierella and Penicillium. However, even though recently deposited snow would be expected to contain microbial airborne particles entrained from the air column as the snow fell, fungal diversity in the snow sampled was very different to that in the air over the same location. In snow sample, the dominant taxa found included representatives of Cladosporium, Pseudogymnoascus, Penicillium, Meyerozyma, Lecidea, Malassezia, Hanseniaspora, Austroplaca, Mortierella, Rhodotorula, Penicillium, Thelebolus, Aspergillus, Poaceicola, Glarea and Lecanora. The diversity present in both the air and snow samples also included dominant taxa that could only be assigned to higher taxonomic levels such as Fungal sp., Ascomycota sp., Basidiomycota sp., Agaricales sp., Chaetothyriales sp., Helotiales sp., Lecanorales sp. and Polyporales sp. These may represent currently undescribed or otherwise unsequenced species, further supporting the assertion that much of the true fungal diversity present in Antarctica is currently unknown.
Pseudogymnoascus were detected as dominant fungi in both air and snow samples. Pseudogymnoascus (previously known as Geomyces) is a genus often detected in cold environments including those of polar, alpine, and temperate regions11,44–47. In Antarctica, it has been reported from soils44,48–50, associated with plants51–54 and macroalgae55, in freshwater lakes56, and associated with lichens57. Cladosporium and Penicillium also represent common airborne fungi reported globally, including Antarctica. Cladosporium is a dematiaceous fungal group with global distribution58. In Antarctic microhabitats, Cladosporium has mainly been detected in association with plants and soil11. Penicillium is a ubiquitous genus, again detected in multiple substrates in Antarctica including soils50,59,60, permafrost61,62 and associated with macroalgae63. The abundant presence of Pseudogymnoascus, Cladosporium, and Penicillium both in air and snow sampled indicated that these fungi may circulate at least around the Antarctic Peninsula.
The genus Mortierella includes about 85 species, which occur mainly in soils64. Mortierella species are found worldwide, particularly in temperate and polar regions. Representatives of the genus are abundant in Antarctica and reported in association with plants51,52, macroalgae63, lichens57, soils65, freshwater56, and permafrost62. Some species of Mortierella are known as snow moulds and have the capability to growth and produce spores at 0°C66. They occur abundantly in the interstitial water in Antarctic snow where snow melting occurs in summer, for instance in association with snow algal communities.
The genus Malassezia includes 17 species of basidiomycetous pigmented black yeast species generally present in the skin and mucosa microbiome of humans and other warm-blooded animals67. According to Prohic et al.68, several Malassezia species found on human and animal skin are commensals, but they can also be associated with Pityriasis versicolor, Malassezia folliculitis, seborrheic dermatitis/dandruff, atopic dermatitis, and psoriasis. The detection of Malassezia in Antarctica is unusual. Rosa et al.54 detected different Malassezia taxa in soil samples from undisturbed and disturbed (by human activity) sites on Deception Island (South Shetland Islands) using HTS metabarcoding techniques.
The genus Meyerozyma includes species that are typically widely distributed or cosmopolitan69. Species of Meyerozyma have previously been isolated from aquatic environments in Antarctica69,70 and associated with macroalgae63. The genus Hanseniaspora (anamorph Kloeckera) includes ascomycete yeast species commonly associated with alcoholic fermentation, but is also recorded from soil, plants, fruit-eating insects, birds, and seafood71. Some Hanseniaspora species have been reported as unusual opportunistic superficial mycosis in humans72–75.
The genus Rhodotorula includes cosmopolitan pigmented yeast species and is often dominant in extreme environments76, including those of Antarctica63,70. Our study represents the first report of high abundance of R. muscilagionsa in Antarctic snow samples, although de Menezes et al.13 reported the species among the dominant fungi detected in snow samples from several Antarctic islands. The genus Thelebolus is distributed globally and representatives occur in diverse habitats77. Species of Thelebolus have been reported in Arctic and Antarctic environments78,79, as being abundant in lakes, and in association with birds (skuas)80, in freshwater56,81 and in ice15. Finally, from the air and snow sampled in Livingston Island, Antarctica, we detected 11 unidentified species hypotheses in the list of the top 50 most wanted fungi31, suggesting the both habitats may shelter rare species that merit further taxonomic attention.
Conclusions
We used DNA metabarcoding to catalogue the fungi present in air and snow samples from Livingston Island, South Shetland Islands. This revealed a diverse fungal community comprising taxa from the phyla Ascomycota, Basidiomycota, Mortierellomycota and Mucoromycota. The assemblages were dominated by cold-adapted and cosmopolitan (psychrophilic) taxa, including members of the genera Pseudogymnoascus, Malassezia and Rhodotorula, which include taxa reported as opportunistic fungi. Our results confirm the presence of fungi in the airspora, supporting the possibility of dispersal over different geographical scales around Antarctica in the air column. Given that many of the taxa identified in this study are known from Antarctic fungal communities, a local source for those present in the air column is plausible. The large proportion of unassigned taxa highlight the poor level of baseline knowledge of Antarctic fungal diversity, and further aeromicrobiology and diversity studies are required to understand the dynamics of fungal dispersal within and beyond Antarctica. However, as metabarcoding detects environmental DNA, the technique can also detect DNA from dead fungi or otherwise non-viable material. Further studies will be necessary to develop strategies to isolate these fungi into culture.
Supplementary information
Acknowledgements
This study received financial support from CNPq, PROANTAR, FAPEMIG, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), and INCT Criosfera 2. P. Convey is supported by NERC core funding to the British Antarctic Survey’s ‘Biodiversity, Evolution and Adaptation’ Team. We are also grateful for the generous support of the Spanish Polar Committee and its staff at Gabriel de Castilla base. PEASC also thanks congresswoman Jô Moraes. Finally, we appreciate the valuable suggestions of anonymous reviewers.
Author contributions
L.H.R., P.E.A.S.C., T.S. conceived the study. L.H.R. and P.E.A.S.C. performed DNA extraction from snow and air. L.H.R., P.E.A.S.C., O.H.B.P., T.S., P.C., M.C.S., and C.A.R. analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78630-6.
References
- 1.Siegert MJ, et al. Recent advances in understanding Antarctic climate evolution. Antarct. Sci. 2008;4:313–325. doi: 10.1017/S0954102008000941. [DOI] [Google Scholar]
- 2.Archer SDJ, et al. Airborne microbial transport limitation to isolated Antarctic soil habitats. Nat. Microbiol. 2019;4:925–932. doi: 10.1038/s41564-019-0370-4. [DOI] [PubMed] [Google Scholar]
- 3.Marshall WA. Aerial dispersal of lichen soredia in the maritime Antarctic. New Phytol. 1996;134:523–530. doi: 10.1111/j.1469-8137.1996.tb04370.x. [DOI] [Google Scholar]
- 4.Marshall WA. Seasonality in Antarctic airborne fungal spores. Appl. Environ. Microbiol. 1997;63:2240–2245. doi: 10.1128/AEM.63.6.2240-2245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes KA, et al. A preliminary study of airborne microbial biodiversity over peninsular Antarctica. Cell. Mol. Biol. 2004;50:537–542. [PubMed] [Google Scholar]
- 6.Vincent WF. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct. Sci. 2000;12:374–385. doi: 10.1017/S0954102000000420. [DOI] [Google Scholar]
- 7.Pearce DA, et al. Biodiversity of airborne microorganisms at Halley Station, Antarctica. Extremophiles. 2010;14:145–159. doi: 10.1007/s00792-009-0293-8. [DOI] [PubMed] [Google Scholar]
- 8.Bottos EM, et al. Airborne bacterial populations above desert soils of the McMurdo Dry Valleys, Antarctica. Microb. Ecol. 2014;67:120–128. doi: 10.1007/s00248-013-0296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce DA, et al. Aerobiology over Antarctica - A new initiative for atmospheric ecology. Front. Microbiol. 2016;16:7–16. doi: 10.3389/fmicb.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womack, A. M., Bohannan, B. J., and Green, J. L. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. B Biol. Sci.365, 3645–3653 (2010). [DOI] [PMC free article] [PubMed]
- 11.Rosa, L.H. et al. Fungi in Antarctica: diversity, ecology, effects of climate change, and bioprospection for bioactive compounds in Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications (ed. Rosa, L.H.) 1–18 (2019).
- 12.de Menezes, G.C.A. et al. Fungi in snow and glacial ice of Antarctica in Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications (ed. Rosa, L.H.) 127–146 (2019a).
- 13.de Menezes GCA, et al. Diversity, distribution, and ecology of fungi in the seasonal snow of Antarctica. Microorganisms. 2019;7:445. doi: 10.3390/microorganisms7100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers SO, et al. Comparisons of protocols for decontamination of environmental ice samples for biological and molecular examinations. Appl. Environ. Microbiol. 2004;70:2540–2544. doi: 10.1128/AEM.70.4.2540-2544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Menezes GCA, et al. Fungi in glacial ice of Antarctica: diversity, distribution and bioprospecting of bioactive compounds. Extremophiles. 2020;24:367–376. doi: 10.1007/s00792-020-01161-5. [DOI] [PubMed] [Google Scholar]
- 16.Šantl-Temkiv T, et al. High-flow-rate impinger for the study of concentration, viability, metabolic activity, and ice-nucleation activity of airborne bacteria. Environ. Sci. Technol. 2017;51:11224–11234. doi: 10.1021/acs.est.7b01480. [DOI] [PubMed] [Google Scholar]
- 17.Šantl-Temkiv, T. et al. Aeolian dispersal of bacteria in southwest Greenland: their sources, abundance, diversity and physiological states. FEMS Microbiol. Ecol. 94, 10.1093 (2018). [DOI] [PubMed]
- 18.Lever MA, et al. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front. Microbiol. 2015;6:476. doi: 10.3389/fmicb.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE. 2010;5:e8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson RT, et al. Application of ITS2 metabarcoding to determine the provenance of pollen collected by honey bees in an agroecosystem. Appl. Plant Sci. 2015;3:1400066. doi: 10.3732/apps.1400066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White, T.J. et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics in PCR Protocols: a guide to methods and applications (ed. Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J.) 315–322 (1990).
- 22.Joshi, N.A., Fass, J.N. Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.33) [Software]. https://github.com/najoshi/sickle (2011).
- 23.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods13, 581–583 (2016). [DOI] [PMC free article] [PubMed]
- 25.Bokulich NA, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abarenkov, K. et al. UNITE QIIME release for eukaryotes. Version 04.02.2020. UNITE Community. 10.15156/BIO/786386 (2020).
- 27.Medinger R, et al. Diversity in a hidden world: potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol. Ecol. 2010;19:32–40. doi: 10.1111/j.1365-294X.2009.04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber AA, Pawlowski J. Can abundance of protists be inferred from sequence data: a case study of Foraminifera. PLoS ONE. 2013;8:e56739. doi: 10.1371/journal.pone.0056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giner CR, et al. Environmental sequencing provides reasonable estimates of the relative abundance of specific picoeukaryotes. Appl. Environ. Microbiol. 2016;82:4757–4766. doi: 10.1128/AEM.00560-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hering D, et al. Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res. 2018;138:192–205. doi: 10.1016/j.watres.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson RH, et al. Top 50 most wanted fungi. Myco-Keys. 2016;12:29–40. doi: 10.3897/mycokeys.12.7553. [DOI] [Google Scholar]
- 32.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 33.Abarenkov, K. et al. UNITE general FASTA release for Fungi. Version 04.02.2020. UNITE Community. 10.15156/BIO/786368 (2020).
- 34.Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 35.Bardou P, et al. An interactive Venn diagram viewer. BMC Bioinform. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen NH, et al. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016;20:241–248. doi: 10.1016/j.funeco.2015.06.006. [DOI] [Google Scholar]
- 37.Bridge PD, Spooner BM. Non-lichenized Antarctic fungi: Transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012;5:381–394. doi: 10.1016/j.funeco.2012.01.007. [DOI] [Google Scholar]
- 38.Duncan SM, et al. Monitoring and identification of airborne fungi at historic locations on Ross Island, Antarctica. Polar Sci. 2010;4:275–283. doi: 10.1016/j.polar.2010.03.008. [DOI] [Google Scholar]
- 39.Baeza M, et al. Amplicon-metagenomic analysis of fungi from Antarctic terrestrial habitats. Front. Microbiol. 2017;8:2235. doi: 10.3389/fmicb.2017.02235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durán P, et al. Occurrence of soil fungi in Antarctic pristine environments. Front. Bioeng. Biotechnol. 2019;7:28. doi: 10.3389/fbioe.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa, L.H. et al. DNA metabarcoding high-throughput sequencing uncovers cryptic fungal diversity in soils of protected and non-protected areas on Deception Island, Antarctica. Sci. Rep. Submitted (2020). [DOI] [PMC free article] [PubMed]
- 42.Robinson CH. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001;151:341–353. doi: 10.1046/j.1469-8137.2001.00177.x. [DOI] [Google Scholar]
- 43.Braga GUL, et al. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015;61:405–425. doi: 10.1007/s00294-015-0483-0. [DOI] [PubMed] [Google Scholar]
- 44.Mercantini R, Marsella R, Cervellati MC. Keratinophilic fungi isolated from Antarctic soil. Mycopathologia. 1989;106:47–52. doi: 10.1007/BF00436926. [DOI] [PubMed] [Google Scholar]
- 45.Lorch JM, et al. A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia. 2013;105:237–252. doi: 10.3852/12-207. [DOI] [PubMed] [Google Scholar]
- 46.Minnis, A.M., Lindner, D.L. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 117, 638–649 (2013). [DOI] [PubMed]
- 47.Ali SH, et al. Studies on diversity of soil microfungi in the Hornsund area, Spitsbergen. Pol. Res. 2014;35:203–224. [Google Scholar]
- 48.Arenz BE, Blanchette RA. Distribution and abundance of soil fungi in Antarctica at sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys. Soil Biol. Biochem. 2011;43:308–315. doi: 10.1016/j.soilbio.2010.10.016. [DOI] [Google Scholar]
- 49.Krishnan A, et al. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar Biol. 2011;34:1535–1542. doi: 10.1007/s00300-011-1012-3. [DOI] [Google Scholar]
- 50.Gomes EC, et al. Cultivable fungi present in Antarctic soils: taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles. 2018;22:381–393. doi: 10.1007/s00792-018-1003-1. [DOI] [PubMed] [Google Scholar]
- 51.Tosi S, Casado B, Gerdol R. Fungi isolated from Antarctic mosses. Polar Biol. 2002;25:262–268. doi: 10.1007/s00300-001-0337-8. [DOI] [Google Scholar]
- 52.Rosa, L.H. et al. Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol. Ecol. 73, 178–189 (2010). [DOI] [PubMed]
- 53.Carvalho CR, et al. Fungi associated with the briosphere of the bipolar mosses Polytrichastrum alpinum and Polytrichum juniperinum in Antarctica. Polar Biol. 2020;43:545–553. doi: 10.1007/s00300-020-02658-7. [DOI] [Google Scholar]
- 54.Rosa LH, et al. Opportunistic fungal assemblages present on fairy rings spread on different moss species in the Antarctic Peninsula. Polar Biol. 2020;43:587–596. doi: 10.1007/s00300-020-02663-w. [DOI] [Google Scholar]
- 55.Loque CP, et al. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33:641–648. doi: 10.1007/s00300-009-0740-0. [DOI] [Google Scholar]
- 56.Gonçalves VN, et al. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol. Ecol. 2012;82:459–471. doi: 10.1111/j.1574-6941.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- 57.Santiago IF, et al. Lichenosphere: a protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles. 2015;19:1087–1097. doi: 10.1007/s00792-015-0781-y. [DOI] [PubMed] [Google Scholar]
- 58.Bensch K, et al. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales) Stud. Mycol. 2010;67:1–94. doi: 10.3114/sim.2010.67.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McRae CF, Hocking AD, Seppelt RD. Penicillium species from terrestrial habitats in the Windmill Islands, East Antarctica, including a new species, Penicillium antarcticum. Polar Biol. 1999;21:97–111. doi: 10.1007/s003000050340. [DOI] [Google Scholar]
- 60.Godinho VM, et al. Diversity and bioprospection of fungal community present in oligotrophic soil of continental Antarctica. Extremophiles. 2015;19:585–596. doi: 10.1007/s00792-015-0741-6. [DOI] [PubMed] [Google Scholar]
- 61.Zucconi L, et al. Searching for eukaryotic life preserved in Antarctic permafrost. Polar Biol. 2012;35:749–757. doi: 10.1007/s00300-011-1119-6. [DOI] [Google Scholar]
- 62.Silva TH, et al. Diversity, distribution, and ecology of viable fungi in permafrost and active layer of Maritime Antarctica. Extremophiles. 2020;24:565–576. doi: 10.1007/s00792-020-01176-y. [DOI] [PubMed] [Google Scholar]
- 63.Godinho VM, et al. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013;7:1434–1451. doi: 10.1038/ismej.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirk, P.M., Cannon, P.F., Minter, D.W., Stalpers, J.A. Dictionary of the Fungi, 10th ed., CAB International, Wallingford, UK, p. 784 (2008).
- 65.Bridge PD, Newsham KK. Soil fungal community composition at Mars Oasis, a southern maritime Antarctic site, assessed by PCR amplification and cloning. Fungal Ecol. 2009;2:66–74. doi: 10.1016/j.funeco.2008.10.008. [DOI] [Google Scholar]
- 66.Onofri S, et al. Antarctic microfungi as models for exobiology. Planet Space Sci. 2004;52:229–237. doi: 10.1016/j.pss.2003.08.019. [DOI] [Google Scholar]
- 67.Theelen, B. et al. Malassezia ecology, pathophysiology, and treatment. Med. Mycol.56, S10–S25 (2018). [DOI] [PubMed]
- 68.Prohic A, et al. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016;55:494–504. doi: 10.1111/ijd.13116. [DOI] [PubMed] [Google Scholar]
- 69.Brandão LR, et al. Diversity and biogeographical patterns of yeast communities in Antarctic, Patagonian and tropical lakes. Fungal Ecol. 2017;28:33–43. doi: 10.1016/j.funeco.2017.04.003. [DOI] [Google Scholar]
- 70.Vaz ABM, et al. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011;42:937–947. doi: 10.1590/S1517-83822011000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurtzman, C., Fell, J.W., Boekhout, T. The Yeasts: A taxonomic study, 5th ed., Elsevier: Amsterdam, The Netherlands, p. 2354 (2011).
- 72.Emmanouil-Nikoloussi, E. et al. Hanseniaspora uvarum the ultrastructural morphology of a rare ascomycete, isolated from oral thrush. Bull. Group Int. Rech. Sci. Stomatol. Odontol.37, 13–7 (1994). [PubMed]
- 73.García-Martos P, et al. Isolation of Hanseniaspora uvarum (Kloeckera apiculata) in humans. Mycopathologia. 1999;144:73–75. doi: 10.1023/A:1006900909455. [DOI] [PubMed] [Google Scholar]
- 74.Severo-Gomes, B.S. et al. Pathogenic characteristics of yeasts isolated from vaginal secretion preserved under mineral oil. J Venom Anim. Toxins Incl. Trop. Dis.17, 460–6 (2011).
- 75.Jankowski, M. et al. Hand dermatitis with Hanseniaspora uvarum as a plausible causative agent. Adv. Dermatol. Allergol.XXXV, 641–643 (2018). [DOI] [PMC free article] [PubMed]
- 76.Margesin, R. et al. Rhodotorula psychrophila sp. nov., Rhodotorula psychrophenolica sp. nov. and Rhodotorula glacialis sp. nov., novel psychrophilic basidiomycetous yeast species isolated from alpine environments. Int. J. Syst. Evol. Microbiol.57, 2179–2184 (2007). [DOI] [PubMed]
- 77.Crous PW, et al. MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 2004;50:19–22. [Google Scholar]
- 78.Kobayasi Y, et al. Mycological studies of the Alaskan Arctic. Annu Rep. Inst. Ferment. Osaka. 1967;3:1–138. [Google Scholar]
- 79.Montemartini A, Caretta G, Del Frate G. Notes on Thelebolus microsporus isolated in Antarctica. Mycotaxon. 1993;48:343–358. [Google Scholar]
- 80.de Hoog GS, et al. Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud. Mycol. 2005;51:33–76. [Google Scholar]
- 81.Brunati M, et al. Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar. Gen. 2009;2:43–50. doi: 10.1016/j.margen.2009.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.