Abstract
COVID-19 pandemic has affected more than 200 countries and 1.3 million individuals have deceased within eleven months. Intense research on COVID-19 occurrence and prevalence enable us to understand that comorbidities play a crucial role in spread and severity of SARS-CoV-2 infection. Chronic kidney disease, diabetes, respiratory diseases and hypertension are among the various morbidities that are prevalent in symptomatic COVID-19 patients. However, the effect of altered thyroid-driven disorders cannot be ignored. Since thyroid hormone critically coordinate and regulate the major metabolism and biochemical pathways, this review is on the potential role of prevailing thyroid disorders in SARS-CoV-2 infection. Direct link of thyroid hormone with several disorders such as diabetes, vitamin D deficiency, obesity, kidney and liver disorders etc. suggests that the prevailing thyroid conditions may affect SARS-CoV-2 infection. Further, we discuss the oxidative stress-induced aging is associated with the degree of SARS-CoV-2 infection. Importantly, ACE2 protein which facilitates the host-cell entry of SARS-CoV-2 using the spike protein, are highly expressed in individuals with abnormal level of thyroid hormone. Altogether, we report that the malfunction of thyroid hormone synthesis may aggravate SARS-CoV-2 infection and thus monitoring the thyroid hormone may help in understanding the pathogenesis of COVID-19.
Keywords: Thyroid, COVID-19, Hypothyroid, Hyperthyroid, Oxidative stress, Aging, Biochemistry, Endocrinology, Toxicology, Epidemiology, SARS-CoV-2
Thyroid; COVID-19; Hypothyroid; Hyperthyroid; Oxidative stress; Aging; Biochemistry; Endocrinology; Toxicology; Epidemiology; SARS-CoV-2
1. Introduction
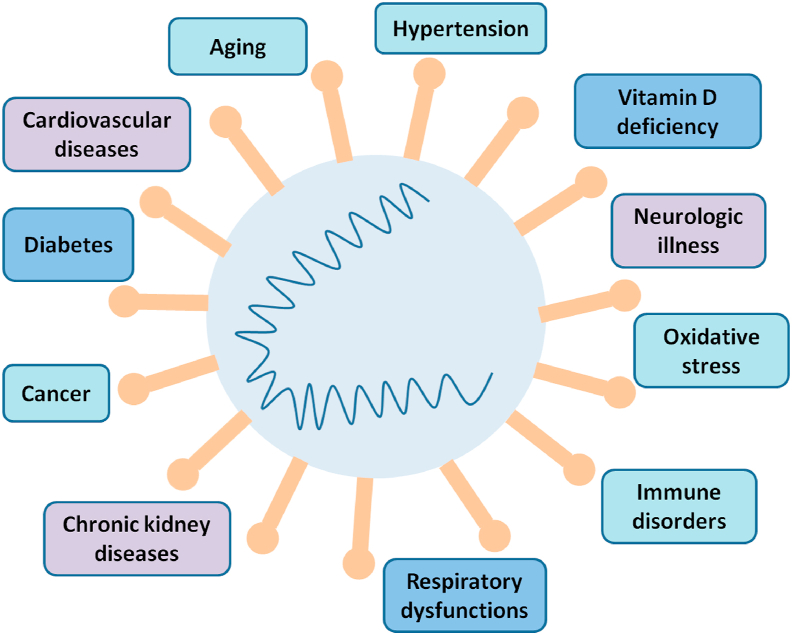
An outbreak of betacoronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began in China in December 2019. COVID-19, the disease associated with SARS-CoV-2 infection rapidly spread across the Globe. Since then the world is experiencing an outbreak of COVID-19 pandemic with 1.3 million deaths and 57 million symptomatic cases worldwide as on 20 November 2020 [1]. Individuals infected with SARS-CoV-2 show various types of clinical signs including fever, respiratory tract infections, diarrhea, breathlessness, memory loss etc. depending on person's health conditions [2]. Progression of SARS-CoV-2 infection increases in case of comorbidities such as diabetes [3], cardiovascular diseases [4], hypertension, chronic kidney diseases and respiratory disorders [5]. Figure 1 displays the comorbidities found in COVID-19 symptomatic patients and Table 1 describes the prevalence of these diseases or symptoms in different cohort studies [6, 7, 8, 9, 10, 11, 12, 13, 14]. Based on thyroid hormone functions and their association with other ailments, in this review a link has been established between aggravations of SARS-CoV-2 infection with thyroid disorders.
Figure 1.
COVID-19 and associated comorbidities. Risk of severe COVID-19 symptoms increases in case of multiple disorders that includes respiratory dysfunctions, hypertension, cardiovascular diseases, chronic kidney disease, diabetes, deregulated immune response, oxidative stress, reduced level of vitamin D, neurologic illness and cancer.
Table 1.
Prevalence of comorbidities in COVID-19 patients.
| Symptoms | [6] Zhu et. al. 2020 | [7] Zhang et. al. 2020 | [8] Liu et.al. 2020 | [9] Chen et. al. 2020 | [10] Jin et.al. 2020 | [11] Li Jitian et. al. 2020 | [12] Jiang et. al. 2020 | [13] Li et. al. 2020 | [14] Guan et. al. 2020 |
|---|---|---|---|---|---|---|---|---|---|
| Fever | 80.40% | 91.70% | 81.80% | 83% | 85.14% | 92.1% | 98% | 88.50% | 43.80% |
| Elevated C-reactive protein levels | 73.60% | Significantly high (all P < 0.001) | 83.90% | - | - | - | - | 44.30% | - |
| Lesions involving bilateral lungs | 75.70% | 89.60% | 84.70% | 75% | - | - | - | - | - |
| Cough | 63.10% | 75.00% | 48.20% | 82% | 71.62% | 42.10% | 76% | 68.60% | 67.80% |
| Lymphopenia | 56.50% | 75.40% | - | - | - | - | - | 64.50% | 83.20% |
| Fatigue | 46% | 75.00% | 32.10% | 31.08 | - | 28.10% | 44% | 35.80% | - |
| Expectoration | 41.80% | - | 4.40% | - | 39.19% | 25.80% | 28% | 28.20% | - |
| Anorexia | 38.80% | - | - | - | - | - | - | - | - |
| Elevated D-dimer | 37.20% | Significantly high (all P < 0.001) | - | - | - | - | - | - | - |
| Chest tightness | 35.70% | - | - | 2% (Pain in chest) | - | 12.70% | - | - | - |
| Shortness of breath | 35% | - | 7.3% (Heart palpitations) | 31% | 10.81% | 5% | - | - | - |
| Dyspnea | 33.90% | - | 19% | - | - | - | 55% | 21.90% | - |
| Leukopenia | 25.90% | - | - | - | - | - | - | - | - |
| Abnormal liver function | 29% | - | - | - | - | - | - | - | - |
| Lesions involving single lung | 25.80% | - | - | - | - | - | - | - | - |
| Abnormal renal function | 25.50% | - | - | - | - | - | 7% (Acute kidney injury) | - | - |
| Elevated procalcitonin | 17.50% | - | - | - | - | - | - | - | - |
| Headache | 15.40% | - | 9.50% | 8% | 21.62% | - | 8% | 12.10% | - |
| Pharyngalgia | 13.10% | - | - | - | - | - | - | - | - |
| Diarrhea | 12.90% | - | 8% | 2% | - | 5% | 3% | 4.80% | 3.80% |
| Leukocytosis | 12.60% | - | - | - | - | - | - | 29.40% | - |
| Shivering | 10.90% | - | - | - | - | - | - | - | - |
| Eosinopenia | - | 52.90% | - | - | - | - | - | - | - |
| Gastrointestinal symptoms | - | 39.60% | - | - | - | - | - | - | - |
| Hypertension | - | 30.00% | 9.50% | - | 16.22% | - | - | - | 23.70% |
| Diabetes mellitus | - | 12.10% | 10.20% | - | 9.46% | - | - | - | - |
| Drug hypersensitivity | - | 11.40% | - | - | - | - | - | - | - |
| Fatty liver and abnormal liver function | - | 5.70% | - | - | 10.81% | - | - | - | - |
| Chronic gastritis and gastric ulcer | - | 5% | - | - | - | - | - | - | - |
| Coronary heart disease | - | 5% | - | - | 1% | - | - | - | - |
| Cholelithiasis | - | 4.30% | - | - | - | - | - | - | - |
| Arrhythmia | - | 3.60% | - | - | - | - | - | - | - |
| Thyroid diseases | - | 3.60% | - | - | - | - | - | - | - |
| Urticaria | - | 1.40% | - | - | - | - | - | - | - |
| Chronic obstructive pulmonary disease | - | 1.40% | - | - | - | - | - | - | - |
| Smokers | - | 1.40% | - | - | 4.23% | - | - | - | - |
| Malignancy | - | - | 1.50% | - | - | - | - | - | - |
| Hemoptysis | - | - | 5.10% | - | 4.05% | - | 5% | - | - |
| Cardiovascular disease | - | - | 7.30% | - | - | 59.30% | 12% | - | - |
| Chronic obstructive pulmonary disease | - | - | 1.50% | - | - | - | - | - | - |
| Acute respiratory distress syndrome | - | - | - | 17% | - | - | 29% | - | - |
| Multiple mottling and ground-glass opacity | - | - | - | 14% | - | - | - | - | 56.40% |
| Muscle ache | 33% | - | - | 11% | 13.51% | 11.90% | - | - | - |
| Confusion | - | - | - | 9% | - | - | - | - | - |
| Sore throat | - | - | - | 5% | 8.11% | 10.70% | - | - | - |
| Rhinorrhoea | - | - | - | 4% | - | 6.30% | - | - | - |
| Nausea | - | - | - | 1% | - | 3.40% | 3.90% | ||
| Vomiting | - | - | - | 1% | - | 2.90% | 3.90% | 5.00% | |
| Pneumothorax | - | - | - | 1% | - | - | - | - | - |
| Nasal obstruction | - | - | - | - | 2.70% | 4.60% | - | - | - |
| Increase of lactic dehydrogenase | - | - | - | - | - | - | - | 28.30% | - |
| Elevated ESR | 65.60% | - | - | - | - | - | - | - | - |
| Oxygenation index decreased | 63.60% | - | - | - | - | - | - | - | - |
Thyroid disorders such as hypothyroidism and hyperthyroidism are more common in India being 11%, compared to only 2% in the UK and 4.6% in the USA [15, 16, 17, 18]. The thyroid hormone is secreted from thyroid gland that controls the growth, development of an organism and plays a vital role in maintaining normal human physiology and homeostasis. The primary hormone secreted by the thyroid gland is thyroxine (T4) but the gland also secretes smaller amounts of T3 (tri-iodothyronine). An active thyroid gland produces appropriate amounts of T3 and T4 under the influence of thyroid stimulating hormone (TSH), which is secreted from the pituitary gland [19]. The function of thyroid gland is influenced during hypothyroidism, hyperthyroidism, follicular thyroid carcinoma, papillary thyroid carcinoma and undifferentiated thyroid carcinoma. In hyper- and hypothyroidism, the thyroid gland is over- or underactive respectively and fails to produce appropriate hormone level that leads to deregulated secretion of T3 and T4 into the blood [20]. Altered thyroid state is a type of thyroid dysfunction that shows potentially devastating health consequences and is more common in women than men [21, 22]. The common symptoms of hypothyroidism such as fatigue, muscle weakness, dry skin, excessive hair fall, weight gain, increased sensitivity to cold etc. (Table 2) [23, 24, 25, 26, 27, 28], are drastically affect the professional productivity of people [29, 30, 31, 32]. Thyroid disorder may occur due to several factors that include I) Autoimmunity: The most common cause of hyper- and hypothyroidism is an immune disorder called Graves' and Hashimoto's thyroiditis. Here, the immune system produces antibodies that attack their own body tissues thereby named autoantibodies. In autoimmune hyper- and hypothyroidism, the immune system attacks the cells in the thyroid gland thereby the production of thyroid hormone is compromised [33]. II) Thyroid surgery: In some people, part or the entire thyroid gland is removed due to the detection of thyroid nodules that leads to deregulated thyroid hormone levels or even thyroid carcinoma [34]. III) Radiation therapy: People with Graves' disease, nodular goiter, or thyroid cancer are treated with radioactive iodine (131I). Radiation may affect the thyroid gland that can result into thyroid dysfunction [35]. IV) Congenital hypothyroidism: Babies that are born with defective thyroid, without thyroid or partly formed thyroid gland, are born with either permanent or transient congenital hypothyroidism at birth. Transient hypothyroidism at birth is caused due to placental transfer of maternal anti-thyroid antibodies inhibiting fetal thyroid function [36]. Defects in thyroid hormone biosynthesis, resistance to thyrotropin, developmental defects, central hypothyroidism, thyroid hormone resistance, abnormal thyroid hormone transport into the cell and thyroid dysgenesia which includes thyroid ectopia, athyreosis, hypoplastic gland in situ are major reasons for permanent congenital hypothyroidism [37]. V) Damage to the pituitary gland: If pituitary gland is damaged by injury, radiation, tumor or surgery, it will not be able to produce TSH that directs thyroid gland to produce hormones. VI) Treatment and medications: Some drugs can interfere with the normal functioning of thyroid gland such as Lithium that are prescribed for certain psychiatric disorders. These medications may lead to abnormalities of thyroid gland [38] and VII) Iodine deficiency: Thyroid gland produces thyroxin by utilizing iodine that comes into our body from foods. Decreased iodine intake can lead to hypothyroidism and on the otherhand too much of iodine may worsen the disease. The present treatment approach is to normalize thyroxin hormone levels, specifically T4 and TSH by prescribing oral thyroid hormone (L-thyroxine) preparation to alleviate the hormone deficiency in case of hypothyroidism. Similarly, hyperthyroid patients are treated with n-propyl thiouracil (PTU) or methimazole to lower the thyroid level. Although the present treatment of thyroid disorders substantially maintains the level of hormone in the blood, it fails to improve well-being and quality of life of patients that have to be under medication for life-time [39].
Table 2.
Clinical manifestations of patients with hypothyroidism.
| Symptoms and Signs | [23] Kostoglou-Athanassiou and Ntalles, 2010 |
[24] Paudel, 2014 |
[25] El-Shafie, 2003 |
[26] Dutta et.al. 2019 |
[27] Paul and Dasgupta, 2012 |
[28] Sethi et.al. 2017 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypothyroidism | Subclinical Hypothyroid (n = 17) | Subclinical Hypothyroid (n = 30) | Severe Primary Hypothyroism (n = 91) | Non-severe overt Primary Hypothroidism (n = 130) | Subclinical Hypothyroidism (n = 240) | Hypothyroid (n = 41) | Overt Hypothyroid (20) | Subclinical Hypothyroid (21) | Hypothyroidism (n = 1499) | |
| Fatigue | 88% | 58.80% | 25% | 91.20% | 68.46% | 56.67% | - | - | - | 60.17% |
| Cold intolerance | 84% | 58.80% | - | 69.23% | 13.07% | 1.67% | - | - | - | 4.40% |
| Dry skin | 77% | 70.60% | 10% | - | - | - | - | - | - | - |
| Voice hoarseness | 74% | - | - | 69.23% | 16.92% | 5.41% | - | - | - | - |
| Decreased hearing | 40% | - | - | - | - | - | - | - | - | - |
| Sleepiness | 68% | - | 3% | - | - | - | - | - | - | - |
| Impaired memory | 66% | 29.40% | - | 79.12% | 25.38% | 22.08% | - | - | - | 19.81% |
| Weight gain | 72% | - | 10% | 48.35% | 40% | 32.08% | - | - | - | 36.22% |
| Paresthesia | 56% | - | - | - | - | - | - | - | - | - |
| Constipation | 52% | 58.80% | 20% | - | - | - | - | - | - | 18.15% |
| Hair loss | 41% | 5.90% | - | 76.92% | 26.92% | 32.08% | - | - | - | 30.89% |
| Dry coarse skin | 90% | - | - | 72.52% | 3.07% | 7.08% | - | - | - | 17.01% |
| Asthma | - | - | - | - | - | - | 34.10% | 40% | 28.57% | - |
| Diabetes | - | - | - | - | - | - | 31.70% | 30% | 33.33% | 13.54% |
| Obesity | - | - | - | - | - | - | 31.70% | 25% | 38.10% | 1.27% |
| Hypertension | - | - | - | - | - | - | 29.30% | 45% | 14.28% | 11.34% |
| Voice hoarseness | 87% | - | - | - | - | - | - | - | - | 8.74% |
| Facial periorbital edema | 76% | - | - | - | - | - | - | - | - | - |
| Slowed movements/Delayed tendon reflex | 73% | - | - | 69.23% | - | - | - | - | - | 7.47% |
| Mental impairment | 54% | - | - | - | - | - | - | - | - | - |
| Bradycardia <60/min | 10% | - | - | - | - | - | - | - | - | 3.20% |
| Bradycardia>60/min | 90% | - | - | - | - | - | - | - | - | - |
| Anxiety/Depression | - | 29.40% | - | - | - | - | - | - | - | - |
| Tingling sensation | - | 52.90% | - | - | - | - | - | - | - | - |
| Muscle pain/muscle weakness | - | 47.10% | - | - | - | - | - | - | - | - |
| Dysarthria | - | - | 3% | - | - | - | - | - | - | - |
| Dysphagia | - | - | 3% | - | - | - | - | - | - | - |
| Snoring | - | - | 3% | - | - | - | - | - | - | - |
| Goiter | - | - | 10% | - | - | - | - | - | - | - |
| Menstrual irregularities | - | - | - | 73.62% | 28.46% | 16.25% | - | - | - | 10.41% |
| Amenorrhea | - | - | - | 14.29% | - | - | - | - | - | - |
| Oligomenorrhea | - | - | - | 27.47% | 28.46% | 16.25% | - | - | - | - |
| Meno-metrorrhagia | - | - | - | 9.89% | - | - | - | - | - | - |
| Menorrhagia | - | - | 3% | 21.97% | - | - | - | - | - | - |
| Carpal tunnel syndrome | - | - | 10% | 13.18% | - | - | - | - | - | 1.27 |
| Odema of lower limbs | - | - | 10% | 81.31% | 43.07% | 28.33% | - | - | - | 18.08% |
| Shortness of breath | - | - | - | 93.40% | 40.76% | 32.92% | - | - | - | 16.74% |
| Pero-orbital edema | - | 76.50% | - | 90.10% | 26.15% | 18.33% | 11.47% | |||
| Feeling cold | - | - | - | - | - | - | - | - | - | 15.21% |
| Hypervitaminosis | - | - | - | - | - | - | - | - | - | 5.94% |
| Dyslipidemia | - | - | - | - | - | - | - | - | - | 4.27% |
| Hypocalcemia | - | - | - | - | - | - | - | - | - | 2.87% |
| Vitamin D deficiency | - | - | - | - | - | - | - | - | - | 1.73% |
| Anemia | - | - | - | - | - | - | - | - | - | 1.27% |
| Lethargy | - | - | - | - | - | - | - | - | - | 1.07% |
| Mineral deficiency | - | - | - | - | - | - | - | - | - | 0.87% |
| Iron deficiency | - | - | - | - | - | - | - | - | - | 0.80% |
| Basedow's disease | - | - | - | - | - | - | - | - | - | 0.53% |
| Neuralgia | - | - | - | - | - | - | - | - | - | 0.53% |
| Vitamin B12 deficiency | - | - | - | - | - | - | - | - | - | 0.53% |
| Pericardial effusion | - | 52.90% | - | - | - | - | - | - | - | - |
| Pleural effusion | - | 52.90% | - | - | - | - | - | - | - | - |
| Ascites | - | 41.20% | - | - | - | - | - | - | - | - |
Thyroid hormones are critically involved in the metabolism and thus their deregulated levels lead to abnormal metabolic state which may be hypermetabolic or hypometabolic. Action of thyroid hormone determines the function of heart, lung, liver, kidney and other organs thus playing a central role in human healthcare system. With increasing age, prevalence of thyroid disorders such as subclinical hypothyroidism is increased [40, 41]. Furthermore, hypermetabolic rate in hyperthyroid individuals is believed to be due to increased mitochondrial reactive oxygen and nitrogen species, thereby indicating the role of thyroid hormone in oxidative stress management [42] which in turn is linked with aging [43, 44]. Besides, oxidative stress leads to several age-related disorders that include diabetes, arthritis, osteoporosis, dementia, cancer and metabolic syndromes [45, 46, 47]. Intricate relation of thyroid hormones with aging, oxidative stress and other diseases suggests the crucial role of thyroid hormone in adverse outcome of SARS-CoV-2 infection. Herein, we discuss in detail the association of thyroid disorders with comorbidities including oxidative stress and aging.
2. Disorders and diseases associated with thyroid dysfunction
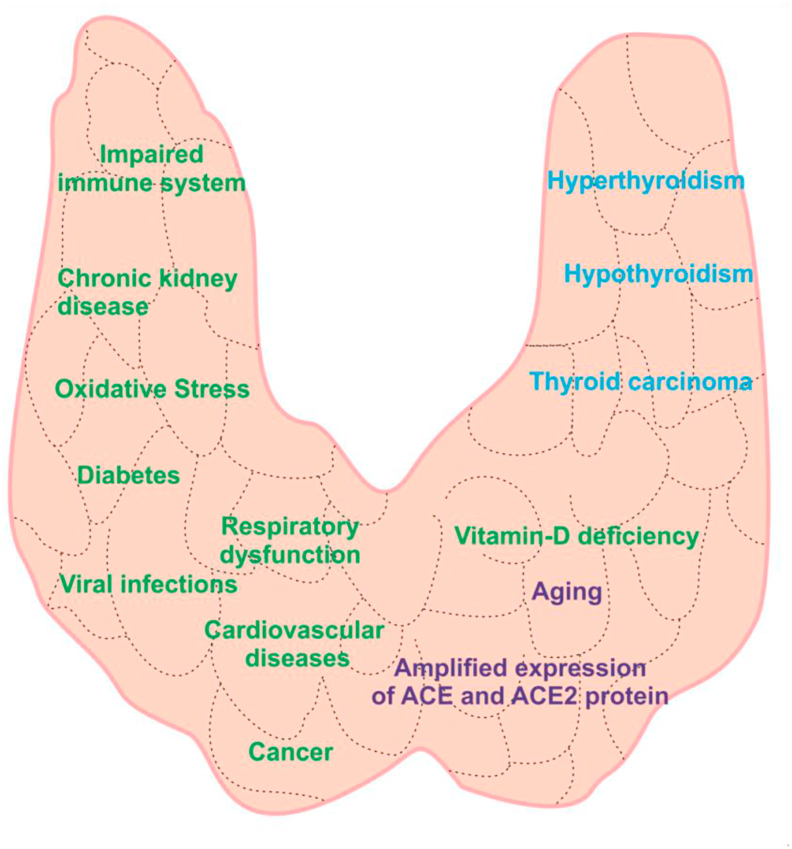
Risk of morbidities and mortalities in individuals with thyroid disorders is well established [48, 49, 50]. Several reports suggest critical association of thyroid dysfunction with diabetes [[51], [52]], cardiovascular diseases [53, 54], respiratory dysfunctions [55, 56], cancer [57, 58], chronic kidney diseases, hypertension, bacterial and viral infections. In the following sections, we discuss the association of several physiological disorders with thyroid dysfunctions (Figure 2).
Figure 2.
Physiological disorders and diseases associated with thyroid dysfunctions. Abnormal thyroid hormone action leads to several ailments including diabetes, cardiovascular diseases, respiratory dysfunctions, immune disorders, chronic kidney disease, vitamin D deficiency, deregulated RAS system, oxidative stress, hypertension, aging and various cancer. Importantly, thyroid hormone action regulates the expression of ACE and ACE2 protein on endothelial cells.
2.1. Immune dysfunctions
Immune response in viral infections play determining role in disease outcome and thus immunotherapy can be promising treatment strategy [59]. Bidirectional link between neuroendocrine system and immune system is well established from previous research [60, 61]. Thyroid hormones play important role in modulating immune cell activities at cellular level. The intimate relationship between increased levels of T3 and proinflammatory activities such as the respiratory burst, nitric oxide synthase activity, and tumor necrosis factor-α expression in Kupffer cells has been extensively studied [62]. Thyroid hormones are also known to play vital role in maturation of dendritic cell and increased secretion of interleukin-12 (IL-12), which suggests their profound implications on immunopathology [63]. Nevertheless, immune system is known to regulate the function of thyroid hormone [64]. Autoimmune thyroiditis is a T-cell mediated autoimmune disease characterized by lymphocytic infiltration followed by fibrosis and atrophy of the gland [65, 66, 67]. Similarly, hyperthyroidism affects the functional aspects of monocytes and macrophages by inhibiting pro-inflammatory actions. The pro-inflammatory markers such as macrophage inflammatory protein-1a (MIP-1α) and IL-1β was found to be masked in T4-induced hyperthyroidism [68]. Action of thyroid hormone is significantly associated with innate immune response that suggests the complex adaptive responses in immunopathology [69]. Abnormal production of antibody was reported in patients with hyperthyroidism [70]. Similarly, a drastic reduction of lymphocytic function has been observed in patients with severe hypothyroidism [71] and congenital hypothyroidism [72]. Increased adherence of neutrophils and their reduced migration was detected in patients with primary hypothyroidism in comparison to healthy individuals [73] which strongly suggest increased risk of bacterial infections in hypothyroid individuals.
2.2. Major physiological disorders
Thyroid hormone have a central role in cardiovascular homeostasis, thus supplementation of thyroid hormone have been suggested in ischemic heart disease [74]. On the other hand, hypothyroidism is a known risk factor for coronary artery diseases [75] and elevated cardiac troponins are reported to be associated with severe hypothyroidism [76]. At the same time, high serum level of brain natriuretic peptide has been detected in hyperthyroid subjects compared to euthyroid controls [77]. Similarly, the levels of D-dimers, and fibrinogen in impaired thyroid individuals have been negatively correlated with free-thyroxin levels [78, 79]. Hypothyroidism increases the risk of atherosclerotic cardiovascular diseases by increasing the levels of low-density-lipoprotein cholesterol particles, affecting smooth muscle and inducing diastolic hypertension [80]. This risk is exaggerated with insulin resistance and cigarette smoking.
Thyroid malfunctioning is associated with insulin resistance and is a known comorbid disorder for type-2 diabetes mellitus [81, 82, 83, 84]. Thyroid dysfunction is prevalent in individuals with diabetes and shows significantly increased serum levels of T3 and TSH [85]. Diabetic patients with hypothyroidism are susceptible to periodic hypoglycemic episodes. In addition, severe hyperthyroidism may lead to hyperglycemia [86, 87, 88]. Reports suggest that diabetic individuals are more likely to develop thyroid nodules [89]. Intimate relation between insulin resistance and thyroid cancer is apparently responsible for increased prevalence of thyroid cancer worldwide [90] and vice versa [91, 92].
Endocrine diseases in general have critical association with respiratory system [93] and thyroid hormones in particular play an important role in development of lungs and maturation of pulmonary surfactant [94]. Functional lung impairment has been observed in patients with hypothyroidism [95, 96, 97]. Moreover, respiratory dysfunctions has been studied to be more prevalent in hypothyroid patients [98]. For instance, reduced percentage of diffusing capacity of the lungs for carbon monoxide (DLCO), force expiratory flow (FEF) and force vital capacity (FVC) was detected in individuals with hypothyroidism [99, 100]. Under or overactive thyroid leads to diaphragmatic dysfunction [101] and weak reversible respiratory muscle [102, 103, 104].
Hypertension, which may be defined as systolic and/or diastolic blood pressure more than 160/95 mm Hg, was found more common in hypothyroid patients than in euthyroid individuals [105]. Nevertheless, hyperthyroidism is also a causal factor of hypertension. Atherosclerotic changes due to deregulated lipid metabolism in altered thyroid conditions may be the basis of vasculature changes in the blood vessels thus leading to high blood pressure. Along with this, some genetic mutations may be contributing towards thyroid disorders-mediated hypertension [106].
Thyroid hormones regulate renal function directly, thus modulate renal blood flow and glomerular filtration rate (GFR) [107]. Association of hypothyroidism and hyperthyroidism with GFR and increased renin-angiotensin-aldosterone activation underscores the link of thyroid diseases and kidney dysfunctions [108, 109]. Chronic kidney disease (CKD) caused by hypothyroidism is characterized by reduced levels of hormone T3 and can lead to renal fibrosis and renal failure with severe thyroid conditions [110]. Hypothyroidism has been reported to increase the risk of CKD incidence, progression and mortality in individuals with kidney dysfunction [111].
Thyroid dysfunctions and cancer of breast, prostate and thyroid are closely associated [112, 113]. Increased risk in the incidence of colorectal and thyroid carcinoma as well as increased cancer mortality has been reported in individuals with subclinical hypothyroidism [114]. Recently, overt thyroid dysfunction has been found to predict response to anti-PD-1 immunotherapy in association with anti-thyroid antibodies and is of clinical relevance for overall patient survival of non-small-cell lung carcinoma patients [115]. In recent times, a strong association between primary malignancies of thyroid and melanoma has been established [116]. At the same time increased TSH level is associated with poor prognosis for endometrial carcinoma patients [117]. A meta-analysis study reported an elevated risk of colorectal cancer in patients with hypothyroidism and thyroid replacement therapy has a protective role [118].
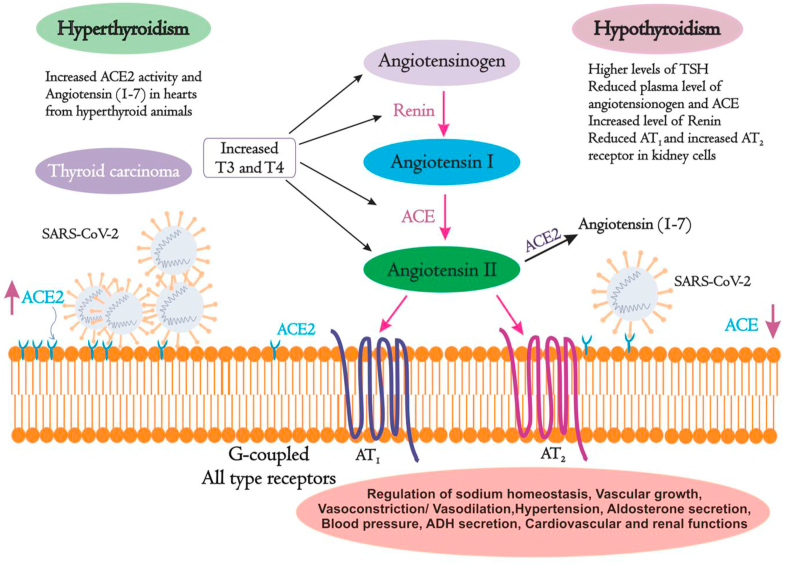
2.3. Renin-angiotensinogen system and thyroid disorders
Blood pressure and sodium homeostasis of electrolytes in the body are coordinately balanced by the renin-angiotensinogen system (RAS) which in turn is influenced by hormones in general and thyroid hormones in particular [119, 120]. RAS also controls cardiovascular and renal functions. In this system, angiotensinogen is cleaved by an aspartyl protease called renin into a decapeptide angiotensin I (AI) which is further cleaved into an octapeptide angiotensin II (AII) by angiotensin-converting enzyme (ACE). ACE cleaves AI into AII by removing the Carboxyl-terminal His-Leu residues from AI [121]. ACE2 is the homolog of ACE that exhibits 60% homology and is resistant to ACE inhibitors [122]. The primary function of ACE2 is to counterbalance the effect of ACE. ACE2 cleaves the Phe from carboxyl-terminal of AII and hydrolyzes into the vasodilator angiotensin (1–7) thereby lowering the blood pressure. AII is the active peptide that acts on the body tissues by binding to G-protein coupled receptors such as AII type receptor 1 (AT1) and AII type receptor 2 (AT2) (Figure 3). ACE2 counteracts the activity of the ACE by reducing the amount of AII and increasing Ang (1–7) which makes ACE2 a promising drug target for treating cardiovascular diseases [123]. All the components of RAS are influenced by the thyroid hormones and thus RAS is deregulated in individuals with altered thyroid states. Moreover, thyroid hormones play an essential role in development of lung and kidney which are the major sites of ACE and renin synthesis. The levels of thyroid hormones in plasma modulate both the synthesis and secretion of RAS components [119, 124, 125]. Though ACE is primarily located on pulmonary vascular endothelium, ACE is also found in tissues of heart, kidney, lungs, liver and brain. ACE2 plays critical role in SARS-CoV-2 infection by facilitating the virus entry into the host [126]. Serum level of ACE2 determines pathophysiological process of viral spread leading to lung injury. Li et. al. 2020 studied the expression of ACE2 in 31 healthy human tissues in both male and females of different age groups. ACE2 was highly expressed in several organs including thyroid, kidney, heart, small intestine, testis and adipose tissues. Nevertheless, a reduced expression of ACE2 was observed in blood vessels, blood, spleen, bone marrow, muscle and brain [127]. On the other hand, activity of ACE is tissue-specific and is highly influenced by thyroid dysfunctions [128]. For instance, treatment with glucocorticoids such as dexamethasone induces the activity of ACE in endothelial cells and rat lungs in vivo [129]. Moreover, during experimental hypothyroidism, significantly reduced ACE activity was found in serum and liver but was unaltered in lungs and kidney. However, in hyperthyroid animals, increased ACE activity was observed in kidney and reduced activity was detected in lungs with no effect on serum and liver [130]. Activity of ACE in serum is found to be increased in patients with hyperthyroidism [131, 132, 133] and reduced in hypothyroid state during treatment with anti-thyroid drugs [122]. A strong positive correlation between serum ACE activity and T3 hormone concentration was observed in patients with thyroid dysfunctions. Moreover, in a recent study, amplified expression of ACE and ACE2 has been witnessed in thyroid carcinoma cases [134].
Figure 3.
Effect of thyroid hormones on Renin-Angiotensinogen System. RAS plays a crucial role in normal human physiology. Thyroid hormones directly regulate the levels of RAS components. Hyper- and hypothyroid individuals show deregulated levels of RAS components. T3: Tri-iodothyronine; T4: Thyroxine; TSH: Thyroid stimulating hormone; ACE: Angiotensin-converting enzyme; AT1: All type receptors 1; AT2: All type receptors 2.
2.4. Viral infections
Farina et al. 2020 reports the pharmacology and kinetics of viral clearance in COVID-19 patients [135]. Evidences for presence of viruses such as retroviruses, human T-lymphotropic virus type 1 (HTLV-1), rubella, mumps and many more in thyroid disorders such as Graves' and Hashimoto's disease [136] advocates the relationship between thyroid gland dysfunction and viral infection. Previously, it was reported that patients who recovered from SARS showed low serum T3 and T4 levels which were due to damage caused by the SARS coronavirus to follicular cells of the thyroid gland [137]. Viral disease caused by hepatitis C virus (HCV) is also associated with rise in incidence and prevalence of thyroiditis [138]. A recent report suggests the prevalence of thyroid dysfunction in HIV infected adults [139]. Neuroendocrine functions of the thyroid gland affect the entire body including the immune system [66]. Earlier studies suggest that people with hypothyroidism have impaired immunity and are at higher risk of viral infection. Patients with hypothyroidism are at a significant risk of Human herpes virus-6 (HHV-6) infection [140, 141, 142]. A significantly increased prevalence of remote cytomegalovirus [143] infection was observed in primary hypothyroid subjects [144]. Increased risk of thyroid cancer is potentially associated with viral infections as suggested by recent meta-analysis studies [105, 145]. According to a recently conducted study, thyroid function abnormalities was observed in COVID-19 patients as compared to control group [146]. A significantly lower level of T3 and TSH was observed in COVID-19 patients compared to control group. Viral attack and damage to thyroid-pituitary axis might be the primary reason for thyroid dysfunction in COVID-19 patients. Interestingly, after recovery, no significant difference in TSH, TT3, T4, FT3 and FT4 levels is observed between COVID-19 patients and control group [147].
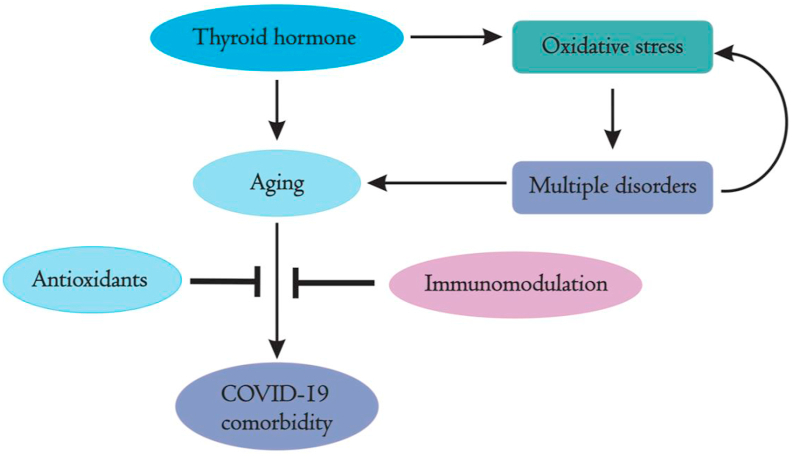
2.5. Oxidative stress and aging
The effect of oxidative stress is evident in many disorders along with hormonal imbalance. The elevated level of reactive oxygen species (ROS) was found during altered hormone level in hyperthyroidism that promotes oxidative stress and downregulates efficiency of antioxidants through the activation of the nuclear factor erythroid 2–related factor 2 (Nrf-2) [148]. Immune response to various pathogens is strongly regulated by oxidative stress and inflammatory processes [149]. The innate immune response was also found to be stimulated by oxidative stress through activation of the toll-like receptors and the nuclear factor kappa-B (NF-κB) during viral infections [150]. Oxidative stress occurs due to imbalance between oxidants and antioxidants. The disproportion between ROS, nitric oxides, lipid peroxidases and exogenous antioxidants (polyphenols, Vitamin E, Vitamin C, and carotenoids), endogenous antioxidants (reduced glutathione, urea, and albumin) as well as endogenous antioxidant enzymes (Superoxide dismutase, Glutathione peroxidase, and Catalase) leads to oxidative stress [151]. In long lasting viral infections such as Human immunodeficiency virus [152] and Epstein-Bar Virus [153], chronically elevated oxidative stress occurs and is associated with damaged immune system [154]. Viral infections cause increase in free radicals production and deplete antioxidants [155]. Both inflammation and endothelial damage found to play an important role in SARS-CoV-2 infection [156]. ROS production in COVID-19 patients was evaluated by cytokine shock with release of Il-2, Il-6, Il-7, TNF-α etc. that leads to hyperinflammation [157]. Oxidative stress-induced increased ROS accumulation is accompanied by reduced level of nicotinamide adenine dinucleotide (NAD+) that is a single substrate for poly-ADP-ribose polymerase and act as a crucial electron transporter in mitochondrial respiration and oxidative phosphorylation [158]. Recognition, addition and removal of ADP-ribosylation are critical in host-virus encounters [159]. SARS-CoV-2 deregulates NAD+ synthesis and utilization as well as induces expression of PARP [160]. Additionally, in response to SARS-CoV-2 infection, ROS has been observed to act as initiator of induced toxic innate immune response in aging population [161]. A critical analysis reveals drastic impact of COVID-19 on elderly population than young and adults [162]. Elderly people suffer from various diseases such as Alzheimer's, pulmonary dysfunction, cardiac malfunctioning, kidney failure, diabetics and others [163]. Most of such age-related diseases are reported as cause or effect of oxidative stress [43]. As shown in Figure 4, oxidative stress is critically associated with multiple disorders including aging under the influence of thyroid hormone that can be associated with COVID-19 comorbidities.
Figure 4.
Intricate relation of thyroid hormone with oxidative stress, age-related multiple disorders and thus with COVID-19. Thyroid hormones are the master regulators of metabolism and its malfunctions induce oxidative stress which in turn leads to multiple disorders including aging. Deregulated thyroid hormones are closely associated with aging and SARS-CoV-2 infection. Administration of anti-oxidations and/or immunomodulators may prove to be helpful in COVID-19 disease management.
Since the endocrine functions in general and thyroid in particular is compromised with progress of age, collectively they influence oxidative stress status in individuals [164, 165, 166]. Increase or decrease in production of free radicals in hypermetabolic and hypometabolic state [167] have been noticed in hyperthyroidism [168, 169] and hypothyroidism [170] respectively. Both hypo- as well as hyperthyroid states are shown to influence antioxidant defence system in brain [171, 172, 173], liver [174, 175], heart [176], kidney [177] and testis [178]. Furthermore, thyroid hormones have been found to prevent lung congestion and improve cardiac functions by reducing the levels of reactive oxygen species in male Wistar rats [179]. Although supplementation of antioxidants do not modify thyroid hormone levels in hypo or hyperthyroid states, but they are capable of rescuing organs from oxidative stress [173, 175, 177, 180, 181, 182, 183, 184, 185]. Reduced oxidative stress was recently observed in rat upon treatment with Vitamin E/curcumin under altered thyroid state via NF-kB signaling [186]. Thus, use of exogenous antioxidants against oxidative stress-induced disorders may help the patients in general and aged population in particular to fight against COVID-19.
3. Management of thyroid dysfunctions during COVID-19
Thyroid dysfunctions are strongly associated with comorbid conditions that are found to be significantly associated with COVID-19 disease outcome which includes diabetes, cardiovascular disorders, chronic kidney disease, and hypertension etc. (Tables 1 and 2). Prevailing disease conditions determine the severity of COVID-19 disease outcome. Thus, management of endocrine diseases that affect the function in other glands including pituitary, thyroid, adrenal and gonad during SARS-CoV-2 infection is a prerequisite [187, 188]. Deregulated levels of cardiac troponin I, brain natriuretic peptide (BNP), D-dimers, and fibrinogen have been used as diagnostic markers to assess the risk of cardiovascular disorders in COVID-19 patients [189] and are equally imbalanced in patient with thyroid disorders. Similarly, Vitamin D is thought to play an important role in SARS-CoV-2 infection. For illustration, people having deficiency of Vitamin D are said to be at a higher risk of SARS-CoV-2 infection [190, 191]. Other conventional link for existing thyroid disorders and COVID-19 disease progression may be acceptable with the fact that low Vitamin D status is significantly associated with thyroid dysfunction [192, 193]. Above argument signifies that Vitamin D supplements may reduce the severity of viral infections such as influenza and SARS-CoV-2 [194,195]. Moreover, mortality of COVID-19 patients has been observed more in elderly patients than in young and middle-aged persons [196]. At the same time, old-aged hyper- or hypothyroid patients are more prevalent to hypertension [197]. Notably, thyroid hormones play indispensable role on cardiovascular system and altered thyroid accompanies congestive heart failure [198]. Oxidants play important pathogenic role in acute and chronic lung diseases [199, 200, 201] and administration of antioxidants may be effective against chronic obstructive pulmonary disease (COPD) [202]. Level of NADPH oxidase-2 (NOX-2)-induced oxidative stress is closely associated with troponin level and play important role in myocardial damage in COVID-19 patients [203]. Antioxidants can be considered as a plausible approach to reduce oxidative stress in viral infections including SARS-CoV-2. The efficacy of antioxidants such as melatonin, Vitamin E and Vitamin C is suggested and reported against obstructive pulmonary disease and multiple newborn diseases including COVID-19 [157,203,204]. Host cell entry of SARS-CoV-2 is facilitated by the spike (S) protein which binds to host ACE2 along with transmembrane serine-protease-2 (TMPRSS2). Differential ACE activity in hypothyroid and hyperthyroid experimental animals and patients elucidates the indispensable role of thyroid hormones in renin-angiotensinogen system that is critically involved in cardiovascular and renal functions [112]. Relation of ACE2 expression level during SARS-CoV-2 infection is intricate. Higher expression of ACE2 favors increased SARS-CoV-2 host cell entry, whereas, reduced expression of ACE2 after infection may lead to severe illness. Yang et. al. 2014, reported that reduced ACE2 resulted in severe H7N9-induced lung injury [205]. At the same time, recombinant ACE was found to improve pulmonary hemodynamics in human pulmonary arterial hypertension [206]. Studies suggest the role of genetic background as well as the level of hormones like estrogen in differential expression of ACE2 in the body [207, 208]. Level of ACE and ACE2 is the determining factor for the regulated functioning of RAS. Thyroid hormones play critical role in defining the expression of ACE and ACE2 in plasma and different tissues that in turn might contribute in SARS-CoV-2 infection as well as disease severity. Increased expression of ACE2 and other components of RAS in hyperthyroidism, suggest the modulatory role of thyroid hormones on RAS-regulated metabolic pathways (Figure 3) which advocates the possible role of thyroid hormone in SARS-CoV-2 outcome. Levels of thyroid hormones T3, T4 and TSH not only govern the thyroid condition but also help to assess the function of other organs of the body. Moreover, reports suggest that increased incidence of subclinical thyroid dysfunctions is noticed in elderly people [209]. Thyroid hormone has been suggested for treatment of critically ill COVID-19 patients based on the experimental evidence that deregulated thyroid hormone metabolism occurs during acute illness such as sepsis, trauma and myocardial infarction [210, 211]. According to a recent report, thrombocytopenia was observed in COVID-19 patient having autoimmune hypothyroidism or hyperthyroidism after treatment with amoxicillin–clavulanic acid, heparin and oxygen [212]. Thyroid hormone related dysfunctions including immune response may keep individuals at a higher risk for adverse outcome for SARS-CoV-2 infection. Nevertheless, association of COVID-19 and thyroid dysfunctions is not well studied and documented due to which there are no guidelines from World Health Organization [1] for assessment of thyroid function during COVID-19 treatment. However, reports and editorials suggest the possible connection of existing thyroid-related disorders and SARS-CoV-2 infection [213, 214, 215, 216]. Therefore, like other risk factors, deregulated thyroid may significantly influence the severity of SARS-CoV-2 infection.
4. Conclusions
Since thyroid hormones play major role in metabolism, growth and development of human body, dysfunctions due to thyroid hormone may have significant clinical consequences on immune response and thus on human health. Increased prevalence of other comorbidities has been observed in individuals with abnormal thyroid that keep them at a higher risk for susceptibility towards bacterial or viral infections. Thus, deregulated thyroid hormone may have considerable risk in aggravating the infection and spread of SARS-CoV-2. Intricate association of thyroid hormone and oxidative stress suggest that supplementation of antioxidants in elderly COVID-19 patients with thyroid diseases may strengthen the immune system. Moreover, in view of existing global disaster due to COVID-19, monitoring of thyroid hormones may help in understanding the pathogenesis of COVID-19.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Council for Scientific and Industrial Research and Department of Biotechnology, Government of India, New Delhi.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.WHO . 2020. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19whoint/ [Google Scholar]
- 2.Pathak S.K., Pandey S., Pandey A., Salunke A.A., Thivari P., Ratna H.V.K., Chawla J. Focus on uncommon symptoms of COVID-19: potential reason for spread of infection. Diabetes Metab Syndr. 2020;14(6):1873–1874. doi: 10.1016/j.dsx.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomgarden Z.T. Diabetes and COVID-19. J. Diabetes. 2020;12(4):347–348. doi: 10.1111/1753-0407.13027. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., Zhang J., Zhao C. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J. Med. Virol. 2020;92:1902–1914. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D., Xu K.J., Wang X.Y., Gu J.Q., Zhang S.Y., Ye C.Y., Jin C.L., Lu Y.F., Yu X., Yu X.P., Huang J.R., Xu K.L., Ni Q., Yu C.B., Zhu B., Li Y.T., Liu J., Zhao H., Zhang X., Yu L., Guo Y.Z., Su J.W., Tao J.J., Lang G.J., Wu X.X., Wu W.R., Qv T.T., Xiang D.R., Yi P., Shi D., Chen Y., Ren Y., Qiu Y.Q., Li L.J., Sheng J., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Chen Z., Nie Y., Ma Y., Guo Q., Dai X. Prognostic symptoms on COVID-19 severity. J. Med. Internet Res. 2020;22(6) doi: 10.2196/19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B., Zhang H.Y., Sun W., Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagcchi S. Hypothyroidism in India: more to be done. Lancet Diabet Endocrinol. 2014;2(10):778. doi: 10.1016/S2213-8587(14)70208-6. [DOI] [PubMed] [Google Scholar]
- 16.Marwaha R.K., Tandon N., Ganie M.A., Kanwar R., Sastry A., Garg M.K., Bhadra K., Singh S. Status of thyroid function in Indian adults: two decades after universal salt iodization. J. Assoc. Phys. India. 2012;60:32–36. [PubMed] [Google Scholar]
- 17.Unnikrishnan A.G., Menon U.V. Thyroid disorders in India: an epidemiological perspective. Indian J. Endocrinol. Metabol. 2011;15(Suppl 2):S78–81. doi: 10.4103/2230-8210.83329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doubleday A.R., Sippel R.S. Hyperthyroidism. Gland Surg. 2020;9(1):124–135. doi: 10.21037/gs.2019.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teeling H., Fuchs B.M., Becher D., Klockow C., Gardebrecht A., Bennke C.M., Kassabgy M., Huang S., Mann A.J., Waldmann J., Weber M., Klindworth A., Otto A., Lange J., Bernhardt J., Reinsch C., Hecker M., Peplies J., Bockelmann F.D., Callies U., Gerdts G., Wichels A., Wiltshire K.H., Glockner F.O., Schweder T., Amann R. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science. 2012;336(6081):608–611. doi: 10.1126/science.1218344. [DOI] [PubMed] [Google Scholar]
- 20.Premawardhana L.D., Lazarus J.H. Management of thyroid disorders. Postgrad. Med. 2006;82(971):552–558. doi: 10.1136/pgmj.2006.047290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velayutham K., Selvan S.S., Unnikrishnan A.G. Prevalence of thyroid dysfunction among young females in a South Indian population. Ind. J. Endocrinol. Metabol. 2015;19(6):781–784. doi: 10.4103/2230-8210.167546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Rodriguez L.A., Felici-Giovanini M.E., Haddock L. Thyroid dysfunction in an adult female population: a population-based study of Latin American Vertebral Osteoporosis Study (LAVOS) - Puerto Rico site. Puert. Rico Health Sci. J. 2013;32(2):57–62. [PMC free article] [PubMed] [Google Scholar]
- 23.Kostoglou-Athanassiou I., Ntalles K. Hypothyroidism - new aspects of an old disease. Hippokratia. 2010;14(2):82–87. [PMC free article] [PubMed] [Google Scholar]
- 24.Paudel K. Prevalence and clinical characteristics of hypothyroidism in a population undergoing maintenance hemodialysis. J. Clin. Diagn. Res. 2014;8(4):MC01–4. doi: 10.7860/JCDR/2014/7821.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Shafie K.T. Clinical presentation of hypothyroidism. J. Fam. Commun. Med. 2003;10(1):55–58. [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta D., Garg A., Khandelwal D., Kalra S., Mittal S., Chittawar S. Thyroid symptomatology across the spectrum of hypothyroidism and impact of levothyroxine supplementation in patients with severe primary hypothyroidism. Ind. J. Endocrinol. Metabol. 2019;23(3):373–378. doi: 10.4103/ijem.IJEM_78_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul J., Dasgupta S. Co-morbidities in hypothyroid patients in a tertiary health care hospital in India. J. Thyroid Disord. Ther. 2012;1(2):1–3. [Google Scholar]
- 28.Sethi B., Barua S., Raghavendra M.S., Gotur J., Khandelwal D., Vyas U. The thyroid registry: clinical and hormonal characteristics of adult Indian patients with hypothyroidism. Ind. J. Endocrinol. Metabol. 2017;21(2):302–307. doi: 10.4103/ijem.IJEM_387_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandt F., Thvilum M., Hegedus L., Brix T.H. Hyperthyroidism is associated with work disability and loss of labour market income. A Danish register-based study in singletons and disease-discordant twin pairs. Eur. J. Endocrinol. 2015;173(5):595–602. doi: 10.1530/EJE-15-0306. [DOI] [PubMed] [Google Scholar]
- 30.Thvilum M., Brandt F., Brix T.H., Hegedus L. Hypothyroidism is a predictor of disability pension and loss of labor market income: a Danish register-based study. J. Clin. Endocrinol. Metabol. 2014;99(9):3129–3135. doi: 10.1210/jc.2014-1407. [DOI] [PubMed] [Google Scholar]
- 31.Nexo M.A., Watt T., Pedersen J., Bonnema S.J., Hegedus L., Rasmussen A.K., Feldt-Rasmussen U., Bjorner J.B. Increased risk of long-term sickness absence, lower rate of return to work, and higher risk of unemployment and disability pensioning for thyroid patients: a Danish register-based cohort study. J. Clin. Endocrinol. Metabol. 2014;99(9):3184–3192. doi: 10.1210/jc.2013-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thvilum M., Brandt F., Almind D., Christensen K., Brix T.H., Hegedus L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid. 2014;24(5):802–808. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 33.Weetman A.P. Autoimmune thyroid disease: propagation and progression. Eur. J. Endocrinol. 2003;148(1):1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 34.Folkestad L., Brandt F., Brix T., Vogsen M., Bastholt L., Grupe P., Krogh Petersen J., Hegedus L. Total thyroidectomy for thyroid cancer followed by thyroid storm due to thyrotropin receptor antibody stimulation of metastatic thyroid tissue. Eur. Thyroid. J. 2017;6(5):276–280. doi: 10.1159/000479061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnema S.J., Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr. Rev. 2012;33(6):920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 36.Radetti G., Zavallone A., Gentili L., Beck-Peccoz P., Bona G. Foetal and neonatal thyroid disorders. Minerva Pediatr. 2002;54(5):383–400. [PubMed] [Google Scholar]
- 37.Ordooei M., Rablei A., Soleimanizad R., Mirjalili F. Prevalence of permanent congenital hypothyroidism in children in Yazd, Central Iran. Iran. J. Public Health. 2013;42(9):1016–1020. [PMC free article] [PubMed] [Google Scholar]
- 38.Kibirige D., Luzinda K., Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(3):1–5. doi: 10.1186/1756-6614-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh J.P., Shiels L., Lim E.M., Bhagat C.I., Ward L.C., Stuckey B.G., Dhaliwal S.S., Chew G.T., Bhagat M.C., Cussons A.J. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J. Clin. Endocrinol. Metabol. 2003;88(10):4543–4550. doi: 10.1210/jc.2003-030249. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal N., Razvi S. Thyroid and aging or the aging thyroid? An evidence-based analysis of the literature. J. Thyroid Res. 2013:481287. doi: 10.1155/2013/481287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boucai L., Surks M.I. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin. Endocrinol. 2009;70(5):788–793. doi: 10.1111/j.1365-2265.2008.03390.x. [DOI] [PubMed] [Google Scholar]
- 42.Venditti P., Di Meo S. Thyroid hormone-induced oxidative stress. Cell. Mol. Life Sci. 2006;63(4):414–434. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romano A.D., Serviddio G., de Matthaeis A., Bellanti F., Vendemiale G. Oxidative stress and aging. J. Nephrol. 2010;23(Suppl 15):S29–S36. [PubMed] [Google Scholar]
- 45.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxidative Med. Cell Long. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw P.X., Werstuck G., Chen Y. Oxidative stress and aging diseases. Oxidative Med. Cell. Long. 2014:569146. doi: 10.1155/2014/569146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan B.L., Norhaizan M.E., Liew W.P., Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 2018;9(1162):1–28. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laulund A.S., Nybo M., Brix T.H., Abrahamsen B., Jorgensen H.L., Hegedus L. Duration of thyroid dysfunction correlates with all-cause mortality: the openthyro Register Cohort. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn R.W., Macdonald T.M., Jung R.T., Morris A.D., Leese G.P. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J. Clin. Endocrinol. Metabol. 2006;91(6):2159–2164. doi: 10.1210/jc.2005-1833. [DOI] [PubMed] [Google Scholar]
- 50.Brandt F., Almind D., Christensen K., Green A., Brix T.H., Hegedus L. Excess mortality in hyperthyroidism: the influence of preexisting comorbidity and genetic confounding: a Danish nationwide register-based cohort study of twins and singletons. J. Clin. Endocrinol. Metabol. 2012;97(11):4123–4129. doi: 10.1210/jc.2012-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair A., Jayakumari C., Jabbar P.K., Jayakumar R.V., Raizada N., Gopi A., George G.S., Seena T.P. Prevalence and associations of hypothyroidism in Indian patients with type 2 diabetes mellitus. J. Thyroid Res. 2018;5386129:1–7. doi: 10.1155/2018/5386129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han C., He X., Xia X., Li Y., Shi X., Shan Z., Teng W. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0135233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorr M., Volzke H. Cardiovascular morbidity and mortality in thyroid dysfunction. Minerva Endocrinol. 2005;30(4):199–216. [PubMed] [Google Scholar]
- 54.Osman F., Franklyn J.A., Holder R.L., Sheppard M.C., Gammage M.D. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J. Am. Coll. Cardiol. 2007;49(1):71–81. doi: 10.1016/j.jacc.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 55.Szabo J., Foris G., Mezosi E., Nagy E.V., Paragh G., Sztojka I., Leovey A. Parameters of respiratory burst and arachidonic acid metabolism in polymorphonuclear granulocytes from patients with various thyroid diseases. Exp. Clin. Endocrinol. Diabetes. 1996;104(2):172–176. doi: 10.1055/s-0029-1211440. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez V., Videla L.A. On the mechanism of thyroid hormone-induced respiratory burst activity in rat polymorphonuclear leukocytes. Free Radic. Biol. Med. 1995;19(3):359–363. doi: 10.1016/0891-5849(95)00016-q. [DOI] [PubMed] [Google Scholar]
- 57.Goldman M.B., Monson R.R., Maloof F. Cancer mortality in women with thyroid disease. Can. Res. 1990;50(8):2283–2289. [PubMed] [Google Scholar]
- 58.Khan S.R., Chaker L., Ruiter R., Aerts J.G., Hofman A., Dehghan A., Franco O.H., Stricker B.H., Peeters R.P. Thyroid function and cancer risk: the rotterdam study. J. Clin. Endocrinol. Metabol. 2016;101(12):5030–5036. doi: 10.1210/jc.2016-2104. [DOI] [PubMed] [Google Scholar]
- 59.Mansourabadi A.H., Sadeghalvad M., Mohammadi-Motlagh H.R., Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci. 2020;118185:1–14. doi: 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imura H., Fukata J., Mori T. Cytokines and endocrine function: an interaction between the immune and neuroendocrine systems. Clin. Endocrinol. 1991;35(2):107–115. doi: 10.1111/j.1365-2265.1991.tb03506.x. [DOI] [PubMed] [Google Scholar]
- 61.Gaillard R.C. Immuno-endocrine interactions at the hypothalamo-hypophyseal level. Ann. Endocrinol. 1995;56(6):561–566. [PubMed] [Google Scholar]
- 62.De Vito P., Incerpi S., Pedersen J.Z., Luly P., Davis F.B., Davis P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 63.Mascanfroni I., Montesinos Mdel M., Susperreguy S., Cervi L., Ilarregui J.M., Ramseyer V.D., Masini-Repiso A.M., Targovnik H.M., Rabinovich G.A., Pellizas C.G. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22(4):1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- 64.Klein J.R. The immune system as a regulator of thyroid hormone activity. Exp. Biol. Med. 2006;231(3):229–236. doi: 10.1177/153537020623100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klecha A.J., Genaro A.M., Gorelik G., Barreiro Arcos M.L., Silberman D.M., Schuman M., Garcia S.I., Pirola C., Cremaschi G.A. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 2006;189(1):45–55. doi: 10.1677/joe.1.06137. [DOI] [PubMed] [Google Scholar]
- 66.Klecha A.J., Genaro A.M., Lysionek A.E., Caro R.A., Coluccia A.G., Cremaschi G.A. Experimental evidence pointing to the bidirectional interaction between the immune system and the thyroid axis. Int. J. Immunopharm. 2000;22(7):491–500. doi: 10.1016/s0192-0561(00)00012-6. [DOI] [PubMed] [Google Scholar]
- 67.Calder E.A., Penhale W.J., McLeman D., Barnes E.W., Irvine W.J. Lymphocyte-dependent antibody-mediated cytotoxicity in Hashimoto thyroiditis. Clin. Exp. Immunol. 1973;14(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- 68.Perrotta C., Buldorini M., Assi E., Cazzato D., De Palma C., Clementi E., Cervia D. The thyroid hormone triiodothyronine controls macrophage maturation and functions: protective role during inflammation. Am. J. Pathol. 2014;184(1):230–247. doi: 10.1016/j.ajpath.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Montesinos M.D.M., Pellizas C.G. Thyroid hormone action on innate immunity. Front. Endocrinol. 2019;10(350):1–9. doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jara E.L., Munoz-Durango N., Llanos C., Fardella C., Gonzalez P.A., Bueno S.M., Kalergis A.M., Riedel C.A. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol. Lett. 2017;184:76–83. doi: 10.1016/j.imlet.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Schoenfeld P.S., Myers J.W., Myers L., LaRocque J.C. Suppression of cell-mediated immunity in hypothyroidism. South. Med. J. 1995;88(3):347–349. doi: 10.1097/00007611-199503000-00019. [DOI] [PubMed] [Google Scholar]
- 72.Pillay K. Congenital hypothyroidism and immunodeficiency: evidence for an endocrine-immune interaction. J. Pediatr. Endocrinol. Metab. 1998;11(6):757–761. doi: 10.1515/jpem.1998.11.6.757. [DOI] [PubMed] [Google Scholar]
- 73.Hrycek A. Functional characterization of peripheral blood neutrophils in patients with primary hypothyroidism. Folia Biol. (Praha) 1993;39(6):304–310. [PubMed] [Google Scholar]
- 74.von Hafe M., Neves J.S., Vale C., Borges-Canha M., Leite-Moreira A. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr. Connect. 2019;8(5):R76–R90. doi: 10.1530/EC-19-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toft A.D., Boon N.A. Thyroid disease and the heart. Heart. 2000;84(4):455–460. doi: 10.1136/heart.84.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buschmann I.R., Bondke A., Elgeti T., Kuhnle Y., Dietz R., Mockel M. Positive cardiac troponin I and T and chest pain in a patient with iatrogenic hypothyroidism and no coronary artery disease. Int. J. Cardiol. 2007;115(2):e83–e85. doi: 10.1016/j.ijcard.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Ertugrul D.T., Gursoy A., Sahin M., Unal A.D., Pamuk B., Berberoglu Z., Ayturk S., Tutuncu N.B., Demirag N.G. Evaluation of brain natriuretic peptide levels in hyperthyroidism and hypothyroidism. J. Natl. Med. Assoc. 2008;100(4):401–405. doi: 10.1016/s0027-9684(15)31272-4. [DOI] [PubMed] [Google Scholar]
- 78.Chadarevian R., Bruckert E., Ankri A., Beucler I., Giral P., Turpin G. Relationship between thyroid hormones and plasma D-dimer levels. Thromb. Haemostasis. 1998;79(1):99–103. [PubMed] [Google Scholar]
- 79.Chadarevian R., Bruckert E., Giral P., Turpin G. Relationship between thyroid hormones and fibrinogen levels. Blood Coagul. Fibrinolysis: Int. J. Haemostasis Thromb. 1999;10(8):481–486. doi: 10.1097/00001721-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Cappola A.R., Ladenson P.W. Hypothyroidism and atherosclerosis. J. Clin. Endocrinol. Metabol. 2003;88(6):2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 81.Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;390534:1–9. doi: 10.1155/2013/390534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hage M., Zantout M.S., Azar S.T. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011;439463:1–7. doi: 10.4061/2011/439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coller F.A., Huggins C.B. Effect of hyperthyroidism upon diabetes mellitus: striking improvement in diabetes mellitus from thyroidectomy. Ann. Surg. 1927;86(6):877–884. doi: 10.1097/00000658-192712000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ezeani I., Ogbonna S. Burden of thyroid dysfunction among type 2 Diabetes mellitus patients with emphasis on its prevalence and pattern of presentation: a Case Controlled Study. Curr. Diabetes Rev. 2020 doi: 10.2174/1573399816666200206113203. [DOI] [PubMed] [Google Scholar]
- 85.Elgazar E.H., Esheba N.E., Shalaby S.A., Mohamed W.F. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metabol. Syndrome. 2019;13(4):2513–2517. doi: 10.1016/j.dsx.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Kadiyala R., Peter R., Okosieme O.E. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int. J. Clin. Pract. 2010;64(8):1130–1139. doi: 10.1111/j.1742-1241.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 87.Vondra K., Vrbikova J., Dvorakova K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. 2005;30(4):217–236. [PubMed] [Google Scholar]
- 88.Ogbonna S.U., Ezeani I.U., Okafor C.I., Chinenye S. Association between glycemic status and thyroid dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2019;12:1113–1122. doi: 10.2147/DMSO.S204836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H.M., Feng Q.W., Niu Y.X., Su Q., Wang X. Thyroid nodules in type 2 diabetes mellitus. Curr. Med. Sci. 2019;39(4):576–581. doi: 10.1007/s11596-019-2076-5. [DOI] [PubMed] [Google Scholar]
- 90.Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med. Hypotheses. 2010;74(1):35–36. doi: 10.1016/j.mehy.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 91.Rezzonico J.N., Rezzonico M., Pusiol E., Pitoia F., Niepomniszcze H. Increased prevalence of insulin resistance in patients with differentiated thyroid carcinoma. Metab. Syndr. Relat. Disord. 2009;7(4):375–380. doi: 10.1089/met.2008.0062. [DOI] [PubMed] [Google Scholar]
- 92.Arduc A., Isik S., Ozuguz U., Tutuncu Y.A., Kucukler F.K., Ozcan H.N., Berker D., Guler S. Relationship between thyroid nodules and non-functioning adrenal incidentalomas and their association with insulin resistance. Endocr. Res. 2014;39(3):99–104. doi: 10.3109/07435800.2013.840653. [DOI] [PubMed] [Google Scholar]
- 93.Lencu C., Alexescu T., Petrulea M., Lencu M. Respiratory manifestations in endocrine diseases. Clujul Med. 2016;89(4):459–463. doi: 10.15386/cjmed-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zimmerman L. Murray and Nadel’s textbook of respiratory medicine. sixth ed. Elsevier Saunders; Philadelphia: 2016. Pulmonary complications of endocrine diseases; pp. 1671–1678. chapter 95. [Google Scholar]
- 95.Ali E.R. Assessment of functional lung impairment in patients with thyroid disorders. Egypt J. Bronchol. 2016;10:337–347. [Google Scholar]
- 96.Chaitanya P.S.K., Suresh V., Mohan A., Sachan A. Respiratory dysfunction in hypothyroidism. J. Clin. Sci. Res. 2019;8:89–94. [Google Scholar]
- 97.Roel S., Punyabati O., Prasad L., Salam R., Ningshen K., Shimray A.J., Sangma M.A., Hungyo H., Devi A.N. Assessment of functional lung impairment in hypothyroidism. IOSR J. Dental Med. Sci. (IOSR-JDMS) 2014;13(9):4–7. [Google Scholar]
- 98.Birring S.S., Morgan A.J., Prudon B., McKeever T.M., Lewis S.A., Falconer Smith J.F., Robinson R.J., Britton J.R., Pavord I.D. Respiratory symptoms in patients with treated hypothyroidism and inflammatory bowel disease. Thorax. 2003;58(6):533–536. doi: 10.1136/thorax.58.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadek S.H., Khalifa W.A., Azoz A.M. Pulmonary consequences of hypothyroidism. Ann. Thorac. Med. 2017;12(3):204–208. doi: 10.4103/atm.ATM_364_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iyer S.K., Menon S.K., Bahuleyan B. An analysis of dynamic pulmonary functions of hypothyroid patients. J. Clin. Diagn. Res. 2017;11(3):CC10–CC12. doi: 10.7860/JCDR/2017/24653.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez F.J., Bermudez-Gomez M., Celli B.R. Hypothyroidism. A reversible cause of diaphragmatic dysfunction. Chest. 1989;96(5):1059–1063. doi: 10.1378/chest.96.5.1059. [DOI] [PubMed] [Google Scholar]
- 102.Laroche C.M., Cairns T., Moxham J., Green M. Hypothyroidism presenting with respiratory muscle weakness. Am. Rev. Respir. Dis. 1988;138(2):472–474. doi: 10.1164/ajrccm/138.2.472. [DOI] [PubMed] [Google Scholar]
- 103.Weiner M., Chausow A., Szidon P. Reversible respiratory muscle weakness in hypothyroidism. Br. J. Dis. Chest. 1986;80(4):391–395. doi: 10.1016/0007-0971(86)90093-8. [DOI] [PubMed] [Google Scholar]
- 104.Siafakas N.M., Salesiotou V., Filaditaki V., Tzanakis N., Thalassinos N., Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189–194. doi: 10.1378/chest.102.1.189. [DOI] [PubMed] [Google Scholar]
- 105.Saito I., Ito K., Saruta T. Hypothyroidism as a cause of hypertension. Hypertension. 1983;5(1):112–115. doi: 10.1161/01.hyp.5.1.112. [DOI] [PubMed] [Google Scholar]
- 106.Berta E., Lengyel I., Halmi S., Zrinyi M., Erdei A., Harangi M., Pall D., Nagy E.V., Bodor M. Hypertension in thyroid disorders. Front. Endocrinol. 2019;10(482):1–11. doi: 10.3389/fendo.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G., investigators I.C. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:1–12. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basu G., Mohapatra A. Interactions between thyroid disorders and kidney disease. Ind. J. Endocrinol. Metabol. 2012;16(2):204–213. doi: 10.4103/2230-8210.93737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mariani L.H., Berns J.S. The renal manifestations of thyroid disease. J. Am. Soc. Nephrol. 2012;23(1):22–26. doi: 10.1681/ASN.2010070766. [DOI] [PubMed] [Google Scholar]
- 110.Li Z.W.Y. Chronic kidney disease caused by hypothyroidism. J. Integr. Nephrol. Androl. 2015;2:93–95. [Google Scholar]
- 111.Rhee C.M. The interaction between thyroid and kidney disease: an overview of the evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2016;23(5):407–415. doi: 10.1097/MED.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran T.V., Kitahara C.M., de Vathaire F., Boutron-Ruault M.C., Journy N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocr. Relat. Cancer. 2020;27(4):245–259. doi: 10.1530/ERC-19-0417. [DOI] [PubMed] [Google Scholar]
- 113.Yuan S., Kar S., Vithayathil M., Carter P., Mason A.M., Burgess S., Larsson S.C. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: a two-sample Mendelian randomization study. Int. J. Cancer. 2020;147:1895–1903. doi: 10.1002/ijc.32988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gomez-Izquierdo J., Filion K.B., Boivin J.F., Azoulay L., Pollak M., Yu O.H.Y. Subclinical hypothyroidism and the risk of cancer incidence and cancer mortality: a systematic review. BMC Endocr. Disord. 2020;20(83):1–10. doi: 10.1186/s12902-020-00566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basak E.A., van der Meer J.W.M., Hurkmans D.P., Schreurs M.W.J., Oomen-de Hoop E., van der Veldt A.A.M., Bins S., Joosse A., Koolen S.L.W., Debets R., Peeters R.P., Aerts J., Mathijssen R.H.J., Medici M. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30(7):966–973. doi: 10.1089/thy.2019.0726. [DOI] [PubMed] [Google Scholar]
- 116.Lazzara D.R., Zarkhin S.G., Rubenstein S.N., Glick B.P. Melanoma and thyroid carcinoma: our current understanding. J. Clin. Aesthet. Dermatol. 2019;l12(9):39–41. [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y., Zhou R., Wang J. Relationship between hypothyroidism and endometrial cancer. Aging Dis. 2019;10(1):190–196. doi: 10.14336/AD.2018.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rostkowska O., Spychalski P., Dobrzycka M., Wilczynski M., Lachinski A.J., Obolonczyk L., Sworczak K., Kobiela J. Effects of thyroid hormone imbalance on colorectal cancer carcinogenesis and risk - a systematic review. Endokrynol. Pol. 2019;70(2):190–197. doi: 10.5603/EP.a2019.0007. [DOI] [PubMed] [Google Scholar]
- 119.Vargas F., Rodriguez-Gomez I., Vargas-Tendero P., Jimenez E., Montiel M. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J. Endocrinol. 2012;213(1):25–36. doi: 10.1530/JOE-11-0349. [DOI] [PubMed] [Google Scholar]
- 120.Lavoie J.L., Sigmund C.D. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144(6):2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 121.Sparks M.A., Crowley S.D., Gurley S.B., Mirotsou M., Coffman T.M. Classical Renin-Angiotensin system in kidney physiology. Compr. Physiol. 2014;4(3):1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chappel M.C., Ferrario C.M. ACE and ACE2: their role to balance the expression of angiotensin II and angiotensin-(1-7) Kidney Int. 2006;70(1):8–10. doi: 10.1038/sj.ki.5000321. [DOI] [PubMed] [Google Scholar]
- 123.Chamsi-Pasha M.A., Shao Z., Tang W.H. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Curr. Heart Fail. Rep. 2014;11(1):58–63. doi: 10.1007/s11897-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee D.S., Chung J.K., Cho B.Y., Koh C.S., Lee M. Changes of serum angiotensin-converting enzyme activity during treatment of patients with Graves' disease. Korean J. Intern. Med. 1986;1(1):104–112. doi: 10.3904/kjim.1986.1.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilbert M.T., Sun J., Yan Y., Oddoux C., Lazarus A., Tansey W.P., Lavin T.N., Catanzaro D.F. Renin gene promoter activity in GC cells is regulated by cAMP and thyroid hormone through Pit-1-dependent mechanisms. J. Biol. Chem. 1994;269(45):28049–28054. [PubMed] [Google Scholar]
- 126.Liu M., Wang T., Zhou Y., Zhao Y., Zhang Y., Li J. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J .Transl. Int. Med. 2020;8(1):9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9(45):1–7. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carneiro-Ramos M.S., Silva V.B., Santos R.A., Barreto-Chaves M.L. Tissue-specific modulation of angiotensin-converting enzyme (ACE) in hyperthyroidism. Peptides. 2006;27(11):2942–2949. doi: 10.1016/j.peptides.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 129.Mendelsohn F.A., Lloyd C.J., Kachel C., Funder J.W. Induction by glucocorticoids of angiotensin converting enzyme production from bovine endothelial cells in culture and rat lung in vivo. J. Clin. Invest. 1982;70(3):684–692. doi: 10.1172/JCI110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montiel M., Ruiz M., Jimenez E., Morell M. Angiotensin converting enzyme in hyper- and hypothyroid rats. Horm. Metab. Res. 1987;19(2):90–92. doi: 10.1055/s-2007-1011749. [DOI] [PubMed] [Google Scholar]
- 131.Silverstein E., Schussler G.C., Friedland J. Elevated serum angiotensin-converting enzyme in hyperthyroidism. Am. J. Med. 1983;75(2):233–236. doi: 10.1016/0002-9343(83)91198-1. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura Y., Takeda T., Ishii M., Nishiyama K., Yamakada M., Hirata Y., Kimura K., Murao S. Elevation of serum angiotensin-converting enzyme activity in patients with hyperthyroidism. J. Clin. Endocrinol. Metabol. 1982;55(5):931–934. doi: 10.1210/jcem-55-5-931. [DOI] [PubMed] [Google Scholar]
- 133.Yotsumoto H., Imai Y., Kuzuya N., Uchimura H., Matsuzaki F. Increased levels of serum angiotensin-converting enzyme activity in hyperthyroidism. Ann. Intern. Med. 1982;96(3):326–328. doi: 10.7326/0003-4819-96-3-326. [DOI] [PubMed] [Google Scholar]
- 134.Narayan S.S., Lorenz K., Ukkat J., Hoang-Vu C., Trojanowicz B. Angiotensin converting enzymes ACE and ACE2 in thyroid cancer progression. Neoplasma. 2020;67(2):402–409. doi: 10.4149/neo_2019_190506N405. [DOI] [PubMed] [Google Scholar]
- 135.Farina N., Ramirez G.A., De Lorenzo R., Di Filippo L., Conte C., Ciceri F., Manfredi A.A., Rovere-Querini P. COVID-19: pharmacology and kinetics of viral clearance. Pharmacol. Res. 2020;161:105114. doi: 10.1016/j.phrs.2020.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Desailloud R., Hober D. Viruses and thyroiditis: an update. Virol. J. 2009;6(5):1–14. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei L., Sun S., Xu C.H., Zhang J., Xu Y., Zhu H., Peh S.C., Korteweg C., McNutt M.A., Gu J. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007;38(1):95–102. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen Y., Wang X.L., Xie J.P., Shao J.G., Lu Y.H., Zhang S., Qin G. Thyroid disturbance in patients with chronic hepatitis C infection: a systematic review and meta-analysis. J. Gastrointest. Liver Dis. 2016;25(2):227–234. doi: 10.15403/jgld.2014.1121.252.chc. [DOI] [PubMed] [Google Scholar]
- 139.Ji S., Jin C., Hoxtermann S., Fuchs W., Xie T., Lu X., Wu H., Cheng L., Skaletz-Rorowski A., Brockmeyer N.H., Wu N. Prevalence and influencing factors of thyroid dysfunction in HIV-infected patients. BioMed Res. Int. 2016;2016:3874257. doi: 10.1155/2016/3874257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seyyedi N., Dehbidi G.R., Karimi M., Asgari A., Esmaeili B., Zare F., Farhadi A., Dabbaghmanesh M.H., Saki F., Behzad-Behbahani A. Human herpesvirus 6A active infection in patients with autoimmune Hashimoto's thyroiditis. Braz. J. Infect. Dis. 2019;23(6):435–440. doi: 10.1016/j.bjid.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varedi M., Moattari A., Amirghofran Z., Karamizadeh Z., Feizi H. Effects of hypo- and hyperthyroid states on herpes simplex virus infectivity in the rat. Endocr. Res. 2014;39(2):50–55. doi: 10.3109/07435800.2013.808208. [DOI] [PubMed] [Google Scholar]
- 142.Di Crescenzo V., D'Antonio A., Tonacchera M., Carlomagno C., Vitale M. Human herpes virus associated with Hashimoto's thyroiditis. Infez Med. 2013;21(3):224–228. [PubMed] [Google Scholar]
- 143.Meurer M.C., Mees M., Mariano L.N.B., Boeing T., Somensi L.B., Mariott M., da Silva R., Dos Santos A.C., Longo B., Santos Franca T.C., Klein-Junior L.C., de Souza P., de Andrade S.F., da Silva L.M. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr. Res. 2019;66:95–106. doi: 10.1016/j.nutres.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 144.Chakraborty J. Viral and other infections in community and primary hypothyroidism – cause or coincidence? Clin. Med. (Lond) 2019;19:126. [Google Scholar]
- 145.Mostafaei S., Keshavarz M., Sadri Nahand J., Farhadi Hassankiadeh R., Moradinazar M., Nouri M., Babaei F., Ahadi M., Payandeh M., Salari Esker A., Hajighadimi S., Mirzaei H., Moghoofei M. Viral infections and risk of thyroid cancer: a systematic review and empirical bayesian meta-analysis. Pathol. Res. Pract. 2020;216(4):152855. doi: 10.1016/j.prp.2020.152855. [DOI] [PubMed] [Google Scholar]
- 146.Weibin Wang X.S., Ding Yongfeng, Fan Weina, Su Junwei, Chen Zhendong, Zhao Hong, Xu Kaijin, Ni Qin, Xu Xiaowei, Qiu Yunqing, Teng Lisong. Thyroid function abnormalities in COVID-19 patients. MedRXiv. 2020 doi: 10.3389/fendo.2020.623792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen M., Zhou W., Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2020 doi: 10.1089/thy.2020.0363. (In press) [DOI] [PubMed] [Google Scholar]
- 148.Costilla M., Macri Delbono R., Klecha A., Cremaschi G.A., Barreiro Arcos M.L. Oxidative stress produced by hyperthyroidism status induces the antioxidant enzyme transcription through the activation of the Nrf-2 factor in lymphoid tissues of Balb/c mice. Oxidative Med. Cell Long. 2019;2019:7471890. doi: 10.1155/2019/7471890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poultry Sci. 2019;98(10):4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- 150.Caruso A.A., Del Prete A., Lazzarino A.I. Hydrogen peroxide and viral infections: a literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypotheses. 2020;144:109910. doi: 10.1016/j.mehy.2020.109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chattopadhyay S., Sahoo D.K., Subudhi U., Chainy G.B.N. Differential expression profiles of antioxidant enzymes and glutathione redox status in hyperthyroid rats: A temporal analysis. Comp. Biochem. Physiol. Part C. 2007;146:383–391. doi: 10.1016/j.cbpc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 152.Holley R.J., Ellison S.M., Fil D., O'Leary C., McDermott J., Senthivel N., Langford-Smith A.W.W., Wilkinson F.L., D'Souza Z., Parker H., Liao A., Rowlston S., Gleitz H.F.E., Kan S.H., Dickson P.I., Bigger B.W. Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy. Brain: J. Neurol. 2018;141(1):99–116. doi: 10.1093/brain/awx311. [DOI] [PubMed] [Google Scholar]
- 153.Roy J., Lefebvre J., Maltais R., Poirier D. Inhibition of dehydroepiandosterone sulfate action in androgen-sensitive tissues by EM-1913, an inhibitor of steroid sulfatase. Mol. Cell. Endocrinol. 2013;376(1-2):148–155. doi: 10.1016/j.mce.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 154.Beck M.A., Handy J., Levander O.A. The role of oxidative stress in viral infections. Ann. N. Y. Acad. Sci. 2000;917:906–912. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 155.Camini F.C., da Silva Caetano C.C., Almeida L.T., de Brito Magalhaes C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017;162(4):907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 156.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr. Med. J. 2020;35(Suppl 2):12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Poljsak B., Milisav I. NAD+ as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity, and health span. Rejuvenation Res. 2016;19(5):406–415. doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 159.Daugherty M.D., Young J.M., Kerns J.A., Malik H.S. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 2014;10(5) doi: 10.1371/journal.pgen.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heer C.D., Sanderson D.J., Alhammad Y.M.O., Schmidt M.S., Trammell S.A.J., Perlman S., Cohen M.S., Fehr A.R., Brenner C. Coronavirus and PARP expression dysregulate the NAD Metabolome: a potentially actionable component of innate immunity. BioRxiv. 2020 doi: 10.1074/jbc.RA120.015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nasi A., McArdle S., Gaudernack G., Westman G., Melief C., Rockberg J., Arens R., Kouretas D., Sjolin J., Mangsbo S. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.UN . 2020. Policy Brief:The Impact of COVID-19 on Older Persons. [Google Scholar]
- 163.Franceschi C., Garagnani P., Morsiani C., Conte M., Santoro A., Grignolio A., Monti D., Capri M., Salvioli S. The continuum of aging and age-related diseases: common mechanisms but different rates. Front. Med. 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sar P., Rath B., Subudhi U., Chainy G.B.N., Supakar P.C. Alterations in expression of senescence marker protein-30 gene by 3,3’,5-triiodo-L-thyronone (T3) Mol. Cell. Biochem. 2007;303:239–242. doi: 10.1007/s11010-007-9462-1. [DOI] [PubMed] [Google Scholar]
- 165.van den Beld A.W., Kaufman J.M., Zillikens M.C., Lamberts S.W.J., Egan J.M., van der Lely A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018;6(8):647–658. doi: 10.1016/S2213-8587(18)30026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chainy G.B.N., Sahoo D.K. Hormones and oxidative stress: an overview. Free Radic. Res. 2020;54(1):1–26. doi: 10.1080/10715762.2019.1702656. [DOI] [PubMed] [Google Scholar]
- 167.Resch U., Helsel G., Tatzber F., Sinzinger H. Antioxidant status in thyroid dysfunction. Clin. Chem. Lab. Med. 2002;40(11):1132–1134. doi: 10.1515/CCLM.2002.198. [DOI] [PubMed] [Google Scholar]
- 168.Fernandez V., Barrientos X., Kipreos K., Valenzuela A., Videla L.A. Superoxide radical generation, NADPH oxidase activity, and cytochrome P-450 content of rat liver microsomal fractions in an experimental hyperthyroid state: relation to lipid peroxidation. Endocrinology. 1985;117(2):496–501. doi: 10.1210/endo-117-2-496. [DOI] [PubMed] [Google Scholar]
- 169.Asayama K., Dobashi K., Hayashibe H., Megata Y., Kato K. Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: a possible mechanism of injury to heart and skeletal muscle in hyperthyroidism. Endocrinology. 1987;121(6):2112–2118. doi: 10.1210/endo-121-6-2112. [DOI] [PubMed] [Google Scholar]
- 170.Swaroop A., Ramasarma T. Heat exposure and hypothyroid conditions decrease hydrogen peroxide generation in liver mitochondria. Biochem. J. 1985;226(2):403–408. doi: 10.1042/bj2260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Das K., Chainy G.B. Thyroid hormone influences antioxidant defense system in adult rat brain. Neurochem. Res. 2004;29(9):1755–1766. doi: 10.1023/b:nere.0000035812.58200.a9. [DOI] [PubMed] [Google Scholar]
- 172.Bhanja S., Chainy G.B. PTU-induced hypothyroidism modulates antioxidant defence status in the developing cerebellum. Int. J. Dev. Neurosci. 2010;28(3):251–262. doi: 10.1016/j.ijdevneu.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 173.Jena S., Dandapat J., Chainy G.B. Curcumin differentially regulates the expression of superoxide dismutase in cerebral cortex and cerebellum of L-thyroxine (T(4))-induced hyperthyroid rat brain. Neurol. Sci. 2013;34(4):505–510. doi: 10.1007/s10072-012-1084-z. [DOI] [PubMed] [Google Scholar]
- 174.Das K., Chainy G.B. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim. Biophys. Acta. 2001;1537(1):1–13. doi: 10.1016/s0925-4439(01)00048-5. [DOI] [PubMed] [Google Scholar]
- 175.Subudhi U., Das K., Paital B., Bhanja S., Chainy G.B. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Biol. Interact. 2008;173(2):105–114. doi: 10.1016/j.cbi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 176.Chattopadhyay S., Zaidi G., Das K., Chainy G.B. Effects of hypothyroidism induced by 6-n-propylthiouracil and its reversal by T3 on rat heart superoxide dismutase, catalase and lipid peroxidation. Indian J. Exp. Biol. 2003;41(8):846–849. [PubMed] [Google Scholar]
- 177.Jena S., Chainy G.B., Dandapat J. Expression of antioxidant genes in renal cortex of PTU-induced hypothyroid rats: effect of vitamin E and curcumin. Mol. Biol. Rep. 2012;39(2):1193–1203. doi: 10.1007/s11033-011-0849-4. [DOI] [PubMed] [Google Scholar]
- 178.Sahoo D.K., Roy A., Bhanja S., Chainy G.B. Experimental hyperthyroidism-induced oxidative stress and impairment of antioxidant defence system in rat testis. Indian J. Exp. Biol. 2005;43(11):1058–1067. [PubMed] [Google Scholar]
- 179.de Castro A.L., Tavares A.V., Campos C., Fernandes R.O., Siqueira R., Conzatti A., Bicca A.M., Fernandes T.R., Sartorio C.L., Schenkel P.C., Bello-Klein A., da Rosa Araujo A.S. Cardioprotective effects of thyroid hormones in a rat model of myocardial infarction are associated with oxidative stress reduction. Mol. Cell. Endocrinol. 2014;391(1-2):22–29. doi: 10.1016/j.mce.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 180.Subudhi U., Das K., Paital B., Bhanja S., Chainy G.B. Supplementation of curcumin and vitamin E enhances oxidative stress, but restores hepatic histoarchitecture in hypothyroid rats. Life Sci. 2009;84:372–379. doi: 10.1016/j.lfs.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 181.Subudhi U., Chainy G.B. Curcumin and vitamin E modulate hepatic antioxidant gene expression in PTU-induced hypothyroid rats. Mol. Biol. Rep. 2012;39(11):9849–9861. doi: 10.1007/s11033-012-1851-1. [DOI] [PubMed] [Google Scholar]
- 182.Jena S., Chainy G.B. Regulation of expression of antioxidant enzymes by vitamin E and curcumin in L-thyroxine-induced oxidative stress in rat renal cortex. Mol. Biol. Rep. 2011;38(2):1047–1054. doi: 10.1007/s11033-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 183.Choudhury S., Chainy G.B., Mishro M.M. Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defence system in adult rat testis. Andrologia. 2003;35(3):131–140. doi: 10.1046/j.1439-0272.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- 184.Sahoo D.K., Roy A., Chainy G.B. Rat testicular mitochondrial antioxidant defence system and its modulation by aging. Acta Biol. Hung. 2008;59(4):413–424. doi: 10.1556/ABiol.59.2008.4.3. [DOI] [PubMed] [Google Scholar]
- 185.Subudhi U., Chainy G.B. Expression of hepatic antioxidant genes in L-thyroxine-induced hyperthyroid rats: regulation by vitamin E and curcumin. Chem. Biol. Interact. 2010;183(2):304–316. doi: 10.1016/j.cbi.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 186.Mishra P., Paital B., Jena S., Swain S.S., Kumar S., Yadav M.K., Chainy G.B.N., Samanta L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFkB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019;9(1):7408. doi: 10.1038/s41598-019-43320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Velayoudom F.L., Alwis Wijewickrama P.S., Ranathunga H.I., Somasundaram N. Endocrine vigilance in COVID-19. J. Pakistan Med. Assoc. 2020;70(Suppl 3):S83–S86. doi: 10.5455/JPMA.16. (5) [DOI] [PubMed] [Google Scholar]
- 188.Shaha A.R. Thyroid surgery during COVID-19 pandemic: principles and philosophies. Head Neck. 2020;42(6):1322–1324. doi: 10.1002/hed.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aboughdir M., Kirwin T., Abdul Khader A., Wang B. Prognostic value of cardiovascular biomarkers in COVID-19: a review. Viruses. 2020;12(5):527. doi: 10.3390/v12050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pal R., Bhadada S.K. Managing common endocrine disorders amid COVID-19 pandemic. Diabetes Metab. Syndrome. 2020;14(5):767–771. doi: 10.1016/j.dsx.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim C.Y., Lee Y.J., Choi J.H., Lee S.Y., Lee H.Y., Jeong D.H., Choi Y.J. The association between low vitamin D status and autoimmune thyroid disease in Korean premenopausal women: the 6th Korea national health and nutrition examination survey, 2013-2014. Korean J. Fam. Med. 2019;40(5):323–328. doi: 10.4082/kjfm.18.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ferrari S.M., Fallahi P., Antonelli A., Benvenga S. Environmental issues in thyroid diseases. Front. Endocrinol. 2017;8(50):1–8. doi: 10.3389/fendo.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grant W.B. Hypothesis--ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochem. Photobiol. 2008;84(2):356–365. doi: 10.1111/j.1751-1097.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 196.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020;80(6):e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saito I., Saruta T. Hypertension in thyroid disorders. Endocrinol Metab. Clin. N. Am. 1994;23(2):379–386. [PubMed] [Google Scholar]
- 198.Klein I., Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 199.Mannam P., Srivastava A., Sugunaraj J.P., Lee P.J., Sauler M. Oxidants in acute and chronic lung disease. J. Blood Lymph. 2014;4:1–16. doi: 10.4172/2165-7831.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Christofidou-Solomidou M., Muzykantov V.R. Antioxidant strategies in respiratory medicine. Treat. Respir. Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 201.Lang J.D., McArdle P.J., O'Reilly P.J., Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122(6 Suppl):314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 202.Rahman I. Antioxidant therapies in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006;1(1):15–29. doi: 10.2147/copd.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Loffredo L., Violi F. COVID-19 and cardiovascular injury: a role for oxidative stress and antioxidant treatment? Int. J. Cardiol. 2020;312:136. doi: 10.1016/j.ijcard.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Simonson W. Vitamin C and coronavirus. Geriatr. Nurs. 2020;41(3):331–332. doi: 10.1016/j.gerinurse.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hemnes A.R., Rathinasabapathy A., Austin E.A., Brittain E.L., Carrier E.J., Chen X., Fessel J.P., Fike C.D., Fong P., Fortune N., Gerszten R.E., Johnson J.A., Kaplowitz M., Newman J.H., Piana R., Pugh M.E., Rice T.W., Robbins I.M., Wheeler L., Yu C., Loyd J.E., West J. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018;51:1702638. doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.da Silva J.S., Gabriel-Costa D., Wang H., Ahmad S., Sun X., Varagic J., Sudo R.T., Ferrario C.M., Dell Italia L.J., Sudo G.Z., Groban L. Blunting of cardioprotective actions of estrogen in female rodent heart linked to altered expression of cardiac tissue chymase and ACE2. J Renin-Angiotensin-Aldosterone System. 2017;18(3):1–14. doi: 10.1177/1470320317722270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yu Zhao Z.Z., Wang Yujia, Zhou Yueqing, Ma Yu, Zuo Wei. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chaker L., Cappola A.R., Mooijaart S.P., Peeters R.P. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6(9):733–742. doi: 10.1016/S2213-8587(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 210.Pantos C., Tseti I., Mourouzis I. Use of triiodothyronine to treat critically ill COVID-19 patients: a new clinical trial. Crit. Care. 2020;24(1):209. doi: 10.1186/s13054-020-02934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Padhi R., Kabi S., Panda B.N., Jagati S. Prognostic significance of nonthyroidal illness syndrome in critically ill adult patients with sepsis. Int. J. Crit. Illn. Inj. Sci. 2018;8(3):165–172. doi: 10.4103/IJCIIS.IJCIIS_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zulfiqar A.A., Lorenzo-Villalba N., Hassler P., Andres E. Immune thrombocytopenic purpura in a patient with covid-19. N. Engl. J. Med. 2020;382(18):e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ippolito S., Dentali F., Tanda M.L. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Invest. 2020;43(8):1171–1172. doi: 10.1007/s40618-020-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brancatella A., Ricci D., Viola N., Sgro D., Santini F., Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J. Clin. Endocrinol. Metabol. 2020;105(7):2367–2370. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dworakowska D., Grossman A.B. Thyroid disease in the time of COVID-19. Endocrine. 2020;68(3):471–474. doi: 10.1007/s12020-020-02364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bellastella G., Maiorino M.I., Esposito K. Endocrine complications of COVID-19: what happens to the thyroid and adrenal glands? J. Endocrinol. Invest. 2020;43(8):1169–1170. doi: 10.1007/s40618-020-01311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.