Abstract
Purpose of Review
Allergic rhinitis (AR) is a chronic inflammatory immunoglobulin (Ig) E-mediated disease of the nasal mucosa that can be triggered by the inhalation of seasonal or perennial allergens. Typical symptoms include sneezing, rhinorrhea, nasal itching, nasal congestion and symptoms of allergic conjunctivitis. AR affects a quarter of the population in the United States of America and Europe.
Recent Findings
AR has been shown to reduce work productivity in 36–59% of the patients with 20% reporting deteriorated job attendance. Moreover, 42% of children with AR report reduced at-school productivity and lower grades. Most importantly, AR impacts the patient’s quality of life, due to sleep deprivation. However, a proportion of patients fails to respond to conventional medication and opts for the allergen immunotherapy (AIT), which currently is the only disease-modifying therapeutic option. AIT can be administered by either subcutaneous (SCIT) or sublingual (SLIT) route. Both routes of administration are safe, effective, and can lead to tolerance lasting years after treatment cessation. Both innate and adaptive immune responses that contribute to allergic inflammation are suppressed by AIT. Innate responses are ameliorated by reducing local mast cell, basophil, eosinophil, and circulating group 2 innate lymphoid cell frequencies which is accompanied by decreased basophil sensitivity. Induction of allergen-specific blocking antibodies, immunosuppressive cytokines, and regulatory T and B cell phenotypes are key pro-tolerogenic adaptive immune responses.
Conclusion
A comprehensive understanding of these mechanisms is necessary for optimal selection of AIT-responsive patients and monitoring treatment efficacy. Moreover, it could inspire novel and more efficient AIT approaches.
Keywords: Allergen immunotherapy, Allergic rhinitis, Innate and adaptive immune response, T cells, B cell, Innate lymphoid cells, Dendritic cells
Introduction
Allergic rhinitis (AR) is a chronic inflammatory disease of the lining of nasal mucosa induced by type I hypersensitivity response upon exposure to common inhaled allergens in sensitized individuals [1]. AR affects up to 40% of worldwide population with an increasing prevalence over the past 20 years [2–8]. It is associated with significantly lower quality of life due to impaired sleep, learning difficulties, deterioration of at-work performance and social functioning, which highlights AR as a substantial economic burden and a serious global health problem [8–11].
AR manifests itself through rhinorrhea, sneezing, nasal itching and congestion. Seasonal AR (SAR) is periodically triggered by outdoor allergens, in particular grass, tree or weed pollen. Symptoms of perennial AR (PAR) last throughout the year in response to persistently present indoor allergens, such as house dust mite (HDM), animal dander, insects and mold [12, 13]. It is noteworthy that a proportion of patients displaying nasal reactivity do not exhibit systemic sensitization evidenced by negative skin prick tests (SPT) and undetectable serum-specific IgE (sIgE), an endotype consequently defined as local AR (LAR) [14, 15]. Recent evidence shows that both sensitization patterns in response to different allergens can coexist within the same individual, the proposed term for which is dual AR (DAR) [16].
Currently employed pharmacotherapy approaches include antihistamines and intranasal corticosteroids which only provide a temporary symptomatic relief [12, 13]. Furthermore, such treatments fail to attenuate symptoms in 30% to 60% of the patients [17, 18]. Allergen immunotherapy (AIT) is a proposed treatment strategy for such individuals being the single disease-modifying strategy to date. Subcutaneous immunotherapy (SCIT) involves administration of incremental doses of sensitizing allergen for 8–12 weeks followed by high dose monthly interval over the course of 3–5 years. Sublingual immunotherapy (SLIT) involves administration of high doses of the allergen under the tongue. SCIT and SLIT confer long-term clinical benefit and immunologic tolerance after cessation treatment. While SCIT is highly efficient at inducing tolerance to both seasonal and perennial allergens, SLIT is regarded as a safer and more convenient alternative generally in managing SAR [19–21].
This review will focus on historical and recent advances in understanding the mechanisms of allergy and AIT in the context of AR. Furthermore, novel AIT approaches and predictive/ indicative biomarkers of treatment success will be discussed. Collectively, these findings can support new potential treatment approaches.
Mechanisms of Allergic Rhinitis
Sensitization
The primary step in the cascade of allergic inflammation is orchestrated by intricate interactions between epithelial (EC) and dendritic cells (DCs) which ultimately lead to the initiation of early and late phase responses. Initially, an inhaled allergen passes through ECs of the nasal mucosa. Once activated, these cells shed a range of chemokines, particularly CCL20, in an ADAM10-mediated manner, which in turn promotes the recruitment of immature DCs [22, 23].
A dysfunctional epithelial barrier can contribute to the pathophysiology of AR. Upregulated activity of histone deacetylase (HDAC) compromises epithelial integrity by impairing tight junction proteins, possibly escalating allergen challenge [24•]. Furthermore, necroptosis-induced release of nuclear IL-33 as well as secretion of TSLP and IL-25 from ECs introduce stimuli required for the development of a pro-allergic dendritic cell of type 2 (DC2) phenotype, defined by the expression of CD141, GATA-3, OX40L, and RIPK4 [25, 26]. A recent study highlighted that epithelium-derived cytokines exert their pro-inflammatory effects in the most severe manner when in combination, suggesting their functions are additive [27]. In addition, these mediators facilitate the development of group 2 innate lymphoid cells (ILC2s), which, together with DC2s, amplify local T helper type 2 (TH2)-mediated allergic inflammation [26, 28]. TSLP and IL-33 can also directly activate TH2 cells, as seen in a murine model of AR [29]. Recently, IL-33 was also established as a key facilitator of mast cell degranulation, resulting from the inhibition of ST2/PI3K/mTOR-mediated autophagy, thereby amplifying early phase responses [30].
After aeroallergen internalization, activated DCs migrate to local lymph nodes. There, major histocompatibility complex (MHC) II-dependent antigen presentation and CD80/CD86-mediated co-stimulation prompt naïve CD4+ T lymphocyte polarization into effector cells [31]. In atopic subjects, presence of IL-4 is key for the development of the TH2 subset [32]. Healthy subjects can also develop an allergen-specific T cell response, though contrastingly, interferon-γ (IFN-γ)-secreting T helper type 1 (TH1) polarization is favored [33]. Furthermore, non-coding RNA GAS5, secreted by the exosomes of the nasal epithelium in AR patients, has been found to downregulate expression of TH1-related transcription factor T bet henceforth suppressing TH1 differentiation [34•].
T follicular helper (TFH) cells are another key subset to arise in the germinal centers (GCs) of lymph nodes in response to DC-mediated antigen presentation. These CD4+ lymphocytes express a surface marker CXCR5 and a transcription factor Bcl6. CXCR5, mutually expressed by B lymphocytes, is crucial for B follicle formation and T and B cell interaction [35]. Besides the lymph nodes, effector TFH cells can also enter the circulation or migrate to the nasal mucosa, where they can acquire TH2-like characteristics and induce local IgE production. Type 2 follicular helper T cells (TFH2) express transcription factor GATA-3 and secrete a TH2 cytokine repertoire [36, 37]. A recent study in mice by Gowthaman et al. showed that IL-13-secreting TFH cells are required to facilitate affinity maturation and differentiation of IgE+ B cells [38]. Moreover, AR patients with or without asthma show significant elevation in circulating TFH2 numbers [39].
TFH-secreted IL-4, IL-13 and IL-21 together with TH2-derived IL-4 and IL-13 promote B cell ε-germline transcription. Class-switch recombination and B cell activation is finalized by CD40/CD40L interaction between TFH and B cells [40]. The phenomenon of sensitization to allergen occurs when IgE+ plasma cells produce sIgE which binds to high affinity FcεRI receptors on the surface of mast cells and basophils. Upon subsequent exposure, the allergen cross-links neighboring IgE molecules and induces degranulation, leading to the early phase responses [38, 41].
Early Phase Responses
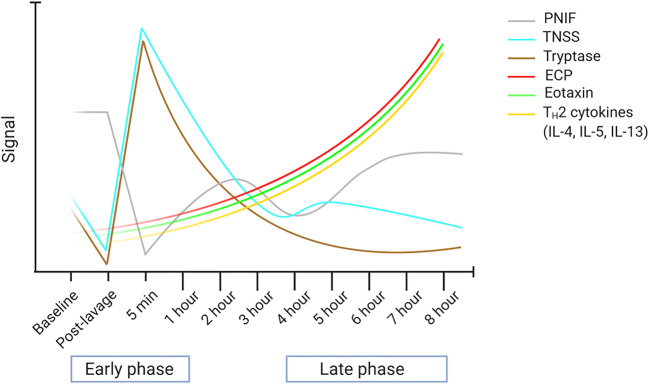
The early phase responses (EPR) take place for up to 60 min post-nasal allergen challenge (NAC). During EPR, levels of tryptase in nasal fluid following NAC significantly peak at 5 min post exposure. This elevation is accompanied by severe rhinorrhea, sneezing, itching and nasal obstruction, evident by peaks in total nasal symptom score (TNSS). Moreover, a significant deterioration of peak nasal inspiratory flow (PNIF), a surrogate of nasal congestion, gradually recovers to baseline at 3–4 h post NAC (Fig. 1) [42].
Fig. 1.
The biphasic response of allergic rhinitis following nasal allergen challenge (NAC). In the early phase, tryptase and total nasal symptom scores (TNSS) peak at 5 min post challenge which is accompanied by deterioration of peak nasal inspiratory flow (PNIF). Eosinophilic cationic protein (ECP), eotaxin, and TH2-related cytokines gradually increase and peak at 8 h during the late phase response which is paralleled by ongoing nasal congestion. Created with BioRender.com
A key mechanistic feature of the early phase reactions is IgE-dependent mast cell and basophil degranulation [43]. High affinity IgE receptors preferentially bind free IgE molecules, whereas IgE-allergen immune complexes are cleared upon binding to low-affinity receptor CD23 on B cells [44]. FcεRI are expressed not only on basophils and mast cells, but DCs as well. The expression of high-affinity receptors can be elevated in response to increasing serum IgE. Irrespective of allergic status, IgE primarily binds to basophil rather than DC receptors [45]. Just recently, it was demonstrated that upon allergen exposure, increases in serum IgE are accompanied by expansion of IgE+ plasmablasts. Moreover, IgE-related memory cells were found to reside in the allergen-specific IgG+ B cell fraction [46•].
Following the allergen cross-linking of adjacent IgE molecules, the mediator release from intracytoplasmic granules in the airway is orchestrated by phosphodiesterase 3 which mediates intracellular signaling of cGMP and cAMP [47]. The contents released include histamine, tryptase, cysteinyl leukotrienes and prostaglandin D2 [43, 47]. Notably, overexpression of CD300c, which acts in a co-stimulatory manner for IgE-dependent basophil activation, is seen in patients with AR [48].
Stimulation of histamine receptors H1 and H2 located on sensory neurons causes itching and sneezing, whereas stimulation of these receptors on ECs results in downregulation of tight junctions, thus increasing vascular permeability. Locally produced histamine in nasal secretions of AR patients is sufficient to compromise epithelial integrity in vitro, which is essentially a mechanism of continuous exacerbation of the allergic cascade [49]. This, together with cysteinyl leukotriene and prostaglandin D2-mediated chemoattraction, promotes immune cell influx to the nasal mucosa [50].
Although basophils and mast cells share numerous functional similarities, recent findings have elucidated regulatory differences of the effector functions. Several studies suggest that basophils and mast cells have different thresholds of stimulation to achieve FcεRI-dependent activation [51–53]. Moreover, studies show that mast cell survival is actively promoted by monomeric IgE binding to FcεRI which in turn induces an autocrine secretion of IL-3. While basophils similarly respond to IL-3, the induction of the cytokine is not subjected to monomeric IgE [54]. Besides promoting basophil survival, pre-exposure to IL-3 can also enhance histamine and pro-TH2 cytokine production upon IgE-allergen cross-linking [55]. Recent in vitro studies have demonstrated that peripheral basophils can be activated in an allergen-independent manner by cell-cell contact with ECs [56] or by high concentrations of serum IgE [57].
Late Phase Responses
Late phase responses (LPR) occur at 4 to 12 h post allergen challenge and are generally characterized by tissue recruitment of eosinophil, TH2 cells, and ILC2s [43, 58]. Significant increases in nasal eotaxin, eosinophil cationic protein (ECP), and TH2-related cytokines IL-4, IL-5, IL-9, and IL-13 are detected within 8 h of NAC. Elevation of IL-5 and IL-13 inversely correlates with PNIF, making nasal obstruction the clinical hallmark of late phase reactions (Fig. 1) [42].
A wide repertoire of TH2-derived cytokines (IL-4, IL-5, IL-9, IL-13) orchestrates a variety of critical allergic reactions. Although TH2 phenotype develops in response to basophil-derived IL-4, subsequently differentiated TH2 cells secrete IL-4 in an autocrine manner to maintain their identity [32, 59]. A study in a mouse model of HDM-allergic airway inflammation showed that TH2-derived IL-4, similarl to histamine, can contribute to the disruption of mucosal barrier by tight junction downregulation [43, 49]. IL-4 and IL-13 upregulate endothelial adhesion molecules, such as ICAM-1 and VCAM-1, to facilitate migration of effector cells to the nasal mucosa [60]. IL-5 is critical for tissue eosinophilia, as it not only promotes their release from bone marrow but also inhibits their apoptosis [61, 62]. A similar cell survival-promoting effect of this cytokine was recently observed in CD4+ T cells of AR patients [63]. Locally recruited eosinophils secrete toxic mediators which damage the nasal epithelium [64, 65]. Finally, TH2-derived IL-9 promotes mast cell differentiation and maturation [66].
TH2 cells express CRTH2 which is a receptor for prostaglandin D2 secreted during the EPR. The expression of this receptor on TH2 cells is regulated by tyrosine kinase and was found to be elevated 6 h post NAC suggesting it is the peak of TH2 migration [67]. Furthermore, a study exploring the spectrum of AR has identified TH2 cells highly expressing ST2, a receptor for IL-33, as the most pathological subset, which is not present in asymptomatic sensitized patients. The evolvement of this phenotype could be the immunological shift required for the clinical manifestation of AR [68•]. A relatively novel subset of allergen-specific TH2 cells (TH2A), virtually absent in non-atopic subjects, was found to be key in promoting a swift response to an allergen [69•]. Furthermore, TH17-derived IL-17 was found to be elevated in the serum of AR patients. This cytokine can potentially contribute to the pathology of AR by promoting sIgE synthesis in B cells [70, 71].
Accumulating evidence suggests that type 2 inflammation is not the sole driver of the LPR. A study involving nasal mucosa of grass pollen (GP) allergic patients showed that late phase responses also involve upregulation of genes related to alternative complement pathway (factor P and C5AR1) and the inflammasome components (IL-1α and IL-1β), the latter being shown to promote neutrophil recruitment [72]. Moreover, a study on birch pollen-allergic peripheral blood neutrophils demonstrated their capability to fully process and present the allergen subsequently promoting Bet v 1-specific T cell proliferation and cytokine production [73•]. In a HDM-sensitized murine asthma model, inhibition of the complement component C5a was shown to reduce TH2 infiltration and IL-4 concentration in the lung tissue without affecting their counterparts ILC2s, suggesting it has a role in amplifying type 2 inflammation [74].
Recent studies in the context of AR have highlighted the emerging role of PD-1/PD-L1 axis among its immune checkpoint counterparts. Soluble PD-L1, significantly more abundant in the circulation of healthy subjects compared to AR, was shown to negatively correlate with IL-4 as opposed to being positively associated with IFN-γ which suggests that T cell exhaustion is a potential protective mechanism against the disorder [75]. The blockade of this co-inhibitory axis can indeed promote allergen-specific CD4+ T responses in cases of both PAR and SAR. However, given that it affects both TH1 and TH2 cells in vitro, the contributions of this mechanism could be studied further considering possible local or cell-cell interactions [76].
ILC2s are innate counterparts of TH2 cells. They share the expression of CRTH2 but are lineage negative and act in an antigen-independent manner [28]. It was shown that upon stimulation with epithelial cell-derived TSLP and IL-33, ILC2s actively reset their miRNA repertoire, similar to the behavior of T cells after antigen stimulation. miR-19a in particular is crucial for the regulation of IL-13 production [77]. A study by Miao et al. demonstrated an increase in circulating IL-13+ ILC2s together with a greater capacity of this subset to produce IL-13 in response to IL-33 and IL-25 which was observed during natural pollen season in mug-worth sensitized asthmatics [78]. Similarly, SAR patients were shown to have elevated frequencies of total ILC2 and IL-13+ ILC2 during grass pollen season, compared to out-of-season which correlated with seasonal symptom severity [79]. To support that, an in-season study involving grass pollen-sensitized patients of allergic rhinoconjunctivitis showed a significant increase in circulating ILC2 numbers. Here, ILC2s did not demonstrate an enhanced ability to produce IL-4 and IL-13 after in vitro stimulation with phorbol 12-myristate 13-acetate and ionomycin. In the allergic group, the functional impairment was rather observed in ILC1s, the counterparts of the TH1 subset, as evidenced by reduced IFN-γ production [80].
Mechanisms of AIT in Allergic Rhinitis
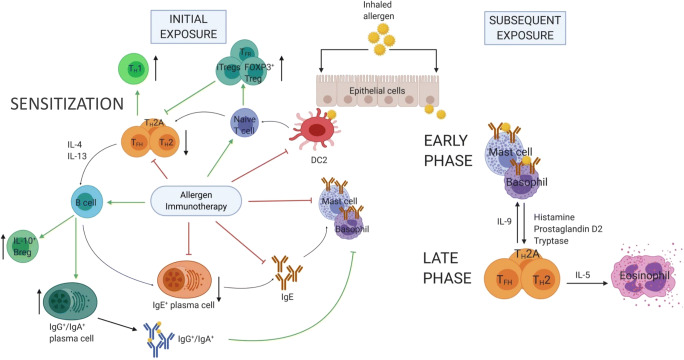
AIT is a standard therapeutic approach that is indicated for those AR patients whose symptoms persist in spite of consumption of conventional anti-allergic medication. Numerous double-blind, placebo-controlled clinical studies have demonstrated that both SCIT and SLIT are effective options for managing seasonal and perennial allergies. Critically, on-going treatment-induced desensitization can translate into long-term allergen-specific tolerance and clinical benefit, lasting for 2 to 3 years after its cessation [19–21]. Accumulating evidence fuels a more comprehensive understanding of AIT-related mechanisms of tolerance (Fig. 2; Table 1).
Fig. 2.
Mechanisms of allergic sensitization and allergen immunotherapy (AIT). (A) Upon inhalation of the allergen, ECs recruit DCs and polarize them to a pro-allergic DC2 phenotype. These cells uptake the allergen and migrate to lymph nodes, where they present it to naïve T cells and promote the development of TH2 and TFH subsets. TFH and TH2 cells collectively facilitate B cell maturation and class-switch recombination which leads to allergen-specific IgE production. These IgE molecules bind to high-affinity receptors on basophil and mast cell surfaces this way sensitizing the patient. The early phase reactions are triggered when a sensitized individual is subsequently exposed to the allergen, which in turn cross-links neighboring IgE molecules on basophil and mast cell surfaces and prompts the release of vasodilatory and chemoattractive mediators. This facilitates the recruitment of late phase effector T cells and eosinophils. (B) AIT suppresses the development of DC2 phenotype and promotes naïve T cell differentiation to regulatory phenotypes (iTregs, FOXP3+Tregs, TFR cells). These subsets in turn suppress TH2, TH2A and TFH responses and favor the differentiation of TH1. Inhibition of TH2 responses results in reduced local eosinophilia and prevents the development of IgE+ plasma cells. AIT also induces Bregs and IgG+/IgA+ plasma cells which produce blocking antibodies that compete with IgE for binding to the allergen, preventing the cross-linking of high-affinity receptors on mast cell and basophil surfaces and inhibiting their degranulation. Red arrows represent inhibition of effector cells; green arrows represent AIT-induced regulatory phenotypes and their effects; black arrows represent increases or decreases of the population frequencies. EC, epithelial cells; DC, dendritic cells; TH2, T helper type 2 cell; TFH, T follicular helper cell; Treg, T regulatory cell; Breg, B regulatory cell. Created with BioRender.com
Table 1.
A summary of key cell subsets, their role in allergic rhinitis (AR), and allergen immunotherapy (AIT)-induced effects on their responses. EC, epithelial cells; DC2, dendritic cells of type 2; ILC2s, group 2 innate lymphoid cells; TH2, T helper type 2 cell; TFH2, type 2 follicular helper T cells
| Stage of allergy | Cell subset | Key functional molecules | Role in allergic rhinitis | Effects of allergen immunotherapy |
|---|---|---|---|---|
| Sensitization | EC |
RNA GAS5 [35] |
DC recruitment [24•] Induction of ILC2 [29] and DC2 [27] cell subsets Promotion of TH2 [30] and inhibition of TH1 [35] polarization Promotion of mast cell degranulation [31] |
Restoration of EC integrity in murine models [81] |
| DC2 |
MHC II CD80/CD86 [32] |
TH2 polarization Antigen presentation [32] |
Promotion of DCreg polarization [27, 82] Increased FcεRI expression [46•] |
|
| TFH2 | Surface CD40 secreted IL-4, IL-13, IL-21 [36, 39] | Induction of B cell ε-germline transcription, differentiation and affinity maturation and sIgE production [39, 41] |
Decreased circulating numbers [38, 83] Restored function of suppressive counterparts TFR cells [84] |
|
| B cells | IgE [42] |
Mast cell and basophil sensitization [42] Antigen presentation [85••] |
Induction of IgG+ B cells [39, 47, 86–88•] Induction of IL-10+Bregs [87–90] Reduced CD23 expression [85••] |
|
| Early phase responses | Basophils | Histamine, tryptase, cysteinyl leukotrienes, PGD2 [43, 44, 50, 51] |
Promotion of TH2 polarization [33] Activation of ILC2s [51] Increasing vascular permeability and immune cell influx [50] Induction of symptoms: itching, sneezing, rhinorrhea [44] |
|
| Mast cells | ||||
| Late phase responses | TH2 | IL-4, IL-5, IL-9, IL-13 [44, 50, 64, 67, 99] |
Induction of B cell ε-germline transcription, differentiation and affinity maturation [41] Downregulation of tight junctions of nasal epithelium [50] Upregulation of endothelial adhesion molecules [61] Promotion of basophil and mast cell differentiation [67] |
Induction of iTregs [100–103] and nTregs [103–106] Reduced TH2 and increased TH1 frequencies and corresponding cytokines [100, 107–112] |
| ILC2s | Reduced circulating frequencies [80, 113•] | |||
| Eosinophils | LTC4, PGE2, EDN, ECP [66] | Damage of the nasal epithelium [65] | Reduced infiltration [92, 104, 107, 114, 115] | |
| Neutrophils | MHC II [74] | Antigen presentation [74] | Reduced activation (157) |
Effect of AIT on Innate Immune Responses
Innate responses are triggered by initial allergen interactions with the physical barrier—the nasal epithelium. To date, no studies in humans have demonstrated a reduction of epithelium-derived pro-inflammatory cytokines following AIT. However, a recent study of SCIT in a murine Der f-sensitized model demonstrated a reduced IL-25 secretion and restoration of epithelial integrity by ameliorating said cytokine-related endoplasmic reticulum stress and EC apoptosis [99]. However, such findings remain to be replicated in human models. The subsequent allergen presentation by DCs bridges the innate and adaptive immunity. Historically, AIT-induced elevation of complement component 1Q (C1Q)-expressing DCreg phenotype has been associated with the response to treatment. This DC subset favors the development of regulatory T cell phenotype [26, 81]. More recently, HDM-SCIT was shown to induce a temporary increase in FcεRI expression on DCs, suggesting that IgE/FcεRI signaling in this subset can contribute to the development of tolerance [45].
The EPR are mediated by mast cells and basophils which act in an antigen-independent manner. AIT reduces the infiltration of these effector cells which is followed by decreases of histamine and tryptase in the nasal mucosa of AR patients [82, 91–93]. A recent study involving SCIT for GP allergy demonstrated a 447-fold decrease in basophil sensitivity to the allergen 1 year into the treatment. The trend remained similar a year following treatment cessation. Remarkably, long-term clinical efficacy correlated with the reduction of basophil sensitivity 3 weeks into the treatment suggesting this could be a potential predictive biomarker [94]. A strong correlation between CD203c expression and clinical efficacy of SLIT for Parietaria was documented in a randomized 12-month trial. Interestingly, the reference group treated with conventional medication exhibited slightly reduced threshold of basophil activation, which highlights that AIT can serve to not only treat the disease but also prevent its progression [95]. Recently, an alternative to surface marker expression for evaluating basophil function has been described. Principally, the histamine amount released from the cell inversely correlates with intracellular fluorochrome-labeled diamine oxidase (DAO). Upon ex vivo basophil stimulation with GP allergen, the frequency of DAO+ basophils was significantly higher in SCIT and SLIT-treated compared to the untreated group. Critically, this suppression of histamine release correlated with the alleviation of clinical symptoms [96••]. A proteomics approach has been implemented in a study investigating cedar pollen (CP)-SLIT effects on mast cell degranulation, as responder and non-responder groups were not distinguishable by serum IgE, IgG or relevant cytokines. Thrombospondin 1 (THRS-1) was identified as a significant suppressant of mast cell degranulation elevated in the responder group compared to non-responders [97].
In the following LPR, the main innate cell populations are eosinophils and ILCs. Reduced local eosinophil infiltration is a characteristic feature resulting from AIT-induced dampening of upstream TH2 responses together with reduced eotaxin concentrations in nasal fluids [91, 98, 104, 114, 115]. ILCs are the sole innate immunity component of the lymphoid lineage. A murine airway inflammation model demonstrated a reduced proportion of IL5+ ILC2s in the circulation without affecting innate ILC-activating cytokines IL-33 and IL-25 after a 2-month birch pollen-SCIT [107]. In contrast, a 4-month GP-SCIT in humans failed to induce alterations of ILC2 frequencies in the periphery [80]. The first evidence of the effects of AIT on circulating ILC2s in humans was reported by Lao-Araya et al. Here, GP-SCIT for 8 months and more reduced circulating total and IL-13+ ILC2 frequencies in SAR patients during the pollen season which was accompanied by reduced seasonal symptoms [79]. In the context or PAR, responders to a 2-year HDM-SCIT exhibited a significant decrease in circulating ILC2 and elevation of ILC1 frequencies compared to non-responders and the untreated group, ultimately achieving a similar ILC2/ILC1 proportion as seen in healthy subjects. Moreover, ex vivo ILC2 stimulation with Der p1, IL-33 and IL-2 showed that ILC2s in AIT-treated group exhibit a reduced expression of activation marker CD69, however, without any impairment of cytokine secretion [116]. Very recently, a novel regulatory ILC (ILCreg) subset has been described. ILCregs were demonstrated to suppress lung [113•] and intestinal [117] inflammation by secreting IL-10. Following this, Morita et al. were able to demonstrate an in vitro induction of ILCreg subset derived from ILC2s upon exposure to retinoic acid (RA) and the presence of IL-2 and IL-33. ILCregs demonstrated a dose-dependent IL-10 production in response to RA, while IL-5 and IL-13 were not elevated. Transcriptome profiling of sorted IL-10+ ILCs revealed a downregulation of ILC2-related genes, such as CRTH2 and CD127 with conversely elevated Treg-related CD25 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) expression. However, IL-10 production rather than CTLA-4 activity was critical for ILCreg-dependent anti-proliferative activity toward CD4+ T cells and ILC2s. Concordantly, in human nasal tissue, epithelial cell-derived RA was also shown to promote ILCreg development from ILC2s. Conclusively, this novel subset dampens excessive inflammation [118]. The potential role of ILCregs in AIT-induced tolerance remains to be investigated in future studies.
Effect of AIT on Adaptive Immune Responses
Effect of AIT on TH2 and TFH Cells
Given the pivotal role of the TH2 subset in mediating allergic inflammation, dampening their response is a preferred outcome of AIT. Typically, immune deviation toward TH1 polarization is one of the mechanisms related to tolerance, particularly given that such response to an allergen is evident in healthy patients [33]. Generally, TH2 and TH2A numbers and their cytokines can be reduced systemically and locally following conventional AIT approaches [100, 108, 109, 119]. Complementary to this, AIT is associated with an increase in TH1 numbers, related chemokines and IFN-γ [109–111, 115].
SLIT for Artemisia annua pollen showed a reduction in TH2 proportion as early as 16 weeks which was accompanied by clinical symptomatic relief. Although the treatment course was comparably shorter than described in other studies, tolerance persisted throughout the following pollen season [108]. A recent study in timothy grass pollen-allergic children confirms that 3-year treatment can lead to a sustained clinical benefit. Two years after treatment discontinuation, systemic and local tolerance was paralleled by low ratios of IL-5/IFN-γ, and IL-13/IFN-γ. The study also detected elevated TH1-related chemokines CXCL10 and CXCL11, suggesting that sufficient migration of the subset to the site of inflammation can also be crucial for tolerance [111]. An indirect role for the regulation of TH1-favored immune deviation was attributed to type I IFN signaling. Gene expression analysis in SAR patients after 1 year of SLIT showed a significant downregulation of type I IFN signaling pathway-related genes (STAT1, STAT2, IFN-α, IFN-β) which are otherwise known to facilitate de novo TH1 formation [112]. A microarray-based multivariate analysis in the context of cedar pollinosis showed a differential apoptosis-related gene expression upregulation in CD4+T subset of the SLIT-treated group which suggests that induction of programmed cell death is a potential mechanism orchestrating the balance of all T cell subsets. However, it remains unclear which subpopulations were affected [120].
While the shifts in TH2/TH1 balance are often observed during or post treatment, it has also been found to have predictive potential at baseline. In the example of Dermatophagoides pteronyssinus (DP)-SCIT, patients who were responsive had a highly reactive pre-treatment state evidenced by low TH1 and high TH2 frequencies accompanied by higher concentrations of IL-5, IL-9 and IL-13. Contrastingly, non-responders showed a low reactivity profile at baseline defined by high TH1 and low TH2 proportions. The responsive group displayed a significant reduction in the proportion of TH2 cells after 12 months of SCIT while no shift was seen in non-responsive patients [100].
Besides affecting the overall TH2 subpopulation, a 1-year SLIT study demonstrated a reduction of particularly pathogenic HDM-reactive memory TH2 subsets defined as IL-5+IL-13+CD27−CD161+CD4+ and ST2+CD45RO+CD4+ the latter of which correlated with clinical benefit. Interestingly, these subpopulations in the non-responder group remained elevated making the subsets potential markers for treatment monitoring [121].
Critical TFH role in the induction of IgE production constitutes them as a key subset to target by AIT. ICOS+ TFH cells, IL-4+ TFH cells, IL-21+ TFH cells and dual IL-4+IL-21+ TFH cells can be reduced by SCIT and SLIT [122•]. A recent study found that circulating Der p 1–specific IL-4+ TFH cells can decrease as a result of AIT which correlated with the improvement of clinical symptoms. This shift was accompanied by a reduction of serum sIgE; however, no significant correlation between them suggests the contribution of alternative factors, such as regulatory counterparts T follicular regulatory (TFR) cells [37].
Effect of AIT on Regulatory T Cells
Induction of regulatory T cell subsets is another key mechanism favoring tolerance. Both natural Tregs (nTregs) and induced Tregs (iTregs) modulate allergen-specific TH2 immunity with their respective mechanisms. nTregs is a thymus-derived subset defined by the expression of a transcription factor FOXP3 and surface marker CD25. The subpopulation is known to dampen the immune responses by direct cell-cell interaction in both healthy and allergic subjects. iTreg subpopulation arises in the periphery in response to the cytokine milieu and antigen stimulation and exerts their immunosuppressive abilities by secreting IL-10 and TGF-β accordingly comprising Tr1 and TH3 subsets [83]. IL-10, in particular, contributes to effector T cell anergy and is also crucial for the induction of allergen-specific IgG production [123]. Recently, the immunosuppressive capacity of newly identified IL-35 derived from the inducible regulatory T (iTR35) cells has been demonstrated following SLIT for grass pollen. In the treated group, the elevation of this subset was accompanied by IL-35-mediated dampening of cellular and humoral TH2 responses. Moreover, this cytokine exerted a suppressive capacity on IgE production in B cells [124••].
Current studies show that AIT can induce increases in circulating iTregs [101, 108], whereas FOXP3+CD25+ T cell role is rather attributed to increased local infiltration [102] or allergen-specific nTreg frequency and phenotypic shifts within the subpopulation [105]. Moreover, the immune response shift toward allergen-specific Tregs after 1 year of DP-SCIT is seen in patients who are responsive to the treatment [100].
A recent study proposed a regulatory role for IL-10-related transcription factor E4BP4, as its mRNA and IL-10 producing Tr1 were elevated in the blood of CP-SCIT-treated patients [103]. Special AT-rich sequence binding protein 1 (SATB1) has been identified as a transcriptional suppressor of FOXP3+ which in turn regulates Treg function. In an AIT cross-sectional study, both SCIT and SLIT were shown to increase DNA methylation levels at the SATB1 locus, subsequently resulting in reduced SATB1 mRNA expression, compared to untreated SAR group. Critically, SATB1 but not FOXP3 expression in Tregs correlated with the alleviation of clinical symptoms [125••]. Reduced sensitivity to apoptosis in FOXP3+ Tregs is another proposed AIT-induced mechanism of propelling tolerance, as seen in a murine asthma model [106].
Serine/threonine kinase CK2 is a constitutively active enzyme found to be crucial for the maintenance of a functional Treg phenotype in both humans and mice. Genetic ablation of the β-subunit of CK2 prompts the development of ILT3+ Treg subpopulation which is unable to modulate the airway TH2 response [126]. A study on Der p 1-specific FOXP3+ T cells after SCIT demonstrated a change of functional heterogeneity in the nTreg subset. The proportion of circulating activated allergen-specific FOXP3+Helios+ Tregs increased following a 30-week treatment and slightly declined at the 3-year mark, whereas the dysfunctional ILT3+ proportion was reduced. Importantly, these shifts correlated with improved allergic symptoms. The study also detected elevations of the Der p 1-specific IL-10+CD4+ T subset [105]. TH2 proliferation can directly be amplified by introducing antibodies against IL-10 and TGF-β into the supernatant of a co-culture system with effector and regulatory T cells isolated from patient PBMCs after 12 months of DP-SCIT. In the experiment, Tregs were also shown to have a greater capacity of suppressing IL-4 production in effector cells than those isolated pre-treatment [101]. A 3-year treatment with a grass sublingual tablet was shown to be key at inducing a lasting activated memory Treg phenotype defined as CD127−CD45RA−CD25high in the circulation of 2/3 of the patients. Moreover, the response was associated with reduced eosinophil numbers as well as sIgE titers [98].
TFR cells are regulatory counterparts of TFH that express CXCR5, transcription factor FOXP3 and analogous surface molecules seen in Tregs which facilitate cell-cell contact-dependent immunosuppression. Interestingly, TFR cells can migrate to germinal centers in a CXCR5-independent manner [127]. In AR patients, this subset is not only less abundant in the circulation, but also exhibits a reduced capacity to suppress TFH-mediated sIgE production. A 12-month HDM-SCIT demonstrated a significant restoration of the suppressive capacity on IgE production and an increase in circulating TFR proportion. The latter correlated significantly with clinical improvement which potentially represents a biomarker for monitoring the treatment response [128]. A very recent study suggested that impaired chromatin accessibility and therefore immunosuppression-related gene transcription can contribute to the pathogenesis of SAR which importantly can be reversed by SCIT and SLIT [122•].
B Cell-Related Tolerance
Upon AIT, allergen-specific IgE+ B cells switch isotype and start producing blocking antibodies with the same antigen specificity [84, 123]. This shift not only reduces the pathological IgE+ B cell subset, but also supplements antibodies that compete with the remaining sIgE for binding to the allergen.
IgE responses during AIT have been investigated in numerous studies. A study involving GP-SLIT demonstrated that IgE repertoire during the first year of treatment remains stable which suggests that there is no production of novel allergen-specific antibody clonotypes and therefore treatment-induced progression of the disease [46•]. Interestingly, in a DP-SCIT-responsive group, a significant sIgE elevation during initial months, which returned to baseline at year 1 of the treatment, was observed, whereas such shift was not seen in nonresponsive patients [100]. In a GRASS randomized clinical trial, SLIT was associated with a transient serum sIgE increase at year 1, while in SCIT group’s allergen-specific IgE concentration was not affect at the same time point and only decreased throughout years 2 and 3. Critically, this study demonstrated that 2 years of GP-SLIT is not sufficient to induce lasting clinical benefit at a 3-year follow up [129]. Alternatively, IgE responses can also be dampened by the downregulation of low affinity IgE receptor CD23 on switched memory B cells which was associated with positive clinical outcome after 12-month HDM-SCIT. This mechanism serves to impair T and B cell interaction which weakens antigen presentation and IgE synthesis [130•].
The ability to inhibit the effects of IgE resides mainly in IgG and IgA immunoglobulin fractions. AIT-induced IgG4 in particular is capable of binding to allergen epitopes otherwise recognized by IgE [84]. Thus, IgG dampens allergic inflammation by preventing further IgE production in B cells, mast cell and basophil degranulation and IgE-mediated antigen presentation by B and dendritic cells [85••, 89, 131]. AIT can induce blocking antibodies as early as 4 weeks into the treatment, while remaining elevation of this fraction at year 1 can predict patient responsiveness [100, 132]. After a 2-year increase in serum IgG4, the levels of this immunoglobulin stabilize during the third year and decrease post-therapy by 90%. However, the remaining circulating IgG exerts sufficient inhibitory capacity toward the activity of IgE which promotes lasting tolerance [90, 98]. Shamji et al. demonstrated that nasal secretions display a greater IgG-associated inhibitory activity than that of the serum in SCIT-treated patients. Remarkably, clinical efficacy of the treatment was shown to closely correlate with nasal IgG4 inhibitory capacity [85••]. The evidence of SLIT inducing B cell memory was observed in ryegrass-allergic patients as evidenced by elevation of allergen-specific IgG2 and IgG4 with corresponding IgG2+ and IgG4+ memory B cell frequencies which was associated with clinical improvement [133]. Timothy grass pollen SLIT treated children exhibited significantly higher levels of Phl p 1-specific salivary IgA and serum IgG4, along with lower SPT positivity after 3 years of treatment which proceeded throughout 2 years after treatment cessation [111].
Accumulating evidence highlights the importance of B regulatory cells (Bregs) for the induction of tolerance following AIT. A study by van de Veen et al. involving a 16-week bee venom-AIT elucidated key features and functions of this cell subset. Primarily defined by the expression of IL-10, these Breg cells were shown to have a CD25highCD71highCD73low phenotype. During AIT, IL-10+ Breg cells become sole producers of allergen-specific IgG4, which demonstrates the dual capacity of this cell subset to induce tolerance both via allergen-specific T cell suppression and IgG-associated inhibitory processes [86]. Following this, numerous studies regarding seasonal and perennial inhaled allergens have reported AIT-induced increases in peripheral Breg frequencies and sIgG4. After a 2-year Der p1-SCIT, a significant elevation of serum allergen-specific and IgG4+ B cells was accompanied by the expansion of IL-10 and IL-1RA producing Bregs, which, taken together, correlated with a positive clinical outcome [87]. A 3-week therapy with linear rye-grass peptides was sufficient to alleviate seasonal symptoms in SAR patients. Aside from the induction of blocking IgG4 antibodies, the treatment also induced IL-10 production in Breg cells which was attributed to IL-35 secreted by the iTR35 subset [88•]. A cross-sectional controlled study demonstrated that SCIT-induced increases in IL-10+ Bregs were significantly higher during the natural pollen season than out of it. In a co-culture experiment, these CpG primed Bregs suppressed proliferation as well as IL-5 and IL-13 production in CD4+ T memory cells which was reversed by introducing anti-IL-10. Moreover, Breg frequencies in SCIT group correlated with serum sIgG4 and to a lesser extent—with nasal sIgG4 [85••]. Furthermore, a correlation between peripheral increases in grass pollen-specific IgG4 and IL-10+ Breg cells was observed after SCIT which potentially supports a previously observed notion that sIgG4 is produced by IL-10+ B cells [85••]. Induction of circulating IL-10+ Breg subpopulation has been suggested to have predictive value for anticipating treatment success, as increases of Breg/Th17 ratio at the initiation of the treatment significantly correlated with induction of tolerance at 3-year mark after GP-SCIT [134].
Biomarkers of AIT
In order to ensure the maximum clinical benefit of AIT, there is a need to identify biomarkers to pre-select patients who are likely to become responders as well as biomarkers of desensitization, efficacy and tolerance. Currently, there are no confirmed biomarkers that can predict these parameters on an individual patient level [135]. Nevertheless, a recently published European Academy of Allergy and Clinical Immunology (EAACI) Position Paper reviewed current candidate biomarkers used to monitor the clinical efficacy of AIT in AR patients with or without asthma (Table 2) [142•].
Table 2.
Six domains for monitoring the efficacy of allergen immunotherapy: antibodies, serum inhibitory activity for IgE, cytokines and chemokines, basophil activation, cellular, and in vivo biomarkers. IgE-FAB, IgE-facilitated antigen binding; ELIFAB, enzyme-linked immunosorbent-facilitated antigen binding; DAO, diamine oxidase
| Domains | Biomarkers | References |
|---|---|---|
| Antibodies |
IgE (tIgE, sIgE, sIgE/tIgE) sIgG4 sIgA |
[112] |
| Serum inhibitory activity for IgE |
IgE-FAB ELIFAB |
[139] |
| Cytokines and chemokines |
TH2-related: IL-4, IL-5, IL-9, IL-13 TH1-related: IFN-γ, CCL10, CCL11 Treg-related: IL-10, IL-35 |
|
| Basophil activation |
CD63/CD203c DAO |
[97] |
| Cellular biomarkers |
Treg Breg DCreg |
|
| In vivo |
Allergen provocation tests Chamber studies |
[141] |
Mechanisms of AIT Determine Potential Biomarkers
Biomarkers for AIT monitoring consist of humoral, cellular and in vivo compartments. In serum, elevation of sIgE is considered to be the gold standard for both diagnosis of allergy and recruitment for AIT [19, 45, 98, 136]. Moreover, strong evidence supports the use of sIgE/tIgE ratio for predicting treatment efficacy [143]. Increases of serum sIgG4 have inconsistently been linked with the clinical outcome [100, 101], whereas the elevation of the same fraction and its inhibitory activity in the nasal mucosa were associated with ameliorated allergic response [85, 137]. To assess the overall serum inhibitory activity, attributed to IgG, IgA and IgD fractions, IgE-facilitated antigen binding (IgE-FAB) assay is conducted [85, 132, 138]. However, this assay is complex and may be limited to specialized facilities. An enzyme-linked immunosorbent-facilitated antigen binding (ELIFAB) is a more feasible assay for routine applications [139]. Reduction of inflammatory and increases in immunosuppressive chemokines and cytokines in serum are not seen consistently [100, 109, 111, 115], whilst nasally located mediators correlate with clinical benefit more frequently [93, 109]. In regard to cellular changes, AIT can alter cell function as well as subset frequencies and phenotypes. Reduction of basophil activation, determined by surface marker CD63 and CD203c expression, is inconsistently associated with lasting clinical improvement [94, 95, 100, 140]. Alternatively, reduction of basophil histamine release, determined by DAO assay, can correlate with AIT success [96••]. AIT-induced cellular changes, such as deviation toward DCreg [26, 81] and Treg phenotypes [98, 103, 105], are associated with induction of tolerance by skewing the immune response from TH2 to TH1. More recently, several studies have been able to associate clinical efficacy with the induction of IgG-producing memory B cells [133] or downregulation of their surface CD23 [130•]. Other recently arisen cellular candidates for monitoring AIT efficacy are pathogenic memory TH2 subsets [121], dysfunctional ILT3+ Tregs [105], Breg/Th17 ratio [134]. Notably, these findings remain to be replicated in future studies. Finally, in vivo biomarkers, such as allergen provocation tests and chamber studies, can be used to identify systemic or local sensitization to a relevant allergen [93, 100, 111, 141].
Addressing the Unmet Needs and the Current Challenges in Biomarkers for AIT
Universal biomarkers for monitoring efficacy of AIT have yet to be identified and plenty remains to be understood. Nevertheless, with the recent advances in molecular and computational biology techniques, research limitations are as trivial as ever. The rise of machine learning-assisted unbiased clustering algorithms has revolutionized the interpretation of complex flow and mass cytometry data. As recent as they are, such computational tools have already elucidated mechanisms in fundamental immunology [144], cancer [145, 146], cell cycle [147], vaccination and HIV [148]. Importantly, unbiased analysis has substantially contributed to the understanding of immunological biomarkers in food allergy [149], and its usage for AR-related research in the future is more than likely. In the field of transcriptomics analysis, single-cell RNA sequencing (scRNA-seq) is the future of comprehensive and well-rounded understanding of biological processes. This analysis platform enables the assessment of gene expression in each individual cell which is superior to bulk analysis that often overlooks shifts in transcriptomic patterns by averaging the mRNA expression data [150]. In the field of allergy, scRNA-seq has already propelled knowledge of B cell class switching [151], B cell memory [46•], airway T cell metabolism [152], transcriptional differences between asthmatics with and without allergy [153], IgE responses [154], T cell clonotypes [155], and functional heterogeneity within allergen-specific T cell subset [156].
Conclusions
AR is a serious health problem, affecting the quality of life of a vast proportion of the world’s population. Patients that do not respond to conventional anti-allergic medication can benefit from AIT which induces lasting clinical benefit post treatment cessation. Currently, there is a need for biomarkers to optimally select potentially responsive patients and monitoring the efficacy of the treatment. An in-depth understanding of both allergic inflammation and mechanisms of immunotherapy informs potential candidates for monitoring these parameters. Following nasal allergen challenge, EC-derived cytokines prime the development of pro-allergic DC2 and TH2 phenotypes. In the early phase, basophils and mast cells release their mediators upon surface IgE cross-linking by the allergen. These mediators recruit late phase effector TH2 cells which promote local eosinophilia. SCIT and SLIT for perennial and seasonal allergens dampen both innate and adaptive immune responses. Following AIT, reduced local infiltration of basophils, mast cells, eosinophils as well as their corresponding mediators is observed. Furthermore, induction of regulatory DC phenotype leads to the rise of Tregs which skew the immune response from TH2 to TH1 and give rise to the Breg subset. Breg and Treg-derived IL-10 prompts B cells isotype switch and subsequent IgG4 production. Informed by these mechanisms, current candidate biomarkers for monitoring AIT efficacy are classified into domains of sIgE, sIgG4, serum inhibitory activity, chemokines and cytokines, basophil activation, cellular changes, and in vivo biomarkers. However, there is no biomarker for monitoring clinical benefit on an individual patient level to date. Further studies need to be conducted to identify and confirm biomarkers, suitable for AIT efficacy monitoring and recognizing potential responders and non-responders.
Abbreviations
- AIT
Allergen immunotherapy
- AR
Allergic rhinitis
- Breg
B regulatory cell
- CP
Cedar pollen
- DAO
Diamine oxidase
- DC
Dendritic cell
- DP
Dermatophagoides pteronyssinus (en. house dust mite)
- EC
Epithelial cell
- ECP
Eosinophil cationic protein
- FOXP3+
Forkhead box protein P3
- GP
Grass pollen
- HDM
House dust mite
- IgE-FAB
IgE-facilitated antigen binding
- ILC2
Group 2 innate lymphoid cell
- NAC
Nasal allergen challenge
- PAR
Perennial allergic rhinitis
- PBMC
Peripheral blood mononuclear cell
- PNIF
Peak nasal inspiratory flow
- SAR
Seasonal allergic rhinitis
- SATB1
Special AT-rich sequence binding protein 1
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- SPT
Skin prick test
- TFH
T follicular helper cells
- TH1
T helper type 1 cell
- TH2
T helper type 2 cell
- TH2A
Allergen-specific TH2 cells
- TNSS
Total nasal symptom score
- Treg
T regulatory cell
- TSLP
Thymic stromal lymphopoietin
Compliance with Ethics Standard
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rhinitis, Conjunctivitis, and Sinusitis
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378(9809):2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 2.Mortz CG, Andersen KE, Poulsen LK, Kjaer HF, Broesby-Olsen S, Bindslev-Jensen C. Atopic diseases and type I sensitization from adolescence to adulthood in an unselected population (TOACS) with focus on predictors for allergic rhinitis. Allergy. 2019;74(2):308–317. doi: 10.1111/all.13630. [DOI] [PubMed] [Google Scholar]
- 3.Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, Ge SQ, Zhang N, Zhang L, Bachert C. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X-Y, Ma T-T, Wang X-Y, Zhuang Y, Wang X-D, Ning H-Y, et al. Prevalence of pollen-induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73(6):1232–1243. doi: 10.1111/all.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan D, Sibbald B, Weiland S, Ait-Khaled N, Anabwani G, Anderson HR, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatr Allergy Immunol. 1997;8(4):161–168. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Patil VK, Kurukulaaratchy RJ, Venter C, Grundy J, Roberts G, Dean T, et al. Changing prevalence of wheeze, rhinitis and allergic sensitisation in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy. 2015;45(9):1430–1438. doi: 10.1111/cea.12534. [DOI] [PubMed] [Google Scholar]
- 7.Alsowaidi S, Abdulle A, Shehab A, Zuberbier T, Bernsen R. Allergic rhinitis: prevalence and possible risk factors in a Gulf Arab population. Allergy. 2010;65(2):208–212. doi: 10.1111/j.1398-9995.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 8.Canonica GW, Mullol J, Pradalier A, Didier A. Patient perceptions of allergic rhinitis and quality of life. World Allergy Organ J. 2008;1(9):138–144. doi: 10.1097/WOX.0b013e3181865faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet J, Demarteau N, Mullol J, Akker-van Marle ME, Van Ganse E, Bachert C. Costs associated with persistent allergic rhinitis are reduced by levocetirizine. Allergy. 2005;60(6):788–794. doi: 10.1111/j.1398-9995.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Shedden A. Impact of nasal congestion on quality of life and work productivity in allergic rhinitis. Treat Respir Med. 2005;4(6):439–446. doi: 10.2165/00151829-200504060-00007. [DOI] [PubMed] [Google Scholar]
- 11.Blanc PD, Trupin L, Eisner M, Earnest G, Katz P, Israel L, et al. The work impact of asthma and rhinitis. J Clin Epidemiol. 2001;54(6):610–618. doi: 10.1016/S0895-4356(00)00349-8. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008*. Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 13.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, Correia de Sousa J, Cruz AA, Cuello-Garcia CA, Demoly P, Dykewicz M, Etxeandia-Ikobaltzeta I, Florez ID, Fokkens W, Fonseca J, Hellings PW, Klimek L, Kowalski S, Kuna P, Laisaar KT, Larenas-Linnemann DE, Lødrup Carlsen KC, Manning PJ, Meltzer E, Mullol J, Muraro A, O’Hehir R, Ohta K, Panzner P, Papadopoulos N, Park HS, Passalacqua G, Pawankar R, Price D, Riva JJ, Roldán Y, Ryan D, Sadeghirad B, Samolinski B, Schmid-Grendelmeier P, Sheikh A, Togias A, Valero A, Valiulis A, Valovirta E, Ventresca M, Wallace D, Waserman S, Wickman M, Wiercioch W, Yepes-Nuñez JJ, Zhang L, Zhang Y, Zidarn M, Zuberbier T, Schünemann HJ. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Rondón C, Fernández J, López S, Campo P, Doña I, Torres MJ, et al. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J Allergy Clin Immunol. 2009;124(5):1005–1011. doi: 10.1016/j.jaci.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975;306(7926):148–150. doi: 10.1016/S0140-6736(75)90056-2. [DOI] [PubMed] [Google Scholar]
- 16.Eguiluz-Gracia I, Fernandez-Santamaria R, Testera-Montes A, Ariza A, Campo P, Prieto A, et al. Coexistence of nasal reactivity to allergens with and without IgE sensitization in patients with allergic rhinitis. Allergy. 2020;75(7):1689–1698. doi: 10.1111/all.14206. [DOI] [PubMed] [Google Scholar]
- 17.White S, Baker D, Frew Symptom control in patients with hay fever in UK general practice: how well are we doing and is there a need for allergen immunotherapy? Clin Exp Allergy. 1998;28(3):266–270. doi: 10.1046/j.1365-2222.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3):S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Durham SR, Emminger W, Kapp A, de Monchy JGR, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 20.Durham SR, Walker SM, Varga E-M, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 21.Pfaar O, Nell MJ, Boot JD, Versteeg SA, van Ree R, Roger A, et al. A randomized, 5-arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy. 2016;71(7):967–976. doi: 10.1111/all.12860. [DOI] [PubMed] [Google Scholar]
- 22.Post S, Rozeveld D, Jonker MR, Bischoff R, van Oosterhout AJ, Heijink IH. ADAM10 mediates the house dust mite-induced release of chemokine ligand CCL20 by airway epithelium. Allergy. 2015;70(12):1545–1552. doi: 10.1111/all.12730. [DOI] [PubMed] [Google Scholar]
- 23.Vanbervliet B, Homey B, Durand I, Massacrier C, Aït-Yahia S, de Bouteiller O, et al. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur J Immunol. 2002;32(1):231–242. doi: 10.1002/1521-4141(200201)32:1<231::AID-IMMU231>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.•Steelant B, Wawrzyniak P, Martens K, Jonckheere A-C, Pugin B, Schrijvers R, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(5):1242–53 e7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674919306153. Accessed 17 July 2020. This paper describes a mechanism contributing to epithelial barrier dysregulation in patients with allergic rhinitis. [DOI] [PubMed]
- 25.Shlomovitz I, Erlich Z, Speir M, Zargarian S, Baram N, Engler M, Edry-Botzer L, Munitz A, Croker BA, Gerlic M. Necroptosis directly induces the release of full-length biologically active <scp>IL</scp> -33 in vitro and in an inflammatory disease model. FEBS J. 2019;286(3):507–522. doi: 10.1111/febs.14738. [DOI] [PubMed] [Google Scholar]
- 26.Gueguen C, Bouley J, Moussu H, Luce S, Duchateau M, Chamot-Rooke J, et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol. 2016;137(2):545–558. doi: 10.1016/j.jaci.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Zheng R, Chen Y, Shi J, Wang K, Huang X, Sun Y, et al. Combinatorial IL-17RB, ST2, and TSLPR signaling in dendritic cells of patients with allergic rhinitis. Front Cell Dev Biol. 2020;8:207. doi: 10.3389/fcell.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 29.Akasaki S, Matsushita K, Kato Y, Fukuoka A, Iwasaki N, Nakahira M, et al. Murine allergic rhinitis and nasal T h2 activation are mediated via TSLP- and IL-33-signaling pathways. Int Immunol. 2015;dxv055. Available from: https://academic.oup.com/intimm/article-lookup/doi/10.1093/intimm/dxv055 [DOI] [PMC free article] [PubMed]
- 30.Nian J-B, Zeng M, Zheng J, Zeng L-Y, Fu Z, Huang Q-J, et al. Epithelial cells expressed IL-33 to promote degranulation of mast cells through inhibition on ST2/PI3K/mTOR-mediated autophagy in allergic rhinitis. Cell Cycle. 2020;19(10):1132–1142. doi: 10.1080/15384101.2020.1749402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinjan A, Willart M, Vanrijt L, Braunstahl G, Leman K, Jung S, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118(5):1117–1125. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4(3):313–319. doi: 10.1016/S1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 33.Van Overtvelt L, Wambre E, Maillère B, von Hofe E, Louise A, Balazuc AM, et al. Assessment of bet v 1-specific CD4 + T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J Immunol. 2008;180(7):4514–4522. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 34.•Zhu X, Wang X, Wang Y, Zhao Y. Exosomal long non-coding RNA GAS5 suppresses Th1 differentiation and promotes Th2 differentiation via downregulating EZH2 and T-bet in allergic rhinitis. Mol Immunol. 2020;118:30–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S016158901930584X. Accessed 17 July 2020. A novel mediator contributing to T helper type 2 polarization in allergic rhinitis. [DOI] [PubMed]
- 35.Crotty S. Follicular helper CD4 T cells (T FH) Annu Rev Immunol. 2011;29(1):621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y-N, Song J, Wang H, Wang H, Zeng M, Zhai G-T, et al. Nasal IL-4+CXCR5+CD4+ T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016;137(2):462–473. doi: 10.1016/j.jaci.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Yao Y, Chen C-L, Wang N, Wang Z-C, Ma J, Zhu R-F, et al. Correlation of allergen-specific T follicular helper cell counts with specific IgE levels and efficacy of allergen immunotherapy. J Allergy Clin Immunol. 2018;142(1):321–324.e10. doi: 10.1016/j.jaci.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science (80-) 2019;365(6456):eaaw6433. doi: 10.1126/science.aaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015;158(2):204–211. doi: 10.1016/j.clim.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Punnonen J, Yssel H, Devries J. The relative contribution of IL-4 and IL-13 to human IgE synthesis induced by activated CD4 or CD8 T cells. J Allergy Clin Immunol. 1997;100(6):792–801. doi: 10.1016/S0091-6749(97)70276-8. [DOI] [PubMed] [Google Scholar]
- 41.Knol EF. Requirements for effective IgE cross-linking on mast cells and basophils. Mol Nutr Food Res. 2006;50(7):620–624. doi: 10.1002/mnfr.200500272. [DOI] [PubMed] [Google Scholar]
- 42.Scadding GW, Eifan A, Penagos M, Dumitru A, Switzer A, McMahon O, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy. 2015;45(3):613–623. doi: 10.1111/cea.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watts AM, Cripps AW, West NP, Cox AJ. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol. 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fphar.2019.00294/full [DOI] [PMC free article] [PubMed]
- 44.Engeroff P, Caviezel F, Mueller D, Thoms F, Bachmann MF, Vogel M. CD23 provides a noninflammatory pathway for IgE-allergen complexes. J Allergy Clin Immunol. 2020;145(1):301–311.e4. doi: 10.1016/j.jaci.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 45.Berings M, Gevaert P, De Ruyck N, Derycke L, Holtappels G, Pilette C, et al. FcεRI expression and IgE binding by dendritic cells and basophils in allergic rhinitis and upon allergen immunotherapy. Clin Exp Allergy. 2018;48(8):970–980. doi: 10.1111/cea.13157. [DOI] [PubMed] [Google Scholar]
- 46.•Hoof I, Schulten V, Layhadi JA, Stranzl T, Christensen LH, de la Mata Herrera S, et al. Allergen-specific IgG+ memory B cells are temporally linked to IgE memory responses. J Allergy Clin Immunol. 2020;146(1):180–91 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674919326028. Accessed 17 July 2020. Insights into IgE-related memory. [DOI] [PMC free article] [PubMed]
- 47.Beute J, Ganesh K, Nastiti H, Hoogenboom R, Bos V, Folkerts J, et al. PDE3 inhibition reduces epithelial mast cell numbers in allergic airway inflammation and attenuates degranulation of basophils and mast cells. Front Pharmacol. 2020;11. Available from: https://www.frontiersin.org/article/10.3389/fphar.2020.00470/full [DOI] [PMC free article] [PubMed]
- 48.Vitallé J, Terrén I, Orrantia A, Segurola A, Seras Y, Gamboa PM, et al. Increased expression levels of CD300c on basophils from allergic individuals. World Allergy Organ J. 2019;12(9):100060. doi: 10.1016/j.waojou.2019.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farré R, Wawrzyniak P, et al. Histamine and T helper cytokine–driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951–963.e8. doi: 10.1016/j.jaci.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashiwakura J, Yamashita S, Yoshihara M, Inui K, Saitoh K, Sekine Y, et al. STAP-2 positively regulates FcεRI-mediated basophil activation and basophil-dependent allergic inflammatory reactions. Int Immunol. 2019;31(5):349–356. doi: 10.1093/intimm/dxz013. [DOI] [PubMed] [Google Scholar]
- 52.Sekine Y, Nishida K, Yamasaki S, Muromoto R, Kon S, Kashiwakura J, et al. Signal-transducing adaptor protein-2 controls the IgE-mediated, mast cell–mediated anaphylactic responses. J Immunol. 2014;192(8):3488–3495. doi: 10.4049/jimmunol.1300886. [DOI] [PubMed] [Google Scholar]
- 53.Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, et al. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175(10):6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zellweger F, Buschor P, Hobi G, Brigger D, Dahinden CA, Villiger PM, et al. IL-3 but not monomeric IgE regulates FcεRI levels and cell survival in primary human basophils. Cell Death Dis. 2018;9(5):–510 Available from: http://www.nature.com/articles/s41419-018-0526-9. [DOI] [PMC free article] [PubMed]
- 55.Rignault-Bricard R, Machavoine F, Mecheri S, Hermine O, Schneider E, Dy M, et al. IL-3-producing basophils are required to exacerbate airway hyperresponsiveness in a murine inflammatory model. Allergy. 2018;73(12):2342–2351. doi: 10.1111/all.13480. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder JT, Bieneman AP. Activation of human basophils by A549 lung epithelial cells reveals a novel IgE-dependent response independent of allergen. J Immunol. 2017;199(3):855–865. doi: 10.4049/jimmunol.1700055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanase Y, Matsuo Y, Kawaguchi T, Ishii K, Tanaka A, Iwamoto K, et al. Activation of human peripheral basophils in response to high IgE antibody concentrations without antigens. Int J Mol Sci. 2018;20(1):45. doi: 10.3390/ijms20010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992;148(8):2390–2394. [PubMed] [Google Scholar]
- 59.Stark JM, Tibbitt CA, Coquet JM. The metabolic requirements of Th2 cell differentiation. Front Immunol. 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.02318/full [DOI] [PMC free article] [PubMed]
- 60.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Cytokines differentially regulate ICAM-1 and VCAM-1 expression on human gingival fibroblasts. Clin Exp Immunol. 2006;144(3):494–502. doi: 10.1111/j.1365-2249.2006.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada S, Hagan JB, Kato M, Bankers-Fulbright JL, Hunt LW, Gleich GJ, et al. Lidocaine and its analogues inhibit IL-5-mediated survival and activation of human eosinophils. J Immunol. 1998;160(8):4010–4017. [PubMed] [Google Scholar]
- 63.Luo X, Ma F, Wang S, Zhao M, Shao J, Geng X, et al. Interleukin-5 induces apoptotic defects in CD4 + T cells of patients with allergic rhinitis. J Leukoc Biol. 2019;105(4):719–727. doi: 10.1002/JLB.3A0718-287RR. [DOI] [PubMed] [Google Scholar]
- 64.Avars GH, Altman LC, Mcmanus MM, Agosti JM, Baker C, Luchtel DL, et al. Injurious effect of the eosinophil peroxide-hydrogen peroxide-halide system and major basic protein on human nasal epithelium in vitro. Am Rev Respir Dis. 1989;140(1):125–131. doi: 10.1164/ajrccm/140.1.125. [DOI] [PubMed] [Google Scholar]
- 65.Blanchard C, Rothenberg ME. Chapter 3 Biology of the eosinophil. In 2009. p. 81–121. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0065277608010031 [DOI] [PMC free article] [PubMed]
- 66.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136(2):433–440.e1. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Shazly A, Roncarati P, Lejeune M, Lefebvre P, Delvenne P. Tyrosine kinase inhibition is an important factor for gene expression of CRTH2 in human eosinophils and lymphocytes: a novel mechanism for explaining eosinophils recruitment by the neuro-immune axis in allergic rhinitis. Int Immunopharmacol. 2017;45:180–186. doi: 10.1016/j.intimp.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Iinuma T, Okamoto Y, Morimoto Y, Arai T, Sakurai T, Yonekura S, et al. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy. 2018;73(2):479–489. doi: 10.1111/all.13295. [DOI] [PubMed] [Google Scholar]
- 69.•• Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen Q-A, Speake C, et al. A phenotypically and functionally distinct human T H 2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401):eaam9171 Available from: https://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aam9171. Accessed 17 July 2020. A particuarly relevant memory T helper type 2 cell subset found exclusively in atopic subjects. [DOI] [PMC free article] [PubMed]
- 70.Amin K, Issa SM, Ali KM, Aziz MI, Hama Amieen HM, Bystrom J, et al. Evidence for eosinophil and IL-17 mediated inflammation in allergic rhinitis. Clin Mol Allergy. 2020;18(1):6. doi: 10.1186/s12948-020-00117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milovanovic M, Drozdenko G, Weise C, Babina M, Worm M. Interleukin-17A promotes IgE production in human B cells. J Invest Dermatol. 2010;130(11):2621–2628. doi: 10.1038/jid.2010.175. [DOI] [PubMed] [Google Scholar]
- 72.Leaker BR, Malkov VA, Mogg R, Ruddy MK, Nicholson GC, Tan AJ, Tribouley C, Chen G, de Lepeleire I, Calder NA, Chung H, Lavender P, Carayannopoulos LN, Hansel TT. The nasal mucosal late allergic reaction to grass pollen involves type 2 inflammation (IL-5 and IL-13), the inflammasome (IL-1β), and complement. Mucosal Immunol. 2017;10(2):408–420. doi: 10.1038/mi.2016.74. [DOI] [PubMed] [Google Scholar]
- 73.•Polak D, Hafner C, Briza P, Kitzmüller C, Elbe-Bürger A, Samadi N, et al. A novel role for neutrophils in IgE-mediated allergy: evidence for antigen presentation in late-phase reactions. J Allergy Clin Immunol. 2019;143(3):1143–1152.e4 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674918308583. Accessed 17th July 2020. A novel neutrophil role in mediating allergic inflammation. [DOI] [PMC free article] [PubMed]
- 74.Yang J, Ramirez Moral I, van’t Veer C, de Vos AF, de Beer R, Roelofs JJTH, et al. Complement factor C5 inhibition reduces type 2 responses without affecting group 2 innate lymphoid cells in a house dust mite induced murine asthma model. Respir Res. 2019;20(1):165. doi: 10.1186/s12931-019-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nasiri Kalmarzi R, Fakhimi R, Manouchehri F, Ataee P, Naleini N, Babaei E, et al. The relationship between B7 homologous 1 with interleukin-4, interleukin-17 and interferon gamma in patients with allergic rhinitis. Expert Rev Clin Immunol. 2019;15(8):897–901. doi: 10.1080/1744666X.2019.1637256. [DOI] [PubMed] [Google Scholar]
- 76.Rosskopf S, Jahn-Schmid B, Schmetterer KG, Zlabinger GJ, Steinberger P. PD-1 has a unique capacity to inhibit allergen-specific human CD4+ T cell responses. Sci Rep. 2018;8(1):13543. doi: 10.1038/s41598-018-31757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh PB, Pua HH, Happ HC, Schneider C, von Moltke J, Locksley RM, Baumjohann D, Ansel KM. MicroRNA regulation of type 2 innate lymphoid cell homeostasis and function in allergic inflammation. J Exp Med. 2017;214(12):3627–3643. doi: 10.1084/jem.20170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miao Q, Wang Y, Liu Y, Ren Y, Guan H, Li Z, et al. Seasonal variation in circulating group 2 innate lymphoid cells in mugwort-allergic asthmatics during and outside pollen season. Allergy, Asthma Clin Immunol. 2018;14(1):6. doi: 10.1186/s13223-018-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134(5):1193–1195.e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 80.Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016;138(1):305–308. doi: 10.1016/j.jaci.2015.12.1325. [DOI] [PubMed] [Google Scholar]
- 81.Zimmer A, Bouley J, Le Mignon M, Pliquet E, Horiot S, Turfkruyer M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1020–1030. doi: 10.1016/j.jaci.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 82.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;116(1):73–79. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Shevyrev D, Tereshchenko V. Treg heterogeneity, function, and homeostasis. Front Immunol 2020;10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.03100/full [DOI] [PMC free article] [PubMed]
- 84.Groh N, von Loetzen CS, Subbarayal B, Möbs C, Vogel L, Hoffmann A, et al. IgE and allergen-specific immunotherapy-induced IgG 4 recognize similar epitopes of Bet v 1, the major allergen of birch pollen. Clin Exp Allergy. 2017;47(5):693–703. doi: 10.1111/cea.12835. [DOI] [PubMed] [Google Scholar]
- 85.•• Shamji MH, Kappen J, Abubakar-Waziri H, Zhang J, Steveling E, Watchman S, et al. Nasal allergen-neutralizing IgG4 antibodies block IgE-mediated responses: Novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1067–76 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674918315951. Accessed 17 July 2020. A relevant relationship between B regulatory cells and blocking antibodies and identification of a novel biomarker that correlates with the clinical response following subcutaneous grass pollen immunotherapy. [DOI] [PubMed]
- 86.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 87.Boonpiyathad T, van de Veen W, Wirz O, Sokolowska M, Rückert B, Tan G, et al. Role of Der p 1–specific B cells in immune tolerance during 2 years of house dust mite–specific immunotherapy. J Allergy Clin Immunol. 2019;143(3):1077–1086.e10. doi: 10.1016/j.jaci.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 88.•Sharif H, Singh I, Kouser L, Mösges R, Bonny M-A, Karamani A, et al. Immunologic mechanisms of a short-course of Lolium perenne peptide immunotherapy: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2019;144(3):738–49. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674919302878. Accessed 17 July 2020. Mechanisms of a novel peptide immunotherapy for seasonal allergic rhinitis. [DOI] [PubMed]
- 89.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163(5):2944–2952. [PubMed] [Google Scholar]
- 90.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol 2011 Feb;127(2):509–516.e5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S009167491003040X [DOI] [PubMed]
- 91.Wilson DR, Irani A-M, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, et al. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clin Exp Allergy. 2001;31(11):1705–1713. doi: 10.1046/j.1365-2222.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 92.Creticos PS, Marsh DG, Proud D, Kagey-Sobotka A, Adkinson NF, Friedhoff L, et al. Responses to ragweed-pollen nasal challenge before and after immunotherapy. J Allergy Clin Immunol. 1989;84(2):197–205. doi: 10.1016/0091-6749(89)90325-4. [DOI] [PubMed] [Google Scholar]
- 93.Scadding GW, Eifan AO, Lao-Araya M, Penagos M, Poon SY, Steveling E, Yan R, Switzer A, Phippard D, Togias A, Shamji MH, Durham SR. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy. 2015;70(6):689–696. doi: 10.1111/all.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid JM, Würtzen PA, Siddhuraj P, Jogdand P, Petersen CG, Dahl R, et al. Basophil sensitivity reflects the long-term clinical outcome of subcutaneous immunotherapy in grass pollen allergic patients. Allergy. 2020;all.14264. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/all.14264 [DOI] [PubMed]
- 95.Caruso M, Cibella F, Emma R, Campagna D, Tringali G, Amaradio MD, et al. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2018;60:50–58. doi: 10.1016/j.intimp.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 96.•• Shamji MH, Layhadi JA, Scadding GW, Cheung DKM, Calderon MA, Turka LA, et al. Basophil expression of diamine oxidase: a novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol. 2015;135(4):913–921.e9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674914014778. Accessed 17 July 2020. An alternative approach to assess basophil degranulation that can be used to monitor the efficacy of allergen immunotherapy for allergic rhinitis. [DOI] [PubMed]
- 97.Kaminuma O, Kitamura N, Gotoh M, Shindo M, Watanabe N, Saeki M, et al. Thrombospondin 1-mediated suppression of mast cell degranulation is involved in the efficacy of sublingual immunotherapy. Allergol Int. 2019;68:S9–10. doi: 10.1016/j.alit.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 98.Varona R, Ramos T, Escribese MM, Jimeno L, Galán A, Würtzen PA, et al. Persistent regulatory T-cell response 2 years after 3 years of grass tablet SLIT: links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74(2):349–360. doi: 10.1111/all.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan X, Wang J, Li Y, He X, Niu B, Wu D, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep. 2018;8(1):7950. doi: 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gómez E, Fernández TD, Doña I, Rondon C, Campo P, Gomez F, et al. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin Exp Allergy. 2015;45(10):1542–1553. doi: 10.1111/cea.12578. [DOI] [PubMed] [Google Scholar]
- 101.Gonzalez M, Doña I, Palomares F, Campo P, Rodriguez MJ, Rondon C, et al. Dermatophagoides pteronyssinus immunotherapy changes the T-regulatory cell activity. Sci Rep. 2017;7(1):11949. doi: 10.1038/s41598-017-12261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121(6):1467–1472.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Matsuda M, Terada T, Tsujimoto N, Morie Y, Ishida T, Takahashi H, Hamaguchi J, Tabuchi Y, Doi K, Noro K, Kikuoka Y, Omura S, Yoshida T, Ayani Y, Suzuki M, Ichihara T, Inaka Y, Inui T, Kawata R, Nabe T. Regulatory T and B cells in peripheral blood of subcutaneous immunotherapy-treated Japanese cedar pollinosis patients. Immunotherapy. 2019;11(6):473–482. doi: 10.2217/imt-2018-0170. [DOI] [PubMed] [Google Scholar]
- 104.Furin MJ, Norman PS, Creticos PS, Proud D, Kagey-Sobotka A, Lichtenstein LM, et al. Immunotherapy decreases antigen-induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991;88(1):27–32. doi: 10.1016/0091-6749(91)90297-2. [DOI] [PubMed] [Google Scholar]
- 105.Boonpiyathad T, Sokolowska M, Morita H, Rückert B, Kast JI, Wawrzyniak M, et al. Der p 1-specific regulatory T-cell response during house dust mite allergen immunotherapy. Allergy. 2019;74(5):976–985. doi: 10.1111/all.13684. [DOI] [PubMed] [Google Scholar]
- 106.Datta A, Moitra S, Das PK, Mondal S, Omar Faruk SM, Hazra I, et al. Allergen immunotherapy modulates sensitivity of Treg cells to apoptosis in a rat model of allergic asthma. Immunotherapy. 2017;9(15):1239–1251. doi: 10.2217/imt-2017-0038. [DOI] [PubMed] [Google Scholar]
- 107.van Rijt LS, Logiantara A, Canbaz D, van Ree R. Birch pollen-specific subcutaneous immunotherapy reduces ILC2 frequency but does not suppress IL-33 in mice. Clin Exp Allergy. 2018;48(11):1402–1411. doi: 10.1111/cea.13254. [DOI] [PubMed] [Google Scholar]
- 108.Lou H, Huang Y, Ouyang Y, Zhang Y, Xi L, Chu X, et al. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: A randomized controlled trial. Allergy. 2020;all.14218. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/all.14218 [DOI] [PubMed]
- 109.Wachholz PA, Nouri-Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1 : Th2 cytokine ratios. Immunology. 2002;105(1):56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phi p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27(9):1007–1015. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 111.Huoman J, Papapavlou G, Pap A, Alm J, Nilsson LJ, Jenmalm MC. Sublingual immunotherapy alters salivary IgA and systemic immune mediators in timothy allergic children. Kalaycı Ö, editor. Pediatr Allergy Immunol 2019;pai.13047. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/pai.13047 [DOI] [PubMed]
- 112.Mattson L, Lentini A, Gawel DR, Badam TVS, Benson M, Ledin T, et al. Potential involvement of type I interferon signaling in immunotherapy in seasonal allergic rhinitis. J Immunol Res. 2016;2016:1–6. doi: 10.1155/2016/5153184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.•Seehus CR, Kadavallore A, de la Torre B, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017;8(1):1900 Available from: http://www.nature.com/articles/s41467-017-02023-z. Accessed 17 July 2020. A novel regulatory subset, relevant for future allergen immunotherapy research. [DOI] [PMC free article] [PubMed]
- 114.Hesse L, van Ieperen N, Habraken C, Petersen AH, Korn S, Smilda T, et al. Subcutaneous immunotherapy with purified Der p1 and 2 suppresses type 2 immunity in a murine asthma model. Allergy. 2018;73(4):862–874. doi: 10.1111/all.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-γ. J Allergy Clin Immunol. 1996;97(6):1356–1365. doi: 10.1016/S0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 116.Mitthamsiri W, Pradubpongsa P, Sangasapaviliya A, Boonpiyathad T. Decreased CRTH2 expression and response to allergen re-stimulation on innate lymphoid cells in patients with allergen-specific immunotherapy. Allergy, Asthma Immunol Res. 2018;10(6):662. doi: 10.4168/aair.2018.10.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. 2017;171(1):201–216.e18. doi: 10.1016/j.cell.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 118.Morita H, Kubo T, Rückert B, Ravindran A, Soyka MB, Rinaldi AO, et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143(6):2190–2201.e9. doi: 10.1016/j.jaci.2018.12.1018. [DOI] [PubMed] [Google Scholar]
- 119.Nomura T, Suzuki M, Yokota M, Nakamura Y, Ozeki K, Ito Y, et al. Effect of Japanese cedar–specific sublingual immunotherapy on allergen-specific TH2 cell counts in blood. Ann Allergy Asthma Immunol. 2016;117(1):72–78.e4. doi: 10.1016/j.anai.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Gotoh M, Kaminuma O, Hiroi T, Okubo K. Microarray-based multivariate analysis of the effectiveness of sublingual immunotherapy for cedar pollinosis. Allergy, Asthma Immunol Res. 2018;10(5):562. doi: 10.4168/aair.2018.10.5.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ihara F, Sakurai D, Yonekura S, Iinuma T, Yagi R, Sakurai T, et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy. 2018;73(9):1823–1832. doi: 10.1111/all.13436. [DOI] [PubMed] [Google Scholar]
- 122.•Sharif H, Acharya S, Dhondalay GKR, Varricchi G, Krasner-Macleod S, Laisuan W, et al. Grass pollen immunotherapy alters chromatin landscape in circulating T follicular and regulatory cells. J Allergy Clin Immunol. 2020 Nov 5:S0091-6749(20)31561-X. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091-6749(20)31561-X. Epub ahead of print. Accessed 25 Nov 2020. Allergen immunotherapy for seasonal allergic rhinitis can decrease cytokine-expressing T follicular helper cell frequencies.
- 123.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102(1):98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.•• Shamji MH, Layhadi JA, Achkova D, Kouser L, Perera-Webb A, Couto-Francisco NC, et al. Role of IL-35 in sublingual allergen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1131–1142.e4 Available from: https://linkinghub.elsevier.com/retrieve/pii/S009167491831056X. Accessed 17 July 2020. Novel induced T regulatory cell subset following sublingual immunotherapy for grass pollen allergy. [DOI] [PubMed]
- 125.•• Froese van Dijck A, Kousar L, Layhadi J, Fedina O, Calvosa S, Scadding GW, et al. SATB1 is repressed in FoxP3+Tregs following grass pollen subcutaneous and sublingual immunotherapy and correlates with clinical efficacy. J Allergy Clin Immunol. 2017;139(2):AB192 Available from: https://linkinghub.elsevier.com/retrieve/pii/S009167491632142X. Accessed 17 July 2020. Novel T regulatory cell-related biomarker for monitoring the efficacy of allergen immunotherapy for seasonal allergic rhinitis.
- 126.Ulges A, Klein M, Reuter S, Gerlitzki B, Hoffmann M, Grebe N, et al. Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat Immunol. 2015;16(3):267–275. doi: 10.1038/ni.3083. [DOI] [PubMed] [Google Scholar]
- 127.Vanderleyden I, Fra-Bido SC, Innocentin S, Stebegg M, Okkenhaug H, Evans-Bailey N, et al. Follicular regulatory T cells can access the germinal center independently of CXCR5. Cell Rep. 2020;30(3):611–619.e4. doi: 10.1016/j.celrep.2019.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yao Y, Wang Z-C, Wang N, Zhou P-C, Chen C-L, Song J, et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(1):118–128. doi: 10.1016/j.jaci.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 129.Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 Years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis. JAMA. 2017;317(6):615. doi: 10.1001/jama.2016.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.•Yao Y, Wang N, Chen C-L, Pan L, Wang Z-C, Yunis J, et al. CD23 expression on switched memory B cells bridges T-B cell interaction in allergic rhinitis. Allergy. 2020; Available from: http://doi.wiley.com/10.1111/all.14288. Accessed 17 July 2020. The role of T and B cell interaction in allergic rhinitis. [DOI] [PubMed]
- 131.Feng M, Zeng X, Su Q, Shi X, Xian M, Qin R, et al. Allergen immunotherapy–induced immunoglobulin G4 reduces basophil activation in house dust mite–allergic asthma patients. Front Cell Dev Biol. 2020 20;8. Available from: https://www.frontiersin.org/article/10.3389/fcell.2020.00030/full [DOI] [PMC free article] [PubMed]
- 132.Mösges R, Koch AF, Raskopf E, Singh J, Shah-Hosseini K, Astvatsatourov A, Hauswald B, Yarin Y, Corazza F, Haazen L, Pirotton S, Allekotte S, Zadoyan G, Legon T, Durham SR, Shamji MH. Lolium perenne peptide immunotherapy is well tolerated and elicits a protective B-cell response in seasonal allergic rhinitis patients. Allergy. 2018;73(6):1254–1262. doi: 10.1111/all.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heeringa JJ, McKenzie CI, Varese N, Hew M, Bakx ATCM, Aui PM, et al. Induction of IgG 2 and IgG 4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75(5):1121–1132. doi: 10.1111/all.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zissler UM, Jakwerth CA, Guerth FM, Pechtold L, Aguilar-Pimentel JA, Dietz K, et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. EBioMedicine. 2018;36:475–488. doi: 10.1016/j.ebiom.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bousquet J, Pfaar O, Togias A, Schünemann HJ, Ansotegui I, Papadopoulos NG, Tsiligianni I, Agache I, Anto JM, Bachert C, Bedbrook A, Bergmann KC, Bosnic-Anticevich S, Bosse I, Brozek J, Calderon MA, Canonica GW, Caraballo L, Cardona V, Casale T, Cecchi L, Chu D, Costa E, Cruz AA, Czarlewski W, Durham SR, du Toit G, Dykewicz M, Ebisawa M, Fauquert JL, Fernandez-Rivas M, Fokkens WJ, Fonseca J, Fontaine JF, Gerth van Wijk R, Haahtela T, Halken S, Hellings PW, Ierodiakonou D, Iinuma T, Ivancevich JC, Jacobsen L, Jutel M, Kaidashev I, Khaitov M, Kalayci O, Kleine Tebbe J, Klimek L, Kowalski ML, Kuna P, Kvedariene V, la Grutta S, Larenas-Linemann D, Lau S, Laune D, le L, Lodrup Carlsen K, Lourenço O, Malling HJ, Marien G, Menditto E, Mercier G, Mullol J, Muraro A, O’Hehir R, Okamoto Y, Pajno GB, Park HS, Panzner P, Passalacqua G, Pham-Thi N, Roberts G, Pawankar R, Rolland C, Rosario N, Ryan D, Samolinski B, Sanchez-Borges M, Scadding G, Shamji MH, Sheikh A, Sturm GJ, Todo Bom A, Toppila-Salmi S, Valentin-Rostan M, Valiulis A, Valovirta E, Ventura MT, Wahn U, Walker S, Wallace D, Waserman S, Yorgancioglu A, Zuberbier T, the ARIA Working Group 2019 ARIA Care pathways for allergen immunotherapy. Allergy. 2019;74(11):2087–2102. doi: 10.1111/all.13805. [DOI] [PubMed] [Google Scholar]
- 136.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–1296.e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 137.Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116(2):347–354. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 138.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317(1–2):71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shamji MH, Francis JN, Würtzen PA, Lund K, Durham SR, Till SJ. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: inhibition by blocking antibody responses after immunotherapy. J Allergy Clin Immunol. 2013;132(4):1003–1005.e4. doi: 10.1016/j.jaci.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 140.Van Overtvelt L, Baron-Bodo V, Horiot S, Moussu H, Ricarte C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011;66(12):1530–1537. doi: 10.1111/j.1398-9995.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 141.Nolte H, Maloney J, Nelson HS, Bernstein DI, Lu S, Li Z, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135(6):1494–1501.e6. doi: 10.1016/j.jaci.2014.12.1911. [DOI] [PubMed] [Google Scholar]
- 142.•Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156–73 Available from: http://doi.wiley.com/10.1111/all.13138. Accessed 17 July 2020. A comprehensive overview of current biomarker candidates for monitoring the clinical response during allergen immunotherapy. [DOI] [PubMed]
- 143.Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123(5):1103–1110.e4. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 144.Huse K, Wogsland CE, Polikowsky HG, Diggins KE, Smeland EB, Myklebust JH, et al. Human germinal center B cells differ from naïve and memory B cells in CD40 expression and CD40L-induced signaling response. Cytom Part A. 2019;95(4):442–449. doi: 10.1002/cyto.a.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marsh-Wakefield F, Kruzins A, McGuire HM, Yang S, Bryant C, Fazekas de St. Groth B, et al. Mass cytometry discovers two discrete subsets of CD39−Treg which discriminate MGUS from multiple myeloma. Front Immunol. 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.01596/full [DOI] [PMC free article] [PubMed]
- 146.Erhart F, Blauensteiner B, Zirkovits G, Printz D, Soukup K, Klingenbrunner S, et al. Gliomasphere marker combinatorics: multidimensional flow cytometry detects CD44+/CD133+/ITGA6+/CD36+ signature. J Cell Mol Med. 2019;23(1):281–292. doi: 10.1111/jcmm.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rein ID, Notø HØ, Bostad M, Huse K, Stokke T. Cell cycle analysis and relevance for single-cell gating in mass cytometry. Cytom Part A. 2020;cyto.a.23960. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cyto.a.23960 [DOI] [PubMed]
- 148.Leite Pereira A, Tchitchek N, Lambotte O, Le Grand R, Cosma A. Characterization of leukocytes from HIV-ART patients using combined cytometric profiles of 72 cell markers. Front Immunol 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.01777/full [DOI] [PMC free article] [PubMed]
- 149.Neeland MR, Andorf S, Manohar M, Dunham D, Lyu S-C, Dang TD, et al. Mass cytometry reveals cellular fingerprint associated with IgE+ peanut tolerance and allergy in early life. Nat Commun. 2020;11(1):1091. doi: 10.1038/s41467-020-14919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.See P, Lum J, Chen J, Ginhoux F. A single-cell sequencing guide for immunologists. Front Immunol. 2018;9. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2018.02425/full [DOI] [PMC free article] [PubMed]
- 151.Wu YL, Stubbington MJT, Daly M, Teichmann SA, Rada C. Intrinsic transcriptional heterogeneity in B cells controls early class switching to IgE. J Exp Med. 2017;214(1):183–196. doi: 10.1084/jem.20161056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tibbitt CA, Stark JM, Martens L, Ma J, Mold JE, Deswarte K, et al. Single-cell RNA sequencing of the T helper cell response to house dust mites defines a distinct gene expression signature in airway Th2 cells. Immunity. 2019;51(1):169–184.e5. doi: 10.1016/j.immuni.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Seumois G, Ramírez-Suástegui C, Schmiedel BJ, Liang S, Peters B, Sette A, et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci Immunol. 2020;5(48):eaba6087. doi: 10.1126/sciimmunol.aba6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Croote D, Darmanis S, Nadeau KC, Quake SR. High-affinity allergen-specific human antibodies cloned from single IgE B cell transcriptomes. Science (80-) 2018;362(6420):1306–1309. doi: 10.1126/science.aau2599. [DOI] [PubMed] [Google Scholar]
- 155.Tu AA, Gierahn TM, Monian B, Morgan DM, Mehta NK, Ruiter B, et al. TCR sequencing paired with massively parallel 3′ RNA-seq reveals clonotypic T cell signatures. Nat Immunol. 2019;20(12):1692–1699. doi: 10.1038/s41590-019-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiang D, Chen X, Jones SM, Wood RA, Sicherer SH, Burks AW, et al. Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector TH2 subsets. J Allergy Clin Immunol. 2018;141(6):2107–2120. doi: 10.1016/j.jaci.2017.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]