Abstract
Objectives
The main objective of this study was to determine the incidence of invasive pulmonary aspergillosis (IPA) in patients with coronavirus disease 2019 (COVID-19) admitted to the intensive care unit (ICU), and to describe the patient characteristics associated with IPA occurrence and to evaluate its impact on prognosis.
Methods
We conducted a retrospective cohort study including all successive COVID-19 patients, hospitalized in four ICUs, with secondary deterioration and one or more respiratory samples sent to the mycology department. We used a strengthened IPA testing strategy including seven mycological criteria. Patients were classified as probable IPA according to the European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group Education and Research Consortium (MSGERC) classification if immunocompromised, and according to the recent COVID-19-associated IPA classification otherwise.
Results
Probable IPA was diagnosed in 21 out of the 366 COVID-19 patients (5.7%) admitted to the ICU and in the 108 patients (19.4%) who underwent respiratory sampling for deterioration. No significant differences were observed between patients with and without IPA regarding age, gender, medical history and severity on admission and during hospitalization. Treatment with azithromycin for ≥3 days was associated with the diagnosis of probable IPA (odds ratio 3.1, 95% confidence interval 1.1–8.5, p = 0.02). A trend was observed with high-dose dexamethasone and the occurrence of IPA. Overall mortality was higher in the IPA patients (15/21, 71.4% versus 32/87, 36.8%, p < 0.01).
Conclusion
IPA is a relatively frequent complication in severe COVID-19 patients and is responsible for increased mortality. Azithromycin, known to have immunomodulatory properties, may contribute to increase COVID-19 patient's susceptibility to IPA.
Keywords: Aspergillus, Azithromycin, Coronavirus, Corticosteroids, COVID-19, Critical care, Risk factors, SARS-CoV-2
Introduction
Although pulmonary invasive fungal disease is typically described in the immunocompromised host, invasive pulmonary aspergillosis (IPA) has been increasingly reported in critically ill patients, including patients without classical risk factors of immunosuppression [1]. In acute respiratory distress syndrome (ARDS) patients, ~12.5% of the patients had IPA as shown by random post-mortem histopathological examination of lung tissue [2]. Coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) has been increasingly reported [[3], [4], [5]]. Whether the use of immunomodulatory therapies (such as corticosteroids prescribed to dampen detrimental inflammatory response) and antibiotics (to treat and/or prevent bacterial superinfections) is responsible for increased susceptibility of COVID-19 patients to pulmonary invasive fungal disease remains incompletely studied [6,7]. The aim of our study was to evaluate the incidence of IPA and the risk factors associated with IPA in patients with severe COVID-19 admitted to the intensive care unit (ICU), and to evaluate the impact of IPA on patient outcome.
Methods
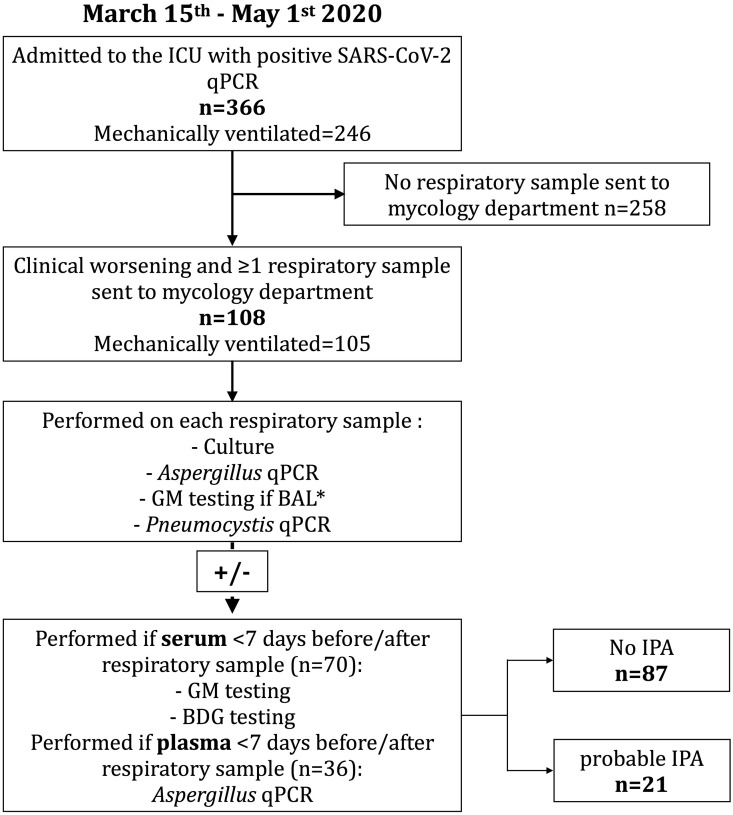
We conducted a retrospective observational cohort study. All successive COVID-19 patients admitted to the four ICUs of our two university hospitals between 15th March and 1st May 2020 with a positive PCR for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Cobas® SARS-CoV-2 Test, Roche) and one or more respiratory samples (bronchoalveolar lavage (BAL), tracheal aspirate, sputum) sent to the mycology department were included (Fig. 1 ). Of note, the 27 first patients included had previously been partially analysed [3]. On respiratory samples, culture, galactomannan (GM) (BAL only) and Aspergillus quantitative PCR (qPCR) were systematically performed. In concomitantly received blood sample, GM, β-D-glucan (BDG) and Aspergillus qPCR were performed on serum/plasma. Patients were classified as probable IPA according to the European Organization for Research and Treatment of Cancer (EORTC) and the Mycoses Study Group Education and Research Consortium (MSGERC) consensus criteria in immunocompromised patients [8] and according to the consensus case definition proposal for influenza-/COVID-19-associated pulmonary aspergillosis (CAPA) in ICU patients otherwise (Supplementary Material Table S1) [9]. An extensive list of clinical data was collected as part of the initial protocol (Table 1 ). The cumulative dose of corticosteroids, azithromycin and β-lactams was determined as the total dose of drug received prior to the day of sampling. Prescription of azithromycin >1500 mg and β-lactams >3 days were predefined as exposure variables. Azithromycin was systematically prescribed before or on the day of admission to the ICU, except for two patients for whom it was introduced at day 4 and day 5, respectively. If no fungal infection was diagnosed, the latest sample was used. Corticosteroid doses were quantified as dexamethasone-equivalents [10].
Fig. 1.
Study flowchart. Direct examination of respiratory samples was performed only on samples collected after the 27th March as initial data regarding the contamination risk of lab technicians were not available. BAL, bronchoalveolar lavage; BDG, β-D-glucans; GM, galactomannan; ICU, intensive care unit; qPCR, quantitative polymerase chain reaction. ∗As recommended by the manufacturer.
Table 1.
Comparison of patients with severe coronavirus disease 2019 (COVID-19) with and without probable invasive pulmonary aspergillosis (IPA)
| Total (n = 108) | Without IPA (n = 87) | With IPA (n = 21) | OR | 95%CI | p | |
|---|---|---|---|---|---|---|
| Malen (%) | 88 (81.5) | 72 (82.8) | 16 (76.2) | 0.7 | 0.2–2.1 | — |
| Agemedian (Q1–Q3) | 62 (56–68) | 62 (56–68) | 63 (56.75–68.25) | — | — | 0.63 a |
| Mechanical ventilationn (%) | 105 (97.2) | 85 (97.7) | 20 (95.2) | 0.5 | 0.04–5.3 | |
| COVID risk factors | ||||||
| HTAn (%) | 64 (59.3) | 50 (57.5) | 14 (66.7) | 1.5 | 0.5–4.0 | — |
| Diabetesn (%) | 40 (37.0) | 31 (35.6) | 9 (42.9) | 1.4 | 0.5–3.6 | — |
| Obesityn (%) | 35 (32.4) | 31 (35.6) | 4 (19.0) | 0.4 | 0.1–1.3 | — |
| Coronary diseasen (%) | 15 (13.9) | 13 (14.9) | 2 (9.5) | 0.6 | 0.1–2.9 | — |
| BMImedian (Q1–Q3) | 28 (25–31) | 28 (26–32) | 28 (25–29) | — | — | 0.70 a |
| Other patient characteristics | ||||||
| Asthman (%) | 5 (4.6) | 3 (3.4) | 2 (9.5) | 2.9 | 0.5–18.9 | — |
| COPDn (%) | 2 (1.9) | 2 (2.3) | 0 (0.0) | 0.8 | 0.04–17.2 | — |
| Immunocompromised patientn (%) | 10 (9.3) | 8 (9.2) | 2 (9.5) | 0.6 | 0.1–2.9 | — |
| Long-term corticosteroidsn (%) | 11 (10.2) | 8 (9.2) | 3 (14.3) | 1.6 | 0.4–6.8 | — |
| Severity at admission | ||||||
| PaO2/FiO2mean (SD) | 173.47 (123.19) | 169.63 (125.85) | 187.79 (114.74) | — | — | 0.34 a |
| Vasopressors in first 48 hn (%) | 65 (60.2) | 52 (59.8) | 13 (61.9) | 1.1 | 0.4–2.9 | |
| Creatininaemia (mg/dL)mean (SD) | 103.34 (74.01) | 92.64 (47.19) | 149.85 (132.96) | — | — | 0.08 a |
| D-dimermedian (Q1–Q3) | 2395 (1193– 4635) | 2325 (1163– 4563) | 2515 (1610–10917) | — | — | 0.63 a |
| LDHmean (SD) | 755.11 (312.15) | 759.49 (303.61) | 740.20 (350.52) | — | — | 0.80 a |
| SAPS2mean (SD) | 39.93 (14.40) | 40.4 (14.6) | 38.1 (13.8) | — | — | 0.58 a |
| SOFAmean (SD) | 6.02 (3.79) | 5.8 (3.6) | 7.1 (4.5) | — | — | 0.28 a |
| Severity during hospitalization | ||||||
| Nadir PaO2/FiO2mean (SD) | 79.75 (37.21) | 81.54 (39.01) | 72.50 (28.40) | — | — | 0.50 a |
| ECMOn (%) | 10 (9.3) | 9 (10.3) | 1 (4.8) | 0.4 | 0.1–3.6 | — |
| Renal replacement therapyn (%) | 38 (35.2) | 30 (34.5) | 8 (38.1) | 1.2 | 0.4–3.1 | — |
| Vasopressorsn (%) | 89 (82.4) | 70 (80.5) | 19 (90.5) | 2.3 | 0.5–10.9 | — |
| Specific COVID therapy | ||||||
| Lopinavir–ritonavirn (%) | 16 (14.8) | 10 (11.5) | 6 (28.6) | 3.1 | 0.9–9.8 | |
| Hydroxychloroquinen (%) | 34 (31.5) | 27 (31.0) | 7 (33.3) | 1.1 | 0.4–3.1 | |
| Azithromycin + hydroxychloroquinen (%) | 29 (26.9) | 22 (25.3) | 7 (33.3) | 1.4 | 0.5–4.1 | |
| Immunoglobulinsn (%) | 3 (2.8) | 3 (3.4) | 0 (0.0) | 0.6 | 0.03–11.3 | |
| Sarilumabn (%) | 1 (0.9) | 1 (1.1) | 0 (0.0) | 4.3 | 0.3–71.8 | |
| Eculizumabn (%) | 6 (5.6) | 4 (4.6) | 2 (9.5) | 2.2 | 0.4–12.8 | |
| Tocilizumabn (%) | 4 (3.7) | 2 (2.3) | 2 (9.5) | 4.5 | 0.6–33.8 | |
| Therapy with cumulative dose before sampling | ||||||
| Azithromycin >1500 mg total dosen (%) | 26 (24.1) | 17 (19.5) | 9 (42.9) | 3.1 | 1.1–8.5 | |
| Dexamethasone >1000 mgn (%) | 16 (14.8) | 10 (11.5) | 6 (28.6) | 3.1 | 1.0–9.8 | |
| Any β-lactam >3 daysn (%) | 90 (83.3) | 74 (85.1) | 16 (76.2) | 0.6 | 0.2–1.8 | |
| Respiratory sample characteristics | ||||||
| PaO2/FiO2at samplingmean (SD) | 173.69 (91.70) | 173.18 (96.05) | 175.66 (74.40) | — | — | 0.612 a |
| % BAL macrophagesmean (SD) | 31.23 (21.94) | 31.00 (22.76) | 32.17 (20.21) | — | — | 0.836 a |
| % BAL PMNmean (SD) | 47.37 (30.92) | 47.08 (31.99) | 48.50 (28.89) | — | — | 0.959 a |
| % BAL lymphocytesmean (SD) | 20.63 (18.94) | 21.17 (19.10) | 18.50 (19.92) | — | — | 0.795 a |
| Outcome | ||||||
| Mortalityn (%) | 47 (43.5) | 32 (36.8) | 15 (71.4) | 4.3 | 1.5–12.1 | |
| LOS daysmean (SD) | 24.33 (18.88) | 25.13 (19.18) | 21.05 (17.60) | — | — | 0.313a |
BAL, bronchoalveolar lavage; BMI, body mass index; 95%CI, 95% confidence interval; IPA, invasive pulmonary aspergillosis; LOS, length of stay; OR, odd ratio; PMN, polymorphonuclear; SAPSII, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment.
Wilcoxon test.
Culture of respiratory specimens was performed as previously described [11]. For Aspergillus qPCR, DNA was extracted from 1 mL of plasma or from a bead-beaten pellet of the respiratory sample and resuspended in 1000 μL of DNA-free water using the Qiasymphony DSP virus/Pathogen Mini kit (Qiagen) and a QIAsymphony apparatus (Qiagen). PCR assay has been reported previously [12]. GM and BDG detection were performed using Platelia BioRad kit (BioRad Laboratories) and Fungitell assay (Cape Cod Diagnostics) respectively according to the manufacturers' instructions.
Statistics
Data were reported in percentages, means and standard deviations (SDs) or medians and interquartiles (Q1–Q3) as appropriate. Univariate analyses were performed to assess an association between clinical factors and IPA using Fisher's exact, χ2 and Wilcoxon tests as appropriate. Odds ratios (ORs) with 95% confidence intervals (95%CI) were calculated for each significant variable based on univariate logistic regression. All analyses were performed using R software, version 3.5.3 (http://www.r-project.org).
Ethical statements
Our institutional ethics committee approved the study (IDRCB, 2020-A00256-33; CPP, 11-20-20.02.04.68737).
Results
A total of 366 patients with positive SARS-CoV2 qPCR were admitted to the four intensive care units between 15th March and 1st May 2020. Among these, 246 were intubated and mechanically ventilated (Fig. 1). The mycology department received 193 respiratory samples from 108 patients whose conditions deteriorated despite appropriate initial care. Patient characteristics are described in Supplementary Material Table S2. Male/female sex ratio was 4.4 and median age was 61 years.
Twenty-one patients developed probable IPA according to CAPA criteria stricto sensu (n = 19) and EORTC/MSGERC definitions (n = 2; one solid organ transplant recipient and one myeloma patient). Overall, incidence was 5.7% (21/366) in severe COVID-19 patients admitted to the ICU and 8.5% (21/246) in those mechanically ventilated. IPA incidence in patients whose conditions worsened despite appropriate care was 19.4% (21/108). The median times from symptom onset to IPA diagnosis and from ICU admission to IPA diagnosis were 16 (10–23) days and 6 (1–15) days, respectively.
When comparing patients who developed probable IPA (n = 21) or not (n = 87), no significant differences were observed regarding general population characteristics and severity upon admission (Table 1). Prescription of hydroxychloroquine (n = 34) did not differ between the two groups. Administration of azithromycin for more than 3 days (cumulative dose ≥1500 mg) was associated with probable IPA (OR 3.1, 95%CI 1.1–8.5, p 0.025) (Supplementary Material Fig. S1). Of note, 34 patients received azithromycin, which was discontinued prematurely on day 1 or day 2 in eight patients because of QT interval prolongation. Administration of high-dose corticosteroids was not significantly associated with IPA (11.5 versus 28.6%, p 0.08), although the cumulative dose ≥100 mg tended to be higher among IPA patients (OR 3.7, 95%CI 1.0–9.7). Details for the incidence of IPA among patients who received azithromycin and/or corticosteroids is available in the Supplementary Material Table S3. Mortality was significantly higher in the probable IPA group (15/21, 71.4% versus 32/87, 36.8%, p < 0.01). Further details on each IPA patient are available in Supplementary Material Table S4.
Discussion
In our study, the incidence of IPA in COVID-19 patients was 5.7% in all ICU patients and 8.5% in those mechanically ventilated, yet this may be an underestimate considering that only patients with clinical worsening were tested.
A cumulative azithromycin dose ≥1500 mg was associated with IPA. Azithromycin has an antiviral effect in vitro and is a broad-spectrum antibiotic with immunomodulatory properties, and could have both prevented bacterial superinfections and reduced inflammation [13]. A recent meta-analysis found an increased mortality when hydroxychloroquine is associated with azithromycin [14]. Any azithromycin-related impact on the risk of secondary infections has been only incompletely studied. Azithromycin has been shown to decrease serum interleukin-6, induce delayed down-regulation of neutrophil oxidative burst and increased neutorphil apoptosis up to 28 days after three doses of azithromycin (i.e. 1500 mg) in humans [15]. Neutrophils and oxidative burst represent the first and most important immune system barrier against aspergillosis [16]. Furthermore, azithromycin may promote Aspergillus colonization by altering the lung microbiome [17].
Corticosteroids are known to increase susceptibility to invasive fungal disease due to complex quantitative and qualitative immune deregulation [10]. High-dose corticosteroid, although not precisely defined, has previously been found to be associated with CAPA [4,5]. Although not significantly associated with IPA in our study, probably because of insufficient statistical power, a trend was observed after a cumulative dose of ≥100 mg dexamethasone-equivalent (OR 3.7; 95%CI 1.0–9.7), Recent studies such as the RECOVERY trial and various meta-analyses showed that corticosteroid administration is beneficial in COVID-19 patients requiring hospitalization [18]. Interestingly, the cumulative dose of dexamethasone using the RECOVERY trial regimen does not exceed 60 mg.
Although no severity score or variables, including oxygenation parameters, were found to be associated to IPA occurrence, we cannot rule out the extension of the lesion to be an associated risk factor. Indeed, the extension of lung lesions quantified by computed tomography has been shown to be a predictor of COVID-19 severity and early death [19].
Susceptibility to IPA in previously immunocompetent critically ill patients is most likely multifactorial. In ARDS patients, epithelial damage, impaired mucociliary clearance and temporary immune deregulation, starting with excess release of danger-associated molecular patterns (DAMPs) secondary to COVID-19 damages, may be initiating factors [20]. The addition of known or suspected risk factors, such as corticosteroid or azithromycin—further inhibiting neutrophils and the innate immune response—may tilt the balance in favour of IPA development. The risk of IPA associated with corticosteroids compellingly depends on its cumulative dose, although cut-offs are not clearly defined and depend on the underlying host factor [8]. Our findings raise questions regarding the possible connection between azithromycin use and the observed increased susceptibility to IPA, which needs to be further explored.
Author contributions
Writing original draft: SD and AA. Writing review & editing: all. Conceptualization: SD, AA and SB. Investigation: SD, ED, SF, SV and MC. Data curation: SD, ED, SF, SV, MC and TFG. Formal analysis: PAO and MS. Visualization: SD, AA, BM, EA and AM. Supervision: AA, BM, EA and AM.
Transparency declaration
The authors declare no conflicts of interest related to the content of the present study. No external funding was received for the present study.
Acknowledgements
We thank Dr Pierre Gazeau for providing follow-up information regarding patients transferred to Brest University Hospital. We thank all staff, nurses and lab technicians who were essential to patient care in intensive care and medical mycology from Saint Louis Hospital and Lariboisière Hospital.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tudesq J.-J., Peyrony O., Lemiale V., Azoulay E. Invasive pulmonary aspergillosis in nonimmunocompromised hosts. Semin Respir Crit Care Med. 2019;40:540–547. doi: 10.1055/s-0039-1696968. [DOI] [PubMed] [Google Scholar]
- 2.de Hemptinne Q., Remmelink M., Brimioulle S., Salmon I., Vincent J.-L. ARDS: A clinicopathological confrontation. Chest. 2009;135:944–949. doi: 10.1378/chest.08-1741. [DOI] [PubMed] [Google Scholar]
- 3.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schauwvlieghe A.F., Rijnders B.J., Philips N., Verwijs R., Vanderbeke L., Van Tienen C. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 7.Erjavec Z., Kluin-Nelemans H., Verweij P.E. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin Microbiol Infect. 2014;15:625–633. doi: 10.1111/j.1469-0691.2009.02929.x. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E. Revision and update of the consensus definitions of invasive fungal disease from the European organization for Research and treatment of cancer and the Mycoses study group education and Research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 11.Alanio A., Denis B., Hamane S., Raffoux E., Peffault de la Tour R., Touratier S. New therapeutic strategies for invasive aspergillosis in the era of azole resistance: how should the prevalence of azole resistance be defined? J Antimicrob Chemother. 2016;71:2075–2078. doi: 10.1093/jac/dkw036. [DOI] [PubMed] [Google Scholar]
- 12.Alanio A., Menotti J., Gits-Muselli M., Hamane S., Denis B., Rafoux E. Circulating Aspergillus fumigatus DNA is quantitatively correlated to galactomannan in serum. Front Microbiol. 2017;8:405–408. doi: 10.3389/fmicb.2017.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldenburg C.E., Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396:936–937. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiolet T., Guihur A., Rebeaud M., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culić O., Eraković V., Cepelak I., Barisić K., Brajsa K., Ferencić Z. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450:277–289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 16.Latgé J.-P., Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev. 2019;33:310–375. doi: 10.1128/CMR.00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson R.P., Morris A. Macrolides, inflammation and the lung microbiome: untangling the web of causality. Thorax. 2017;72:10–12. doi: 10.1136/thoraxjnl-2016-209180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020:1–12. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruch Y., Kaeuffer C., Ohana M., Labani A., Fabacher T., Bilbault P. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect. 2020;26:1417.e5–1417.e8. doi: 10.1016/j.cmi.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi. 2020;6:91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.