Abstract
Subchondral bone cysts represent an early postoperative sign associated with many articular cartilage repair procedures. They may be defined as an abnormal cavity within the subchondral bone in close proximity of a treated cartilage defect with a possible communication to the joint cavity in the absence of osteoarthritis. Two synergistic mechanisms of subchondral cyst formation, the theory of internal upregulation of local proinflammatory factors, and the external hydraulic theory, are proposed to explain their occurrence. This review describes subchondral bone cysts in the context of articular cartilage repair to improve investigations of these pathological changes. It summarizes their epidemiology in both preclinical and clinical settings with a focus on individual cartilage repair procedures, examines an algorithm for subchondral bone analysis, elaborates on the underlying mechanism of subchondral cyst formation, and condenses the clinical implications and perspectives on subchondral bone cyst formation in cartilage repair.
Keywords: bone cyst, cartilage repair, osteochondral unit, subchondral bone
Subchondral bone cysts commonly occur adjacent to a treated focal cartilage defect and are possibly connected to the joint cavity. A radiographic‐based algorithm allows for a detailed analysis of postoperative subchondral bone cysts and other alterations of the subchondral bone. Formation of subchondral bone cysts might result from synergistic effects of both external and internal contributors.

1. BACKGROUND
Articular cartilage, the resilient and flexible connective tissue covering the articulating surfaces of joints, has a limited regenerative capacity. 1 Regeneration of chondral (limited to the cartilage) and osteochondral defects (extending into the subchondral bone) refers to an identical reconstruction of the original osteochondral unit. However, in adults, only different degrees of repair occur, all resulting in a structurally and functionally inferior (osteo)chondral repair tissue. 2 , 3 , 4 , 5 Present major reconstructive surgical interventions for focal cartilage defects include marrow stimulation, osteochondral allograft or autograft transplantation (OCT), and autologous chondrocyte implantation (ACI). 2 , 3 , 4 , 5 Indications for these approaches are symptomatic cartilage defects with unsatisfactory outcomes after sufficient conservative therapies, aiming at preventing secondary degenerative processes. 6 , 7 , 8 , 9 To identify an appropriate surgical modality, the following critical issues need to be considered: etiology of the defect, patient's age, body mass index, physical activity level and expectations, mechanical axis, possible comorbidities, and defect characteristics such as size, number, and location. 10 , 11 , 12 , 13 , 14 If correctly indicated, such cartilage repair techniques yield largely satisfactory outcomes. 15 Clinical outcomes of cartilage repair are usually assessed using different joint function scores, patient reported outcome measures, and structural evaluations such as the nondestructive MRI 16 and Arthro‐CT 17 imaging. Rarely, macroscopic or even microscopic evaluations (based on biopsies) of the repair tissue during second‐look arthroscopy are performed. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31
Currently, a focus of research has been expanded from exclusively regarding the cartilaginous repair tissue to a more complex view including also postoperative structural alterations of the subchondral bone, as they have emerged as a source of considerable clinical problems and thus are being recognized as additional factors influencing long‐time clinical outcomes of various cartilage repair procedures. 32 Among those, cyst formation in the subchondral bone has been recently described and identified as an important postoperative pathology that may affect the articular cartilage repair.
Bone cysts in general are one of the most widely reported bone changes. From a clinical perspective, they are often causing pain and may reduce the range of motion and of the overall joint function. Bone cysts might result from external (trauma) or multiple internal etiologies such as osteoarthritis (OA), the major degenerative joint disease, 33 , 34 , 35 , 36 rheumatoid arthritis (RA), 37 , 38 intraosseous ganglia, 39 , 40 , 41 aneurysmal bone cysts (ABC), 42 , 43 , 44 , 45 and articular cartilage defects. 46 , 47 , 48 As such bone cysts alter the structural support for weightbearing, they potentially undermine the biomechanics of the joint, inducing degeneration of the overlying articular cartilage, subchondral collapse, and fracture, all leading to a possible extension into formerly unaffected areas in the form of OA. In the worst case, such changes may progress and necessitate a total knee arthroplasty. 15
Nevertheless, a clear definition and comprehensive analysis focusing on subchondral bone cyst formation in the context of focal, non‐OA articular cartilage defects and their repair are largely lacking. The aims of this review are to present an algorithm for analysis and a definition of subchondral bone cysts following cartilage repair, discuss mechanism of their formation, and provide a comprehensive overview of such cysts reported in preclinical and clinical studies of cartilage repair.
Highlights
Subchondral bone cysts commonly occur adjacent to a treated focal cartilage defect and are possibly connected to the joint cavity.
A radiographic‐based algorithm allows for a detailed analysis of postoperative subchondral bone cysts and other alterations of the subchondral bone.
Formation of subchondral bone cysts might result from synergistic effects of both external and internal contributors.
2. A SYSTEMATIC ANALYTIC ALGORITHM FOR CYST FORMATION IN THE SUBCHONDRAL BONE FOLLOWING CARTILAGE REPAIR
In the field of clinical knee OA, algorithms to predict structural progression without specifically addressing subchondral bone cysts support the general concept of subchondral bone evaluations, for example, by quantifying periarticular bone mineral density. 49 , 50 Previous analyses of subchondral bone changes in the context of cartilage repair exposed variable patterns, including the formation of subchondral bone cysts (Figure 1), intralesional osteophytes, generalized upward migration of the subchondral bone plate, and the presence of residual marrow stimulation hole(s), together with peri‐hole or generalized bone resorption (Table 1). 51 , 52 With a view of systematically exploring each of these morphologic changes in both preclinical 46 , 47 , 53 , 54 , 55 , 56 , 57 and clinical 25 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 settings, an adjustable algorithm has been recently proposed to radiographically analyze them (Figure 2). 52 In this algorithm, the projected tidemark and cement line serve as topographical landmarks. In the special case of microfracture treatment for cartilage repair, the algorithm utilizes the diameter of the microfracture awl as a constant reference and the dimension of bone void relative to the original microfracture hole as a quantitative standard. The algorithm has been validated and proved to be reliable and reproducible to analyzes datasets from preclinical models of articular cartilage repair, allowing for a precise distinction between each category of subchondral bone changes. 52 It thus may serve as a useful tool to analyze postoperative subchondral bone cysts and other alterations in an objective and reproducible manner.
FIGURE 1.

Radiographic images of a 30‐year‐old male patient with osteochondritis dissecans (OCD) (A) at the left medial femoral condyle initially treated with the subchondral drilling and subsequent symptomatic subchondral bone cyst formation (B, C) at 60 months postoperatively. The white (A), yellow (B), and red arrows (C) indicate the subchondral bone cyst. The yellow arrowheads (B) designate the area of the OCD lesion surrounding the subchondral bone cyst in the CT image. The red arrowheads (C) denote the high signal intensity of the diffuse bone marrow edema (BME) around the cyst in the T2‐weighted MRI image
TABLE 1.
Definitions of subchondral bone alterations.51,52
| Type | Definition |
|---|---|
| Complete reconstitution | Completely restored subchondral bone underlying the treated defect |
| Upward migration of subchondral bone plate | Osteochondral junction broadly expanding above its original level, thus subchondral bone plate elevating into cartilaginous repair tissue |
| Intralesional osteophyte | Focal, newly‐formed bone located apical to its original cement line and projected into cartilaginous repair tissue layer |
| Generalized upward migration of the subchondral bone plate | Universal expansion of the osteochondral junction above its original level into the cartilaginous repair tissue |
| Residual marrow stimulation hole | Residual holes or canals originating from marrow stimulation procedures with visible border and opening towards the joint space |
| Peri‐hole bone resorption | Intermediate bone resorption surrounding the marrow stimulation hole or canal with a possible large opening towards the joint space (may lead to large defects when marrow stimulation holes merge) |
| Generalized subchondral bone resorption | Generalized weakening of the subchondral bone below the cartilage defect without cyst formation |
| Subchondral bone cyst | Isolated round or irregular shaped cavity within the subchondral bone with or without connection with the joint space encased by subchondral bone sclerosis |
FIGURE 2.
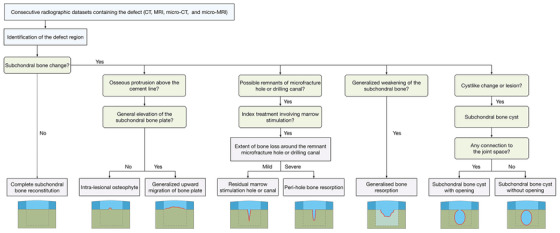
Adapted algorithm for a precise analysis of subchondral bone alterations in translational models and in patients. 52 The bottom schematics show each pattern of subchondral bone changes with articular cartilage and subchondral bone denoted in dark blue and dark green, respectively. The cartilaginous repair tissue and subchondral bone underlying the defect are depicted in light blue and with dashed border, respectively. The margin of the subchondral bone changes is outlined with red lines. A diffuse bone weakness (light green) is only seen in the generalized bone resorption
3. DEFINITION OF A SUBCHONDRAL BONE CYST IN THE CLINICAL CONTEXT OF CARTILAGE REPAIR
Bone cysts may be categorized according to different pathophysiologies. 67 , 68 Osteoarthritic cysts commonly occur in large or small joints with advanced OA, 33 , 34 , 35 , 36 and are often present within regions of maximal joint space narrowing without or with remaining connections to the joint and thus the synovial fluid. The cysts usually appear within the subchondral bone region, are of spherical or ellipsoid shape, and are associated to other subchondral bone alterations and articular cartilage degeneration. 69 Sanal et al described them to be located in the subchondral bone below degenerated articular cartilage, lacking a synovial lining. 70 Associations between subchondral bone cysts and pain along with OA progression have been described especially well in the knee. 34 , 71 , 72 Cyst formation without OA is possible, albeit infrequent. 73 Such cysts may be present in late RA, 37 , 38 pigmented villonodular synovitis (PVNA), 74 where the invasive inflammatory granulation tissue replaces the subchondral bone. Intraosseous ganglia are benign nonneoplastic intramedullary cysts without signs of OA. 39 These cysts are usually located in the epiphysis and contain myxomatous fibrous tissue and viscous mucous fluid. 40 Chondroblastoma is a rare benign tumor, typically leading to a cystic lesion in the epiphyses of long bones. 75 ABCs represent a different and distinct entity because of their destructive and expansible nature. 43 They are characterized by a proliferation of connective tissue within blood‐filled cavities. 42 Sometimes they are accompanied by potentially benign lesions such as chondroblastoma or giant cell tumors. 44 Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy, also termed Nasu‐Hakola disease, refers to a rare combination of bilateral lytic lesions within the bones of extremities and presenile dementia. 76
A recent consensus statement from the Society of Skeletal Radiology Subchondral Bone Nomenclature Committee proposed a nomenclature of nonneoplastic conditions involving the subchondral bone and recommended to report the radiological and magnetic resonance imaging (MRI) characteristics of subchondral cyst‐like nonneoplastic conditions with the term "cystlike changes’’ or "cystlike lesion’’ irrespective of their diverse pathologies. 77 Schajowicz et al used the term “juxta‐articular bone cyst (intra‐osseous ganglion),” for a “benign cystic and often multiloculated lesion made up of fibrous tissue, with extensive mucoid changes, located in the subchondral bone adjacent to a joint." 78 In the context of joint injury, subchondral bone cysts have been similarly defined by Ziino and Safran as benign cystic and often multiloculated lesions consisting of fibrous tissue located in the subchondral bone adjacent to a joint. 73 As such subchondral bone cysts are lacking a lining of synovium, the term “synovial cyst” is incorrect. 78 However, a consensus definition of a subchondral bone cyst in the context of articular cartilage repair has yet to be established. 79
We propose to define a subchondral bone cyst associated with cartilage repair as an abnormal cavity within the subchondral bone in close proximity of a (treated) cartilage defect with a possible communication to the joint cavity, in the absence of OA. The cyst contains mixed osteo‐chondral‐fibrous tissue with a varying degree of bone remodeling and is often encased with sclerotic subchondral bone. It can be visualized as a pathologic region with well‐defined areas of a fluid signal on MRI corresponding to distinct areas of lucency with a sclerotic rim visible on radiographic or computed tomography (CT) images reflective of the reactive wall around the cyst. Subchondral bone cysts associated with cartilage repair procedures are distinctly different from the many other forms of bone cysts as described above. Compared to OA cysts, their natural history is dissimilar as they are located below a cartilaginous repair tissue, and OA represents a major contraindication for many cartilage repair procedures. Because of the absence of an invasive inflammatory granulation tissue, they are also distinctive from RA‐ and PVNS‐associated cysts. In contract to ABCs, they lack a lytic nature.
4. MECHANISMS OF SUBCHONDRAL BONE CYST FORMATION
Understanding the mechanism and pathogenesis of diseases is crucial to identify possible therapeutic targets. 80 The underlying mechanisms of subchondral cyst formation in the specific context of cartilage repair, albeit of utmost importance, are not yet well understood. 81 , 82 , 83 , 84 , 85 Accumulative evidences suggest the synergistic effect of two processes that have been proposed as mechanisms of cyst formation in OA and RA, termed the external hydraulic theory and the internal inflammatory theory (Figure 3). 86 , 87
FIGURE 3.
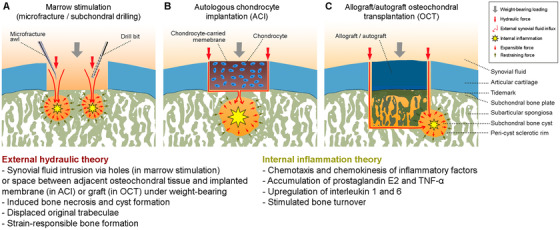
Schematic of the synergistic mechanism of external hydraulic intrusion and internal inflammatory for the subchondral bone cyst formation following articular cartilage repair procedures. The morphological change of the cyst is determined by the equilibrium status between the expansible force due to the synergistic drives and the restraining force from the peri‐cyst sclerotic rim at the cyst‐bone interface. The external hydraulic theory features an intrusion of synovial fluid into the subchondral bone through the canals generated by marrow stimulation techniques (A) or the canals that are possibly opened as a result from the surgically debrided subchondral bone plate in autologous chondrocyte implantation (B) or in a possible gap between the osteochondral unit of the graft and host in allograft/autograft transplantation (C) during the postoperative phase. The pathophysiological characteristics mainly include subchondral bone necrosis, peri‐cyst sclerotic rim formation, displaced original trabeculae and strain‐responsible formation of new bone. The internal inflammatory theory involves mechanisms such as chemotaxis and chemokinesis of inflammatory factors (e.g. PEG2, TNF‐α, IL1, and IL6) as well as bone turnover stimulated by bone necrosis
4.1. External hydraulic theory
The theory of “blow out" of synovial fluid into the subchondral bone as propounded by Freund 88 and Landells 89 requires that a defect in the articular cartilage exists. Prager et al identified a communication of bone cysts with the articular cavity in the form of channels using tomography in 57% of examined cases, although such communication may not always be identifiable. 90 Such channels might escape their detection using conventional X‐rays or may have become eliminated through bone remodeling. 91 The external hydraulic theory was supported by work of Ray et al, who demonstrated subchondral bone cyst formation below untreated osteochondral defects at the medial femoral condyles in horses at 24 weeks postoperatively. 92 The synovial fluid pressure at the exposed subchondral bone generated during the spontaneous postoperative weight‐bearing might be sufficient to induce subchondral bone necrosis and cysts in a step‐wise manner. 89 , 93 Besides a physical effect, the synovial fluid itself might contain acellular and cellular elements that may interfere with the subchondral bone. An inhibitory effect of synovial fluid on the tendon healing of a bone tunnel in the context of ligament reconstruction of the knee has been suggested based on preclinical data. 94 , 95 , 96 A short‐term low‐grade synovial inflammation may possibly be induced by the invasive nature of the cartilage repair procedure, shifting the composition of the synovial fluid into a more catabolic and pro‐inflammatory direction. For OA or RA, the deleterious effects of such synovial fluid are well known. 97 , 98 , 99 , 100 However, a focal cartilage defect represents a comparably less inflammatory and largely nondegenerative condition, a setting in which the aforementioned effects have not yet been investigated. Moreover, a peripheral rim of sclerotic tissue around the cyst is generated during the displacement of the original trabeculae, reflecting a strain‐responding constitution of new bone. 86
The use of reconstructive surgical repair procedures for cartilage defects deserves special attention in this context. Here, subchondral bone cysts probably originate from the iatrogenic association of the subchondral bone marrow space with the synovial fluid, introduced either by drilling or microfracture techniques (Figure 3A), subchondral bone plate débridement, which potentially opens small vascular channels crossing into the (removed) calcified cartilage layer 32 when preparing the defect for ACI or marrow stimulation (Figure 3B), 101 or during OCT in cases of insufficient graft integration (Figure 3C). When performing marrow stimulation for cartilage repair, the penetrations of the subchondral bone plate generate, by definition, communications between the joint space and subchondral bone, which allows the synovial fluid to enter, serving as a possible important contributor for a subsequent subchondral bone cyst formation if these canals are preserved and not closed with an osteochondral repair tissue. 47 In the context of OCT, the formation of subchondral bone cysts at the peripheral graft‐host interface also underscores the role of such a synovial fluid intrusion into the subchondral bone through this interface and/or eroded cartilage, resulting in subchondral bone cyst formation at an early postoperative phase. 102 , 103 , 104 , 105 Pallante‐Kichura et al found that the deterioration of the cartilaginous component of allograft OCTs seen at 1 year in adult goats was associated with subchondral cyst formation. The data suggested that a persisting lateral cartilage‐subchondral bone communication following OCT may favor fluid intrusion as a mechanism for their development, highlighting the need for further mechanistic studies to elucidate the mode of such cyst formation. 105
Interestingly, subchondral bone cysts caused by OA in malaligned knees may regress if the mechanical overload is surgically reduced, as recently shown in a study of patients where the number of cysts located in the previously overloaded tibiofemoral compartment decreased at 5 years after unloading high tibial osteotomy. 91 In some cases, new cysts appeared in the now overloaded lateral compartment at 5 years. 91 These findings highlight the role of local biomechanical overload in the context of the external hydraulic theory.
4.2. Internal inflammatory theory
The internal inflammatory theory is based on cellular and molecular processes with upregulation of local proinflammatory factors that induce a focal area of cystic degeneration caused by an aseptic bone necrosis (as commonly seen in OCT). Local accumulation of the proinflammatory mediator prostaglandin E2 (PGE2) was identified in analyses of tissues harvested from subchondral bone cysts in horses. 68 , 106 Moreover, upregulation of interleukin 1 (IL‐1) and IL‐6 was detected within subchondral bone cysts. 107 Besides, osteoclast recruiting and their activation was provoked in neonate rats when their osteoclasts were cultured in conditioned medium of the fibrous tissue and cystic fluid harvested from the center of subchondral bone cysts, 68 which also accords to the increased number of osteoclasts and resorbed trabeculae identified at the periphery of the cystic lesions. 47 These data might be explained by the combined effect of the inflammatory factors (e.g. PGE2, IL‐1, IL‐6, and tumor necrosis factor‐alpha), which are usually elevated in clinical cases of pathologic bone resorption. 107 , 108 , 109 , 110 , 111 , 112 , 113 Placed in the context of the proposition of Woods that repetitive minor trauma to a localized area of bone results in subchondral cyst formation, 114 it is possible that such events instigated the activation of the internal inflammatory processes. 105
Taken together, subchondral cysts may result from the two mechanisms as described above. Posttraumatic subchondral bone cysts may develop through both mechanisms at the sites of joint injuries (e.g., fracture), possibly due to bone resorption by synovial fluid, reflected in bone marrow edema (BME), 115 and also mechanical stress and repeated microtrauma that subsequently lead to vascular disruption, local bone necrosis, and subsequent cyst formation. 73 As studies on OCTs have shown, channels in the lateral osteochondral graft‐host interface generated by the technique provide a communication to the joint space, which may induce subchondral bone cysts by allowing pressurized synovial fluid to enter the subchondral bone. 105 Next, bone resorption occurs and results in the formation and expansion of a cavity that originates from the communicative canal. The host bone responds by peri‐wall bone thickening and sclerosis, which resembles the cellular and molecular processes of the internal inflammatory theory such as a local proinflammatory state with osteoclast activation, among others. It is possible that subchondral bone cysts may result from a combination of these mechanisms.
5. SUBCHONDRAL CYST FORMATION AFTER CLINICAL ARTICULAR CARTILAGE REPAIR
Subchondral bone cysts related to articular cartilage repair procedures are frequently observed during postoperative radiographic evaluations (Figure 1). They have been traditionally reported after marrow stimulation procedures (e.g., microfracture and subchondral drilling). Penetrations of the subchondral bone resulting from microfracture 58 or subchondral drilling 116 might serve as their basis. Subchondral cyst formation has also been associated with other cartilage repair procedures, among which ACI 25 , 59 , 60 , 61 and autologous or allogeneic OCT (Table 2). 62 , 63 , 64 Stem cell therapy, a promising approach for cartilage repair, has not been associated with subchondral bone cyst formation based on the currently available literature and was therefore excluded from the current review. Also, as the largest number of clinical investigations on cartilage repair with long‐term follow‐ups originates from the knee, a focus is placed on this joint.
TABLE 2.
Overview of reported subchondral bone cyst formation following the clinical use of articular cartilage repair procedures
| Joint | Defect type | Index procedure | Detection method | Follow‐up (months) | Number of patients/defects | Cyst incidence per defect | Cyst characteristics | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Diameter (mm) | Morphology | Location | ||||||||
| Knee | Chondral | Microfracture | MRI | 0.8 | 13 | 0.0% | 0 | n.a. | n.a. | Beneath the repair site | 58 |
| 6 | 13 | 15.4% | 2 | n.a. | n.a. | ||||||
| 12 | 13 | 38.5% | 5 | n.a. | n.a. | ||||||
| 24 | 8 | 37.5% | 3 | n.a. | n.a. | ||||||
| Knee | Chondral | 1st generation ACI | MRI | 155 | 31 | 38.8% | 14 | n.a. | n.a. | Under the lesion area | 59 |
| Knee | Chondral or osteochondral | 1st or 2nd generation ACI | MRI | 12 | 163 | 14.7% | 24 | Small or large; no quantification | n.a. | n.a. | 25 |
| Knee | Chondral; with previous failed MST | 2nd generation ACI | MRI |
8.4 (success); 14.4 (failure) |
30 (success); 8 (failure) |
6.6% (success); 37.5% (failure) |
2 (success); 3 (failure) | n.a. | n.a. | n.a. | 60 |
| Knee. | n.a. | 2nd generation ACI | Micro‐CT; histology | 26.8 (before revision TKA) | 10 | 20.0% | 2 | n.a. | n.a. | n.a. | 61 |
| Knee | Osteochondral | Allograft OCT | MRI | 6 | 29 | 27.6% | 8 | n.a. | n.a. | Within graft or at host‐graft junction | 64 |
| Knee | Osteochondral | Allograft OCT with BMA | MRI | 6 | 29 | 20.7% | 6 | n.a. | n.a. | Within graft or at host‐graft junction | 64 |
| Knee | Chondral or osteochondral | Allograft OCT | MRI | 12 | 16 | 43.8% | 7 | n.a. | n.a. | Within graft or at host‐graft junction | 62 |
| Knee | Chondral or osteochondral | Allograft OCT | MRI | 12 | 15 | 46.7% | 7 | n.a. | n.a. | Within graft or at host‐graft junction | 62 |
| Knee | Osteochondral | Allograft OCT | MRI | 6 | 74 | 21.6% | 16 | n.a. | n.a. | Within graft or at host‐graft junction | 63 |
| Ankle | Osteochondral | Allograft OCT | MRI | 22.3 | 16 | 62.5% | 10 | 6.0 | n.a. | Graft (9); Inferior (2); Peripheral (8) | 65 |
| Ankle | Osteochondral | Autograft OCT | MRI | 26.3 | 25 | 40.0% | 10 | 3.8 | n.a. | Graft (1); Inferior (4); Peripheral (8) | 65 |
| Ankle | Osteochondral | Autograft OCT | MRI | 66.3 | 26 | 76.9% | 20 | 3.8 | n.a. | Graft (7); Inferior (5); Peripheral (14) | 66 |
| Ankle | Osteochondral | Autograft OCT with concentrated BMA | MRI | 60.8 | 28 | 46.4% | 13 | 4.9 | n.a. | Graft (4); Inferior (5); Peripheral (7) | 66 |
BMA, bone marrow aspirate; micro‐CT, micro‐computed tomography; MRI, magnetic resonance imaging; MST, marrow stimulation treatment; OCT, osteochondral transplantation; TKA, total knee arthroplasty; n.a., not available.
5.1. Marrow stimulation
Cole and colleagues reported subchondral cysts beneath the repair tissue after microfracture of isolated full‐thickness chondral defects by MRI in 15.4% (2/13) defects at 6 months, in 38.5% (5/13) defects at 12 months, and in 37.5% (3/8) defects at 24 months postoperatively. Detailed information about cyst number, size, and morphology was not described. Noteworthy, no cysts were observed at 3 weeks postoperatively. These data suggest that subchondral bone cysts develop gradually, appearing perceptible by imaging as early as 6 months postoperatively. 58
5.2. Autologous chondrocyte implantation
McCarthy et al. found subchondral bone cysts under the lesion area in 14.7% patients treated with either first‐ or second‐generation ACI at 1 year postoperatively, 25 considerably lower than the data from previous cohorts treated with microfracture (38.5% at 1 year postoperatively) from Cole et al. 58 Correspondingly, a recent clinical investigation from biopsies of patients undergoing total knee arthroplasty as a salvage procedure for failed second‐generation ACI with an average graft survival period of 26.8 months identified subchondral bone cyst formation within 20% of patients. 61 In a 9‐18 years follow‐up study, subchondral cysts were reported in 38.8% knee defects treated with first‐generation ACI. 59 Merkeley et al. identified the presence of severe BME (grade IV) as a predictive factor for graft failure (n = 8) among patients (n = 38) receiving a salvage second‐generation knee ACI for failed prior marrow stimulation. Interestingly, the incidence of subchondral cysts was not statistically significant between ACI patients without or with a prior marrow stimulation. 60 However, in ACI patients that received a previous marrow stimulation, the incidence of cyst formation was 6.6% (2/30) in successful but 37.5% (3/8) in failed cases. 60 Although not thoroughly addressed, these data suggest that subchondral bone cyst formation might be correlated with ACI failure in patients treated previously with marrow stimulation.
5.3. Osteochondral allograft transplantation
Ackermann et al compared the host‐graft integration outcomes at 1 year postoperatively after knee allograft OCT using two instrumentation sets from different companies. 62 Outcomes were evaluated with the Osteochondral allograft MRI Scoring System, BME size, graft‐host interface distance, graft cartilage integrity, cyst size, graft contour, and effusion presence. Specifically, cysts within the graft or at the host‐graft junction were observed in 43.8% (7/16) and 46.7% (7/15) cases without a statistically significant difference between the two instrumentation sets. These data indicate a considerable incidence of subchondral bone cyst following allograft OCT at 1 year postoperatively that is well within the range reported for microfracture 58 and not affected by the choice of instrumentation.
In patients with focal knee osteochondral defects, cysts within the graft or at the host‐graft junction were observed at 6 months postoperatively in 27.6% (8/29) and 20.7% (6/29) of patients treated with allograft OCT without or with unconcentrated bone marrow aspirate (BMA) without a statistically significant difference between the groups. 64
5.4. Subchondral bone cyst formation in other joints
Of special note, subchondral cyst formation has also been associated with femoroacetabular impingement 117 and following cartilage surgeries in other joints, especially the ankle. By morphological analysis, subchondral talar cysts are either of an irregular or round shape. 118 Cysts with an opening through the subchondral bone plate into the joint space can sometimes be identified. The presence of a sclerotic rim is reflected in the higher peri‐cyst bone volume fraction than in the normal subarticular spongiosa. 119 Allograft 65 or autograft 66 OCTs have been associated with the occurrence of cysts in the ankle joint. Most cysts are located peripheral to or within the grafts. Comparing the clinical and radiographic outcomes of autograft and allograft OCT to treat talar osteochondral defects, Shimozono et al. identified a statistically nonsignificant trend of more subchondral bone cyst formation in 62.5% of cases (10/16) treated with allograft OCT (autograft OCT: 40.0%, 10/25). 65 Interestingly, autograft OCT also yielded a significantly improved ankle function and superior Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score compared with allograft OCT at about 2 years postoperatively, in good agreement with the significantly higher rate of clinical failures following allograft (25%, 4/16) compared with autograft OCT (0%, 0/25). 65
Shimozono et al. also compared the postoperative incidence of cysts in ankle autograft OCT without or with concentrated BMA with a 60 months’ follow‐up. 66 The cyst incidence was significantly lower in autograft OCT with concentrated BMA (46.4%, 13/28) than in OCT without concentrated BMA (76.9%, 20/26). However, the cyst size and location were comparable between both groups. These data show a long‐term favorable inhibitory effectiveness of concentrated BMA against postoperative cyst formation after ankle autograft OCT, which is opposed to the short‐term data from Ackermann et al after knee allograft OCT. 64 These findings suggest that outcomes reported for the ankle joint may not be straightforwardly translated to the knee joint. Also, more investigations are needed to elucidate the possible varied efficacy of combinations with OCT (allograft or autograft) augmented with BMA (unconcentrated or concentrated) in different joints.
5.5. Association of postoperative subchondral cyst formation and clinical outcomes of articular cartilage repair
Although subchondral bone cysts represent an early postoperative sign associated with many articular cartilage repair procedures, a possible association between them and inferior clinical outcomes has not been well established in either the knee or the ankle joint. 51 An association between BME and subchondral bone cysts was already confirmed in the context of OA. Carrino et al showed that cysts always arose from regions of BME‐like signals in knee OA patients (n = 32) after a mean of 17.5 months (range 2.1‐40.1 months). BME were detected in 68 subarticular areas and 23 cysts. Interestingly, increases in size were noted for 25% of the BME and 26.1% of the cysts, 25.0% of BME and 4.4% of cysts decreased, while 21.7% of BME and 1.5% of cysts were unchanged (23.5% of BME were new, 16.2% were resolved). The BME signal size always changed with the cyst development: it increased in 54.5%, decreased in 18.1%, and resolved in 27.2% of cases. Of note, a change in cyst size was constantly accompanied by a change in edema‐like signal size. Moreover, an abnormality of the adjacent articular cartilage was identified for 87.0% of the cysts by MRI. 120 Further evidence supports the association of BME and subchondral bone cysts, and the BME signal on MRI has been also statistically linked with degenerative articular cartilage loss 121 or cartilage defects. 121 Arthroscopic grades of knee articular cartilage defects are positively associated with the prevalence, depth, and cross‐sectional area of subchondral BME on MRI. 122 Also, BME grading has been positively correlated to the presence of knee pain and stiffness, radiographic severity, and the increased rate of OA progression. 123 , 124 , 125 These findings attest to the strong relationships between the BMA and the occurrence of subchondral cysts and suggest an influence on clinical outcomes in the specific context of OA.
However, such a relationship may not be directly inferred to the different settings of the repair of focal cartilage defects in the knee. Currently available cross‐sectional 25 , 59 , 61 , 66 or cohort 58 , 65 studies do not allow identifying a causal relationship between the occurrence of subchondral bone cysts and clinical or radiographic outcomes of articular cartilage repair. For instance, ACI graft failure has been associated with BME, as the rate in patients with severe BME (83.7%) was significantly higher than in patients without severe BME (6.5%) at 60 months postoperatively. 60 In contrast, Vasiliadis et al identified a higher risk for subchondral bone cysts after ACI at 3 years postoperatively, which was not associated with the occurrence of BME but with increasing patient age. 59 These data underscore the pathophysiological and clinical different phenotypes of OA and focal (non‐OA) cartilage defects, which complicate a simple transfer of the rather large evidence gained from the OA field into the context of cartilage repair. They call for more individual investigations into the natural course of cartilage defects and their repair, with a special attention to OA development since these defects are possible triggers to develop secondary OA. 126 , 127 However, the strong evidence identifying BME as a risk factor for structural progression of knee OA, together with the proposition that BME represents a ‘‘pre‐cyst’’ sign 120 (although not every area of BME may give rise to a cyst) 120 warrants clinical alertness to prolonged symptomatic cases following articular cartilage repair, thus necessitating the need for staged MRI to rule out the possibility of BME and, if present, its appropriate treatment to avoid a possible conversion of such a BME into a subchondral bone cyst.
Controversial data have been accumulated for the ankle joint. 66 , 128 , 129 Evidence of cystic changes was identified in 65.8% patients using MRI after autograft OCT for talar osteochondral defects at a short‐term follow‐up of 15 months postoperatively. 129 Interestingly, subchondral bone cyst formation was neither correlated with cartilage integrity nor patient‐reported outcomes. 129 In another study, postoperative cyst formation did not affect clinical outcomes of talar autograft OCT for osteochondral defects. Subchondral cysts were identified via MRI in 64.8% of patients at 15 months (range, 2‐54) postoperatively. 128 Patients with postoperative cysts were significantly older than those without cysts (mean age, 42.7 vs 32.7 years). Among the patients with a cyst, the subchondral bone plate was significantly more involved in old patients (57.3 vs 36.7 years). Interestingly, no other variables associated with cyst formation achieved statistical significance. Patients without postoperative cysts were characterized by lower preoperative Short Form‐12 (SF‐12) and Foot and Ankle Outcome Score (FAOS) and significantly more postoperative improvements in both scores than patients that developed cysts. However, a long‐term study of autograft OCT without or with concentrated bone marrow for talar osteochondral defects reported no significant differences of the postoperative SF‐12 and FAOS between patients without or with cysts at a follow‐up at 5 years postoperatively. 66
6. SUBCHONDRAL CYST FORMATION IN PRECLINICAL CARTILAGE REPAIR
Subchondral bone cyst formation following articular cartilage repair in preclinical models has been regularly recognized as a common postoperative phenomenon. 46 , 47 , 48 , 56 , 57 Such preclinical models offer the elegant possibility of performing ex vivo analyses of the microstructure of the subchondral bone a high resolution using micro‐computed tomography (micro‐CT), allowing to depict subchondral bone cysts at a magnitude of detail that is difficult to obtain in clinical settings. 46 , 47 , 57 , 116 , 130 The prevalence of subchondral bone cyst formation following cartilage repair can be as high as 92.0% in sheep at 6 months postoperatively. 47 Although subchondral bone cyst formation appears to be species and procedure specific, detailed attention to this important issue appears to be warranted (Table 3). 55 , 56
TABLE 3.
Overview of reported subchondral bone cyst formation following the preclinical use of articular cartilage repair procedures
| Preclinicalmodel | Joint | Defect type | Index procedure | Detection method | Follow‐up (months) | Number of animal | Incidence per defect | Reference |
|---|---|---|---|---|---|---|---|---|
| Horse | Knee | Osteochondral | Spontaneous repair | Histomorphometry | 12 | 10 | n.a. | 53 |
| Horse | Knee | Osteochondral | Spontaneous repair | Xeroradiography | 4 | 3 | n.a. | 54 |
| Sheep | Knee | Chondral | Microfracture | Histomorphometry; micro‐CT | 3.3; 6.5 | 12 |
33.3% (3.3 m); 91.7% (6.5 m)* |
47 |
| Sheep | Knee | Chondral | Microfracture | Histomorphometry; micro‐CT | 3 | 8 | 25% | 46 |
| Sheep | Knee | Chondral | Microfracture | Histomorphometry | 6 | 6 | 83% | 56 |
| Horse | Knee | Chondral | Microfracture | Histomorphometry | 4 | 5 | 0% | 55 |
| Horse | Knee | Chondral | Microfracture | Histomorphometry | 12 | 5 | 10% | 55 |
| Sheep | Knee | Chondral | Microfracture | Histomorphometry | 6 | 8 | 63% | 56 |
| Rabbit | Knee | Chondral | Drilling | Histomorphometry; micro‐CT | 3 | 8 | 41% | 46 |
| Sheep | Knee | Chondral | Drilling | Histomorphometry; micro‐CT | 6 | 19 | 63% | 57 |
| Sheep | Knee | Chondral | AMIC | Histomorphometry; micro‐CT | 3.3; 6.5 | 12 |
3.3 m: 50% (3.3 m); 91.7% (6.5 m)* |
47 |
AMIC, Autologous matrix‐induced chondrogenesis; micro‐CT, micro‐computed tomography; n.a., not available. *The mean incidence of subchondral bone cyst formation was 91.7% in samples treated by either microfracture or AMIC at 6.5 month postoperatively, however, no detailed information regarding cyst formation rate for each procedure was separately provided.47.
6.1. Spontaneous cartilage repair
Subchondral cyst formation during the spontaneous repair of osteochondral defects may also be location dependent. In a minipig model, more frequent subchondral bone cyst formation was seen in the medial femoral condyle compared with the medial patellar groove at 12 months postoperatively. 131 Other studies of spontaneous cartilage repair applying either histomorphometry 53 or xeroradiography 54 did not address the issue of subchondral bone cyst formation.
6.2. Microfracture and augmented procedures
The incidence of subchondral bone cysts after microfracture for knee chondral defects was 25% at 3 months postoperatively in rabbits. 46 However, several studies in the ovine model revealed a much higher incidence of 83‐92% at 6 months postoperatively when analyzed with micro‐CT. 47 , 56 , 130 Communication through the microfracture holes between the intraarticular space and the subchondral bone cysts persevered for up to 6 months postoperatively, 47 highlighting the potential role of the surgical penetrations of the subchondral bone with the microfracture instruments as a possible factor that may essentially be involved in subchondral bone cyst formation over time. 102 Prevalence of subchondral bone cyst formation was 50% at 3 months postoperatively and 92% at 6 months postoperatively following autologous matrix‐induced chondrogenesis (AMIC) in sheep, 47 which was comparable to the outcome of microfracture alone at both time points (33.3% and 91.7%, respectively). These data suggest that utilizing an additional bioresorbable membrane scaffold may not reduce the early formation of subchondral bone cysts after microfracture. Also, these early subchondral bone alterations might partly explain the comparable clinical and radiographic outcomes between AMIC and microfracture for knee chondral defects at 5 years postoperatively. 132 , 133
6.3. Subchondral drilling
The occurrence of subchondral bone cysts was as high as 41% in a rabbit model at 3 months postoperatively after subchondral drilling for chondral defects of the knee. 46 Orth et al reported that subchondral drilling for full‐thickness chondral defects in the medial femoral condyle of sheep led to the formation of subchondral bone cysts in 63% of defects at 6 months postoperatively. 57 These bone cysts always originated from the canals generated during the drilling with Kirschner wires. Of note, multiple cysts can concurrently originate from one single defect, and the cyst dimension may also largely exceed the original defect area.
6.4. Autologous chondrocyte implantation
Subchondral bone cyst formation has only rarely been reported in preclinical models of autologous chondrocyte implantation (ACI). A chondrocyte suspension was applied to cartilage defects in a goat model, sealed by a periosteal flap or a collagen membrane, and evaluated after 10 weeks in vivo. If the treated defects were not filled with a repair tissue and if the calcified layer and subchondral bone were damaged at this early time point, bone cracks and subchondral bone cysts below the defect were revealed by histological analysis. Such subchondral bone cyst formation associated with graft failure was limited, although no details on their incidence were reported. 134
7. CLINICAL IMPLICATIONS AND OUTLOOK
Due to its high incidence (38.5% in microfracture 58 ; 38.8% in ACI 59 ; 38.9% in allograft OCT 62 ; 62.5% in allograft OCT 65 ; 76.9% in autograft OCT 66 ) and lasting presence (over 12 years reported for ACI 59 ), subchondral bone cyst formation following articular cartilage repair merits serious attention. Its appearance as early as 6 months postoperatively in over 15% of patients treated with microfracture 58 and 20% of patients treated with OCT 63 , 64 highlights the clinical importance. 57 The currently recommended timeframe for touchdown weight‐bearing within the first 6‐8 postoperative weeks and free full weight‐bearing thereafter, therefore, needs to be respected to constrain early subchondral bone changes, 5 , 135 considering the fact that the rather small bone defects resulting from marrow stimulation will be closed after such a period, but possibly not if earlier weight‐bearing pushes the synovial fluid through the soft repair tissue into the residual (subchondral) canals from the marrow stimulation or, in the case of OCT, into the nonintegrated interface between the graft and the adjacent normal osteochondral unit. This, in turn, might possibly lead to the bone resorption and/or remodeling seen on MRI as BME, followed by subchondral bone cyst formation that ultimately weakens the osteochondral unit that leads to its deterioration over time.
Besides, these data also prompt more clinical observations and radiographic follow‐ups (e.g., MRI or cone‐beam CT 136 ) during the early postoperative phase to identify premature subchondral bone changes for the possible optimization and individualization of the rehabilitation program. As already stated, MRI evaluations over time (e.g. in 6‐week intervals) may be indicated in cases of prolonged pain following the different cartilage repair procedures to rule out BME and/or subchondral bone cysts. Likewise, a long‐term follow‐up of postoperative subchondral bone cysts appears mandatory for many of the cartilage repair techniques.
The substantial inconsistency in the terminology used to describe entities of subchondral bone changes has already been recognized as a frequent disconnect between the used nomenclature and the actual morphological change. 32 , 51 , 52 Standardization of analyses and outcome reporting of postoperative subchondral bone changes, possibly with an established algorithm will assist investigators to report salient characteristics of subchondral bone changes and to improve the transparency and comparability of data from studies regarding articular cartilage repair. 52
A number of other specific issues and possible research questions are worthy to be addressed to further optimize cartilage repair in a clinical setting. First, a better understanding of the mechanisms of subchondral bone cyst development will improve surgical treatment and postoperative rehabilitation and prevent further cyst formation. Second, continuous updating and augmenting the currently available techniques are necessary to reduce or even avoid these deleterious subchondral bone changes. For example, concentrated BMA‐enhanced autograft OCT was shown to decrease subchondral cyst formation rate for talar osteochondral defects, 66 however no information is available about the knee joint. Similarly, it will be interesting to see if the additional coverage of microfractured cartilage defects with biomaterials (e.g., membrane scaffolds) might result in a lower incidence of subchondral cyst formation and possibly ensure better long‐term outcomes compared with the traditional marrow stimulation technique. Third, for ACI, a possible correlation of subchondral bone cyst formation and ACI failure remains to be investigated. Lastly, for an already established subchondral bone cyst, salvage managements (e.g., curettage and autologous cancellous bone grafting) might be beneficial to guarantee the long‐term success of the index cartilage repair procedure. 137
8. CONCLUSION
Subchondral bone cysts are one of the most widely reported subchondral bone changes associated with the repair of focal articular cartilage defects. More investigations into their mechanisms of development and both clinical and radiographic follow‐up in the context of specific cartilage repair procedures will enhance our understanding of the important relationships between the occurrence of postoperative subchondral cysts and clinical outcomes in cartilage repair.
AUTHOR CONTRIBUTIONS
Henning Madry provided the conception and designing. Liang Gao, Magali Cucchiarini, and Henning Madry contributed to literature searching and wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
This research received no external funding. We acknowledge support by the Saarland University within the funding programme Open Access Publishing.
Gao L, Cucchiarini M, Madry H. Cyst formation in the subchondral bone following cartilage repair. Clin Transl Med. 2020;10:e248 10.1049/ctm2248
Funding Information: Saarland University within the funding programme Open Access Publishing.
REFERENCES
- 1. Gao L, Orth P, Müller‐Brandt K, Goebel LK, Cucchiarini M, Madry H. Early loss of subchondral bone following microfracture is counteracted by bone marrow aspirate in a translational model of osteochondral repair. Sci Rep. 2017;7:45189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889‐895. [DOI] [PubMed] [Google Scholar]
- 3. Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2(1):54‐69. [DOI] [PubMed] [Google Scholar]
- 4. Pridie K. A method of resurfacing osteoarthric knee joints. J Bone Joint Surg. 1959;3:618‐619. [Google Scholar]
- 5. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(Suppl):S362‐S369. [DOI] [PubMed] [Google Scholar]
- 6. Madry H, Grün UW, Knutsen G. Cartilage repair and joint preservation: medical and surgical treatment options. Dtsch Arztebl Int. 2011;108(40):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nie X, Chuah YJ, Zhu W, He P, Peck Y, Wang D‐A. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials. 2020;235:119821. [DOI] [PubMed] [Google Scholar]
- 8. Lee C, O'Connell CD, Onofrillo C, Choong PF, Di Bella C, Duchi S. Human articular cartilage repair: sources and detection of cytotoxicity and genotoxicity in photo‐crosslinkable hydrogel bioscaffolds. Stem Cells Transl Med. 2020;9(3):302‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuggle NR, Cooper C, Oreffo RO, et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin Exp Res. 2020;32:547‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faber S, Zinser W, Angele P, et al. Does gender influence outcome in cartilage repair surgery? An analysis of 4,968 consecutive patients from the German cartilage registry (Knorpel Register DGOU). Cartilage. 2020. 10.1177/1947603520923137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kunze KN, Chahla J, Gomoll AH. Defining failure after cartilage preservation surgery: are we expecting too much? Oper Techn Sports Med. 2020;28(1):150708. [Google Scholar]
- 12. Oak SR, Spindler KP. Measuring outcomes in knee articular cartilage pathology. J Knee Surg. 2020. 10.1055/s-0040-1716362 [DOI] [PubMed] [Google Scholar]
- 13. Solheim E, Hegna J, Inderhaug E. Clinical outcome after mosaicplasty of knee articular cartilage defects of patellofemoral joint versus tibiofemoral joint. J Orthop. 2020;18:36‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schreiner MM, Raudner M, Szomolanyi P, et al. Chondral and osteochondral femoral cartilage lesions treated with GelrinC: significant improvement of radiological outcome over time and zonal variation of the repair tissue based on T2 mapping at 24 months. Cartilage. 2020. 10.1177/1947603520926702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madry H, Grün UW, Knutsen G. Cartilage repair and joint preservation: medical and surgical treatment options. Deutsches Ärzteblatt Int. 2011;108(40):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35(10):622‐638. [DOI] [PubMed] [Google Scholar]
- 17. Ferrua P, Tradati D, Berruto M. Computed tomography and arthro‐CT scan in patellofemoral disorders Patellofemoral Pain, Instability, and Arthritis. Springer; 2020:63‐69. [Google Scholar]
- 18. Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow‐stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315‐2324. [DOI] [PubMed] [Google Scholar]
- 19. Hevesi M, Denbeigh JM, Paggi CA, et al. Fresh osteochondral allograft transplantation in the knee: a viability and histologic analysis for optimizing graft viability and expanding existing standard processed graft resources using a living donor cartilage program. Cartilage. 2019. 10.1177/1947603519880330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2009;36(2):235‐246. [DOI] [PubMed] [Google Scholar]
- 21. Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue‐engineered, repaired and osteoarthritic cartilage. Osteoarthr Cartil. 2010;18(1):12‐23. [DOI] [PubMed] [Google Scholar]
- 22. de Windt TS, Vonk LA, Slaper‐Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single‐stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256‐264. [DOI] [PubMed] [Google Scholar]
- 23. Kim MK, Park JS, Jeon YM, Jeon YS. Clinical, radiological, and histological outcomes after the fibrin‐matrix autologous chondrocyte implantation for chondral lesions of the knee in patients more than 50 years old: a prospective case series with minimum 2‐year follow‐up. J Orthop Surg. 2020;28(1). 10.1177/2309499019893509 [DOI] [PubMed] [Google Scholar]
- 24. Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, open‐label, multicenter, phase III Noninferiority trial to compare the clinical efficacy of matrix‐associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCarthy HS, McCall IW, Williams JM, et al. Magnetic resonance imaging parameters at 1 year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop J Sports Med. 2018;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niemeyer P, Uhl M, Salzmann GM, Morscheid YP, Südkamp NP, Madry H. Evaluation and analysis of graft hypertrophy by means of arthroscopy, biochemical MRI and osteochondral biopsies in a patient following autologous chondrocyte implantation for treatment of a full‐thickness‐cartilage defect of the knee. Arch Orthop Trauma Surg. 2015;135(6):819‐830. [DOI] [PubMed] [Google Scholar]
- 27. McCarthy HS, Roberts S. A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr Cartil. 2013;21(12):2048‐2057. [DOI] [PubMed] [Google Scholar]
- 28. Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moriya T, Wada Y, Watanabe A, et al. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12(3):265‐273. [DOI] [PubMed] [Google Scholar]
- 30. Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee—a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12(3):209‐216. [DOI] [PubMed] [Google Scholar]
- 31. de Windt TS, Vonk LA, Slaper‐Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one‐stage cartilage cell transplantion: a first‐in‐man trial in 35 patients. Stem Cells. 2017;35(8):1984‐1993. [DOI] [PubMed] [Google Scholar]
- 32. Madry H, van Dijk CN, Mueller‐Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419‐433. [DOI] [PubMed] [Google Scholar]
- 33. Audrey HX, Abd Razak HR, Andrew TH. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop J. 2014;8:7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanamas SK, Wluka AE, Pelletier JP, et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res Ther. 2010;12(2):R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Y, Wang T, Guan M, et al. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr Cartil. 2015;23(12):2174‐2183. [DOI] [PubMed] [Google Scholar]
- 37. Castillo BA, el Sallab RA, Scott JT. Physical activity, cystic erosions, and osteoporosis in rheumatoid arthritis. Ann Rheum Dis. 1965;24(6):522‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooperberg PL, Tsang I, Truelove L, Knickerbocker WJ. Gray scale ultrasound in the evaluation of rheumatoid arthritis of the knee. Radiology. 1978;126(3):759‐763. [DOI] [PubMed] [Google Scholar]
- 39. Fisk GR. Bone concavity caused by a ganglion. J Bone Joint Surg Br. 1949;31‐B(2):220. [PubMed] [Google Scholar]
- 40. Sakamoto A, Oda Y, Iwamoto Y. Intraosseous ganglia: a series of 17 treated cases. Biomed Res Int. 2013;2013:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crabbe WA. Intra‐osseous ganglia of bone. Br J Surg. 1966;53(1):15‐17. [DOI] [PubMed] [Google Scholar]
- 42. Szendröi M, Cser I, Kónya A, Rényi‐Vámos A. Aneurysmal bone cyst. A review of 52 primary and 16 secondary cases. Arch Orthop Trauma Surg. 1992;111(6):318‐322. [DOI] [PubMed] [Google Scholar]
- 43. Pawar E, Harsoor A, Ramteke U, Yadav AK, Aher G. Management of aneurysmal bone cyst of proximal femur with a pathologic intertrochanteric femur fracture with an intramedullary nail and bone graft. Int J Orthopaed. 2019;5(4):156‐159. [Google Scholar]
- 44. Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921‐2931. [DOI] [PubMed] [Google Scholar]
- 45. Campanacci L. Aneurysmal Bone Cyst (ABC) Diagnosis of Musculoskeletal Tumors and Tumor‐like Conditions. Springer; 2020:97‐100. [Google Scholar]
- 46. Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, Buschmann MD. Characterization of subchondral bone repair for marrow‐stimulated chondral defects and its relationship to articular cartilage resurfacing. Am J Sports Med. 2011;39(8):1731‐1741. [DOI] [PubMed] [Google Scholar]
- 47. Beck A, Murphy DJ, Carey‐Smith R, Wood DJ, Zheng MH. Treatment of articular cartilage defects with microfracture and autologous matrix‐induced chondrogenesis leads to extensive subchondral bone cyst formation in a sheep model. Am J Sports Med. 2016;44(10):2629‐2643. [DOI] [PubMed] [Google Scholar]
- 48. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, Mcllwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824‐1831. [DOI] [PubMed] [Google Scholar]
- 49. LaValley MP, Lo GH, Price LL, Driban JB, Eaton CB, McAlindon TE. Development of a clinical prediction algorithm for knee osteoarthritis structural progression in a cohort study: value of adding measurement of subchondral bone density. Arthritis Res Ther. 2017;19(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lo GH, Schneider E, Driban JB, et al. Periarticular bone predicts knee osteoarthritis progression: data from the osteoarthritis initiative. Semin Arthritis Rheum. 2018;48(2):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orth P, Cucchiarini M, Kohn D, Madry H. Alterations of the subchondral bone in osteochondral repair–translational data and clinical evidence. Eur Cell Mater. 2013;25:299‐316. discussion 314‐296. [DOI] [PubMed] [Google Scholar]
- 52. Gao L, Orth P, Goebel LK, Cucchiarini M, Madry H. A novel algorithm for a precise analysis of subchondral bone alterations. Sci Rep. 2016;6:32982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Howard RD, McIlwraith CW, Trotter GW, et al. Long‐term fate and effects of exercise on sternal cartilage autografts used for repair of large osteochondral defects in horses. Am J Vet Res. 1994;55(8):1158‐1167. [PubMed] [Google Scholar]
- 54. Hanie EA, Sullins KE, Powers BE, Nelson PR. Healing of full‐thickness cartilage compared with full‐thickness cartilage and subchondral bone defects in the equine third carpal bone. Equine Vet J. 1992;24(5):382‐386. [DOI] [PubMed] [Google Scholar]
- 55. Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242‐255. [DOI] [PubMed] [Google Scholar]
- 56. Hoemann CD, Hurtig M, Rossomacha E, et al. Chitosan‐glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671‐2686. [DOI] [PubMed] [Google Scholar]
- 57. Orth P, Goebel L, Wolfram U, et al. Effect of subchondral drilling on the microarchitecture of subchondral bone: analysis in a large animal model at 6 months. Am J Sports Med. 2012;40(4):828‐836. [DOI] [PubMed] [Google Scholar]
- 58. Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single‐stage procedure for cell‐based cartilage repair: a prospective clinical safety trial with 2‐year follow‐up. Am J Sports Med. 2011;39(6):1170‐1179. [DOI] [PubMed] [Google Scholar]
- 59. Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long‐term evaluation with magnetic resonance imaging and delayed gadolinium‐enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38(5):943‐949. [DOI] [PubMed] [Google Scholar]
- 60. Merkely G, Ogura T, Bryant T, Minas T. Severe bone marrow edema among patients who underwent prior marrow stimulation technique is a significant predictor of graft failure after autologous chondrocyte implantation. Am J Sports Med. 2019;47(8):1874‐1884. [DOI] [PubMed] [Google Scholar]
- 61. Beck A, Wood D, Vertullo CJ, et al. Morphological assessment of MACI grafts in patients with revision surgery and total joint arthroplasty. Cartilage. 2019. 10.1177/1947603519890754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ackermann J, Duerr RA, Barbieri Mestriner A, Shah N, Gomoll AH. Effect of graft‐host interference fit on graft integration after osteochondral allograft transplantation: a comparative MRI analysis of two instrumentation sets. Cartilage. 2019. 10.1177/1947603519865314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ackermann J, Merkely G, Shah N, Gomoll AH. Decreased graft thickness is associated with subchondral cyst formation after osteochondral allograft transplantation in the knee. Am J Sports Med. 2019;47(9):2123‐2129. [DOI] [PubMed] [Google Scholar]
- 64. Ackermann J, Mestriner AB, Shah N, Gomoll AH. Effect of autogenous bone marrow aspirate treatment on magnetic resonance imaging integration of osteochondral allografts in the knee: a matched comparative imaging analysis. Arthroscopy. 2019;35(8):2436‐2444. [DOI] [PubMed] [Google Scholar]
- 65. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100(21):1838‐1844. [DOI] [PubMed] [Google Scholar]
- 66. Shimozono Y, Yasui Y, Hurley ET, Paugh RA, Deyer TW, Kennedy JG. Concentrated bone marrow aspirate may decrease postoperative cyst occurrence rate in autologous osteochondral transplantation for osteochondral lesions of the talus. Arthroscopy. 2019;35(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 67. Auer JA; von Rechenberg B. (2012). Subchondral bone cysts In: Auer JA, Stick J. Equine Surgery. St. Louis, Missouri: Elsevier, 1125–1263. 10.1016/B978-1-4377-0867-7.00089-2 [DOI] [Google Scholar]
- 68. von Rechenberg B, McIlwraith C, Auer J. Cystic bone lesions in horses and humans: a comparative review. Vet Comp Orthop Traumatol. 1998;11(01):8‐18. [Google Scholar]
- 69. Chiba K, Burghardt AJ, Osaki M, Majumdar S. Three‐dimensional analysis of subchondral cysts in hip osteoarthritis: an ex vivo HR‐pQCT study. Bone. 2014;66:140‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sanal HT, Chen L, Haghighi P, Trudell DJ, Resnick DL. Carpal bone cysts: mRI, gross pathology, and histology correlation in cadavers. Diagn Interv Radiol. 2014;20(6):503‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crema MD, Roemer FW, Marra MD, et al. Contrast‐enhanced MRI of subchondral cysts in patients with or at risk for knee osteoarthritis: the MOST study. Eur J Radiol. 2010;75(1):e92‐e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McErlain DD, Ulici V, Darling M, et al. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res Ther. 2012;14(1):R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ziino C, Safran MR. Evolution of a posttraumatic femoral head bone cyst: a case study and surgical management. Orthop J Sports Med. 2019;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hughes TH, Sartoris DJ, Schweitzer ME, Resnick DL. Pigmented villonodular synovitis: mRI characteristics. Skeletal Radiol. 1995;24(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 75. Grimm NL, Tainter DM, Eward WC, Brigman BE. Tumors of the epiphyses. JBJS Rev. 2017;5(5):e4. [DOI] [PubMed] [Google Scholar]
- 76. Madry H, Prudlo J, Grgic A, Freyschmidt J. Nasu‐Hakola disease (PLOSL): report of five cases and review of the literature. Clin Orthop Relat Res. 2007;454:262‐269. [DOI] [PubMed] [Google Scholar]
- 77. Gorbachova T, Amber I, Beckmann NM, et al. Nomenclature of subchondral nonneoplastic bone lesions. AJR Am J Roentgenol. 2019;213(5):963‐982. [DOI] [PubMed] [Google Scholar]
- 78. Schajowicz F, Clavel Sainz M, Slullitel JA. Juxta‐articular bone cysts (intra‐osseous ganglia): a clinicopathological study of eighty‐eight cases. J Bone Joint Surg Br. 1979;61‐B(1):107‐116. [DOI] [PubMed] [Google Scholar]
- 79. Menetrey J, Unno‐Veith F, Madry H, Van Breuseghem I. Epidemiology and imaging of the subchondral bone in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):463‐471. [DOI] [PubMed] [Google Scholar]
- 80. Lotz MK, Caramés B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7(10):579‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Howard RD, McIlwraith CW, Trotter GW. Arthroscopic surgery for subchondral cystic lesions of the medial femoral condyle in horses: 41 cases (1988‐1991). J Am Vet Med Assoc. 1995;206(6):842‐850. [PubMed] [Google Scholar]
- 82. McIlwraith C. Sub‐chondral cystic lesions (osteochondrosis) in the horse. Compend Continuing Educ Pract Vet. 1982;4(9):S394. [Google Scholar]
- 83. McIlwraith C. Subchondral cystic lesions in the horse‐the indications, methods and results of surgery. Equine Vet Educ. 1990;2(2):75‐80. [Google Scholar]
- 84. Jeffcott LB, Kold S. Clinical and radiological aspects of stifle bone cysts in the horse. Equine Vet J. 1982;14(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 85. Jeffcott LB. Osteochondrosis—an international problem for the horse industry. J Equine Vet Sci. 1996;16(1):32‐37. [Google Scholar]
- 86. Cox L, Lagemaat M, Van Donkelaar C, et al. The role of pressurized fluid in subchondral bone cyst growth. Bone. 2011;49(4):762‐768. [DOI] [PubMed] [Google Scholar]
- 87. Potty AGR, Gupta A, Rodriguez HC, Stone IW, Maffulli N. Intraosseous bioplasty for a subchondral cyst in the lateral condyle of femur. J Clin Med. 2020;9(5):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Freund E. The pathological significance of intra‐articular pressure. Edin Med J. 1940;47(3):192. [PMC free article] [PubMed] [Google Scholar]
- 89. Landells JW. The bone cysts of osteoarthritis. J Bone Joint Surg Br. 1953;35‐B(4):643‐649. [DOI] [PubMed] [Google Scholar]
- 90. Prager P, Menges V, DiBiase M. The intraosseus ganglion. RoFo. 1975;123(5):458‐461. (Author's Transl). [DOI] [PubMed] [Google Scholar]
- 91. Wang W, Ding R, Zhang N, Hernigou P. Subchondral bone cysts regress after correction of malalignment in knee osteoarthritis: comply with Wolff's law. Int Orthop. 2020. [DOI] [PubMed] [Google Scholar]
- 92. Ray CS, Baxter GM, Mc IC, et al. Development of subchondral cystic lesions after articular cartilage and subchondral bone damage in young horses. Equine Vet J. 1996;28(3):225‐232. [DOI] [PubMed] [Google Scholar]
- 93. Dürr HD, Martin H, Pellengahr C, Schlemmer M, Maier M, Jansson V. The cause of subchondral bone cysts in osteoarthrosis: a finite element analysis. Acta Orthop Scand. 2004;75(5):554‐558. [DOI] [PubMed] [Google Scholar]
- 94. Sun L, Zhou X, Wu B, Tian M. Inhibitory effect of synovial fluid on tendon‐to‐bone healing: an experimental study in rabbits. Arthroscopy. 2012;28(9):1297‐1305. [DOI] [PubMed] [Google Scholar]
- 95. Berg EE, Pollard ME, Kang Q. Interarticular bone tunnel healing. Arthroscopy. 2001;17(2):189‐195. [DOI] [PubMed] [Google Scholar]
- 96. Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra‐articular and extra‐articular ends of a bone tunnel. Hss j. 2009;5(1):51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stefan Lohmander L, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arth Rheum. 1993;36(9):1214‐1222. [DOI] [PubMed] [Google Scholar]
- 98. Kokebie R, Aggarwal R, Lidder S, et al. The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther. 2011;13(2):R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthr Cartil. 2012;20(12):1451‐1464. [DOI] [PubMed] [Google Scholar]
- 100. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Drobnic M, Radosavljevic D, Cör A, Brittberg M, Strazar K. Debridement of cartilage lesions before autologous chondrocyte implantation by open or transarthroscopic techniques: a comparative study using post‐mortem materials. J Bone Joint Surg Br. 2010;92‐B(4):602‐608. [DOI] [PubMed] [Google Scholar]
- 102. Ray C, Baxter G, McIlwraith C, et al. Development of subchondral cystic lesions after articular cartilage and subchondral bone damage in young horses. Equine Vet J. 1996;28(3):225‐232. [DOI] [PubMed] [Google Scholar]
- 103. von Rechenberg B, Akens MK, Nadler D, et al. Changes in subchondral bone in cartilage resurfacing–an experimental study in sheep using different types of osteochondral grafts. Osteoarthr Cartil. 2003;11(4):265‐277. [DOI] [PubMed] [Google Scholar]
- 104. Von Rechenberg B, Akens M, Nadler D, et al. The use of photooxidized, mushroom‐structured osteochondral grafts for cartilage resurfacing–a comparison to photooxidized cylindrical grafts in an experimental study in sheep. Osteoarthritis Cartilage. 2004;12(3):201‐216. [DOI] [PubMed] [Google Scholar]
- 105. Pallante‐Kichura AL, Cory E, Bugbee WD, Sah RL. Bone cysts after osteochondral allograft repair of cartilage defects in goats suggest abnormal interaction between subchondral bone and overlying synovial joint tissues. Bone. 2013;57(1):259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Von Rechenberg B, Guenther H, McIlwraith CW, et al. Fibrous tissue of subchondral cystic lesions in horses produce local mediators and neutral metalloproteinases and cause bone resorption in vitro. Vet Surg. 2000;29(5):420‐429. [DOI] [PubMed] [Google Scholar]
- 107. von Rechenberg B, Leutenegger C, Zlinsky K, McIlwraith CW, Akens MK, Auer JA. Upregulation of mRNA of interleukin‐1 and ‐6 in subchondral cystic lesions of four horses. Equine Vet J. 2001;33(2):143‐149. [DOI] [PubMed] [Google Scholar]
- 108. Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T. Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo. Osteoporos Int. 1993;3(Suppl 1):114‐116. [DOI] [PubMed] [Google Scholar]
- 109. Mundy GR. Inflammatory mediators and the destruction of bone. J Periodontal Res. 1991;26(3 Pt 2):213‐217. [DOI] [PubMed] [Google Scholar]
- 110. Lorenzo JA. The role of cytokines in the regulation of local bone resorption. Crit Rev Immunol. 1991;11(3‐4):195‐213. [PubMed] [Google Scholar]
- 111. Benazzo F, Cadossi M, Cavani F, et al. Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res. 2008;26(5):631‐642. [DOI] [PubMed] [Google Scholar]
- 112. Gowen M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2, and interferon‐gamma on bone resorption in vitro. J Immunol. 1986;136(7):2478‐2482. [PubMed] [Google Scholar]
- 113. Tamura T, Udagawa N, Takahashi N, et al. Soluble interleukin‐6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993;90(24):11924‐11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Woods C. Subchondral bone cysts. J Bone Joint Surg Br. 1961;43‐B(4):758‐766. [DOI] [PubMed] [Google Scholar]
- 115. Moore TE, King AR, Travis RC, Allen BC. Post‐traumatic cysts and cyst‐like lesions of bone. Skeletal Radiol. 1989;18(2):93‐97. [DOI] [PubMed] [Google Scholar]
- 116. Gao L, Goebel LKH, Orth P, Cucchiarini M, Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11(6):dmm034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Foreman SC, Zhang AL, Neumann J, et al. Postoperative MRI findings and associated pain changes after arthroscopic surgery for femoroacetabular impingement. AJR Am J Roentgenol. 2020;214(1):177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Reilingh M, Blankevoort L, van Eekeren I, van Dijk C. Morphological analysis of subchondral talar cysts on microCT. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1409‐1417. [DOI] [PubMed] [Google Scholar]
- 119. Hunter DJ, Gerstenfeld L, Bishop G, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11(1):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB. MRI of bone marrow edema‐like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage. 2006;14(10):1081‐1085. [DOI] [PubMed] [Google Scholar]
- 121. Neumann G, Mendicuti AD, Zou KH, et al. Prevalence of labral tears and cartilage loss in patients with mechanical symptoms of the hip: evaluation using MR arthrography. Osteoarthritis Cartilage. 2007;15(8):909‐917. [DOI] [PubMed] [Google Scholar]
- 122. Kijowski R, Stanton P, Fine J, De Smet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238(3):943‐949. [DOI] [PubMed] [Google Scholar]
- 123. Hofmann S, Kramer J, Vakil‐Adli A, Aigner N, Breitenseher M. Painful bone marrow edema of the knee: differential diagnosis and therapeutic concepts. Orthop Clin North Am. 2004;35(3):321‐333. [DOI] [PubMed] [Google Scholar]
- 124. Andrews CL. From the RSNA refresher courses. Radiological Society of North America. Evaluation of the marrow space in the adult hip. Radiographics. 2000;20:S27‐S42. Spec No. [DOI] [PubMed] [Google Scholar]
- 125. Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non‐cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl A):A87‐94. [DOI] [PubMed] [Google Scholar]
- 126. Everhart JS, Abouljoud MM, Flanigan DC. Role of full‐thickness cartilage defects in knee osteoarthritis (OA) incidence and progression: data from the OA Initiative. Journal of Orthopaedic Research®. 2019;37(1):77‐83. [DOI] [PubMed] [Google Scholar]
- 127. Sanders TL, Pareek A, Obey MR, et al. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16‐year follow‐up. Am J Sports Med. 2017;45(8):1799‐1805. [DOI] [PubMed] [Google Scholar]
- 128. Savage‐Elliott I, Smyth NA, Deyer TW, et al. Magnetic resonance imaging evidence of postoperative cyst formation does not appear to affect clinical outcomes after autologous osteochondral transplantation of the talus. Arthroscopy. 2016;32(9):1846‐1854. [DOI] [PubMed] [Google Scholar]
- 129. Smyth N, Savage‐Elliott I, Deyer T, Murawski C, Do H, Kennedy JG. Post‐operative cyst formation after autologous osteochondral transplanation in the talus: an MRI evaluation (SS‐45). Arthroscopy. 2013;29(6):e22. [Google Scholar]
- 130. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small‐diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209‐219. [DOI] [PubMed] [Google Scholar]
- 131. Jung M, Breusch S, Daecke W, Gotterbarm T. The effect of defect localization on spontaneous repair of osteochondral defects in a Göttingen minipig model: a retrospective analysis of the medial patellar groove versus the medial femoral condyle. Lab Anim. 2009;43(2):191‐197. [DOI] [PubMed] [Google Scholar]
- 132. Anders S, Volz M, Frick H, Gellissen J. Suppl 4: a randomized, controlled trial comparing autologous matrix‐induced chondrogenesis (AMIC®) to microfracture: analysis of 1‐and 2‐year follow‐up data of 2 centers. The open orthopaedics journal. 2013;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix‐induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1):222‐231. [DOI] [PubMed] [Google Scholar]
- 134. Maréchal M, Van Hauwermeiren H, Neys J, Vanderlinden G, Van de Putte T. In vivo evaluation of different surgical procedures for autologous chondrocyte implantation. Cartilage. 2013;4(1):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hurst JM, Steadman JR, O'Brien L, Rodkey WG, Briggs KK. Rehabilitation following microfracture for chondral injury in the knee. Clin Sports Med. 2010;29(2):257‐265. [DOI] [PubMed] [Google Scholar]
- 136. Kokkonen HT, Suomalainen JS, Joukainen A, et al. In vivo diagnostics of human knee cartilage lesions using delayed CBCT arthrography. J Orthop Res. 2014;32(3):403‐412. [DOI] [PubMed] [Google Scholar]
- 137. Lui TH. Arthroscopic bone grafting of talar bone cyst using posterior ankle arthroscopy. J Foot Ankle Surg. 2013;52(4):529‐532. [DOI] [PubMed] [Google Scholar]


