Abstract
Aquaporins (AQPs) aka water channels are a family of conserved transmembrane proteins (~30 kDa monomers) expressed in various organ systems. Of the 13 AQPs (AQP0 through AQP12) in the human body, four (AQPs 1, 3, 4, and 5) are expressed in the respiratory system. These channels are conventionally known for mediating transcellular fluid movements. Certain AQPs (aquaglyceroporins) have the capability to transport glycerol and potentially other solutes. There is an emerging body of literature unveiling the non-conventional roles of AQPs such as in cell proliferation and migration, gas permeation, signal potentiation, etc. Initial gene knock-out studies established a physiological role for lung AQPs, particularly AQP5, in maintaining homeostasis, by mediating fluid secretion from submucosal glands onto the airway surface liquid (ASL) lining. Subsequent studies have highlighted the functional significance of AQPs, particularly AQP1 and AQP5 in lung pathophysiology and diseases, including but not limited to chronic and acute lung injury, chronic obstructive pulmonary disease (COPD), other inflammatory lung conditions, and lung cancer. AQP1 has been suggested as a potential prognostic marker for malignant mesothelioma. Recent efforts are directed toward exploiting AQPs as targets for diagnosis, prevention, intervention, and/or treatment of various lung conditions. Emerging information on regulatory pathways and directed mechanistic research are posited to unravel novel strategies for these clinical implications. Future considerations should focus on development of AQP inhibitors, blockers, and modulators for therapeutic needs, and better understanding the role of lung-specific AQPs in inter-individual susceptibility to chronic lung diseases such as COPD and cancer.
Keywords: Aquaporins, Lung pathophysiology, Regulation, Acute lung injury, Lung inflammation, Chronic Obstructive Pulmonary disease, Lung cancer
1. Introduction
Aquaporins, commonly referred to as “water channels”, are small integral membrane proteins which primarily mediate selective transport of water across the membranes in various organ systems including lungs, kidneys, CNS, heart, skin, eyes, among others [reviewed in [5],[6], [7]]. Water transport across cells and tissues is a physiologically essential process that helps maintain homeostasis. While normal transcellular water transport may occur via diffusion across the lipid layer of the membrane or via passive diffusion with other solutes, aquaporin water channels are involved in areas requiring high water permeabilities for fluid transport [8], [9], [10]. Consequently, not all cells in a given tissue or organ contain AQPs. Fluid transport across cell membranes may be driven by osmotic gradient under normal physiological conditions and/or by differential hydrostatic pressure as may occur in certain pathological conditions such as edema [11] [12]. To cater to these variable fluid transport processes, aquaporins show diverse tissue and cell distribution in different organ systems. This review focuses on their role in respiratory system. In lung airways and parenchyma, transport of water across epithelial-endothelial barrier occurs between airspace and peripheral lung compartments (interstitial and vascular compartments and submucosal glands) for various processes in neonatal and adult lung [reviewed in [13],[12]]. These may include normal physiological processes such as maintenance of airspace hydration and airway surface liquid (ASL) layer, and infant lung fluid clearance at birth, or pathological processes such as pulmonary edema and pleural effusions. Several studies have also revealed role of aquaporins in various lung conditions and diseases such as acute lung injury, chronic obstructive pulmonary disease (COPD), asthma and other inflammatory conditions, and lung cancer.
Aquaporin (AQP) discovery in 1992 by Peter Agre of John Hopkins University won him a Nobel Prize in 2003 [8]. Since then a total of 13 aquaporins (AQP0 through AQP12) have been discovered in humans and this family of proteins has been shown to occur in other animals (vertebrates and invertebrates), plants, and microbes (bacteria, yeasts). Consistent with the original description by Agre that aquaporins are “the plumbing system for cells”, these integral membrane pore proteins mediate selective water transport in and out of the cells and at the same time prevent the trafficking of ions and solutes that may require separate membrane channels [5], [3], [6]. However, certain aquaporins (AQP3, 7, 9, and 10) may also transport glycerol and thus are known as aquaglyceroporins. Additional evidence, though controversial, also points to the role of certain aquaporins (such as AQP9) in transport of other uncharged molecules such as gases (CO2, NH3, NO, O2) and small solutes (H2O2, urea) and even ions (AQP6) [reviewed in [14]]. Besides, there are suggestions on the non-conventional role of certain aquaporins in functions such as cell migration [15] [16], cell-cell adhesion [17], and regulation of other channels [18].
1.1. Structural aspects of aquaporins:
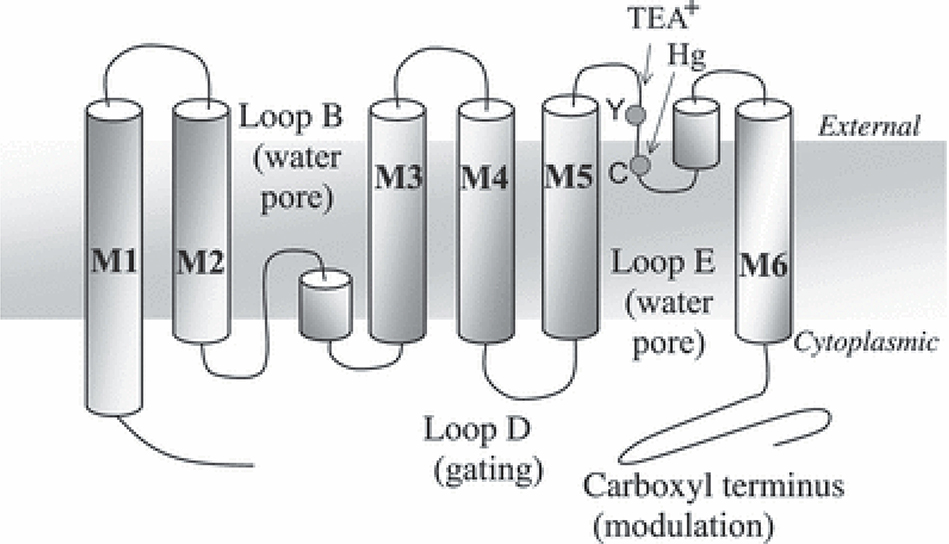
AQPs are small protein monomers (about 30 kDa size each) forming tetramers in the biological membrane though each monomer functions independently. Certain AQPs (AQP0 and AQP4) may also show greater aggregation, forming supramolecular crystalline arrays. AQP monomers consist of 6 transmembrane α-helices and 2 short helical segments that allow formation of a water pore in the membrane (FIGURE 1). The helices are connected by extracellular and intracellular loops to the amino- and carboxy-ends of the monomer. As compared to the water-selective AQPs (AQP0, 1, 2, 4, 5, and 8), aquaglyceroporins contain more hydrophobic amino acids lining the pore and show less constricted pore [1]. Besides the crystal structure of the first discovered aquaporin AQP1 (FIGURE 2), 3D structures of several AQPs have been resolved with high resolution [reviewed in [4].
FIGURE 1. Diagrammatic representation of transmembrane topology of aquaporin-1.
The arrangement depicts six transmembrane domains (M1 to M6), two loops which form the water pore on a subunit (loops B and E), and the regions implicated in gating (loop D) and activity modulation (C-terminus). The figure also illustrates regions blocked by the extracellular agents mercury (Hg) and tetraethylammonium ion (TEA+). Reproduced with permission from Yool et al 2010 [1].
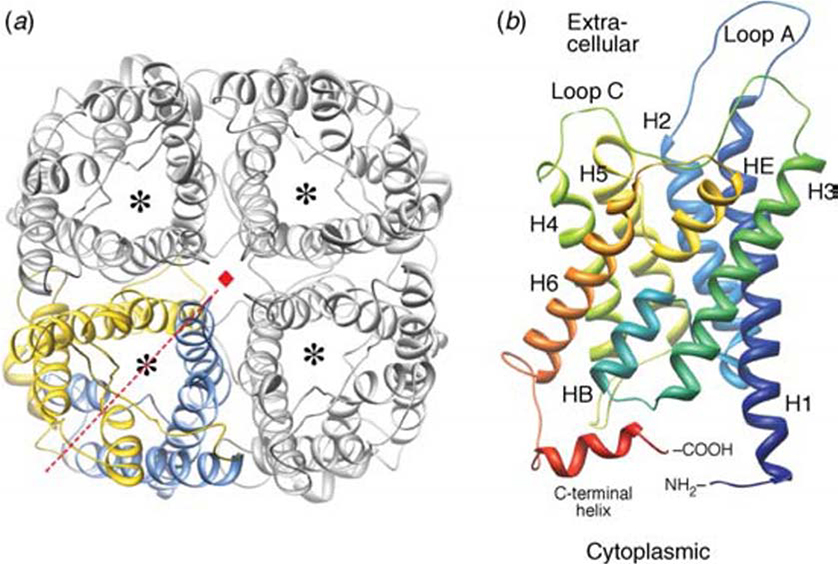
FIGURE 2. 3D structure model and tetrameric arrangement of AQP1.
A: AQP1 tetramer as viewed from the cell surface. One of the four monomers (bottom left) is shown in color to depict its parts: blue to represent the N-terminal and yellow to represent the C-terminal tandem repeat. The dashed line in red depicts the pseudo-2-fold axis relating the two halves of the monomer, and the small red square at the center depicts the 4-fold axis of the tetramer. The asterisk in each monomer represents the water pore.
B: AQP1 monomer as viewed in the direction parallel to the membrane. H1-H6 denote membrane-spanning helices, A-E denote loops, and HB and HE denote the two pore helices formed by loops B and E, respectively. Reproduced with permission from Gonen and Walz 2006 [4]
2. Aquaporins in Lung
2. 1. Tissue- and cell type-specific expression/localization of aquaporins in lung:
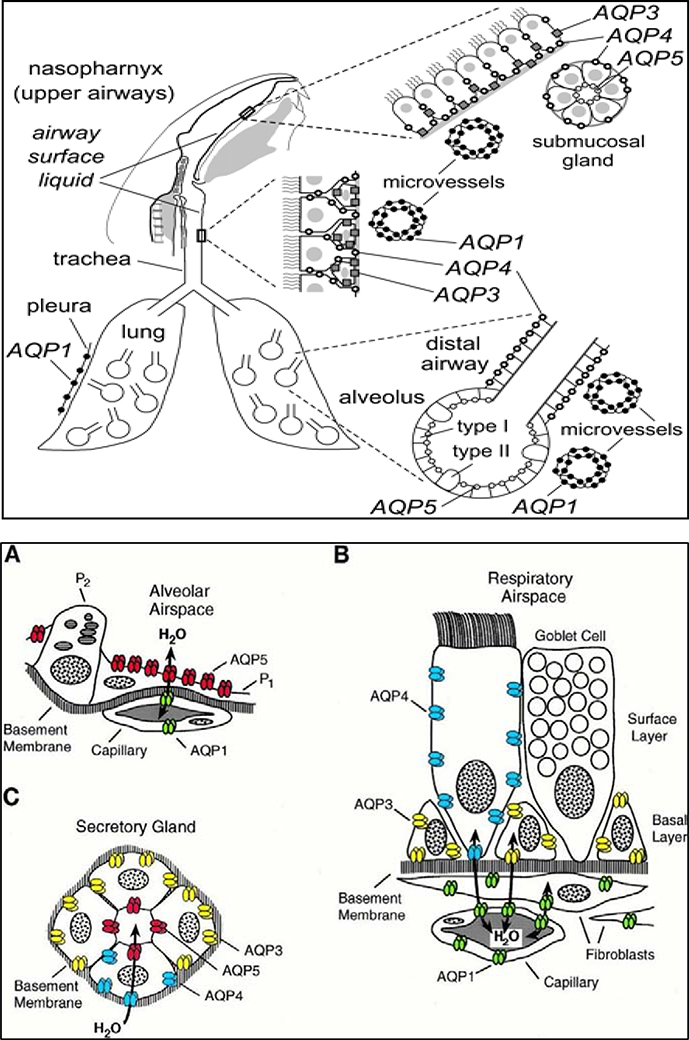
AQPs differ in their tissue distribution and cellular localization pattern in various body tissues depending on the water transportation requirements. For instance, 4 of the 13 AQPs have been known to be expressed in lung as against kidney which expresses 6 of the aquaporins, possibly because of the continuing requirement for a higher water permeability and rapid water transport in kidneys. In terms of cellular distribution, those cell types that require few-fold to about 50-fold higher osmotic water permeabilities express AQPs [6]. AQPs 1, 3, 4, and 5 are expressed in lung. However, these AQPs show species variations in their distribution in the lung compartments [13]. In rodent studies, lung expression of the four AQPs shows wide cellular distribution (FIGURE 3) in various lung cell types including airways epithelia, alveolar epithelia, associated microvasculature endothelia, and submucosal glands [reviewed in [5], [2], [12].
FIGURE 3: AQP expression in airways and lung compartments.
Upper Panel: Schematic diagram showing that mouse AQPs 1, 3, 4 and 5 are expressed in epithelia and endothelia throughout the nasopharyngeal cavity, airways (upper and lower), and alveolar compartment.
Lower Panel: Schematic diagrams showing cellular expression patterns of AQPs in rat airways and lung tissues: A). Subcellular expression pattern of AQP5 in alveolar space. AQP5 (shown in red) is expressed in apical membrane of type 1 pneumocytes (P1) but not expressed in type 2 pneumocytes (P2) and AQP1 is expressed in the alveolar capillaries; B). Cellular expression pattern of AQPs in respiratory epithelial layer, depicting no expression in goblet cells, AQP3 expression in basal cells, AQP4 expression in surface columnar cells, and AQP1 in underlying capillaries and fibroblasts; C). Subcellular expression pattern of AQPs in secretory glands: AQP5 (red) in apical membrane of acinar cells, AQP3 (yellow) and 4 (blue) in basolateral domains of glandular cells of nasopharynx and conchus but not in salivary glands. Reproduced with permission the Upper panel image from Borok and Verkman 2002 [2] and the Lower panel images A, B, and C from Nielsen et al 1997 [10].
AQP1 has been known to express in the endothelial cells (alveolar and airway regions), fibroblasts, and mesothelial cells (pleura). AQP3 is expressed in basal epithelial cells (nasopharynx and large airways). Human small airway epithelia have also been shown to express this protein. AQP4 is expressed in the ciliated columnar cells of various epithelial regions (nasopharyngeal, tracheal, bronchial) in the upper airways. AQP5 is expressed in alveolar type I epithelial cells and acinar epithelial cells in submucosal glands. Reported expression of this protein in large airway epithelia is however debatable. Human lung is known to express AQP5 in alveolar type I epithelial cells and in nasopharyngeal epithelium. In terms of cellular localization, while AQPs 3 and 4 are primarily expressed in the basolateral membrane, AQP5 protein has been shown to localize in the apical membrane of the expressing cells. Such cell-specific expression and localization of the four AQPs in lung tissue help overcome the water transport barriers across various compartments in the lung tissue. For instance, AQP expression in submucosal gland epithelia and airway epithelia allows for secretion of fluid (besides glycoproteins) onto the airway surface to maintain the ASL, a thin layer of liquid lining the airways. Likewise, AQPs in alveolar epithelia and vasculature endothelia allow for movement of the fluid between the alveolar air space and the associated vasculature [reviewed in [12].
2.2. Developmental regulation of AQP expression in lung:
Several studies in laboratory animal models have demonstrated regulation of AQPs during development of lung [reviewed in [13]. For instance, AQP1 has been shown to express right before birth and dramatically increases in perinatal and adult rodent lung [19], [20]. A similar upregulated expression pattern paralleled with the increasing lung water permeability during these developmental stages (perinatal and adulthood) in a rabbit model [21]. AQP4 expression showed dramatic upregulation right after birth whereas AQP5 showed little expression at birth and gradually increased until adulthood.
3. Conventional and Unconventional Roles of Aquaporins
3.1. Conventional Role of AQPs (Water transport function):
AQPs are involved in normal physiological roles in different organ systems wherein they are expressed in epithelia and endothelia facilitating transport of fluid. In addition, they are also expressed in certain other cell types viz. epidermis, adipocytes, and skeletal muscle. In case of respiratory system, tissue- and cell type-specific distribution of AQPs and their developmental regulation imply that aquaporins have a similar role in water transport and normal physiology of the lung. AQPs mediate fluid transport across endothelial and epithelial layers in the lung via a transcellular pathway rather than a paracellular pathway (via tight junctions). Conclusive answers to this question were sought by employing AQP-specific knock out mouse models [reviewed in [12], [22]. Both extrapulmonary and pulmonary roles were investigated in wild type versus the knockout (KO) mice, to understand the importance of aquaporins.
3.1.1. Extrapulmonary role:
The following specific relationships were observed in KO mouse studies:
AQPs 1–4 KO mice showed defect in urine concentrating ability in kidneys [23]
AQP3 KO mice showed decreased skin hydration due to decreased steady state level of glycerol in epidermis and stratum corneum layers due to deficiency of this aquaglyceroporin [24] [25].
AQP5 KO mice showed impairment in near-isomolar fluid secretion in salivary gland [26]
AQP7 KO mice showed adipocyte hypertrophy and greater fat mass due to accumulation of intracellular glycerol (impaired export) and elevated triglyceride, due to deficiency of this aquaglyceroporin [27].
Role of AQPs 1–4 in urine concentrating ability in kidneys was also observed in humans with mutated AQP1 [28] or AQP2 [29]. AQPs are known to play a role in eyes in terms of facilitating exocrine glands secretion of fluid and surface hydration, among other functions. Additionally, AQP3 and AQP4 have been associated with normal physiological processes in skin hydration and CNS, respectively. Human patients carrying E143K mutation in carbonic anhydrase showed a dry mouth syndrome due to mislocalization of AQP5 in the salivary glands [30]. Collectively, mouse KO and other studies have shown a critical role of AQPs in several normal physiological processes in extrapulmonary organs [reviewed in [6].
3.1.2. Pulmonary role:
While insights from nonpulmonary investigations on KO mice implied similar roles in pulmonary physiology, specific studies with AQP null mice yielded both unexpected and expected outcomes. In the airways and peripheral lung, the following normal physiological processes depend on fluid transport: a). Airspace hydration; b). Airspace fluid absorption near birth; c). Airspace fluid absorption in pulmonary edema; d). Fluid secretion by submucosal glands in the airway space. During these processes, there is fluid transport in different parts of the lung viz. airways, distal lung, and pleura as well as fluid secretion by airway mucosal glands. Comparative studies using AQP null mice showed the following specific effects on the fluid transport in these physiological processes.
3.1.2.1. Airways:
Mice lacking AQP3 and/or AQP4 showed reduced osmotic permeability of water in upper airways [31] but had minor effect on humidification of upper or lower airways. Also, the ASL layer characteristics (depth and salt concentration) as well as isomolar fluid absorption in the nasopharyngeal airways did not alter in these AQP-deficient mice [32]. Collectively, while AQP3/AQP4 facilitates movement of osmotically-driven water in the airways, their role appeared to be minor in airway humidification, maintenance of the ASL layer, and isomolar fluid absorption.
3.1.2.2. Peripheral lung.
The two main AQPs in peripheral lung, AQP5 in alveolar type I epithelial cells and AQP1 in endothelial cells, provide the major means for osmotically-driven movement of water across these barriers [33], [34]. Their deletion showed reduced water permeability in the ex-vivo (isolated perfused mouse lung) model [21]. The AQP (AQP1 and AQP5) KO mice showed decreased lung water accumulation and there was no significant effect of deletion of these AQPs on clearance of alveolar fluid in adult stage [33] [34] or just after birth stage. Also, there were no adverse effects on CO2 transport [35] in AQP1 KO mice.
3.1.2.3. Pleura.
Continuous secretion of fluid in the pleural space across its endothelial and mesothelial barrier is counterbalanced by its rapid reabsorption via lymphatic drainage. Studies using mice deficient in AQP1 (which is the key AQP in pleural barriers) showed that, like in peripheral lung, this AQP helps in rapid osmotic equilibration across the surface of the pleura [36], but seems to have no role in pleural fluid accumulation or clearance.
3.1.2.4. Airway submucosal glands.
Unlike the other afore-described fluid transport processes, submucosal gland secretions into the airway were significantly impacted by the AQP deficiency. For instance, based on video imaging of fluid droplets, AQP5 null mice showed more than 2-fold decrease in stimulated fluid secretion but with no loss of total protein and chloride amounts in the fluid [reviewed in [12]. This demonstrated that AQP5 facilitates fluid secretion in submucosal glands which may have physiological implications in secretion of water and solutes mixture (ions and macromolecules) in the ASL lining. This showed that AQP5 plays a role in normal physiology of the lung. AQP5 may therefore serve as a therapeutic target to remedy the pathological conditions that affect the ASL fluid and antimicrobial defense mechanisms of the lung. Nevertheless, in relative terms, limited role of AQPs in lung homeostasis as compared to kidneys is considered to be because of slower fluid transport rates in airways and peripheral lung.
3.2. Unconventional roles of AQPs:
Besides the conventional role in fluid transport processes, AQPs have been shown to be involved in other processes, including cell migration/proliferation, gas permeation, and inflammatory response.
3.2.1. Cell migration and cell proliferation.
Besides the conventional water-transporting role of AQPs in normal physiological processes in different organs including lung, later studies revealed non-conventional (non-water transporting) role of AQP in cell migration and cell proliferation [reviewed in [12]. Saadoun and coworkers [15] [16] discovered this novel role in cell migration for AQPs. In their proposed mechanism for this role, water entry into protruding lamellipodia facilitated by AQP accounted for the cell migration. Specifically, a local osmotic gradient is created at the lamellipodium tip by cleavage of actin and ion uptake which facilitates the movement of the cell. Subsequently, the same research group [37] discovered novel role of aquaglyceroporin AQP3 in cell proliferation via its glycerol transport function. Glycerol availability allows healing of damaged epithelial cells because of its role in ATP generation and lipid biosynthesis and thus could have a direct role in epithelial healing processes following injury in various organs. The unconventional cellular roles of AQPs in cell proliferation and migration could be directly relevant in lung epithelial and endothelial cells during embryonic development (like for other organ systems) as well as under pathological conditions such as lung cancer and remodeling/recovery after lung injury.
3.2.2. Gas permeation:
There are reports on suggested role of aquaporins in gas permeation [reviewed in [14], including AQP9 (CO2, NH3, NO, O2), and AQP1 (CO2, NO, O2). However, AQP1-null mice studies [38] suggested that this AQP is not critical for oxygen uptake in the lung. Latest studies based on molecular dynamics simulations provided valuable insights into the CO2 permeation mechanism [39]. Taken together, this function for AQPs is unclear and needs further investigations [40].
3.2.3. Inflammatory signal potentiator:
Sakamota and coworkers [41] demonstrated role of the water-selective channel protein AQP5 as inflammatory response potentiator. siRNA knockdown of AQP5 expression (alveolar epithelial cell line MLE-12) attenuated TNF-alpha induced transcriptional expression of the chemokine KC whereas AQP5 overexpression (fibroblast cell line NIH-3T3) increased KC expression. This role as inflammatory signal potentiator was suggested to be mediated via ERK and NF-kB activation. Other studies [42] have demonstrated a critical role of aquaporins in inflammasome-related inflammation. In vitro AQP blockage in macrophages activated with NLRP3 activators caused reduced IL-1β production. In vivo experiments using crystals-induced acute lung inflammation model confirmed this activity. Recent studies using an irradiation-induced mouse model and AQP blockage strategy demonstrated role of AQP4 in inflammatory cell infiltration, cytokine release, and activation of M2 macrophages [43]. Emerging perspectives on role of AQPs in inflammatory response and various inflammatory diseases have been the subject of recent reviews [44] [45].
4. Role in Lung Pathophysiology and Disease
While studies on KO mice (lacking specific AQPs) established the fact that these water channel proteins constitute a prominent route for osmotically-driven water transport in lung compartments (airways, alveoli and pleura) across the epithelial and endothelial barriers, their role in normal physiological state of the lung was considered limited to maintaining the ASL homeostasis. However, these studies do not exclude the possibility of onset of compensatory mechanisms in these KO models thus leaving the question of role of AQPs open ended. In this context, another side of the coin is the likely role of aquaporin water channels in lung pathophysiology and disease. Indeed, accumulating information on this aspect points to a larger role of AQPs in lung physiology as discussed in the following sections.
4.1. Aquaporins in acute and chronic lung pathologies.
Provocative data on altered expression of AQPs in several lung pathologies reinforce their role in lung pathophysiology and disease. In this context, several early studies had shown alteration in the expression of AQPs in various lung pathology models such as infection [46], inflammation [47] [48] ], lung injury [49] [50], and fibrosis [51], implying role of AQPs in lung pathologies. Recent developments in this area (reviewed below) have opened new frontiers regarding the role of AQPs in various lung pathologies and diseases.
4.1.1. Pulmonary injury and edema:
Acute lung injury (ALI) is a pulmonary condition characterized by damage of alveolar-capillary wall, inflammatory cell infiltration, and edema. The pulmonary edema characterized by water accumulation in the lung is a distinct phenotype in different lung injury models. Considering that AQPs are primarily water channels, there were anticipated thoughts on their role in pulmonary edema development and/or resolution [reviewed in [2]. However, past studies using AQP null mice imply that AQPs are not essential for alveolar fluid clearance [52]. Nevertheless, the possibility that other compensatory mechanisms take place in these null backgrounds cannot be excluded. Also, it is possible that AQPs have a role in cell volume regulation such as in alveolar epithelial type I cells [53] and in fluid secretion from mucus cells [26]. In an early study using lipopolysaccharide (LPS)-induced acute lung injury mouse model, specific role of AQP-1 was investigated [50]. The results suggested that although AQP1 expression is decreased after the injury, depletion of this aquaporin as in AQP1 null mice does not alter LPS-induced inflammation and edema. In contrast, in a subsequent study using rat model of oleic acid-induced acute lung injury, AQP4 mRNA was found upregulated in the alveolar epithelial type II cells implying its compensatory role in alveolar liquid clearance in the event of damaged Na transport in ALI [50]. In a lung injury model based on infection with Pseudomonas aeruginosa [54], a frequent bacterial pathogen in ventilator-associated pneumonia patients, specific role of AQP5 was examined using AQP null mice. P. aeruginosa is known to cause disruption of epithelial cells in the lung (alveolar and airway) leading to systemic dissemination of the pathogen. AQP deficiency resulted in an increased pathogen dissemination to blood and aggravated injury score as well as reduced mucin production in lung. These results collectively suggested a protective role of AQP5 in maintenance of lung barrier function against infection. In studies on seawater-induced acute lung injury rat model [55], [56], [12], AQP1 and AQP5 were upregulated in the exposed lungs and were shown to contribute to the edema formation in the lung [12]. Additionally, treatment with Tanshinone IIA, an active component of the Chinese herb Danshen [56], or 17β-estradiol [55], resulted in attenuation of lung edema/injury which paralleled with the downregulation of AQP1 and AQP5 in the treated lungs. However, in contrast to the above observations on upregulation of AQP5 in various ALI models, a downregulation of this AQP was observed in a radiation-induced ALI mouse model [57]. In this direction, latest studies focused on different types of lung injury and edema models further clarifying the role of aquaporin expression, are summarized in TABLES 1&2. Collectively, the acute lung injury models suggested the role of AQP1 and AQP5 in lung injury and/or edema development and resolution but the role of specific AQP depended on the target of the injury, epithelial versus endothelial.
TABLE 1:
Role of aquaporin expression in different acute lung injury (ALI) models
| ALI model | Etiological agent | Aquaporin regulation | Primary injury type/target | Related symptoms | Source reference |
|---|---|---|---|---|---|
| LPS-induced ALI mouse model | Lipopolysaccharide (LPS) | AQP5↓ | Capillary endothelium and alveolar epithelium | Lung inflammation (increased vascular permeability and cellular infiltration) | [60] |
| HCl-induced ALI mouse model | Hydrochloric acid (HCl) | AQP4↓ | Alveolar epithelium | Lung inflammation (increased vascular permeability and cellular infiltration) | [60] |
| Ventilator-induced ALI mouse model | Mechanical ventilation | AQP4↓ | Alveolar epithelium | Lung inflammation (increased vascular permeability and cellular infiltration PLUS altered lung mechanics) | [60] |
| Ventilator-induced ALI rat model | Mechanical ventilation at high tidal volume | AQP1↓ | Alveolar-Capillary membrane | Lung injury and edema | [61] |
| AKI-associated ALI rat model | Bilateral nephrectomy (BNx)-induced acute kidney injury (AKI) | AQP5↓ | Interstitial tissue | Interstitial tissue thickening | [62] |
| Sepsis-induced Diffuse Alveolar Damage (DAD)/Acute Respiratory Failure (ARF) in Human patient | Non-pulmonary sepsis | AQP3↑ AQP5↑ |
Alveolar region | DAD/ARF | [63] |
| H1N1 Virus infection-induced DAD/ARF (Human) | H1N1 virus infection | AQP3↑ AQP5↑ |
Alveolar region | DAD/ARF | [63] |
| Leptospirosis-induced DAD/ARF (Human) | Leptospirosis | AQP3↑ AQP5↑ |
Alveolar region | DAD/ARF | [63] |
| High altitude hypoxia-induced ALI rat model | High altitude hypoxia | AQP1↓ | Alveolar wall thickening, outstretching and congestion | Pulmonary edema | [64] |
| CPB-induced Ischemia/Reperfusion acute lung injury (I/R ALI) dog model | Cardiopulmonary bypass (CPB) procedure | AQP1↓ | Lung tissue (different lobes) | Pulmonary structure and function deterioration; Edema | [228] |
| LPS-induced ALI mouse model | LPS | AQP1↓ AQP5↓ |
Lung tissue | Increased inflammatory factors and apoptotic cells | [65] |
| AKI-induced ALI rat model | Continually occluding bilateral renal artery and veins to induce Ischemic AKI. | AQP1↓ | Interstitial and alveolar spaces | AKI; Lung inflammation and edema | [66] |
TABLE 2:
Role of aquaporin regulation in different pulmonary edema models
| Pulmonary edema (PE) model | Etiological agent | Aquaporin regulation | Related information | Source reference |
|---|---|---|---|---|
| H2S-induced acute PE mouse model | Hydrogen sulfide (acute exposure) | AQP5↓ | MAPK pathways (ERK1/2 and p38) implicated in AQP5 down regulation | [67] |
| FES-induced PE mouse model | Fat Embolism Syndrome (FES) | AQP1↑ | P38 MAPK pathway implicated in AQP1 upregulation | [68] |
| LPS-induced DIC-associated PE rat model | Intravenously infused LPS-induced disseminated intravascular coagulation (DIC) syndrome | AQP5↓ | MicroRNAs (miR-96 and miR-330) implicated in AQP5 upregulation in this model | [69,70] |
| Pneumothorax-induced PE rat model | Air injection-induced pneumothorax | Undrained (AQP1↑AQP3↓) Drained (AQP5↑) |
Duration of pneumothorax (5 min vs. 30 min) and fluid drainage regulated the AQP expression | [71] |
| Oral gavage-induced PE mouse model | Oral gavage frequency: a) One-time dose caused alveologenic edema b) Repeated dose caused perivascular edema and hydrostatic pressure edema | AQP1↑ AQP4↑ |
In this lung toxicity evaluation of plant/herb extracts, edema condition was associated with multiple immunological and pathological changes in the lungs | [72] |
4.1.2. Pulmonary infections, stress, and toxicity.
Lung infections (bacterial, viral) and exposures to environmental pollutants (silica, asbestos, irradiation, sulfur dioxide) and climatic conditions (oxygen, temperature, humidity) cause various pulmonary conditions and pathologies. Recent studies using different lung exposure- or infection- models targeting these agents have shown downregulation of lung AQP 1 and/or AQP5 (see TABLE 3); chronic exposure to low oxygen levels however, showed upregulation of AQP1. These observations implied the role of AQPs in these pulmonary conditions.
TABLE 3:
Aquaporin regulation in different pulmonary conditions (Environmental exposures and Infections)
| Experimental model | Lung AQP regulated | Related information | Source reference |
|---|---|---|---|
| Exposure model | |||
| Chronic hypoxia exposure as in Hypoxia pulmonary hypertension (HPH) mouse model | AQP1↑ | AQP1 was upregulated in both endothelia and smooth muscle cells which showed dysfunction and proliferation, respectively. AQP1 deficiency protected from these effects in gene KO mice.. | [73] |
| Lung phlegm-blocking rat model (SO2 inhalation + cold wind exposure) | AQP1↓AQP5↓ | Reversal of the levels of these AQPs using natural products had expectorant effects | [74] |
| Deep hypothermia cardiac arrest -induced Ischemia/reperfusion lung injury in rat model | AQP5↓ | cAMP-PKA signaling pathway implicated in AQP5 regulation | [75] |
| Acute cold exposure rat model (−25 °C) | AQP5↓ | Body core temperature of the cold-exposed rats was 28.07 ± 4.15 °C versus the control rats at 37.33 ± 0.25 °C. | [76] |
| Hot-humid stress and acclimation | AQP5↓ | Mucus hypersecretion in airways | [77] |
| Pulmonary silicosis rat model (silica dust exposure) | AQP1↓ | Suggested role of AQP1 in silicosis development | [78] |
| Asbestos (fluoroadenite fiber)-exposed sheep lung model | AQP1↓ | These amphibole asbestos-like fibers cause chronic inflammation and carcinogenesis in lung tissue | [79] |
| Radiation-induced lung toxicity (RILT) leading to pneumonia or fibrosis | AQP1↓AQP5↓ | Suggested role of AQP1&5 | [80] |
| Infection/sepsis model | |||
| PRRSV virus-infected lung porcine model | AQP1↓AQP5↓ | Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) infection possibly impaired the lung edema resolution via downregulation of these AQPs | [[81] |
| Sepsis rat model | AQP5↓ | Inflammatory mediators (TNF-α, IL-6) and phosphop38 increased. The effect was reversed using Tanshinol (this compound is the primary active component of a Chinese Medicine (Salvia miltiorrhiza Bunge) traditionally used to treat cardiovascular problems) | [82] |
| LPS + Air drawing-induced sepsis rat model | AQP1↓ | Hydrogen treatment improved the pulmonary epithelial barrier function and modulated AQP1 expression | [83] |
| LPS-induced sepsis rat model | AQP1↓AQP5↓ | Hydrogen-rich saline attenuated the impaired lung function via upregulation of AQPs and inhibition of MAPKs (p38 and JNK) | [84] |
4.1.3. Pulmonary fibrosis.
Following various chronic insults, human lung alveolar epithelial cells undergo mesenchymal differentiation (fibroblasts/myofibroblasts), known as epithelial- mesenchymal transition (EMT) process, leading to idiopathic pulmonary fibrosis (IPF). Alveolar epithelial type I (AECI) cells potentially contribute to pulmonary fibrosis [reviewed in [58]. Screening of human IPF lung biopsies revealed clearly elevated AQP1 levels in alveolar epithelia [59]. In vitro analysis in this study comparing IPF patient-derived versus healthy lung-derived fibroblasts showed AQP1 induction in response to TGF-β treatment in the former, implying AQP1 role in the pro-fibrotic TGF-β action and IPF etiology/pathophysiology. This is consistent with earlier studies on bleomycin-induced pulmonary fibrosis mouse model showing dramatic upregulation of AQP1 but down regulation of AQP5 [85] and no effect of AQP4 deficiency [86] in lung fibrosis development.
4.1.4. Asthma.
Role of aquaporins in asthma has been examined in experimental models. In OVA/OVA- induced and IL-13-induced mouse models of asthma, Krane and coworkers [87] noted differential regulation of lung AQPs; AQP5 showed diminished expression whereas AQP3 and AQP4 showed concomitant upregulation. While AQP5 was downregulated in both the models, AQP3 was upregulated in one of them ( IL-13 model). AQP1 and AQP4 transcripts decreased in the OVA/OVA-induced but not in the IL-13-induced model. AQPs are therefore common targets of gene regulation in asthma but are differentially induced in different challenge models, necessitating further confirmation. A subsequent study using AQP5 knockout mice [88], implicated involvement of AQP5 in the development of inflammation and mucus hyperproduction during chronic asthma. Another study using OVA-induced mouse model of asthma [89] showed decreased AQP1, AQP4, and AQP5 but increased AQP3 levels. AQPs 1 and 5 were significantly increased when treated with the anti-asthmatic agents ambroxol (AX), dexamethasone (DM), and terbutaline (TT). Since DM and AX improved pulmonary edema, infiltration of eosinophils, and mucus secretion and TT only improved edema, it was implied that the two AQPs (AQP1 and AQP5) are closely related to edema but not the other two phenotypes in asthma and that these three agents relieved edema through upregulation of these AQPs. However, Ablimit and coworkers [90], using an OVA-induced asthma rat model, observed tissue-specific regulation of lung AQPs. Increased AQP1 and AQP5 in small airways implied their involvement in submucosal edema formation and mucus hypersecretion. On the other hand, decreased expression of these AQPs in alveoli could be related to increased alveolar liquid viscosity and mucus plug formation. A later study [91], using AQP3 deficient mice in an OVA-induced asthma model, reported role of AQP3 in potentiating asthma via mediation of alveolar macrophage chemokine production and T-cell trafficking. OVA-induced bronchial asthma mouse model also showed higher expression levels of AQP1 and AQP2 in kidneys and dysregulation of urine volume which could be reversed using the traditional Chinese natural prescription/formulation San-Ao Decoction [92].
4.1.5. COPD.
Chronic Obstructive Pulmonary Disease (COPD), primarily induced by tobacco smoking and manifested as emphysema and chronic bronchitis, requires continued discovery of novel mechanistic and therapeutic targets. In human patients of COPD, reduction in AQP5 expression was found to correlate with an increased mucus production and compromised lung function [93]. In a gene knockout study, AQP5 deficiency protected against cigarette smoke-induced emphysema by reducing permeability and inflammation (neutrophil), implying the role of this aquaporin in COPD [94]. In a human study based on case-control design [95] targeting smokers with COPD (cases) and smokers with no COPD (controls), AQP1 and AQP5 showed opposing trends; AQP1 expression was upregulated 2.41-fold in the parenchyma whereas AQP5 was upregulated 7.75-fold in the bronchus of the cases than controls. Overall findings implied a role of AQP1 in COPD pathogenesis. In a recent study meant to identify novel target genes involved in COPD, transcriptomic screening of human lung tissue samples led to identification of AQP3 as one of the six candidate genes [96]; AQP3 protein was detectable in alveolar epithelial type I cells (AECI), AECII, macrophages, and bronchi. In a smoking and elastase-induced COPD rat model with cold-dryness symptom pattern, an imbalance in AQPs and mucins affected the mucus function, thereby enhancing airway obstruction phenotype [97]. A recent study using tobacco smoke-induced COPD rat model, showed that administration of a novel antioxidant, hydrogen-rich saline, which upregulated AQP5 while downregulating mucin, conferred significant protection from COPD development [98].
4.1.6. Lung cancer.
AQPs have been known to have a role in cell migration [99]. This function may have implications in tumor growth and metastasis or in endothelial cell migration leading to angiogenesis [100]. Indeed, Hu and Verkman [101] observed that AQP-expressing tumor cells in lung show greater extravasation across microvessels leading to greater metastases and local invasiveness. Additionally, expression of aquaporin in tumor cells has been correlated with edema in astrocytomas. This implies that these water channel proteins allow water penetration in the growing tumor mass (edema) and thus cause tumor expansion. These observations collectively led researchers to propose an oncogenic capacity of aquaporins.
Different lung cancer types have been shown to differentially express AQPs [102] [103], particularly AQP1, AQP3, or/and AQP5 [reviewed in [104] and, the actual explanations underlying these associations are being explored [105] [102] [103], reviewed in [104].
i). AQP1:
AQP1 was shown to predominantly overexpress in non-small cell lung cancer (NSCLC) cell lines of various origins and primary lung tumor specimens [102]. It was detected in adenocarcinomas and bronchoalveolar carcinomas but not in squamous cell carcinoma specimens. In experimental verification of these findings, forced expression of AQP1 in NIH-3T3 cells stimulated cell proliferation and anchorage-independent growth. In a later study using 92 lung biopsies [106], AQP1 was shown to overexpress in primary lung adenocarcinomas (which are the most common and heterogenous form of primary lung cancer) as well as pleural mesotheliomas. Role of AQP1 in tumor angiogenesis was emphasized based on high expression of this protein in small capillaries in areas near or surrounding the tumor, besides expression in walls of alveoli inside or next to tumor tissue. In a study based on a mouse model that spontaneously developed breast adenoma with lung metastases, role of AQP1 as an important determinant of tumor angiogenesis was demonstrated using gene knockout mice [107]. In a more comprehensive screen using 160 lung cancers of different histologic subtypes [108], AQPs 1, 3, and 5 were shown to express in majority of the specimens and their expression patterns showed frequent association with cellular differentiation in cancer cells. AQPs 1 and 5 were upregulated in invading cancer cells (particularly adenocarcinomas) and it was suggested that AQP1 overexpression with loss of subcellular polarization is involved in their potential for invasiveness and metastasis [108] [5]. Xie and coworkers [109] observed overexpression of AQP1 and AQP4 in lung cancer tissues and provided a functional explanation for this expression pattern based on transwell migration assays, confirming their role in lung cancer extravasation and spread.
Besides the role of AQP1 in tumor cell proliferation, cell migration, and angiogenesis, Zhang and coworkers [110] demonstrated role of AQP1 in malignant pleural effusion in a mouse model based on association of elevated expression of AQP1 with increased volume of the effusion. Recent studies based on chemical inhibitor experiments in malignant mesothelioma (MM) model have shown role of AQP1 in cell adhesion, migration and sphere formation [111] and in vasculogenic mimicry, especially under hypoxic conditions [112].
Generally, malignant mesothelioma (MM) has poor survival and prognosis. Overexpression of AQP1 (the main intracellular water transporter in mesothelial cells) was shown to be an independent prognostic factor (favoring survival) in pleural mesothelioma [113]. Gene expression data mining efforts on pleural MM cases showed that AQP1 is overexpressed in epithelioid mesothelioma; in this context, patients with deleted CDKN2A gene, which is a part of the AQP1 gene network predicted based on expression data mining and analyses, showed decreased AQP1 expression [114]. Immunohistochemical labeling of human lung tissue from 100 MM cases emphasized the usefulness of AQP1 as a prognostic marker [115]. AQP1 was also suggested to be a prognostic marker for lung adenocarcinoma based on screening of 505 cases of surgically resected tumors [116].
ii). AQP3.
A functional strategy based on gene knockdown and cell invasion assay led to a suggested role of AQP3 in cell proliferation and adhesion/penetration in a human lung cancer cell line XWLC-05 [117]. Besides inhibiting the cell growth and invasiveness, AQP3 knockdown suppressed MMP2 activity in XWLC-05 cells [118]. AQP3 knockdown suppressed tumor growth as well as angiogenesis and prolonged survival [119] in NSCLC xenograft mouse model. AQP3 knockdown slowed the growth of NSCLC cells via inhibition of HIF-1α/VEGF and Raf/MEK/ERK signaling pathways [120].
iii). AQP5.
AQP5 is expressed at higher levels in NSCLC tissues as compared to the neighboring normal tissue and has been associated with poor prognosis [121]. Specific role of AQP5 in lung carcinogenesis has been demonstrated using in vitro and in vivo strategies. For instance, AQP5 promoted cell migration and angiogenesis in NSCLC as demonstrated by gene knockdown and vascularization experiments in H1299 cell line [122]. mRNA silencing of AQP5 inhibited the growth in vitro and in vivo for A549 lung cancer cells [123]. Both AQP5 and the osmoregulated transcription factor NFAT5 are upregulated in lung adenocarcinoma cells and this TF plays its role in cell proliferation and migration via AQP5 regulation [124].
iv). AQP4.
Autoantibodies against this aquaporin may cause the demyelinating disease of the CNS Neuromyelitis Optica Spectrum Disorders (NMOSD). In elder patients, NMOSD has been reported to occur in the setting of lung adenocarcinoma [125], [126]. In paraneoplastic NMOSD associated with squamous cell lung carcinoma in a 66-year old female, anti-AQP4 antibodies were present in both the serum and cerebrospinal fluid of the patient but absent on the tumor surface [Reviewed in [127]. AQP4 antibody-positive NMOSD has also been reported to be associated with interstitial pneumonia as another rare extra-CNS complication of this disease [128].
4.2. Regulatory Pathways for lung Aquaporins in normal and pathological processes
Water transport through aquaporins may be regulated by three possible mechanisms: 1). regulation by translocation [129]; 2). transcriptional/translational regulation [130]; 3). regulation by conformational change or gating [131]. Arguably, the most dynamic, and selective regulatory mechanism is that by translocation of aquaporins to the plasma membrane in response to specific triggers; this mechanism has been thoroughly reviewed by Conner and coworkers [3].
4.2.1. Regulation via translocation:
Translocation helps cells to adapt to diverse changes in their environment due to triggers or stimuli and thus helps maintain water homeostasis. The various triggers of post-translational translocation vary with the individual AQPs. Diverse regulatory pathways are involved in trigger-induced translocation of AQPs. Particularly, triggers and pathways for those AQPs (primarily AQP1 and AQP5) which are relevant in water transport in respiratory system have been reviewed by Conner and coworkers.
AQP1 has been shown to be important in lung although it has a diverse tissue distribution. While early studies showed that it is constitutively expressed in cell membranes in kidney, recent studies show that it can be expressed in both cell membranes and cytoplasm and can be translocated by hypotonic stimulation mediated by protein kinase C (PKC) and microtubules as triggers [132] and also due to involvement of calmodulin and calcium influx [133]. GPCR agonists are one of the most important triggers of AQPs. The osmoregulatory hormone secretin is one such GPCR agonist that promotes water movement in cholangiocytes by inducing exocytic insertion of AQP1 [134]. This secretin-induced translocation could be mediated by the microtubule network.
AQP5, which has major role in respiratory water permeabilities, can be translocated in lung epithelial cells by induction with lipopolysaccharide [135] or microtubules and cAMP [136]. Protein kinase A (PKA) pathway is involved in the cAMP-induced translocation [136]. It has been suggested that, like that in AQP1, cytoskeletal proteins are involved in AQP5 translocation via microtubules [137]. AQP5 is also translocated to the membrane upon stimulation with GPCR agonists such as acetylcholine or with adrenergic receptor agonist such as adrenaline [138] [139]. Like AQP1, this protein is also triggered by hypertonicity [53].
4.2.2. Transcriptional/translational regulation:
Interestingly, there are emerging recent developments on transcriptional/translational mode of regulation of AQPs. The focus has been on AQPs 1 and 5.
AQP5:
Inflammation-associated mediators and pathways have been reported to regulate lung aquaporins particularly AQP5 [140] [141], [142]. Downregulation of AQP5 (but not AQP3 and AQP4) on LPS treatment of human primary bronchial epithelial cells was ascribed to p38/JNK signaling pathway [141]. A similar role of this pathway in LPS downregulation of AQP5 was earlier observed in human airway submucosal gland cell line [140]. The proinflammatory cytokine TNF-alpha was shown to induce decrease in AQP5 in human bronchial epithelial cells BEAS-2B [142]. In contrast, the anti-inflammatory endogenous lipids lipoxins (particularly lipoxin A4) were demonstrated to regulate AQP5 expression to maintain the clearance of the fluid in acutely injured lung during acute pancreatitis [143]. Another class of endogenous mediators, thyroid hormone, was shown to regulate AQP5, with hypothyroid state inducing and hyperthyroid state diminishing the AQP5 expression [144]. AQP5 expression in alveolar epithelial cells (E10 and MLE-12 cell lines) has been linked with activation of P2X7 receptor [145]. During trans-differentiation of alveolar epithelial cells type II (AECII) into type I (AECI ) regulated by Wnt signaling pathway, the Wnt7a ligand was shown to regulate the expression of AECI marker AQP5 [146]. In terms of epigenetic regulation, it has been shown that cell type-specific expression of AQP5 marker in AECI epithelial cells during trans-differentiation was a result of cooperative interactions among transcription factors (GATA6, Sp1) and histone modifications. Specifically, GATA6 and HDAC3 were shown to regulate transcription of AQP5 via modulation of H3 acetylation/deacetylation, respectively by competing for binding to Sp1 and interaction of GATA6/Sp1 with p300 [147].
AQP1:
This water channel, widely expressed in vascular endothelia including lung endothelia, is regulated by oxygen, with hypoxia and hyperoxia showing differential regulation. AQP1 was induced at both mRNA and protein levels by hypoxia in lung tissue [148] and hypoxia-inducible transcription factor-1alpha (HIF-1α) was shown to participate in this induction. However, it was emphasized [148] that AQP1 activation by hypoxia is a complex and multifactorial process that may involve other transcription factors in the regulatory process. Heme oxygenase-1 (HO-1), a protective enzyme with role in the defense against oxidative and inflammatory insults in the lung, was found to upregulate AQP1 expression in rat primary alveolar epithelial type II cells and the HO-1 effect was ascribed to its anti-oxygenation property [149]. In a recent study, AQP1 was shown to be significantly upregulated in hypoxia-exposed human vascular cells and hypoxia-induced lungs in pulmonary hypertension mouse model [150]. In such hypoxic pulmonary hypertension studies, elevated AQP1 expression was shown to exert its causal effects via upregulation of beta-catenin protein and the transcriptional and translational expression of known beta-catenin targets c-Myc and cyclin D1 [151]. In contrast, hyperoxia induced downregulation of AQP1 which may be the primary mechanism underlying hyperoxic pulmonary edema [152] and disturbance in alveolar fluid clearance under these pathological conditions [152]. In this context, it is interesting that AQP5 was significantly downregulated by hypoxia in alveolar epithelia where this is the primary water flux channel, and this effect was mediated through both HIF-1α and proteose-mediated pathways [153]; the latter appeared to mediate the stability of HIF-1α as well as other unknown transcription factor(s). In this context, AQP1 deficiency (null mice) showed increase in lung ischemia-reperfusion injury that was mediated via lowered stability of HIF-2α [154]. In a recent study, increased AQP1 expression was shown to play a role in aldosterone mediated development of pulmonary arterial hypertension [155]
In acute lung injury (ALI), pre-B cell colony-enhancing factor (PBEF), which is involved in enhancing the inflammatory factors, downregulated AQP1 expression primarily via MAPK pathways [156]. Another study using rat pleural mesothelial cells showed role of p38 MAPK pathway in downregulation of AQP1 by Staphylococcus peptidoglycan [157]. AQP1 expression during LPS-induced ALI was regulated by miR-144–3p miRNA which in turn was shown to be regulated by the long noncoding RNA CASC2 [158], thereby improving lung injury. In hyperbaric hypoxia-induced ALI, protective effect of Puerarin was mediated by down regulation of AQP1 via NF-kB pathway [159].
In lung cancer, AQP1 facilitates cell proliferation and migration in a manner dependent on MMP-2 and MMP-9 [160]. Elevated hydrostatic pressure in lung cancer enhanced cell motility and size via upregulation of AQP1 which in turn was mediated by caveolin-1 and ERK1/2 signaling [161].
5. Clinical Potential of Aquaporins
Research developments in aquaporin biology reflect a significant clinical potential of these channel proteins such as for use as diagnostic and prognostic markers and therapeutic targets, or for their role in heritable human diseases.
5.1. Aquaporins as diagnostic and prognostic targets.
Diagnostic potential of aquaporins may rely on detection of their autoantibodies or direct detection of specific AQP(s) in body fluids and/or tissue specimens as a marker for certain AQP-related conditions. A recognized diagnostic assay based on autoantibodies is the test for AQP4 autoantibody (NMO-IgG) in inflammatory demyelinating disease Neuromyelitis Optica (NMO) or Devic’s disease. This serum-based assay is claimed to be highly sensitive and specific for this disease [162] [163]. Possibly similar autoantibody-based assays could be developed for other autoimmune diseases (e.g. targeting AQP3 in skin diseases; AQP5 in Sjogren’s syndrome) and pathologies. Direct detection of AQP protein in body fluids and tissues and its association with specific diseases and pathologies offers another diagnostic potential with a wider spectrum of applications. An established example is urinary AQP2 assay [164] for Nephrogenic diabetes insipidus (NDI). ELISA-based quantification of AQP1 and AQP5 levels in induced sputum samples from mid to moderate adult-onset asthma patients demonstrated their potential as diagnostic markers for asthma diagnosis [165]. AQP5 expression levels in human COPD lungs were quantitatively correlated with lung function (spirometry measurements) and disease severity, suggesting its potential as a diagnostic marker [166]. In another study, intrapulmonary AQP5 expression was suggested as a possible biomarker for discriminating ‘smothering and choking’ from ‘sudden cardiac death’ [167]. Likewise, comparative expression analysis in rat models of vital drowning versus postmortem-submersion, showed elevated expression of AQPs 1 and 4 [168] or AQP5 [169], suggesting their potential as biomarkers of drowning in forensic applications.
On the other hand, it is interesting that certain AQPs associated with lung have been found to possess prognostic marker potential for malignant pathologies and cancer. For instance, AQP1 has been suggested as an independent prognostic factor [113] [115] [170] [171] in pleural malignant mesothelioma (MM). Considering that its expression in the MM cancer specimens significantly correlated with progress in MM, regardless of treatment or known prognostic factors, it was suggested that AQP1 immunohistochemical labeling could be a part of routine histopathologic workup. Alternatively, other less invasive assays could be developed based on further investigations on human mesothelioma cases. Recent studies have also suggested the prognostic potential of AQP1 in Non-small cell lung cancer [172] and lung adenocarcinoma [116] [173] patients.
5.2. Aquaporins as therapeutic targets
Considering their established and emerging functional properties, aquaporins have a high potential to serve as therapeutic targets. While considerable interest is generated, the progress in this field has been slow. Possible AQP-based therapeutics may include natural products, small molecule pharmacological blockers, small molecule modulators of function, AQP-specific monoclonal antibodies, among others.
5.2.1. Natural products as potential therapeutics.
Identification of natural products from traditional medicine as the AQP-directed therapeutics has been the major focus of research thus far. Several such candidate products have been evaluated for reversing the AQP-associated lung pathologies such as acute lung injury, asthma and other inflammatory conditions, and lung cancers, as summarized in TABLE 4.
TABLE 4:
Aquaporin-directed natural product therapeutics for different lung pathologies
| Natural Therapeutic candidate | Lung Disease Model tested | Therapeutic effects observed | Lung AQP regulated | Source reference |
|---|---|---|---|---|
| 1. Acute Lung Injury | ||||
| Dachengqi decoction (traditional Chinese herbal medicine with anti-inflammatory property) | LPS-induced ALI rat model | Anti-inflammatory effects -Suppressed TLR4 pathway -Inhibited cytokines; Reduction of lung edema |
AQP1↑ AQP5↑ |
[174] |
| Dai-Huang-Fu-Zi-Tang (DHFZT- a Traditional Chinese prescription for GI ailments (Acute pancreatitis, Cholecystalgia, Obstruction) | Na-taurocholate induced Severe acute pancreatitis (SAP)-associated ALI rat model | Anti-inflammatory effects -Suppressed proinflammatory cytokines - Enhanced anti-inflammatory cytokine; Reduction of lung edema |
AQP1↑ AQP5↑ |
[175] |
| Qing Ying Tang (a Traditional Chinese medicine used for septicemia and immune diseases) | SAP-induced ALI rat model | Suppression of inflammation (TNF-α); Reduction of lung edema |
AQP1↑ | [176] |
| Ginkgo biloba leaves extract (Ginaton)- a traditional Chinese medicine used for Ischemic cerebrovascular disease | Ischemic/Reperfusion Lung injury rat model | Pretreatment with Ginaton reduced the ALI symptoms | AQP1↑ | [177] |
| 2. Lung Inflammatory conditions (Asthma, COPD, etc.) | ||||
| Ziziphora clinopodioides extract (Traditional medication plant with anti-asthmatic activity) | OVA-induced allergic asthma mouse model | Anti-inflammatory effects -Downregulated IL-4 and IL-5 -Attenuated leukocytes in blood and BALF Ameliorated lung edema |
AQP1↑ AQP5↑ |
[178] |
| San-Ao Decoction (a traditional Chinese anti-asthmatic prescription developed from Taiping Huimin Heji Jufang) | OVA-induced Bronchial asthma mouse model | Urine volume regulated (increased); AQPs 1&2 in kidneys downregulated via ET-1, NO, and AngII; Weakened airway resistance |
AQP1↓ AQP2↓ (kidneys) |
[92] |
| Pistacia integerrima extract (a traditional Indian medicine for cough, asthma, fever, vomiting, and diarrhea) | OVA-induced allergic asthma mouse model | Anti-asthmatic activity -Reduction in TNF-α, IL-4, and IL-5; Reduction in pulmonary edema |
AQP1↑ AQP5↑ |
[179] |
| Abnormal Savda Munziq (Herbal Preparation of Traditional Uighur/Chinese Medicine used for asthma, digestive cancer, diabetes, cardiovascular diseases) | Fumigation/Elastase-induced COPD rat model | Improved pulmonary function | AQP1↑ AQP4↑ AQP5↑ |
[180] |
Despite the promise in terms of efficacy and safety of some of the natural products evaluated, their translation potential is limited. Consequently, there is a need to focus on their active components to design synthetic analogs as small molecule therapeutics or generate recombinant macromolecule (protein) therapeutics.
5.2.2. Small molecule therapeutics.
Initial efforts toward discovery of small-molecule therapeutic agents for aquaporins indicated the potential of aryl sulfonamides and antiepileptics [181] [182][183]. However, further efficacy studies failed to confirm the translational value of these agents [184]. In a subsequent study [185] involving screening of 45 synthesized bumetanide derivatives, aryl sulfonamide AqB013 was identified as an antagonist for AQP1 and AQP4 with a translational potential. However, further testing and validations were required for developing potential clinical applications. While aquaporins have seemingly poor druggability, available crystal structures of some of these membrane proteins (AQP1, AQP4, and AQP5) are facilitating the ongoing simulation-assisted drug discovery efforts to design better AQP blockers [186]. For instance, simulated docking of AqB13 in AQP4 structural models from rat and human has shown the critical residues (Ser180 and Val/Ile189) determining the blocking effect in this water channel protein.
By now, various AQP modulator candidates have been identified and patented [reviewed in [187] and [188]. Certain heavy metal ions and their derivatives are known to inhibit AQPs such as AQP3 inhibition by Gold(III) complexes [189]. TGN-020 is a small molecule inhibitor of AQP4 which is being evaluated in several recent studies including lung pathologies [190]. Sevoflurane was shown to modulate expression of AQPs 1 and 5 along with inhibition of ER stress response in OVA-induced allergic airway inflammation [191]. Similar evaluations targeting active components of traditional medicines or known anti-inflammatory compounds or repurposing of known drugs have continued in the past decade. Information on such chemical therapeutic candidates in terms of evaluation models, efficacy, and role in modulation of AQPs is summarized in TABLE 5.
TABLE 5:
Chemical therapeutic candidates evaluated for AQP-associated lung pathologies
| Chemical Therapeutic candidate | Lung Disease Model tested | Therapeutic effects observed | Lung AQP regulated | Source reference |
|---|---|---|---|---|
| 1. Lung Injury | ||||
| TGN-020 (AQP4 inhibitor) | LPS-induced ALI mouse model | Attenuated lung injury; Reduced proinflammatory cytokines (IL-1α, IL-1β, IL-6, TNFα, IL-23, IL-17A); Inhibited IL-17A thru down- regulation of P13 K/Akt pathway; Improved survival rate. |
AQP4 inhibited | [190] |
| Glucocorticosteroid (Dexamethasone) | LPS-induced neonatal piglet AEC-II cell injury model (to simulate neonatal lung injury due to bacterial infection) | Pretreatment partially protected cells from injury; Increased SP-C expression |
AQP5↑ | [202] |
| Glucocorticosteroid + Peritoneal dialysis | Blast lung injury rat model (injured using a biological shock tube-I) | Early peritoneal dialysis attenuated edema and inflammatory response and conferred protection from lung injury; dialysis group showed 6.67-fold higher AQP1 expression. | AQP1↑ | [203] |
| N-Acetyl cysteine (a non-specific ROS scavenger) | p-Cresyl sulfate (PCS)-induced human alveolar cell model [modeling chronic kidney disease-associated uremic lung injury (ULI)] | Intracellular ROS abrogated; Protected from PCS-induced cell death |
AQP4↑ | [204] |
| Angiopoietin-1 | Phosgene-induced ALI rat model (treated with Angiopoietin-1 overexpressing Bone marrow Mesenchymal stem cells/MSCs) | Enhanced the therapeutic potential of MSCs; Reduced proinflammatory cytokines (IL-1β, TGF-β1) and increased anti-inflammatory IL-10 in serum and BALF | AQP5↑ | [205] |
| Tanshinol (an aqueous polyphenol from Salvia Miltiorrhiza Bunge) | Sepsis rat model [cecal ligation and puncture (CLP) method] | Reduced the Pro-inflammatory cytokines (TNFα, IL-6) in lung tissue; Decreased p-p38 levels in the lung |
AQP5↑ | [82] |
| Alpinetin (a flavonoid from Alpinia Katsumadai Hayata used in Chinese medicine) | Severe Acute Pancreatitis (SAP)-induced acute lung injury rat model | Alleviated acute lung injury; Inhibited proinflammatory cytokine (TNFα) via upregulation of AQP1 |
AQP↑ | [206] |
| Lipoxin A4 (Endogenous lipid) | Acute Pancreatitis-induced ALI mouse model; LPS injection-induced ALI mouse model | Reduced alveolar fluid exudation by F-actin reconstruction via inhibiting PKC/SSeCKS pathway and apoptosis; Maintained the alveolar fluid clearance via regulation of AQP5 and MMP-9; Restored alveolar fluid clearance capacity via upregulation of AQP5 and inhibition of p-p38 and p-JNK |
AQP5↑ AQP5↑ |
[143] [207] |
| Specialized Pro-resolving Mediators (SPMs) | Acute Respiratory Distress Syndrome (ARDS) | Direct effects on ion channels and pumps of alveolar epithelium; Inhibition of pro-inflammatory cytokines; Repaired alveolar epithelium |
AQP↑ | [208] |
| Celecoxib | Hyperoxia-induced bronchopulmonary dysplasia (BDP)/lung injury rat model | Inhibited NF-kB phosphorylation and nuclear translocation; Upregulated AQP1 expression via COX activity inhibition. |
AQP1↑ | [209] |
| Saquinavir (HIV protease inhibitor) | LPS-induced cell injury model (using human pulmonary AECI cells);LPS-induced ALI mouse model | Reduced injury in human AECI cells with a decrease of NF-kB and increase of AQP5; Prevented experimental ALI and ameliorated glucocorticoid insensitivity |
AQP5↑ | [210] |
| Fasudi (a selective rho kinase inhibitor) | LPS-induced lung injury mouse model | Attenuated injury, edema, and suppressed inflammation via inhibiting NF-kB and upregulating AQP5 | AQP5↑ | [211] |
| SB239063 (p38 MAPK inhibitor) | Intestinal ischemia reperfusion (II/R)-induced ALI rat model | Inhibition of p38 MAPK downregulating AQP4 expression | AQP4↓ | [212] |
| Emodin (trihydroxyanthraquinone compound present in various plants, fungi, lichens) | Severe Acute pancreatitis (SAP)-induced ALI rat model | Reduced pulmonary edema; ameliorated lung injury; upregulated aquaporins. | AQP1↑ AQP5↑ |
[213] |
| Sepsis-induced ALI rat model | Pretreatment effective in reducing edema | AQP5↑ | [214] | |
| CGRP8-37 (an antagonist of α-Calcitonin gene-related peptide receptor) | LPS injection-induced ALI rat model | This CGRP receptor antagonist exacerbated the LPS-ALI phenotype via AQP downregulation (AQP 1 and 5); thus, regulation of endogenous CGRP could upregulate AQP1 and prove a potential treatment for ALI | AQP1 lowered by this antagonist | [215] |
| Glycerol | Intratracheal Naphthalene-induced airway injury mouse model using AQP3 knockout strain | Glycerol corrected the impaired self-healing capacity in the AQP3 KO airway injury model by making up for this aquaglyceroporin deficiency. | AQP3 function substituted | [216] |
| Dobutamine (common Vasoactive drug) | Intravenous LPS injection-induced ALI Rabbit model | Protected against ALI via upregulation of AQP5 via increased cAMP. | AQP5↑ | [217] |
| Dexmedetomidine (a new generation highly selective α2-adrenergic receptor (α2-AR) agonist) | Intravenous LPS injection-induced ALI rat model | Reduced the lung tissue damage, pulmonary edema, and inflammatory response via aquaporin upregulation | AQP1↑ AQP5↑ |
[218] |
| Hyperbaric Oxygen (HBO therapy) | LPS-induced ALI rat model | Palliated lung injury by downregulating TNFα and upregulating AQPs 1 and 5 expression. | AQP1↑ AQP5↑ |
[219] |
| Diammonium glycyrrhizinate | Intratracheal LPS-induced ALI mouse model | Ameliorated lung pathology and edema; upregulated AQP5 possibly via inactivated NF-kB | AQP5↑ | [220] |
| 2. Lung Inflammatory conditions (Asthma, COPD, etc.) | ||||
| Sevoflurane | OVA-induced allergic asthma mouse model | Modulated expression of AQPs 1 and 5; Inhibited ER stress response | AQP1↑AQP5↑ | [191] |
| TGN-020 (AQP4 inhibitor) | Radiation pneumonitis mouse model | Alleviated lung damage development & severity; Attenuated inflammatory response; Inhibited M2 macrophage activation |
AQP4 inhibition | [43] |
| Phosphodiesterase-4 (PDE-4) inhibitor (YM976) | Acrolein atomization inhalation-induced Airway mucus hypersecretion rat model (12 day) | Alleviated pathological damage of lung tissue (mucus gland hyperplasia, airway mucus hypersecretion); Upregulated AQP5 expression | AQP5↑ | [221] |
| 3. Lung cancer | ||||
| Berberine + Cinnamaldehyde (activators of AMP kinase, a sensor of cellular energy status) | Urethane-induced lung cancer mouse model | Reduced susceptibility to cancer induction; Reversed the urethane-induced decrease of AMPK and mTOR and increase of NF-kB and AQP-1 | AQP-1↑ | [222] |
| Preoperative chemotherapy | Human Lung cancer patients subjected to pulmonary resection after 3-cycles of gemcitabine-cisplatin | Chemotherapy was associated with: -upregulation of genes involved in remodeling of alveolar septa and parenchyma scaffold (fostering a fibrosing effect and altered alveolar-capillary membrane); - AQP upregulations (AQP1 by 51% and AQP5 by 36%) |
AQP1↑ AQP5↑ |
[223] |
| Propofol | Human Alveolar epithelial type II cell line A549 | Inhibited cell invasion capacity by downregulating MMP-9 and AQP-3 | AQP3↓ | [224] |
| Cetuximab (EGFR mab) + Afatinib (EGFR and hEGFR2TK irreversible inhibitor) | Human Alveolar epithelial type II cell line A549 | Synergistically inhibited growth and migration of cells and downregulated kinase domain receptor (KDR) and AQP1 | AQP1↓ | [225] |
| Cetuximab + Celecoxib (an NSAID) | Human Alveolar epithelial type II cell line A549 | Synergistically inhibited growth of cells and downregulated KDR and AQP1 | AQP1↓ | [226] |
| 4. Miscellaneous lung pathologies | ||||
| Betamethasone-acetate (antenatal steroid) | Preterm fetal sheep (fetal lung maturation model) | A single dose of Betamethasone acetate is an effective alternative to double dose treatment to induce safe fetal lung maturation; upregulates AQP5 and surfactant proteins. | AQP5↑ | [227] |
Biological therapeutics including antibodies and peptides are other candidate therapeutic agents being investigated. For instance, Aquaporumab monoclonal antibodies that block AQP4-IgG autoantibody binding to its target aquaporin (AQP4) have been developed for neuromyelitis optica spectrum disorders (NMOSD) [192]. These may be used in combination with monoclonals such as pembrolizumab targeting the co-occurring lung adenocarcinoma [193].
Nevertheless, future endeavors in this field may necessitate more extensive drug discovery efforts using high content analysis and development of novel high throughput screening assays for these targets [reviewed in [194]. Desired alternate future approaches could include (i). development of AQP-specific monoclonal antibodies that can outcompete the binding of pathogenic autoantibodies to the same target for AQP-associated auto-immune conditions; (ii). development of expression modulators for specific AQPs for tissue-specific applications in different organs, including those in lung pathologies.
5.3. Aquaporins in human genetic susceptibility to disease
Though there is limited information available on this aspect of AQPs, aquaporin gene variants have been implicated in genetic susceptibility to certain human clinical conditions. For instance, at least two loss-of-function mutations in AQP have been associated with hereditary clinical disease: (i). mutation in AQP2 causes human nephrogenic diabetes insipidus [195]; (ii). mutations in AQP0 have been associated with congenital cataracts in mice [196]. Additionally, human cases deficient in AQP1 have been shown to develop abnormality in urine concentrating ability and water conservation [28]. Notably, there is emerging interest in identifying AQP genetic polymorphisms in human populations, though little has been achieved on functionally-relevant genetic variants. Initial studies have associated single nucleotide polymorphisms (SNPs) in specific AQPs with susceptibility to certain human diseases such as AQP4 variants with severity in brain edema [197], AQP1 variants with diabetic nephropathy and with priapism in sickle cell disease [198], and AQP7 variants with obesity and type II diabetes [199]. A Chinese cohort [200] study associated an AQP5 variant (+2254 A to G in intron 3) with a decreased risk of COPD. In a subsequent study [201], SNPs in AQP5 gene were associated with lung function decline rate in COPD patients who were continuous smokers. In experimental validation using the human bronchial epithelial cell line HBE-16 (comparing the wild type and risk allele of N228K, a coding SNP), the mutation was shown to modulate expression of AQP5 in response to sheer stress and cigarette smoke extract. The study even suggested AQP5 as a potential candidate gene for COPD. These observations on AQP5 polymorphisms may be broadly significant considering that this aquaporin plays a critical role in the maintenance of normal lung homeostasis as well as in lung pathologies due to mucus overproduction and lowered pulmonary function. Future research in this area may reveal critical genetic variations in AQPs expressed in lung and airways.
6. Conclusions and Future Directions
Aquaporins are a family of conserved membrane protein channels that primarily mediate selective transcellular (transepithelial, transendothelial) water transport across cells and tissues. Besides, they have been shown to perform non-conventional roles such as in cell proliferation and cell migration. Of the 13 aquaporins (AQPs) in humans, individual AQPs have been associated with normal physiological and pathological roles in different organs. In terms of their importance in lung, 4 AQPs (AQP1, 3, 4, and 5) are primarily expressed in lung tissue and airways. Of these, AQP5 is important in fluid secretion from the lung-associated submucosal glands to maintain the Airways surface liquid (ASL) lining under normal physiological conditions. Besides the normal physiological role in lung (primarily related to ASL homeostasis), there is accumulating evidence emphasizing the importance of aquaporins especially AQP1 and AQP5 in various lung pathological states and diseases including but not limited to chronic and acute lung injury, COPD, asthma and other inflammatory conditions, and cancer. The recognized role of AQPs in cell proliferation and migration may account for their emerging role in various lung cancers. However, it is too early to speculate whether these non-conventional functions could be extrapolated to account for their role in lung remodeling and repair processes in lung pathologies such as inflammation, infection, and chronic and acute lung injury. Recent studies have highlighted clinical significance of AQP1 and/or AQP5 in various lung conditions. Notably, AQP1 has been suggested as a potential prognostic marker in pulmonary malignant mesothelioma. This and other developments necessitate more directed efforts at understanding the clinical relevance and role of specific AQPs in lung pathophysiology and disease and ways to exploit them to diagnose, prevent, and/or treat various lung conditions. While progress in the area of developing AQP-based therapeutics has been slow, ongoing efforts and new directions involving AQP structure-based simulations coupled with modern high throughput drug screening strategies and assays could hold promise in identifying the more effective AQP-specific therapeutic candidates. Other areas of research on AQPs that need attention include understanding of the molecular mechanisms of AQP regulation and functional relevance of AQP-genetic variations/polymorphisms and their role in human lung diseases. Future developments in these areas may help realize the dream of translating the basic research on AQPs into clinical applications in diagnosis, prognosis, and treatment of AQP-relevant diseases and lead to improved healthcare.
HIGHLIGHTS.
Both basic and clinical aspects of lung aquaporins (AQPs) covered.
Focus on emerging functional roles of AQPs in lung physiology and pathophysiology.
Regulatory pathways governing expression of lung AQPs summarized.
Highlights Potential of AQPs for diagnosis, prognosis, and intervention/treatment.
Expected to stimulate further studies on lung AQPs as druggable targets.
Acknowledgements (funding)
This article was supported in part by funding (to JSY) from the University of Cincinnati’s Center for Environmental Genetics through the NIH / NIEHS award P30ES006096.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yool AJ, Brown EA, Flynn GA, Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer, Clin Exp Pharmacol Physiol 37(4) (2010) 403–9. [DOI] [PubMed] [Google Scholar]
- [2].Borok Z, Verkman AS, Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways, J Appl Physiol (1985) 93(6) (2002) 2199–206. [DOI] [PubMed] [Google Scholar]
- [3].Conner AC, Bill RM, Conner MT, An emerging consensus on aquaporin translocation as a regulatory mechanism, Mol Membr Biol 30(1) (2013) 1–12. [DOI] [PubMed] [Google Scholar]
- [4].Gonen T, Walz T, The structure of aquaporins, Q Rev Biophys 39(4) (2006) 361–96. [DOI] [PubMed] [Google Scholar]
- [5].Agre P, The aquaporin water channels, Proc Am Thorac Soc 3(1) (2006) 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Verkman AS, Aquaporins in clinical medicine, Annu Rev Med 63 (2012) 303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verkman AS, Aquaporins, Curr Biol 23(2) (2013) R52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S, Aquaporin CHIP: the archetypal molecular water channel, Am J Physiol 265(4 Pt 2) (1993) F463–76. [DOI] [PubMed] [Google Scholar]
- [9].Benga G, Popescu O, Borza V, Pop VI, Muresan A, Mocsy I, Brain A, Wrigglesworth JM, Water permeability in human erythrocytes: identification of membrane proteins involved in water transport, Eur J Cell Biol 41(2) (1986) 252–62. [PubMed] [Google Scholar]
- [10].Nielsen S, King LS, Christensen BM, Agre P, Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat, Am J Physiol 273(5) (1997) C1549–61. [DOI] [PubMed] [Google Scholar]
- [11].Mutlu GM, Sznajder JI, Mechanisms of pulmonary edema clearance, Am J Physiol Lung Cell Mol Physiol 289(5) (2005) L685–95. [DOI] [PubMed] [Google Scholar]
- [12].Verkman AS, Role of aquaporins in lung liquid physiology, Respir Physiol Neurobiol 159(3) (2007) 324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu H, Wintour EM, Aquaporins in development -- a review, Reprod Biol Endocrinol 3 (2005) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu B, Beitz E, Aquaporins with selectivity for unconventional permeants, Cell Mol Life Sci 64(18) (2007) 2413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS, Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption, Nature 434(7034) (2005) 786–92. [DOI] [PubMed] [Google Scholar]
- [16].Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS, Involvement of aquaporin-4 in astroglial cell migration and glial scar formation, J Cell Sci 118(Pt 24) (2005) 5691–8. [DOI] [PubMed] [Google Scholar]
- [17].Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y, Implications of the aquaporin-4 structure on array formation and cell adhesion, J Mol Biol 355(4) (2006) 628–39. [DOI] [PubMed] [Google Scholar]
- [18].Ruiz-Ederra J, Zhang H, Verkman AS, Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells, J Biol Chem 282(30) (2007) 21866–72. [DOI] [PubMed] [Google Scholar]
- [19].Ruddy MK, Drazen JM, Pitkanen OM, Rafii B, O’Brodovich HM, Harris HW, Modulation of aquaporin 4 and the amiloride-inhibitable sodium channel in perinatal rat lung epithelial cells, Am J Physiol 274(6) (1998) L1066–72. [DOI] [PubMed] [Google Scholar]
- [20].Umenishi F, Carter EP, Yang B, Oliver B, Matthay MA, Verkman AS, Sharp increase in rat lung water channel expression in the perinatal period, Am J Respir Cell Mol Biol 15(5) (1996) 673–9. [DOI] [PubMed] [Google Scholar]
- [21].Carter EP, Umenishi F, Matthay MA, Verkman AS, Developmental changes in water permeability across the alveolar barrier in perinatal rabbit lung, J Clin Invest 100(5) (1997) 1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Verkman AS, Knock-out models reveal new aquaporin functions, Handb Exp Pharmacol (190) (2009) 359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Verkman AS, Roles of aquaporins in kidney revealed by transgenic mice, Semin Nephrol 26(3) (2006) 200–8. [DOI] [PubMed] [Google Scholar]
- [24].Hara-Chikuma M, Verkman AS, Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins, Cell Mol Life Sci 63(12) (2006) 1386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levin MH, Verkman AS, Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization, Invest Ophthalmol Vis Sci 47(10) (2006) 4365–72. [DOI] [PubMed] [Google Scholar]
- [26].Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS, Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels, J Biol Chem 274(29) (1999) 20071–4. [DOI] [PubMed] [Google Scholar]
- [27].Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS, Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation, J Biol Chem 280(16) (2005) 15493–6. [DOI] [PubMed] [Google Scholar]
- [28].King LS, Choi M, Fernandez PC, Cartron JP, Agre P, Defective urinary concentrating ability due to a complete deficiency of aquaporin-1, N Engl J Med 345(3) (2001) 175–9. [DOI] [PubMed] [Google Scholar]
- [29].Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA, Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine, Science 264(5155) (1994) 92–5. [DOI] [PubMed] [Google Scholar]
- [30].Hwang S, Kang JY, Kim MJ, Shin DM, Hong JH, Carbonic anhydrase 12 mutation modulates membrane stability and volume regulation of aquaporin 5, J Enzyme Inhib Med Chem 34(1) (2019) 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS, Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration, J Gen Physiol 117(6) (2001) 573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS, Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH, J Clin Invest 107(3) (2001) 317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS, Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice, J Clin Invest 103(4) (1999) 555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS, Lung fluid transport in aquaporin-5 knockout mice, J Clin Invest 105(1) (2000) 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakhoul NL, Davis BA, Romero MF, Boron WF, Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes, Am J Physiol 274(2) (1998) C543–8. [DOI] [PubMed] [Google Scholar]
- [36].Song Y, Yang B, Matthay MA, Ma T, Verkman AS, Role of aquaporin water channels in pleural fluid dynamics, Am J Physiol Cell Physiol 279(6) (2000) C1744–50. [DOI] [PubMed] [Google Scholar]
- [37].Hara-Chikuma M, Verkman AS, Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption, Mol Cell Biol 28(1) (2008) 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Al-Samir S, Goossens D, Cartron JP, Nielsen S, Scherbarth F, Steinlechner S, Gros G, Endeward V, Maximal oxygen consumption is reduced in aquaporin-1 knockout mice, Front Physiol 7 (2016) 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alishahi M, Kamali R, A novel molecular dynamics study of CO2 permeation through aquaporin-5, Eur Phys J E Soft Matter 42(11) (2019) 151. [DOI] [PubMed] [Google Scholar]
- [40].Zwiazek JJ, Xu H, Tan X, Navarro-Rodenas A, Morte A, Significance of oxygen transport through aquaporins, Sci Rep 7 (2017) 40411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sakamoto Y, Hisatsune A, Katsuki H, Horie I, Isohama Y, Aquaporin 5 increases keratinocyte-derived chemokine expression and NF-kappaB activity through ERK activation, Biochem Biophys Res Commun 448(4) (2014) 355–60. [DOI] [PubMed] [Google Scholar]
- [42].Rabolli V, Wallemme L, Lo Re S, Uwambayinema F, Palmai-Pallag M, Thomassen L, Tyteca D, Octave JN, Marbaix E, Lison D, Devuyst O, Huaux F, Critical role of aquaporins in interleukin 1beta (IL-1beta)-induced inflammation, J Biol Chem 289(20) (2014) 13937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li Y, Lu H, Lv X, Tang Q, Li W, Zhu H, Long Y, Blockade of aquaporin 4 inhibits irradiation-induced pulmonary inflammation and modulates macrophage polarization in mice, Inflammation 41(6) (2018) 2196–2205. [DOI] [PubMed] [Google Scholar]
- [44].Meli R, Pirozzi C, Pelagalli A, New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflammation, Front Physiol 9 (2018) 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sisto M, Ribatti D, Lisi S, Aquaporin water channels: New perspectives on the potential role in inflammation, Adv Protein Chem Struct Biol 116 (2019) 311–345. [DOI] [PubMed] [Google Scholar]
- [46].Towne JE, Harrod KS, Krane CM, Menon AG, Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection, Am J Respir Cell Mol Biol 22(1) (2000) 34–44. [DOI] [PubMed] [Google Scholar]
- [47].Cher CD, Armugam A, Lachumanan R, Coghlan MW, Jeyaseelan K, Pulmonary inflammation and edema induced by phospholipase A2: global gene analysis and effects on aquaporins and Na+/K+-ATPase, J Biol Chem 278(33) (2003) 31352–60. [DOI] [PubMed] [Google Scholar]
- [48].Towne JE, Krane CM, Bachurski CJ, Menon AG, Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells, J Biol Chem 276(22) (2001) 18657–64. [DOI] [PubMed] [Google Scholar]
- [49].Sato K, Kobayashi K, Aida S, Tamai S, Bronchiolar expression of aquaporin-3 (AQP3) in rat lung and its dynamics in pulmonary oedema, Pflugers Arch 449(1) (2004) 106–14. [DOI] [PubMed] [Google Scholar]
- [50].Su X, Song Y, Jiang J, Bai C, The role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injury, Respir Physiol Neurobiol 142(1) (2004) 1–11. [DOI] [PubMed] [Google Scholar]
- [51].Gabazza EC, Kasper M, Ohta K, Keane M, D’Alessandro-Gabazza C, Fujimoto H, Nishii Y, Nakahara H, Takagi T, Menon AG, Adachi Y, Suzuki K, Taguchi O, Decreased expression of aquaporin-5 in bleomycin-induced lung fibrosis in the mouse, Pathol Int 54(10) (2004) 774–80. [DOI] [PubMed] [Google Scholar]
- [52].Verkman AS, Matthay MA, Song Y, Aquaporin water channels and lung physiology, Am J Physiol Lung Cell Mol Physiol 278(5) (2000) L867–79. [DOI] [PubMed] [Google Scholar]
- [53].Hoffert JD, Leitch V, Agre P, King LS, Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway, J Biol Chem 275(12) (2000) 9070–7. [DOI] [PubMed] [Google Scholar]
- [54].Zhang ZQ, Song YL, Chen ZH, Shen Y, Bai CX, Deletion of aquaporin 5 aggravates acute lung injury induced by Pseudomonas aeruginosa, J Trauma 71(5) (2011) 1305–11. [DOI] [PubMed] [Google Scholar]
- [55].Fan Q, Zhao P, Li J, Xie X, Xu M, Zhang Y, Mu D, Li W, Sun R, Liu W, Nan Y, Zhang B, Jin F, Li Z, 17beta-Estradiol administration attenuates seawater aspiration-induced acute lung injury in rats, Pulm Pharmacol Ther 24(6) (2011) 673–81. [DOI] [PubMed] [Google Scholar]
- [56].Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D, Zhao P, Zhang B, Cao F, Wang Y, Jin F, Li Z, Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung, Respir Physiol Neurobiol 176(1–2) (2011) 39–49. [DOI] [PubMed] [Google Scholar]
- [57].Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W, The role of alveolar epithelium in radiation-induced lung injury, PLoS One 8(1) (2013) e53628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kasper M, Barth K, Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis, Biosci Rep 37(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Galan-Cobo A, Arellano-Orden E, Sanchez Silva R, Lopez-Campos JL, Gutierrez Rivera C, Gomez Izquierdo L, Suarez-Luna N, Molina-Molina M, Rodriguez Portal JA, Echevarria M, The expression of AQP1 is modified in lung of patients with idiopathic pulmonary fibrosis: addressing a possible new target, Front Mol Biosci 5 (2018) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vassiliou AG, Manitsopoulos N, Kardara M, Maniatis NA, Orfanos SE, Kotanidou A, Differential expression of aquaporins in experimental models of acute lung injury, In Vivo 31(5) (2017) 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fabregat G, Garcia-de-la-Asuncion J, Sarria B, Mata M, Cortijo J, de Andres J, Gallego L, Belda FJ, Expression of aquaporins 1 and 5 in a model of ventilator-induced lung injury and its relation to tidal volume, Exp Physiol 101(11) (2016) 1418–1431. [DOI] [PubMed] [Google Scholar]
- [62].Yabuuchi N, Sagata M, Saigo C, Yoneda G, Yamamoto Y, Nomura Y, Nishi K, Fujino R, Jono H, Saito H, Indoxyl sulfate as a mediator involved in dysregulation of pulmonary aquaporin-5 in acute lung injury caused by acute kidney injury, Int J Mol Sci 18(1) (2016) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pires-Neto RC, Del Carlo Bernardi F, Alves de Araujo P, Mauad T, Dolhnikoff M, The expression of water and ion channels in diffuse alveolar damage is not dependent on dad etiology, PLoS One 11(11) (2016) e0166184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu Y, Pan CY, Guo CZ, Dong ZJ, Wu Q, Dong HM, Zhang W, Expression of aquaporin 1 and 4 in rats with acute hypoxic lung injury and its significance, Genet Mol Res 14(4) (2015) 12756–64. [DOI] [PubMed] [Google Scholar]
- [65].Hasan B, Li FS, Siyit A, Tuyghun E, Luo JH, Upur H, Ablimit A, Expression of aquaporins in the lungs of mice with acute injury caused by LPS treatment, Respir Physiol Neurobiol 200 (2014) 40–5. [DOI] [PubMed] [Google Scholar]
- [66].Ma T, Liu Z, Functions of aquaporin 1 and alpha-epithelial Na+ channel in rat acute lung injury induced by acute ischemic kidney injury, Int Urol Nephrol 45(4) (2013) 1187–96. [DOI] [PubMed] [Google Scholar]
- [67].Xu C, Jiang L, Zou Y, Xing J, Sun H, Zhu B, Zhang H, Wang J, Zhang J, Involvement of water channel Aquaporin 5 in H2S-induced pulmonary edema, Environ Toxicol Pharmacol 49 (2017) 202–211. [DOI] [PubMed] [Google Scholar]
- [68].Zhang Y, Tian K, Wang Y, Zhang R, Shang J, Jiang W, Wang A, The effects of aquaporin-1 in pulmonary edema induced by fat embolism syndrome, Int J Mol Sci 17(7) (2016) 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jin Y, Yu G, Peng P, Zhang Y, Xin X, Down-regulated expression of AQP5 on lung in rat DIC model induced by LPS and its effect on the development of pulmonary edema, Pulm Pharmacol Ther 26(6) (2013) 661–5. [DOI] [PubMed] [Google Scholar]
- [70].Zhang Y, Chen M, Zhang Y, Peng P, Li J, Xin X, miR-96 and miR-330 overexpressed and targeted AQP5 in lipopolysaccharide-induced rat lung damage of disseminated intravascular coagulation, Blood Coagul Fibrinolysis 25(7) (2014) 731–7. [DOI] [PubMed] [Google Scholar]
- [71].Elias AS, Oliveira GP, Ornellas DS, Morales MM, Capelozzi VL, Haddad R, Pelosi P, Rocco PR, Garcia CS, Effects of early and late pneumothorax drainage on the development of pulmonary oedema, Respir Physiol Neurobiol 195 (2014) 27–36. [DOI] [PubMed] [Google Scholar]
- [72].Singha O, Kengkoom K, Chaimongkolnukul K, Cherdyu S, Pongponratn E, Ketjareon T, Panavechkijkul Y, Ampawong S, Pulmonary edema due to oral gavage in a toxicological study related to aquaporin-1, −4 and −5 expression, J Toxicol Pathol 26(3) (2013) 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu M, Liu Q, Pei Y, Gong M, Cui X, Pan J, Zhang Y, Liu Y, Liu Y, Yuan X, Zhou H, Chen Y, Sun J, Wang L, Zhang X, Wang R, Li S, Cheng J, Ding Y, Ma T, Yuan Y, Aqp-1 gene knockout attenuates hypoxic pulmonary hypertension of mice, Arterioscler Thromb Vasc Biol 39(1) (2019) 48–62. [DOI] [PubMed] [Google Scholar]
- [74].Yang ZJ, Deng Y, Man Q, Wang Y, Yang XJ, Zhao N, Liu L, [Effects of the fermentation products of endophytes from Glycyrrhiza uralensis on inflammation-associated factors and Aquaporin in rat models with phlegm blocking in lung], Zhongguo Ying Yong Sheng Li Xue Za Zhi 33(5) (2017) 410–414. [DOI] [PubMed] [Google Scholar]
- [75].Wu ZY, Yao Y, Hu R, Dai FF, Zhang H, Mao ZF, Cyclic adenosine monophosphate-protein kinase A signal pathway may be involved in pulmonary aquaporin-5 expression in ischemia/reperfusion rats following deep hypothermia cardiac arrest, Genet Mol Res 15(1) (2016). [DOI] [PubMed] [Google Scholar]
- [76].Lin YS, Zhang L, Yang H, Zhang Y, Li X, Zhang YQ, Yang DF, [Effects of acute cold exposure on the expression of AQP-1 and AQP-5 in rat lung tissues], Zhongguo Ying Yong Sheng Li Xue Za Zhi 32(3) (2016) 234–237. [DOI] [PubMed] [Google Scholar]
- [77].Lai PH, Shan XM, Lu DW, Li PC, He KQ, [Influence of hot-humid stress and acclimation on airway mucus production in lungs of mice], Zhongguo Ying Yong Sheng Li Xue Za Zhi 32(3) (2016) 266–269. [DOI] [PubMed] [Google Scholar]
- [78].Wang H, Hao X, Zhang L, Liu S, Wang Q, Liu N, Zhao J,Q J, Liu H, Decreased expression of aquaporin-1 in lung tissue of silicotic rats, Clin Lab 61(9) (2015) 1163–9. [DOI] [PubMed] [Google Scholar]
- [79].Musumeci G, Loreto C, Szychlinska MA, Imbesi R, Rapisarda V, Aiello FC, Castorina S, Castrogiovanni P, N-Cadherin ADAM -10 and Aquaporin 1 expression in lung tissue exposed to fluoro-edenite fibers: an immunohistochemical study, Histol Histopathol 30(8) (2015) 987–99. [DOI] [PubMed] [Google Scholar]
- [80].Sun CY, Zhao YX, Zhong W, Liu DW, Chen YZ, Qin LL, Bai L, Liu D, The expression of aquaporins 1 and 5 in rat lung after thoracic irradiation, J Radiat Res 55(4) (2014) 683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang J, Yan M, Gu W, Chen A, Liu J, Li L, Zhang S, Liu G, Downregulation of aquaporins (AQP1 and AQP5) and Na,K-ATPase in porcine reproductive and respiratory syndrome virus-infected pig lungs, Inflammation 41(3) (2018) 1104–1114. [DOI] [PubMed] [Google Scholar]
- [82].Xu J, Yang L, Dong L, Tanshinol upregulates the expression of aquaporin 5 in lung tissue of rats with sepsis, Oncol Lett 16(3) (2018) 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Liu LD, Wu XY, Tao BD, Wang N, Zhang J, Protective effect and mechanism of hydrogen treatment on lung epithelial barrier dysfunction in rats with sepsis, Genet Mol Res 15(1) (2016). [DOI] [PubMed] [Google Scholar]
- [84].Tao B, Liu L, Wang N, Wang W, Jiang J, Zhang J, Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs, J Surg Res 202(2) (2016) 291–8. [DOI] [PubMed] [Google Scholar]
- [85].Gao X, Wang G, Zhang W, Peng Q, Xue M, Jinhong H, Expression of pulmonary aquaporin 1 is dramatically upregulated in mice with pulmonary fibrosis induced by bleomycin, Arch Med Sci 9(5) (2013) 916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li XY, Xu XF, Hang J, Liu ZX, Yu SY, Fang SH, Zhang WP, Wei EQ, Lu YB, [Effects of the water channel aquaporin 4 deficiency on bleomycin-induced lung fibrosis in mice], Zhejiang Da Xue Xue Bao Yi Xue Ban 43(3) (2014) 281–6. [DOI] [PubMed] [Google Scholar]
- [87].Krane CM, Deng B, Mutyam V, McDonald CA, Pazdziorko S, Mason L, Goldman S, Kasaian M, Chaudhary D, Williams C, Ho MW, Altered regulation of aquaporin gene expression in allergen and IL-13-induced mouse models of asthma, Cytokine 46(1) (2009) 111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shen Y, Wang Y, Chen Z, Wang D, Wang X, Jin M, Bai C, Role of aquaporin 5 in antigen-induced airway inflammation and mucous hyperproduction in mice, J Cell Mol Med 15(6) (2011) 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dong C, Wang G, Li B, Xiao K, Ma Z, Huang H, Wang X, Bai C, Anti-asthmatic agents alleviate pulmonary edema by upregulating AQP1 and AQP5 expression in the lungs of mice with OVA-induced asthma, Respir Physiol Neurobiol 181(1) (2012) 21–8. [DOI] [PubMed] [Google Scholar]
- [90].Ablimit A, Hasan B, Lu W, Qin W, Wushouer Q, Zhong N, Upur H, Changes in water channel aquaporin 1 and aquaporin 5 in the small airways and the alveoli in a rat asthma model, Micron 45 (2013) 68–73. [DOI] [PubMed] [Google Scholar]
- [91].Ikezoe K, Oga T, Honda T, Hara-Chikuma M, Ma X, Tsuruyama T, Uno K, Fuchikami J, Tanizawa K, Handa T, Taguchi Y, Verkman AS, Narumiya S, Mishima M, Chin K, Aquaporin-3 potentiates allergic airway inflammation in ovalbumin-induced murine asthma, Sci Rep 6 (2016) 25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang M, Sun Y, Zhang SJ, Gao YS, Wei YN, Zhao PN, Liu M, Yang JM, Zheng FJ, Xu H, Li YH, San-ao decoction regulates urine volume on bronchial asthma model mice, Chin J Integr Med (2018). [DOI] [PubMed] [Google Scholar]
- [93].Wang K, Feng YL, Wen FQ, Chen XR, Ou XM, Xu D, Yang J, Deng ZP, Decreased expression of human aquaporin-5 correlated with mucus overproduction in airways of chronic obstructive pulmonary disease, Acta Pharmacol Sin 28(8) (2007) 1166–74. [DOI] [PubMed] [Google Scholar]
- [94].Aggarwal NR, Chau E, Garibaldi BT, Mock JR, Sussan T, Rao K, Rao K, Menon AG, D’Alessio FR, Damarla M, Biswal S, King LS, Sidhaye VK, Aquaporin 5 regulates cigarette smoke induced emphysema by modulating barrier and immune properties of the epithelium, Tissue Barriers 1(4) (2013) e25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Calero C, Lopez-Campos JL, Izquierdo LG, Sanchez-Silva R, Lopez-Villalobos JL, Saenz-Coronilla FJ, Arellano-Orden E, Montes-Worboys A, Echevarria M, Expression of aquaporins in bronchial tissue and lung parenchyma of patients with chronic obstructive pulmonary disease, Multidiscip Respir Med 9(1) (2014) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Heinbockel L, Marwitz S, Schromm AB, Watz H, Kugler C, Ammerpohl O, Schnepf K, Rabe KF, Droemann D, Goldmann T, Identification of novel target genes in human lung tissue involved in chronic obstructive pulmonary disease, Int J Chron Obstruct Pulmon Dis 13 (2018) 2255–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gao Z, Halmurat U, Wang J, Jing J, Li Z, Xu D, Li F, Expression of airway mucus-associated proteins in rats with chronic obstructive pulmonary disease with a cold-dryness symptom pattern, J Tradit Chin Med 36(5) (2016) 671–7. [DOI] [PubMed] [Google Scholar]
- [98].Liu Z, Geng W, Jiang C, Zhao S, Liu Y, Zhang Y, Qin S, Li C, Zhang X, Si Y, Hydrogen-rich saline inhibits tobacco smoke-induced chronic obstructive pulmonary disease by alleviating airway inflammation and mucus hypersecretion in rats, Exp Biol Med (Maywood) 242(15) (2017) 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Verkman AS, More than just water channels: unexpected cellular roles of aquaporins, J Cell Sci 118(Pt 15) (2005) 3225–32. [DOI] [PubMed] [Google Scholar]
- [100].Nico B, Ribatti D, Aquaporins in tumor growth and angiogenesis, Cancer Lett 294(2) (2010) 135–8. [DOI] [PubMed] [Google Scholar]
- [101].Hu J, Verkman AS, Increased migration and metastatic potential of tumor cells expressing aquaporin water channels, FASEB J 20(11) (2006) 1892–4. [DOI] [PubMed] [Google Scholar]
- [102].Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, Mao L, Sidransky D, Moon C, Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth, Am J Pathol 168(4) (2006) 1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu YL, Matsuzaki T, Nakazawa T, Murata S, Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata K, Katoh R, Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues, Hum Pathol 38(1) (2007) 171–8. [DOI] [PubMed] [Google Scholar]
- [104].Moosavi MS, Elham Y, Aquaporins 1, 3 and 5 in different tumors, their expression, prognosis value and role as new therapeutic targets, Pathol Oncol Res 26, 2nd ed (2020), pp. 615–25. [DOI] [PubMed] [Google Scholar]
- [105].Chae YK, Woo J, Kim MJ, Kang SK, Kim MS, Lee J, Lee SK, Gong G, Kim YH, Soria JC, Jang SJ, Sidransky D, Moon C, Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer, PLoS One 3(5) (2008) e2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lopez-Campos JL, Sanchez Silva R, Gomez Izquierdo L, Marquez E, Ortega Ruiz F, Cejudo P, Barrot Cortes E, Toledo Aral JJ, Echevarria M, Overexpression of Aquaporin-1 in lung adenocarcinomas and pleural mesotheliomas, Histol Histopathol 26(4) (2011) 451–9. [DOI] [PubMed] [Google Scholar]
- [107].Esteva-Font C, Jin BJ, Verkman AS, Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice, FASEB J 28(3) (2014) 1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Machida Y, Ueda Y, Shimasaki M, Sato K, Sagawa M, Katsuda S, Sakuma T, Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential, Hum Pathol 42(5) (2011) 669–78. [DOI] [PubMed] [Google Scholar]
- [109].Xie Y, Wen X, Jiang Z, Fu HQ, Han H, Dai L, Aquaporin 1 and aquaporin 4 are involved in invasion of lung cancer cells, Clin Lab 58(1–2) (2012) 75–80. [PubMed] [Google Scholar]
- [110].Zhang JX, Xie CM, Zhu ZW, Huang HY, Zeng ZL, Potential role of AQP1 and VEGF in the development of malignant pleural effusion in mice, Med Oncol 29(2) (2012) 656–62. [DOI] [PubMed] [Google Scholar]
- [111].Jagirdar RM, Apostolidou E, Molyvdas PA, Gourgoulianis KI, Hatzoglou C, Zarogiannis SG, Influence of AQP1 on cell adhesion, migration, and tumor sphere formation in malignant pleural mesothelioma is substratum- and histological-type dependent, Am J Physiol Lung Cell Mol Physiol 310(6) (2016) L489–95. [DOI] [PubMed] [Google Scholar]
- [112].Pulford E, McEvoy J, Hocking A, Prabhakaran S, Griggs K, Klebe S, The effect of aquaporin 1-inhibition on vasculogenic mimicry in malignant mesothelioma, Int J Mol Sci 18(11) (2017) 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kao SC, Armstrong N, Condon B, Griggs K, McCaughan B, Maltby S, Wilson A, Henderson DW, Klebe S, Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma, Cancer 118(11) (2012) 2952–61. [DOI] [PubMed] [Google Scholar]
- [114].Jagirdar R, Solenov EI, Hatzoglou C, Molyvdas PA, Gourgoulianis KI, Zarogiannis SG, Gene expression profile of aquaporin 1 and associated interactors in malignant pleural mesothelioma, Gene 517(1) (2013) 99–105. [DOI] [PubMed] [Google Scholar]
- [115].Driml J, Pulford E, Moffat D, Karapetis C, Kao S, Griggs K, Henderson DW, Klebe S, Usefulness of aquaporin 1 as a prognostic marker in a prospective cohort of malignant mesotheliomas, Int J Mol Sci 17(7) (2016) 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yun S, Sun PL, Jin Y, Kim H, Park E, Park SY, Lee K, Lee K, Chung JH, Aquaporin 1 is an independent marker of poor prognosis in lung adenocarcinoma, J Pathol Transl Med 50(4) (2016) 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang J HY, Zhang Q, Hong ZP, Zhou YC, Chen WL, Down-regulation of aquaporins (AQP3) expression by RNA interference suppresses human lung cancer cell proliferation African J Biotechnol 11 (2012) 3388–3393. [Google Scholar]
- [118].Xiong G, Chen X, Zhang Q, Fang Y, Chen W, Li C, Zhang J, RNA interference influenced the proliferation and invasion of XWLC-05 lung cancer cells through inhibiting aquaporin 3, Biochem Biophys Res Commun 485(3) (2017) 627–634. [DOI] [PubMed] [Google Scholar]
- [119].Xia H, Ma YF, Yu CH, Li YJ, Tang J, Li JB, Zhao YN, Liu Y, Aquaporin 3 knockdown suppresses tumour growth and angiogenesis in experimental non-small cell lung cancer, Exp Physiol 99(7) (2014) 974–84. [DOI] [PubMed] [Google Scholar]
- [120].Hou SY, Li YP, Wang JH, Yang SL, Wang Y, Wang Y, Kuang Y, Aquaporin-3 inhibition reduces the growth of NSCLC cells induced by hypoxia, Cell Physiol Biochem 38(1) (2016) 129–40. [DOI] [PubMed] [Google Scholar]
- [121].Song T, Yang H, Ho JC, Tang SC, Sze SC, Lao L, Wang Y, Zhang KY, Expression of aquaporin 5 in primary carcinoma and lymph node metastatic carcinoma of non-small cell lung cancer, Oncol Lett 9(6) (2015) 2799–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Elkhider A, Wang B, Ouyang X, Al-Azab M, Walana W, Sun X, Li H, Tang Y, Wei J, Li X, Aquaporin 5 promotes tumor migration and angiogenesis in non-small cell lung cancer cell line H1299, Oncol Lett 19(3) (2020) 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhang L, Lu J, Zhou H, Du Z, Zhang G, Silencing of aquaporin 5 inhibits the growth of A549 lung cancer cells in vitro and in vivo, Int J Oncol 52(5) (2018) 1643–1650. [DOI] [PubMed] [Google Scholar]
- [124].Guo K, Jin F, NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression, Biochem Biophys Res Commun 465(3) (2015) 644–9. [DOI] [PubMed] [Google Scholar]
- [125].Iorio R, Rindi G, Erra C, Damato V, Ferilli M, Sabatelli M, Neuromyelitis optica spectrum disorder as a paraneoplastic manifestation of lung adenocarcinoma expressing aquaporin-4, Mult Scler 21(6) (2015) 791–4. [DOI] [PubMed] [Google Scholar]
- [126].Han S, Sharma RA, Sekhon H, Munoz D, Albreiki DH, Aquaporin-4 immunoglobulin-G-positive neuromyelitis optica spectrum disorder associated with small cell lung carcinoma, Can J Ophthalmol 55(4) (2020) e129–e132. [DOI] [PubMed] [Google Scholar]
- [127].Annus A, Bencsik K, Obal I, Kincses ZT, Tiszlavicz L, Hoftberger R, Vecsei L, Paraneoplastic neuromyelitis optica spectrum disorder: A case report and review of the literature, J Clin Neurosci 48 (2018) 7–10. [DOI] [PubMed] [Google Scholar]
- [128].Asato Y, Kamitani T, Ootsuka K, Kuramochi M, Nakanishi K, Shimada T, Takahashi T, Misu T, Aoki M, Fujihara K, Kawabata Y, Transient Pulmonary Interstitial Lesions in Aquaporin-4-positive Neuromyelitis Optica Spectrum Disorder, Intern Med 57(20) (2018) 2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E, Regulation of aquaporin-2 trafficking, Handb Exp Pharmacol (190) (2009) 133–57. [DOI] [PubMed] [Google Scholar]
- [130].Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F, Transcriptional regulation of aquaporin-2 water channel gene by cAMP, J Am Soc Nephrol 8(6) (1997) 861–7. [DOI] [PubMed] [Google Scholar]
- [131].Walz T, Fujiyoshi Y, Engel A, The AQP structure and functional implications, Handb Exp Pharmacol (190) (2009) 31–56. [DOI] [PubMed] [Google Scholar]
- [132].Conner MT, Conner AC, Brown JE, Bill RM, Membrane trafficking of aquaporin 1 is mediated by protein kinase C via microtubules and regulated by tonicity, Biochemistry 49(5) (2010) 821–3. [DOI] [PubMed] [Google Scholar]
- [133].Fotiadis D, Suda K, Tittmann P, Jeno P, Philippsen A, Muller DJ, Gross H, Engel A, Identification and structure of a putative Ca2+-binding domain at the C terminus of AQP1, J Mol Biol 318(5) (2002) 1381–94. [DOI] [PubMed] [Google Scholar]
- [134].Marinelli RA, Pham L, Agre P, LaRusso NF, Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane. Evidence for a secretin-induced vesicular translocation of aquaporin-1, J Biol Chem 272(20) (1997) 12984–8. [DOI] [PubMed] [Google Scholar]
- [135].Ohinata A, Nagai K, Nomura J, Hashimoto K, Hisatsune A, Miyata T, Isohama Y, Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells, Biochem Biophys Res Commun 326(3) (2005) 521–6. [DOI] [PubMed] [Google Scholar]
- [136].Yang F, Kawedia JD, Menon AG, Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway, J Biol Chem 278(34) (2003) 32173–80. [DOI] [PubMed] [Google Scholar]
- [137].Karabasil MR, Hasegawa T, Azlina A, Purwanti N, Purevjav J, Yao C, Akamatsu T, Hosoi K, Trafficking of GFP-AQP5 chimeric proteins conferred with unphosphorylated amino acids at their PKA-target motif ((152)SRRTS) in MDCK-II cells, J Med Invest 56(1–2) (2009) 55–63. [DOI] [PubMed] [Google Scholar]
- [138].Ishikawa Y, Eguchi T, Skowronski MT, Ishida H, Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands, Biochem Biophys Res Commun 245(3) (1998) 835–40. [DOI] [PubMed] [Google Scholar]
- [139].Ishikawa Y, Iida H, Ishida H, The muscarinic acetylcholine receptor-stimulated increase in aquaporin-5 levels in the apical plasma membrane in rat parotid acinar cells is coupled with activation of nitric oxide/cGMP signal transduction, Mol Pharmacol 61(6) (2002) 1423–34. [DOI] [PubMed] [Google Scholar]
- [140].Shen Y, Chen Z, Wang Y, Song Z, Zhang Z, Jin M, Wang X, Bai C, Aquaporin 5 expression inhibited by LPS via p38/JNK signaling pathways in SPC-A1 cells, Respir Physiol Neurobiol 171(3) (2010) 212–7. [DOI] [PubMed] [Google Scholar]
- [141].Shen Y, Wang X, Wang Y, Wang X, Chen Z, Jin M, Bai C, Lipopolysaccharide decreases aquaporin 5, but not aquaporin 3 or aquaporin 4, expression in human primary bronchial epithelial cells, Respirology 17(7) (2012) 1144–9. [DOI] [PubMed] [Google Scholar]
- [142].Mezzasoma L, Cagini L, Antognelli C, Puma F, Pacifico E, Talesa VN, TNF-alpha regulates natriuretic peptides and aquaporins in human bronchial epithelial cells BEAS-2B, Mediators Inflamm 2013 (2013) 159349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shi Z, Ye W, Zhang J, Zhang F, Yu D, Yu H, Chen B, Zhou M, Sun H, LipoxinA4 attenuates acute pancreatitis-associated acute lung injury by regulating AQP-5 and MMP-9 expression, anti-apoptosis and PKC/SSeCKS-mediated F-actin activation, Mol Immunol 103 (2018) 78–88. [DOI] [PubMed] [Google Scholar]
- [144].Pajouhi N, Owji M, Naghibalhossaini F, Omrani GH, Varedi M, Modulation by thyroid hormone of myosin light chain phosphorylation and aquaporin 5 protein expression in intact lung, J Physiol Biochem 71(1) (2015) 99–106. [DOI] [PubMed] [Google Scholar]
- [145].Ebeling G, Blasche R, Hofmann F, Augstein A, Kasper M, Barth K, Effect of P2X7 receptor knockout on AQP-5 expression of type I alveolar epithelial cells, PLoS One 9(6) (2014) e100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Abdelwahab EMM, Rapp J, Feller D, Csongei V, Pal S, Bartis D, Thickett DR, Pongracz JE, Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells, Respir Res 20(1) (2019) 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Flodby P, Li C, Liu Y, Wang H, Rieger ME, Minoo P, Crandall ED, Ann DK, Borok Z, Zhou B, Cell-specific expression of aquaporin-5 (Aqp5) in alveolar epithelium is directed by GATA6/Sp1 via histone acetylation, Sci Rep 7(1) (2017) 3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Abreu-Rodriguez I, Sanchez Silva R, Martins AP, Soveral G, Toledo-Aral JJ, Lopez-Barneo J, Echevarria M, Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha, PLoS One 6(12) (2011) e28385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang YH, Chen M, Wu Y, Qian MJ, Liu HX, Liu GY, [The effect of heme oxygenase-1 on apoptosis and aquaporin-1 expression in type II primary alveolar epithelial cells in rat], Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25(6) (2013) 351–5. [DOI] [PubMed] [Google Scholar]
- [150].Schuoler C, Haider TJ, Leuenberger C, Vogel J, Ostergaard L, Kwapiszewska G, Kohler M, Gassmann M, Huber LC, Brock M, Aquaporin 1 controls the functional phenotype of pulmonary smooth muscle cells in hypoxia-induced pulmonary hypertension, Basic Res Cardiol 112(3) (2017) 30. [DOI] [PubMed] [Google Scholar]
- [151].Yun X, Jiang H, Lai N, Wang J, Shimoda LA, Aquaporin 1-mediated changes in pulmonary arterial smooth muscle cell migration and proliferation involve beta-catenin, Am J Physiol Lung Cell Mol Physiol 313(5) (2017) L889–L898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang QY, Fu JH, Xue XD, Expression and function of aquaporin-1 in hyperoxia-exposed alveolar epithelial type II cells, Exp Ther Med 8(2) (2014) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kawedia JD, Yang F, Sartor MA, Gozal D, Czyzyk-Krzeska M, Menon AG, Hypoxia and hypoxia mimetics decrease aquaporin 5 (AQP5) expression through both hypoxia inducible factor-1alpha and proteasome-mediated pathways, PLoS One 8(3) (2013) e57541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ge H, Zhu H, Xu N, Zhang D, Ou J, Wang G, Fang X, Zhou J, Song Y, Bai C, Increased lung ischemia-reperfusion injury in aquaporin 1-null mice is mediated via decreased hypoxia-inducible factor 2alpha stability, Am J Respir Cell Mol Biol 54(6) (2016) 882–91. [DOI] [PubMed] [Google Scholar]
- [155].Wang Y, Zhong B, Wu Q, Zhu T, Wang Y, Zhang M, Aldosterone contributed to pulmonary arterial hypertension development via stimulating aquaporin expression and pulmonary arterial smooth muscle cells proliferation, Pharmacology 105(7–8) (2020) 405–15. [DOI] [PubMed] [Google Scholar]
- [156].Ming GF, Ma XH, Xu DM, Liu ZY, Ai YH, Liu HX, Shi ZH, PBEF promotes the apoptosis of pulmonary microvascular endothelial cells and regulates the expression of inflammatory factors and AQP1 through the MAPK pathways, Int J Mol Med 36(3) (2015) 890–6. [DOI] [PubMed] [Google Scholar]
- [157].Liu L, Du L, Chen Y, Qin S, Liang Q, Zou X, Liang X, Jiang J, Chen Q, Wang K, Xie C, Down-regulation of Aquaporin1 (AQP1) by peptidoglycan via p38 MAPK pathways in primary rat pleural mesothelial cells, Exp Lung Res 40(4) (2014) 145–53. [DOI] [PubMed] [Google Scholar]
- [158].Li H, Shi H, Gao M, Ma N, Sun R, Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144–3p/AQP1 axis to reduce lung epithelial cell apoptosis, Cell Biosci 8 (2018) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wang C, Yan M, Jiang H, Wang Q, Guan X, Chen J, Wang C, Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-kappaB signaling pathway, Int Immunopharmacol 40 (2016) 300–309. [DOI] [PubMed] [Google Scholar]
- [160].Wei X, Dong J, Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro, Oncol Rep 34(3) (2015) 1440–8. [DOI] [PubMed] [Google Scholar]
- [161].Kao YC, Jheng JR, Pan HJ, Liao WY, Lee CH, Kuo PL, Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling, Oncogene 36(6) (2017) 863–874. [DOI] [PubMed] [Google Scholar]
- [162].Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR, IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel, J Exp Med 202(4) (2005) 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F, Khalil M, Storch MK, Jarius S, Kristoferitsch W, Berger T, Reindl M, Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica, PLoS One 5(5) (2010) e10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ishikawa S, Urinary excretion of aquaporin-2 in pathological states of water metabolism, Ann Med 32(2) (2000) 90–3. [DOI] [PubMed] [Google Scholar]
- [165].Zhang J, Gong L, Hasan B, Wang J, Luo J, Ma H, Li F, Use of aquaporins 1 and 5 levels as a diagnostic marker in mild-to-moderate adult-onset asthma, Int J Clin Exp Pathol 8(11) (2015) 14206–13. [PMC free article] [PubMed] [Google Scholar]
- [166].Zhao R, Liang X, Zhao M, Liu SL, Huang Y, Idell S, Li X, Ji HL, Correlation of apical fluid-regulating channel proteins with lung function in human COPD lungs, PLoS One 9(10) (2014) e109725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wang Q, Ishikawa T, Michiue T, Zhu BL, Guan DW, Maeda H, Intrapulmonary aquaporin-5 expression as a possible biomarker for discriminating smothering and choking from sudden cardiac death: a pilot study, Forensic Sci Int 220(1–3) (2012) 154–7. [DOI] [PubMed] [Google Scholar]
- [168].Zhao B, Yao SQ, Hao XH, [Expression of AQP-1 and AQP-4 in the lungs of drown rats], Fa Yi Xue Za Zhi 32(5) (2016) 321–325. [DOI] [PubMed] [Google Scholar]
- [169].Lee SY, Ha EJ, Cho HW, Kim HR, Lee D, Eom YB, Potential forensic application of receptor for advanced glycation end products (RAGE) and aquaporin 5 (AQP5) as novel biomarkers for diagnosis of drowning, J Forensic Leg Med 62 (2019) 56–62. [DOI] [PubMed] [Google Scholar]
- [170].Angelico G, Caltabiano R, Loreto C, Ieni A, Tuccari G, Ledda C, Rapisarda V, Immunohistochemical expression of aquaporin-1 in fluoro-edenite-induced malignant mesothelioma: a preliminary report, Int J Mol Sci 19(3) (2018) 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ledda C, Senia P, Rapisarda V, Biomarkers for early diagnosis and prognosis of malignant pleural mesothelioma: the quest goes on, Cancers (Basel) 10(6) (2018) 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Jo YM, Park TI, Lee HY, Jeong JY, Lee WK, Prognostic significance of aquaporin 5 expression in non-small cell lung cancer, J Pathol Transl Med 50(2) (2016) 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Bellezza G, Vannucci J, Bianconi F, Metro G, Del Sordo R, Andolfi M, Ferri I, Siccu P, Ludovini V, Puma F, Sidoni A, Cagini L, Prognostic implication of aquaporin 1 overexpression in resected lung adenocarcinoma, Interact Cardiovasc Thorac Surg 25(6) (2017) 856–861. [DOI] [PubMed] [Google Scholar]
- [174].Hu X, Liu S, Zhu J, Ni H, Dachengqi decoction alleviates acute lung injury and inhibits inflammatory cytokines production through TLR4/NF-kappaB signaling pathway in vivo and in vitro, J Cell Biochem 120(6) (2019) 8956–8964. [DOI] [PubMed] [Google Scholar]
- [175].Kang X, Lu XG, Zhan LB, Liang ZK, Guo WX, Ma Q, Wang Y, Song JB, Feng JY, Wang CH, Bai LZ, Song Y, Liu GH, Dai-Huang-Fu-Zi-Tang alleviates pulmonary and intestinal injury with severe acute pancreatitis via regulating aquaporins in rats, BMC Complement Altern Med 17(1) (2017) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Gao Z, Xu J, Sun D, Zhang R, Liang R, Wang L, Fan R, Traditional chinese medicine, qing ying tang, ameliorates the severity of acute lung injury induced by severe acute pancreatitis in rats via the upregulation of aquaporin-1, Exp Ther Med 8(6) (2014) 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Li XN, Yang JY, Pan X, Zhao S, Zhang CY, Zhu DY, Wang P, Influence of extract of Ginkgo biloba leaves tablets on the aquaporin-1 expression in isolated lung ischemia reperfusion, Chin Med J (Engl) 126(24) (2013) 4720–3. [PubMed] [Google Scholar]
- [178].Ahsan F, Shabbir A, Shahzad M, Mobashar A, Sharif M, Basheer MI, Tareen RB, Syed NI, Amelioration of allergic asthma by Ziziphora clinopodioides via upregulation of aquaporins and downregulation of IL4 and IL5, Respir Physiol Neurobiol 266 (2019) 39–46. [DOI] [PubMed] [Google Scholar]
- [179].Rana S, Shahzad M, Shabbir A, Pistacia integerrima ameliorates airway inflammation by attenuation of TNF-alpha, IL-4, and IL-5 expression levels, and pulmonary edema by elevation of AQP1 and AQP5 expression levels in mouse model of ovalbumin-induced allergic asthma, Phytomedicine 23(8) (2016) 838–45. [DOI] [PubMed] [Google Scholar]
- [180].Zhen G, Upur H, Jing W, Jing J, Zheng L, Dan X, Fengsen L, Effect of abnormal savda munziq, a traditional uighur herbal medicine, on pulmonary function and aquaporins of copd rat model with abnormal savda syndrome, Evid Based Complement Alternat Med 2017 (2017) 7176263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Huber VJ, Tsujita M, Nakada T, Identification of aquaporin 4 inhibitors using in vitro and in silico methods, Bioorg Med Chem 17(1) (2009) 411–7. [DOI] [PubMed] [Google Scholar]
- [182].Huber VJ, Tsujita M, Kwee IL, Nakada T, Inhibition of aquaporin 4 by antiepileptic drugs, Bioorg Med Chem 17(1) (2009) 418–24. [DOI] [PubMed] [Google Scholar]
- [183].Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T, Identification of arylsulfonamides as Aquaporin 4 inhibitors, Bioorg Med Chem Lett 17(5) (2007) 1270–3. [DOI] [PubMed] [Google Scholar]
- [184].Yang B, Zhang H, Verkman AS, Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides, Bioorg Med Chem 16(15) (2008) 7489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool AJ, Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site, Mol Pharmacol 76(1) (2009) 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Seeliger D, Zapater C, Krenc D, Haddoub R, Flitsch S, Beitz E, Cerda J, de Groot BL, Discovery of novel human aquaporin-1 blockers, ACS Chem Biol 8(1) (2013) 249–56. [DOI] [PubMed] [Google Scholar]
- [187].Beitz E, Golldack A, Rothert M, von Bulow J, Challenges and achievements in the therapeutic modulation of aquaporin functionality, Pharmacol Ther 155 (2015) 22–35. [DOI] [PubMed] [Google Scholar]
- [188].Soveral G, Casini A, Aquaporin modulators: a patent review (2010–2015), Expert Opin Ther Pat 27(1) (2017) 49–62. [DOI] [PubMed] [Google Scholar]
- [189].Wenzel MN, Mosca AF, Graziani V, Aikman B, Thomas SR, de Almeida A, Platts JA, Re N, Coletti C, Marrone A, Soveral G, Casini A, Insights into the mechanisms of aquaporin-3 inhibition by gold(III) complexes: the importance of non-coordinative adduct formation, Inorg Chem 58(3) (2019) 2140–2148. [DOI] [PubMed] [Google Scholar]
- [190].Guo C, Wu T, Zhu H, Gao L, Aquaporin 4 blockade attenuates acute lung injury through inhibition of th17 cell proliferation in mice, Inflammation 42(4) (2019) 1401–1412. [DOI] [PubMed] [Google Scholar]
- [191].Lv CM, Wu HM, Wu L, Xu GH, Yang ZL, Shen QY, Sevoflurane modulates AQPs (1,5) expression and endoplasmic reticulum stress in mice lung with allergic airway inflammation, Biosci Rep 39(11) (2019) BSR20193282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L, Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO, Brain Pathol 23(6) (2013) 684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Shimada T, Hoshino Y, Tsunemi T, Hattori A, Nakagawa E, Yokoyama K, Hattori N, Neuromyelitis optica spectrum disorder after treatment with pembrolizumab, Mult Scler Relat Disord 37 (2020) 101447. [DOI] [PubMed] [Google Scholar]
- [194].Abir-Awan M, Kitchen P, Salman MM, Conner MT, Conner AC, Bill RM, Inhibitors of mammalian aquaporin water channels, Int J Mol Sci 20(7) (2019) 1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bichet DG, Nephrogenic diabetes insipidus, Adv Chronic Kidney Dis 13(2) (2006) 96–104. [DOI] [PubMed] [Google Scholar]
- [196].Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N, Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice, Genomics 81(4) (2003) 361–8. [DOI] [PubMed] [Google Scholar]
- [197].Kleffner I, Bungeroth M, Schiffbauer H, Schabitz WR, Ringelstein EB, Kuhlenbaumer G, The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion, Stroke 39(4) (2008) 1333–5. [DOI] [PubMed] [Google Scholar]
- [198].Elliott L, Ashley-Koch AE, De Castro L, Jonassaint J, Price J, Ataga KI, Levesque MC, Brice Weinberg J, Eckman JR, Orringer EP, Vance JM, Telen MJ, Genetic polymorphisms associated with priapism in sickle cell disease, Br J Haematol 137(3) (2007) 262–7. [DOI] [PubMed] [Google Scholar]
- [199].Prudente S, Flex E, Morini E, Turchi F, Capponi D, De Cosmo S, Tassi V, Guida V, Avogaro A, Folli F, Maiani F, Frittitta L, Dallapiccola B, Trischitta V, A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities, Diabetes 56(5) (2007) 1468–74. [DOI] [PubMed] [Google Scholar]
- [200].Ning Y, Ying B, Han S, Wang B, Wang X, Wen F, Polymorphisms of aquaporin5 gene in chronic obstructive pulmonary disease in a Chinese population, Swiss Med Wkly 138(39–40) (2008) 573–8. [DOI] [PubMed] [Google Scholar]
- [201].Hansel NN, Sidhaye V, Rafaels NM, Gao L, Gao P, Williams R, Connett JE, Beaty TH, Mathias RA, Wise RA, King LS, Barnes KC, Aquaporin 5 polymorphisms and rate of lung function decline in chronic obstructive pulmonary disease, PLoS One 5(12) (2010) e14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].He L, Dong Y, Wu W, Zhang L, Sun B, Protective role of glucocorticosteroid prior to endotoxin exposure in cultured neonatal type II alveolar epithelial cells, Pulm Pharmacol Ther 52 (2018) 18–26. [DOI] [PubMed] [Google Scholar]
- [203].Chen K, Yang J, Xiao F, Chen J, Hu W, Wang X, Wang L, Du J, Jiang J, He Y, Early peritoneal dialysis ameliorates blast lung injury by alleviating pulmonary edema and inflammation, Shock 53(1) (2020) 95–102. [DOI] [PubMed] [Google Scholar]
- [204].Chang JF, Liang SS, Thanasekaran P, Chang HW, Wen LL, Chen CH, Liou JC, Yeh JC, Liu SH, Dai HM, Lin WN, Translational medicine in pulmonary-renal crosstalk: therapeutic targeting of p-cresyl sulfate triggered nonspecific ROS and chemoattractants in dyspneic patients with uremic lung injury, J Clin Med 7(9) (2018) 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Shao Y, Shen J, Zhou F, He D, Mesenchymal stem cells overexpressing Ang1 attenuates phosgene-induced acute lung injury in rats, Inhal Toxicol 30(7–8) (2018) 313–320. [DOI] [PubMed] [Google Scholar]
- [206].Liang X, Zhang B, Chen Q, Zhang J, Lei B, Li B, Wei Y, Zhai R, Liang Z, He S, Tang B, The mechanism underlying alpinetin-mediated alleviation of pancreatitis-associated lung injury through upregulating aquaporin-1, Drug Des Devel Ther 10 (2016) 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ba F, Zhou X, Zhang Y, Wu C, Xu S, Wu L, Li J, Yin Y, Gu X, Lipoxin A4 ameliorates alveolar fluid clearance disturbance in lipopolysaccharide-induced lung injury via aquaporin 5 and MAPK signaling pathway, J Thorac Dis 11(8) (2019) 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wang Q, Yan SF, Hao Y, Jin SW, Specialized pro-resolving mediators regulate alveolar fluid clearance during acute respiratory distress syndrome, Chin Med J (Engl) 131(8) (2018) 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Liu D, Wang Y, Li L, Zhao H, Li L, Liu Y, Jiang H, Li X, Zhang R, Celecoxib protects hyperoxia-induced lung injury via NF-kappaB and AQP1, Front Pediatr 7 (2019) 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Zhang G, Zhang X, Huang H, Ji Y, Li D, Jiang W, Saquinavir plus methylprednisolone ameliorates experimental acute lung injury, Braz J Med Biol Res 51(10) (2018) e7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Wang JJ, Kong H, Xu J, Wang YL, Wang H, Xie WP, Fasudil alleviates LPS-induced lung injury by restoring aquaporin 5 expression and inhibiting inflammation in lungs, J Biomed Res 33(3) (2019) 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Xiong LL, Tan Y, Ma HY, Dai P, Qin YX, Yang RA, Xu YY, Deng Z, Zhao W, Xia QJ, Wang TH, Zhang YH, Administration of SB239063, a potent p38 MAPK inhibitor, alleviates acute lung injury induced by intestinal ischemia reperfusion in rats associated with AQP4 downregulation, Int Immunopharmacol 38 (2016) 54–60. [DOI] [PubMed] [Google Scholar]
- [213].Xu J, Huang B, Wang Y, Tong C, Xie P, Fan R, Gao Z, Emodin ameliorates acute lung injury induced by severe acute pancreatitis through the up-regulated expressions of AQP1 and AQP5 in lung, Clin Exp Pharmacol Physiol 43(11) (2016) 1071–1079. [DOI] [PubMed] [Google Scholar]
- [214].Sun Y, Sun L, Liu S, Song J, Cheng J, Liu J, Effect of emodin on Aquaporin 5 expression in rats with sepsis-induced acute lung injury, J Tradit Chin Med 35(6) (2015) 679–84. [DOI] [PubMed] [Google Scholar]
- [215].Hong-Min F, Chun-Rong H, Rui Z, Li-Na S, Ya-Jun W, Li L, CGRP 8–37 enhances lipopolysaccharide-induced acute lung injury and regulating aquaporin 1 and 5 expressions in rats, J Physiol Biochem 73(3) (2016) 381–386. [DOI] [PubMed] [Google Scholar]
- [216].Zhu HX, Zhou JB, Zhu XD, Zhou J, Li J, Song YL, Bai CX, Impaired self-healing capacity in airway epithelia lacking aquaporin-3, Respir Physiol Neurobiol 233 (2016) 66–72. [DOI] [PubMed] [Google Scholar]
- [217].Sun CZ, Shen H, He XW, Bao L, Song Y, Zhang Z, Qin HD, Effect of dobutamine on lung aquaporin 5 in endotoxine shock-induced acute lung injury rabbit, J Thorac Dis 7(8) (2015) 1467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Jiang YX, Dai ZL, Zhang XP, Zhao W, Huang Q, Gao LK, Dexmedetomidine alleviates pulmonary edema by upregulating AQP1 and AQP5 expression in rats with acute lung injury induced by lipopolysaccharide, J Huazhong Univ Sci Technolog Med Sci 35(5) (2015) 684–688. [DOI] [PubMed] [Google Scholar]
- [219].Han G, Ma L, Guo Y, Li L, Li D, Liu H, Hyperbaric oxygen therapy palliates lipopolysaccharide-induced acute lung injury in rats by upregulating AQP1 and AQP5 expression, Exp Lung Res 41(8) (2015) 444–9. [DOI] [PubMed] [Google Scholar]
- [220].Xiao M, Zhu T, Wang T, Wen FQ, [Diammonium glycyrrhizinate promotes aquaporin-5 in LPS-induced acute lung injury via inactivation of NF-kappaB], Sichuan Da Xue Xue Bao Yi Xue Ban 45(3) (2014) 376–9, 404. [PubMed] [Google Scholar]
- [221].Jia WH, Yang P, Li J, Tian ZL, [Effects of selective phosphodiesterase 4 inhibitor on expression of aquaporin 5 in airway mucus hypersecretion model of rats], Zhonghua Yi Xue Za Zhi 93(8) (2013) 619–22. [PubMed] [Google Scholar]
- [222].Meng M, Geng S, Du Z, Yao J, Zheng Y, Li Z, Zhang Z, Li J, Duan Y, Du G, Berberine and cinnamaldehyde together prevent lung carcinogenesis, Oncotarget 8(44) (2017) 76385–76397. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [223].Cagini L, Balloni S, Ludovini V, Andolfi M, Matricardi A, Potenza R, Vannucci J, Siggillino A, Tofanetti FR, Bellezza G, Bodo M, Puma F, Marinucci L, Variations in gene expression of lung macromolecules after induction chemotherapy for lung cancer, Eur J Cardiothorac Surg 52(6) (2017) 1077–1082. [DOI] [PubMed] [Google Scholar]
- [224].Ye HJ, Bai JJ, Guo PP, Wang W, Lin CS, [Propofol suppresses invasion of human lung cancer A549 cells by down-regulating aquaporin-3 and matrix metalloproteinase-9], Nan Fang Yi Ke Da Xue Xue Bao 36(9) (2016) 1286–1290. [PubMed] [Google Scholar]
- [225].Liu YH, Zhu WL, Effects of cetuximab combined with afatinib on the expression of KDR and AQP1 in lung cancer, Genet Mol Res 14(4) (2015) 16652–61. [DOI] [PubMed] [Google Scholar]
- [226].Xia H, Ye J, Bai H, Wang C, [Effects of cetuximab combined with celecoxib on apoptosis and KDR and AQP1 expression in lung cancer], Zhongguo Fei Ai Za Zhi 16(12) (2013) 625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Schmidt AF, Kemp MW, Rittenschober-Bohm J, Kannan PS, Usuda H, Saito M, Furfaro L, Watanabe S, Stock S, Kramer BW, Newnham JP, Kallapur SG, Jobe AH, Low-dose betamethasone-acetate for fetal lung maturation in preterm sheep, Am J Obstet Gynecol 218(1) (2018) 132 e1–132 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Cheng C, Li S, Wang Y, Chen S, You L, Zhang H, Ischemic postconditioning alleviates lung injury and maintains a better expression of aquaporin-1 during cardiopulmonary bypass, Chinese Med J 127 (2014), 4012–4018. 10.3760/cma.j.issn.0366-6999.20140852. [PubMed] [Google Scholar]