ABSTRACT
BACKGROUND:
Cochlear implant (CI) recipients with a cochleovestibular malformation (CVM) are at a higher risk of experiencing an intra-operative cerebrospinal fluid (CSF) gusher and, therefore are at greater risk of developing postoperative meningitis than are CI recipients with normal cochlear anatomy. To control CSF gushers, the FORM electrode array was developed.
OBJECTIVES:
To assess the ability of the FORM24 electrode array in managing intraoperative CSF gushers and preventing postoperative CSF leakage in a population of CI recipients.
DESIGN:
Retrospective.
SETTING:
Tertiary health care center.
PATIENTS AND METHODS:
All CIs in which a FORM24 was used between January 2014 and March 2018 were reviewed for demographic and safety results.
MAIN OUTCOME MEASURES:
Safety results were assessed as the intraoperative or postoperative presence of an episode of CSF leakage or meningitis.
SAMPLE SIZE:
177 CI recipients.
RESULTS:
Thirty-six (20.3%) had a CVM and 141 had normal anatomy (79.7%). Of the 36 participants with a CVM, 20 (55.6%) experienced an intraoperative CSF gusher, all of which were resolved. No cases of postoperative leakage or meningitis were recorded after a mean follow-up time of 36 months.
CONCLUSION:
The FORM24 array is able to help surgeons stop intraoperative CSF gushers and prevent postoperative CSF leakage and meningitis in CI recipients with a CVM.
LIMITATIONS:
Further studies are needed.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Cochlea-vestibular malformations (CVMs) are responsible for approximately 20% of cases of severe or severe-to-profound hearing loss.1,2 The most influential classification of CVMs was undertaken by Jackler et al and reclassified by Sennaroglu and Saatci, Sennaroglu et al, and Sennaroglu et al.1–4 Except for Michel's deformity, none of these CVMs are now a contraindication for cochlear implant (CI) provision and people with a CVM have repeatedly been shown to derive significant benefit from CI use.5–7 However, CI recipients with a CVM are at a greater risk of suffering a cerebro-spinal fluid (CSF) gusher than are CI recipients without a CVM,2,8 and are therefore at an increased risk of developing meningitis postoperatively.9 To prevent this, a need was perceived for an electrode array design that could help control perioperative CSF gushers and prevent postoperative CSF leakage. To this end, a custom-made array was developed.2,10 This was the precursor to the FORM array (MED-EL, Innsbruck, Austria) see (Figure 1).
Figure 1. The FORM24 array. The FORM24 is 24 mm long and has an active stimulation range (ASR) of 18.7 mm, otherwise the arrays are the same in every respect. (Image used with permission of MED-EL).

The primary difference between the FORM array and other arrays is that instead of the standard silicone ring at the proximal end of the array, the FORM has a wedge-shaped stopper which, like a cork in a bottle, acts as a seal to close off the cochlear opening. Once the array has been inserted, this makes it easier for the surgeon to apply additional sealing material to prevent postoperative CSF fistulae, leakage, and therefore, possible meningitis.
The two varieties of the FORM (the FORM24 and FORM19, the 19 and 24 indicate the length in mm of the array and are, at present, the only arrays that are designed to help control CSF gushers during surgery and prevent postoperative CSF leakage. Despite this, there are relatively few studies in which the FORM or its custom-made predecessor are used.2,10 The primary aim of this retrospective study was to assess if the FORM24 electrode array helps in managing intraoperative CSF gushers and preventing postoperative CSF leakage.
PATIENTS AND METHODS
All instances between January 2014 and March 2018 in which a person was implanted with a FORM24 electrode array (MED-EL, Innsbruck, Austria) at the center of the study were reviewed for demographic and safety results. Cases of revision implantation were excluded.
The average cochlear duct length (CDL) in the Saudi population is a mean of 31.9 mm (range: 20.3–37.7 mm).11 In our study, the CDL was 29.7 mm (range: 26–33 mm). So, the preoperative assessment indicated the use of the FORM24 to ensure complete insertion.
Feasibility results were assessed as the perioperative or postoperative presence of an episode of CSF leakage or other complication. We reviewed the operative note to report the difficulty of the operation. Furthermore, we have followed up the participants postoperatively during their hospital stay and if they returned to the hospital for any additional treatment.
Ethics committee approval was granted for this study by the Institutional Review Board of the King Saud University – College of Medicine (14/4251/IRB). All participants gave their informed consent. Descriptive statistics such as absolute and relative frequencies were used to describe participants' characteristics and study outcomes.
RESULTS
The FORM24 array was used in all 177 surgeries. Of these, 163 children with a median (interquartile) age of 3 (2) years, range: <1–20 years, and 14 adults with a median (minimum-maximum) age of 31 (21–83); 98 were male (55.4%) and 79 were female (44.6%). 92 participants were implanted on the right side (52.0%); 85 were implanted on the left side (48.0%).
Inner ear anatomy
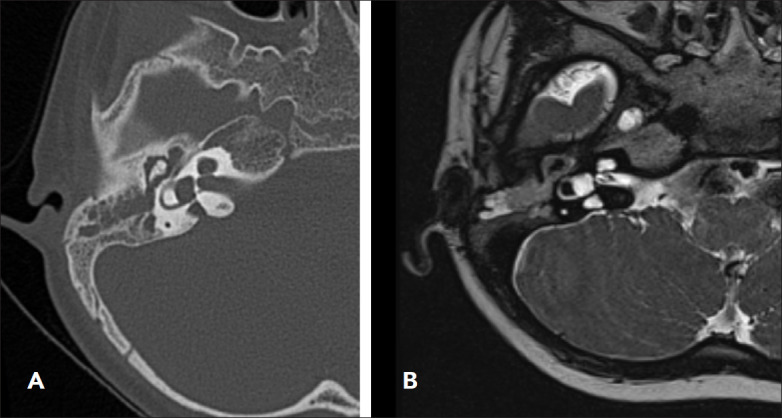
Normal anatomy was present in 141/177 subjects (79.7%); 36/177 had a CVM (20.3%). Of those 36 participants with a CVM: 16 (44.4%) had an enlarged vestibular aqueduct (EVA), 13 (36.1%) had incomplete partition type 2 (IP-II), 3 (8.3%) had Mondini deformity, 2 (5.6%) had cochlear otosclerosis, and 2 (5.6%) had cochlear ossification. The presence of a CVM was determined based on CT scan and MRI (Figures 2, 3, 4). The median age at transplantation for participants with a CVM was 4 years (range 1–64 years); 4 were adults (11.2%) and 32 were children (88.8%).
Figure 2. (A) Axial CT showing dilated osseous vestibular aqueduct (black arrow) and (B) showing MRI bilateral enlarged vestibular aqueduct and left enlarge endolymphatic sac.
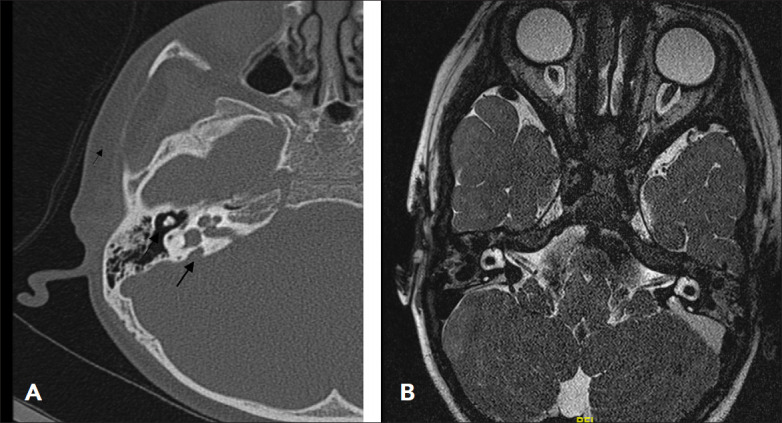
Figure 3. (A) axial CT scan temporal bone showing bilateral appearances of incomplete partition Type 2, note cystic-like appearance of the upper cochlear turn with flattening of the interscalar septum laterally. (B) MRI appearances with the same findings.
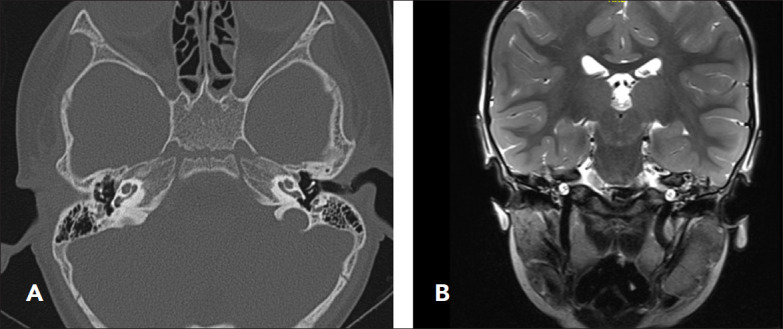
Figure 4. (A) Showing Mondini deformity demonstrated by cystic cochlear apex, a minimally dilated vestibule and a dilated vestibular aqueduct in CT scan. (B) MRI scan with the same findings.
Inner ear approach and type of implant
The insertion was performed via the round window in 171 participants (96.6%) and via cochleostomy in 6 participants (3.4%). All participants reviewed a MED-EL CI: 99 (57.9%) received the CONCERTO, 68 (38.4%) received the SYNCHRONY, and 10 received the SYNCHRONY-PIN (5.6%). The average follow-up period was 36 months (range: 9-72 months). It should be noted that “participants” here refers to individual ears, not individual CI recipients.
CSF gusher
A CSF gusher was experienced in 20/177 (11.3%) participants. The mean (SD) cochlear duct length of this group was 8.1 (0.7) mm (range: 7.2–8.9 mm). Table 1 demonstrates the demographic summary of participants who experienced a CSF gusher. No participants with normal anatomy experienced a CSF gusher; 20/36 (55.6%) participants with a CVM experienced a CSF gusher: 11/16 (55.6%) with an EVA, 8/13 (61.5%) with IP-II, and 1/20 (5%) with Mondini deformity. The mean age at implantation for participants with LVA and had a CSF gusher (11/16) was 7(5) years (range: 4–17 years). Seven participants of this group had their implants in the right side (63.6%), and 4 in the left (36.4%). The mean age at implantation for participants with IP-II who had a CSF gusher (8/13) was 3 (1) years (range: 2-5 years). Seven patients of this group had implants on the right side (87.5%) and only one on the left (12.5%). The participant with Mondini deformity was 2.6 years old and implanted on his left side.
Table 1.
Demographic results for all participants who experienced a cerebrospinal fluid gusher.
| Participant | Age at implantation (years) | Ear | Type of cochleovestibular malformation | Complete insertion? | Insertion via |
|---|---|---|---|---|---|
| 1 | 2.0 | R | IP-II | Yes | Round window |
| 2 | 2.6 | L | IP-II + EVA | Yes | Cochleostomy |
| 3 | 4.8 | R | IP-II | Yes | Round window |
| 4 | 2.3 | L | IP-II | Yes | Round window |
| 5 | 1.8 | R | IP-II | Yes | Round window |
| 6 | 5.0 | R | IP-II | No – 3 contact out | Round window |
| 7 | 7.3 | L | EVA | Yes | Round window |
| 8 | 3.6 | R | EVA | Yes | Round window |
| 9 | L | EVA | Yes | Round window | |
| 10 | 3.8 | R | IP-II | No – 3 contact out | Round window |
| 11 | 4.6 | R | EVA | Yes | Round window |
| 12 | L | EVA | Yes | Round window | |
| 13 | 2.7 | R | IP-II | Yes | Round window |
| 14 | 4.2 | R | EVA | Yes | Round window |
| 15 | 5.5 | R | EVA | Yes | Round window |
| 16 | 12.2 | R | EVA | Yes | Round window |
| 17 | L | EVA | No – 2 contact out | Round window | |
| 18 | 4.7 | R | IP-II | No – 2 contacts out | Round window |
| 19 | 4.1 | R | EVA | Yes | Cochleostomy |
| 20 | 17.1 | R | EVA | Yes | Round window |
R: right, L: left, P-II: incomplete partition, type two, EVA: enlarged vestibular aqueduct.
The cases with CSF gusher were normally sealed with the stopper of FORM24 array. Furthermore, fascia or periosteum was used together with the stopper to seal the gusher in cases when the array could not be completely inserted. The array allowed us to reduce the amount of fascia needed to seal the cochlear entrance. Postoperatively, no participant experienced meningitis or CSF leakage. No intra or postoperative complications were recorded.
Complete insertion
The array could be completely inserted in 166/177 (93.8%) participants: 141/141 participants without a CVM and in 25/36 (69.4%) participants with a CVM. Of the 11 (6.2%) participants that were not completely inserted, 9 participants had two electrodes contacts out and 2 participants had 3 electrodes contacts out. All of these 11 participants were implanted via the round window and 4 (36.4%) experienced a CSF gusher: 3 with IP-II and 1 with EVA. For those participants, fascia or periosteum was used together with the electrode stopper for the sealing.
DISCUSSION
This study shows the ability of the FORM24 to stop intraoperative CSF gushers if they occur and to prevent postoperative CSF leakage, thereby reducing the risk of developing meningitis. While 20/36 (55.6%) of the participants with a CVM experienced a CSF gusher intraoperatively, no cases of CSF leakage or meningitis were reported postoperatively. Further, none of the 141(0%) CI recipients without a CVM experienced an intra-operative gusher or postoperative leaking or meningitis.
Intraoperative CSF gushers can occur in people with normal cochlea but they are more usually seen in people with a CVM, where they occur in an estimated 40-50% of cochlear implantations.2,5 There is some disagreement over whether the type of malformation correlates with the likelihood of experiencing a gusher.2,12 Regardless of whether there is a correlation between the type of malformation and likelihood of a gusher, CI surgeons and their teams must be prepared to encounter and control a CSF gusher, especially when implanting someone with a CVM.
Several ways of controlling an intraoperative gusher have been reported; the most common way is to wait until the gusher stops, insert the array, and meticulously pack the cochlear opening with muscle, fascia, and sometimes also fibrin glue5,7,13,14 or, in cases of severe malformation, fat tissue instead of muscle.14 Indeed, this method continues to be used because it is largely effective at preventing postoperative complications due to CSF leakage, even in CI recipients who did not receive a FORM array.5,7,13,14 However, to the best of our knowledge, Sennaroglu et al is the only study which assessed within one study the effectiveness of the cork-type array (the FORM's predecessor) and standard straight arrays in preventing postoperative CSF leak. They found that 20.7% (6/29) implanted with a straight array experienced postoperative rhinorrhea after a gusher; in contrast, only 4.2% (1/24) implanted with the cork-type array had either of these complications postoperatively. None of our participants had postoperative rhinorrhea or wound collection that required a surgical intervention.
Evidence from published studies on the FORM array (19 or 24, rather than their prototype) is rare but seems to have yielded encouraging results. Gaur et al used the FORM array on one patient with a CVM and reported that the postoperative period was uneventful.16 This led to their calling the FORM array “promising”. Grover et al used the FORM in one CI recipient and, while his/her postoperative result was unclear in the paper, they stated that the senior author finds the FORM “to be an effective and convenient way to handle a CSF gusher”.17
As regards our clinic, we find that using the FORM24 array reduces the requirement of packing material and is very effective in controlling a gusher and preventing future leaks. While we cannot be sure that our use of the FORM24 array prevented postoperative CSF leakage that would have otherwise occurred, the fact that neither postoperative leak nor other complications occurred furthers our belief that the FORM24 array is effective and that we are providing CI recipients with a a good array for their condition. Although the FORM array was specifically designed to be used with CI recipients with a CVM, we suggest using it even in recipients without a CVM because it is our experience that the FORM is easy to handle and to insert. Future studies on the FORM that assess residual hearing preservation and speech understanding would be beneficial.
While this study provides an overview of the FORM24 in a good sample size of CI users, it does have the limitations of having been performed in only one center and in only one country, which may limit its generalizability. Further, as a retrospective study, only clinical observation (and not objective testing) was performed. In conclusion, the FORM24 array is able to help surgeons stop intraoperative CSF gushers and prevent postoperative CSF leakage in CI recipients with a CVM. For the surgeon, this array has the additional advantage of being easy and comfortable to handle.
ACKNOWLEDGMENTS
This project is one of the recommended research projects by the Saudi Oto-rhinolaryngology Society. The authors would like to thank MED-EL for drafting a version of this article.
Funding Statement
None.
REFERENCES
- 1.Jackler RK, Luxford WM, House WF.. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngo-scope. 1987; 97(Pt 2 Suppl. 40):2–14. [DOI] [PubMed] [Google Scholar]
- 2.Sennaroglu L.. Cochlear implantation in inner ear malformations—a review article. Cochlear Implants Int. 2010; 11(1):4–41. [DOI] [PubMed] [Google Scholar]
- 3.Sennaroglu L, Saatci I.. A new classification for cochleovestibular malformations. Laryngoscope. 2002; 112(12):2230–2241. [DOI] [PubMed] [Google Scholar]
- 4.Sennaroglu L, Sarac S, Ergin T.. Surgical results of cochlear implantation in malformed cochlea. Otol Neurotol. 2006; 27(5):615–623. [DOI] [PubMed] [Google Scholar]
- 5.Farhood Z, Nguyen SA, Miller SC, Holcomb MA, Meyer TA, Rizk HG.. Cochlear implantation in inner ear malformations: systematic review of speech perception outcomes and interoperative findings. Otolaryngol Head Neck Surg. 2017; 156(5):783–793. [DOI] [PubMed] [Google Scholar]
- 6.Carner M, Sacchetto A, Bianconi L, Soloperto D, Sacchetto L, Presutti L,. et al. Endoscopic-assisted cochlear implantation in children with malformed ears. Otolaryngol Head Neck Surg. 2019; 161(4):688–693. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Li Y, Gong Y, Chen B, Chen J.. Cochlear implants for patients with inner ear malformations: experience in a cohort of 877 surgeries. Clin Otolaryngol. 2019; 44(4):702–706. [DOI] [PubMed] [Google Scholar]
- 8.Pakdaman MN, Herrmann BS, Curtin HD, Van Beek-King J, Lee DJ.. Cochlear implantation in children with anomalous cochleovestibular anatomy: a systematic review. Otolaryngol Head Neck Surg. 2012; 146(2):180–190. [DOI] [PubMed] [Google Scholar]
- 9.Theunisse HJ, Pennings RJ, Kunst HPM, Mulder JJ, Mylanus EAM.. Risk factors for complications in cochlear implant surgery. Eur Arch Otorhinolaryngol. 2018; 275(4):895–903. [DOI] [PubMed] [Google Scholar]
- 10.Sennaroğlu L, Atay G, Bajin MD.. A new cochlear implant electrode with a “cork”-type stopper for inner ear malformations. Auris Nasus Larynx. 2014; 41(4):331–336. [DOI] [PubMed] [Google Scholar]
- 11.Alanazi Alaa, Alzhrani Farid.. Comparison of cochlear duct length between the Saudi and non-Saudi populations. Ann Saudi Med. 2018. Mar-Apr; 38(2): 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamogashira T, Iwasaki S, Kashio A, Kakigi A, Karino S, Matsumoto Y,. et al. Prediction of intraoperative CSF gusher and postoperative facial nerve stimulation in patients with cochleovestibular malformations undergoing cochlear implantation surgery. Otol Neurotol. 2017; 38(6):e114–e119. [DOI] [PubMed] [Google Scholar]
- 13.Grover M, Sharma S, Bhargava S, Singh SN, Gupta G, Sharma MP.. Cochlear implantation in children with anomalous cochleovestibular anatomy: our experience. Indian J Otolaryngol Head Neck Surg. 2017; 69(4):504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demir B, Cesur S, Sahin A, Binnetoglu A, Ciprut A, Batman C.. 2019. Outcomes of cochlear implantation in children with inner ear malformations. Eur Arch Otorhinolaryngol. 2019, 276(9):2397–2403. [DOI] [PubMed] [Google Scholar]
- 15.Karatas E.. Repair of cerebrospinal fluid leak in cochlear implantation. J Craniofac Surg. 2019; 30(2):535–538. [DOI] [PubMed] [Google Scholar]
- 16.Gaur SK, Dutt SN, Kumar A.. The common cavity vs incomplete partition type I conundrum: Decision making and management with the cochlear implant form electrode. Cochlear Implants Int. 2019; 20(3):158–163. [DOI] [PubMed] [Google Scholar]
- 17.Grover M, Sharma S, Preetam C, Gupta G, Samdani S, Agarwal S,. et al. New SMS classification of cochleovestibular malformation and its impact on decision-making. J Laryngol Otol, 2019; 133:368–375. [DOI] [PubMed] [Google Scholar]


