Abstract
Coronavirus pandemic has created havoc in the world. COVID-19 is now officially labeled as Severe Acute Respiratory Syndrome-related Coronavirus-SARS-CoV-2. Therefore, it is equally important to combat the virus both inside the human body as well as in the environment. These viruses, being RNA viruses, are found to be susceptible to ozone. Ozone being an unstable molecule can breakup into its split products namely reactive oxygen species and ozonides creating a toxic environment for these viruses. Ozone mainly prevents the membrane fusion with the host cell, thus interfering with their replication. With vast applications of the gas, it has created a new spark in the field of medicine in combating these viruses and many other organisms. In this context, this article provides insights from recent clinical and research studies on the problems and possibilities in employing the ozone to combat the coronaviruses.
Keywords: Coronavirus, COVID-19, SARS-coV-2, Ozone therapy, Anti-viral therapeutics
Graphical abstract
1. Introduction
Coronaviruses, the reason for this pandemic, are RNA viruses which are mainly spread by human to human contact by respiratory droplets generated via coughing, sneezing and many other sources as well (Harapan et al., 2020, Coccia, 2020a, Wang et al., 2020b, Rubio-Romero et al., 2020). Typically, these viruses generally do not travel over 6 ft and do not remain much in the air, however some reports also suggest that they remain viable on many inanimate objects up to several hours (Harapan et al., 2020). The penetration capacity of ozone is quantified to be more than that of most of the liquids used as cleansing agents (used to prevent infections) (de Wit et al., 2016). Ozone causes peroxidation of the infected cells and also damages the viral capsid. It thus can disrupt its reproductive cycle. Meanwhile, the infected cells, with peroxidized weak enzyme coatings (which have already succumbed to the virus), eventually get exposed to oxidation in the cellular apparatus of the host and finally get eliminated during the process. They are then replaced by the healthy cells in the body. Ozone can be toxic to lungs and eyes depending on the concentration, temperature, humidity and exposure time, which can result in cough and irritation in the throat (Di Paolo et al., 2004). Furthermore, pulmonary edema can also be caused by high concentrations in bronchial mucosa and pneumocytes (Di Paolo et al., 2004). A concentration of 0.02 g/mL can be fatal and cause death in 4 h (Di Paolo et al., 2004). Blood of elderly and immune compromised may need administration of fortified reducing agents before administration of ozone (Bocci, 1992).
Coronavirus is viable on inanimate surfaces for 9 days, are temperature dependent and can be easily inactivated by oxidation processes induced by oxidizing agents (Rowen and Robins, 2020). It has been proposed by Coccia that high concentration of air pollutants and low wind speed allows longer persistence of viral particles in air. Density of population in a locality and respiratory disorders people suffer from, also plays a major role in spread of infection (Coccia, 2020b). Further, according to this study, the ozonides, which are the byproduct of ozone are themselves potent oxidants with long half-life and can help in degrading viral cysteine residues (Li, 2016). To this end, this review essentially characterizes the suitability of ozone in combating the corona viruses in the perspective of treating the real time COVID-19 patients, which are presented as cases studies and other possible uses establishing the role of ozone in combating the coronaviruses in environment. To begin with, the chemical framework of the virus and the role played by each of its structural components in establishing the disease in humans is discussed. Notably, the ACE2 receptors in humans play important role to get infected with the disease by binding to the spike proteins present in the lungs, respiratory system, which becomes the main target for its pathology (Li, 2016). The TMPRSS211, a serine protease, also plays a vital role in priming the S protein, which is the initiator of disease transmission. Moreover, many cytokines such as IL-6, G-CSF also promote the pathology and are further assisted by many signaling pathways such as nuclear factor kappa beta and JAK-STAT pathways. Similarly, the VEGF and EPO also promote the neoangiogenesis and atherogenesis, and resulting in cardiovascular complications (Zhang et al., 2020). To combat this, it is found that the ozone when given by autohaemotherapy, it dilutes in blood and forms lipid oxidation products and ozonides, which activate many signaling pathways like hypoxia inducible factor 1 – alpha and nuclear factor kappa beta (Zhang et al., 2020). Thereby, it activates many cytokines, glycolytic pathways to enhance ATP, enzymes such as superoxide dismutase and glutathione peroxidase to enhance the intracellular killing of the infected cells. Furthermore, the use of ozone in many conditions including peripheral vascular diseases and neurodegenerative conditions may be of use for future research.
2. Overview of corona viruses
2.1. Structure
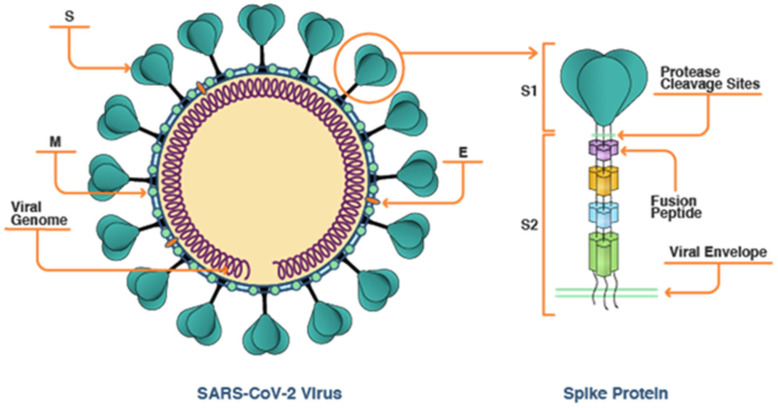
Coronavirus is a positive-sense, single-stranded RNA virus of 27–32 kb in size (Li, 2016), with club-like spikes giving them crown like appearance and a large RNA genome in a helical capsid formed by nucleocapsid protein (N) (Rowen and Robins, 2020, Li, 2016, Cynthia et al., 2020). They are enveloped, pleotropic viruses (Weiss and Navas-Martin, 2005) and found to have cysteine in their membranes essential for membrane fusion (Rowen and Robins, 2020, Cynthia et al., 2020). They belong to the family Coronavirinae subfamily Coronaviridae (Fehr and Perlman, 2015). The virus has mainly 4 structural proteins, known as spike (S), membrane (M), envelope (E) and nucleocapsid proteins (Fig. 1). The M protein and E protein are involved in viral assembly and spike protein is involved in allowing viral entry into the host cells, has a range of viral host, tissue-tropism and is a main stimulator of the immune responses in the host (Li, 2016). Spike protein has 3 segments; a short intracellular tail, a single-pass transmembrane anchor, and a large ectodomain. The ectodomain is composed of receptor-binding subunit (S1) and membrane fusion subunit (S2). S1 subunit binds to the receptor on the host cell surface during the process of viral attachment and a S2 subunit fuses the viral to the host cell membrane (Li, 2016, Catanzaro et al., 2020, Tizaoui, 2020). The envelope also contains arachidonic acid, linoleic acid (important for the disease pathology), palmitic acid, oleic acid and glutamic acid. They also have glycoproteins in the Spike component and are further accompanied by heterogeneous N-linked glycans, which act as the pivotal focus for the action of antibodies (Tizaoui, 2020). Coronaviruses have many sub classifications, among them; SARS Cov and HCov have found their way to the humans. The other subclasses are found to affect many other animals like turkeys, pigs and so on (Weiss and Navas-Martin, 2005).
Fig. 1.
Structure of viral spike protein [https://www.cas.org/blog/covid-19-spike-protein].
2.2. Classification and functions
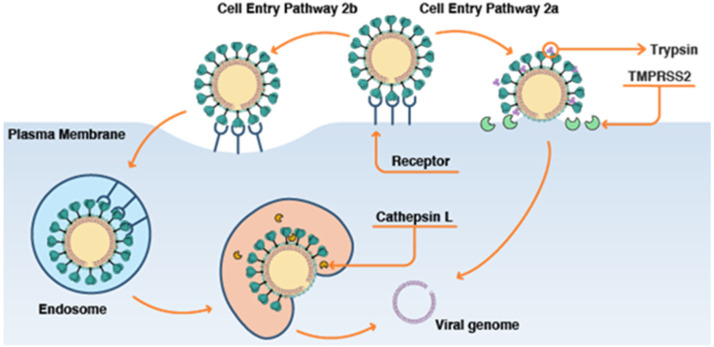
Coronaviruses are mainly classified into 4 classes - , , , subtypes. coronaviruses are further classified into SARS and SARS-CoV, Middle Eastern Respiratory Syndrome virus (MERS and MERS CoV) and COVID-19 causative agent SARS CoV-2. These SARS CoV-2 viruses contain many structural proteins of which the glucosylated spike (S) protein induces the immune response system of the host. S protein binds to a protein receptor known as the Angiotensin Converting Enzyme 2 (ACE 2) situated on the surface-membrane of the host cells mainly in the pulmonary tissues. This binding has a higher affinity when compared to the binding of SARS CoV and hence has higher transmissibility and contagiousness. The process f invasion also requires spike (S) protein priming, which is mainly carried out by host cell which produces serine protease TMPRSS211. The viral-genome has many other non-structural proteins like RNA — papain like protease (PLpro), coronavirus main protease (3CLpro) and dependent RNA polymerase (RdRp). Once the virus invades, it gains entry into the host cell as a single-stranded, positive sense-RNA. After gaining entry into the cytosol, the virus starts replicating inside the cell by using replicase (Fehr and Perlman, 2015). This is followed by translation of viral polyproteins by using host cell protein translator machinery (Fig. 2). The product of translation is then sliced into effector-proteins by viral proteinases 3CLpro and PLpro. PLpro then deubiquitinises particular host cell proteins like INF factor 3 and NF- and causes host immune-suppression. RdRp synthesize full-length negative stranded RNA template to make more viral genome, thus helping in viral replication (Li, 2016, Cynthia et al., 2020, Fehr and Perlman, 2015).
Fig. 2.
Pathways of SARS-CoV-2 [https://www.cas.org/blog/covid-19-spike-protein].
These viruses are also known for homologous and non-homologous recombination (Fehr and Perlman, 2015). Assembly and packaging inside the cell results in the formation of new viruses, ready to be released out and infect other cells. The viruses get released in the form of vesicles and get transported to the neighboring cells, but the S protein does not get incorporated in to the virus particle, making it going undetected by the virus-specific antibodies and helps in the creation of multinucleated giant cells (Fehr and Perlman, 2015).
2.3. Disease pathology
Coronaviruses mainly attack the immune system by stimulating a cytokine storm (hypercytokinemia), which leads to plasma leakage, vascular permeability and disseminated intravascular coagulation. This cytokine storm results in high plasma levels of IL-1, IL-1ra, IL-7, IL-8, IL-9, IL-10, basic FGF, G-CSF, GM-CSF, IFN-, IP-10, MCP-1, MIP-1, MIP-1, PDGF, TNF , VEGF which injure the lung tissue. Hence the uncontrolled release of cytokines results in a ‘cytokine storm’ resembling Systemic Inflammatory Response Syndrome coupled with additional viral and bacterial infections because of dysregulated immunity, finally resulting in multiorgan failure and death (Catanzaro et al., 2020).
ACE2 is mainly expressed in alveolar epithelial type II cells to facilitate replication of coronaviruses. They are also found in extra-pulmonary sites like heart, kidney, endothelium, and intestine (intestinal epithelial cells as a co-receptor for nutrient uptake) (Coccia, 2020b). The binding of viral spike protein to host cells by ACE2 receptor triggers the detection of viral RNAs as Pathogen Associated Molecular Patterns (PAMPs) through pattern recognition receptors. These receptors belong to a family of Toll like receptors. This recognition happens through endosomal RNA receptors like TLR3 and TLR7/8 and cytosolic RNA sensor, retinoic acid-inducible gene (RIG-1)/MDA5. These TLRs activate signaling pathways in human CD 14+ monocytes and CD4+ T cells which include (a) IFN regulatory factor-3 which stimulate cytokines and chemokines, (b) Nuclear factor and (c) JAK (Janus Kinase) STAT (Signal Transducer and Activator of Transcription). Nuclear factor is normally inhibited by inhibitory proteins like I s. SARS CoV2 causes degradation of I , thus activating NF . NF further causes activation of pro-inflammatory cytokines, chemokines, stress-response proteins and anti-apoptotic proteins. NF is also activated by IL-6 and TNF (Catanzaro et al., 2020).
JAK-STAT Pathway: Membrane bound IL-6 receptor and soluble form of IL-6 receptor which interacts with gp130 promotes the activation of JAK-STAT signaling. The signaling pathway is further enhanced by reactivation of IL-6. IL-6 produced by macrophages, endothelial and smooth muscle cells, promotes the secretion of cytokines like MCP-1, which induce atherogenesis further increasing the expression of cell adhesion molecules and stimulating the proliferation and migration of vascular smooth muscle cells. Inflamed cells further increase expression of ATII receptor, and its binding to ATII, which further activates IL-6 in a reciprocal manner, finally activating the JAK-STAT pathway (Catanzaro et al., 2020).
The ACE2 receptor which helps in priming of viral cells is down regulated by the binding of viral spike protein, which results in overproduction of ATII, finally causing vascular inflammation and lung injury as mentioned before. ATII/ATII receptor axis also activates NF- and ADAM17, and ADAM17 inactivates ACE2, hence amplifying ATII. ADAM17 is also known to process membrane form of IL-6 receptor , which forms soluble form SIL-6R via SIL-6R-IL-6 complex which is also mediated by gp130. This reaction further activates STAT3 which finally activate NF- in a variety of negative non-immune cells. Hence the signaling pathways interact with each other, to induce their co-activation. Further IL-6 provides a feedback loop and helps in amplifying many myeloid and lymphoid cells. ADAM17 in presence of ATII,trans-activates EGFR which stimulates the mature form of heparin to bind to EGF-like growth factor in vascular smooth cells which causes vascular remodeling (Catanzaro et al., 2020).
2.4. Symptomatology
Coronaviruses are found to be very contagious and can result in a lot of respiratory symptoms. HCoV-NL63 a strain of human coronavirus can result in acute laryngotracheitis (croup). Many coronaviruses on a long run have found to predispose to Multiple sclerosis. The COVID 19 has mainly affected men, patients with underlying comorbidities like diabetes, hypertension, cardiovascular diseases and other immune compromised states. Median age was found to be around 49 years, but in general any individual above 60 years have been found to be at risk for the infection (Harapan et al., 2020, Chaolin et al., 2020).
Common symptoms were found to be fever, cough, and myalgia or fatigue; less common symptoms were sputum production, headache, hemoptysis, and diarrhea. Dyspnea and lymphopenia were also found to be present in few patients. Most patients were found to have pneumonia picture that is patchy opacity to ground glass appearance on CT chest (Harapan et al., 2020, Chaolin et al., 2020). Imaging suggests that the virus commonly affects lower lobes in their peripheral location bilaterally (Harapan et al., 2020). The complications encountered include acute respiratory distress syndrome, acute cardiac injury and secondary infection. When a comparison was made between non-ICU and ICU patients, the later had higher plasma levels of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNF (Chaolin et al., 2020).
The diagnosis of coronavirus is based on antigen detection of specific strain of coronavirus through the nasopharyngeal swab, and the genetic material RNA is mainly detected via blood and urine (Harapan et al., 2020). Although no specific treatment measures are available so far. However, - Favipiravir, Lopinavir–Ritonavir, Ribavirin and Interferon have shown to provide some relief to few patients with elderly patients requiring ventilators, once diagnosed with the disease (Harapan et al., 2020, Chaolin et al., 2020). Additionally, Remdesivir and hydroxychloroquine have also been found to show potent antiviral activity recently (Harapan et al., 2020, Cynthia et al., 2020).
3. Overview of ozone (O)
3.1. Chemistry of ozone
Ozone is a gas composed of trioxygen and is unstable molecule due to its mesomeric states. It is acrid in odor, a colorless-transparent gas, explosive in liquid and solid form, has a half-life of 40 min at 20 °C and about 140 min at 0 °C. Ozone occurs at less than 20 g/m3 from the surface of the earth, and mainly provides protection against UV radiation coming from the sunrays (Di Paolo et al., 2004). The medical grade ozone is composed of pure and pure in the ratio of 0.1%–5% and 95–99.5% respectively (Rowen and Robins, 2020, Komali, 2012). Ozone can be produced by one of the three means by ultra-violet system (produces only low amount of ), by cold plasma system and by corona discharge system (produces high amount of ) (Komali, 2012, Elvis and Ekta, 2011). Ozone has high affinity for double covalent bonds like C—C, which are present in polyunsaturated fatty acids that are carried by albumin molecules. Hence these results in creation of ozone molecules with longer half life called ozonides — ROS and lipid oxidation products like peroxides, hydroperoxides and aldehydes. They act as messengers of biochemical and immunomodulatory effects of ozone (Hernández et al., 2020).
3.2. Medical uses of ozone
Ozonized water is used as a spray or a compress; and used in dental medicine, geriatric developments, circulatory disorders, viral diseases, macular degeneration, rheumatism/arthritis, infected-wounds, AIDS, SARS and cancer. It also decreases the blood cholesterol, has antioxidant responses, used in treatment of hypoxic, it modifies the oxygenation in resting muscle and used in complementary treatment of ischemic syndromes (Elvis and Ekta, 2011). It has been shown that a single subcutaneous injection of ozone in mouse with spared nerve injury of sciatic nerve reduces neuropathic pain (Elvis and Ekta, 2011).
(a) Peripheral obstructive arterial diseases: Ozone induces glycolysis to increase ATP and 2, 3-DPG. This causes a shift in the sigmoidal O2 – binding curve of hemoglobin to right in turn resulting in release of O2 in ischemic tissues. This results in conversion of H2O2 to H2O in RBC in presence of GSH. This reaction is mainly responsible for healing ischemic tissues and has been shown that even stage IV of the disease, where there is necrosis of toes and unbearable pain can be delayed by ozonated AHT in 50%. Ozone also stimulates HO-1, HSP70 and Nrf2 which induce antioxidants, nuclear transcription factor and hypoxia inducible factor - 1 (HIF – 1). This induces VEGF, EPO and glycolytic enzymes to promote cell proliferation and survival. VEGF induces neo-angiogenesis to increase the blood flow which further increases EPO to enhance oxygen delivery. Moreover the glycolytic enzymes promote metabolism of glucose to increase the biosynthesis of NO and CO which also increase the blood flow and oxygen delivery to hypoxic tissues (Sagai and Bocci, 2011).
(b) Age-related macular degeneration: The condition is mainly caused by death of photoreceptors in fovea centralis and of pigmented retinal epithelium to cause chronic hypoxia. Ozone (AHT) helps by increasing the delivery of oxygen to retina (Sagai and Bocci, 2011).
(c) Diabetes mellitus: Ozone is found to reduce HbA1C, CRP, nonenzymatic glycosylation, aldose reductase activity, advanced glycated end products and antioxidant–prooxidant balance (Sagai and Bocci, 2011).
(d) Cancer: In a study by Sweet et al. it was shown that when an experimental attempt was done to insufflate an ozone and oxygen mixture into the peritoneum, it led to higher rate of survival of treated rabbits (Sagai and Bocci, 2011).
(e) Neurodegenerative diseases: Nrf2 and HIF - 1 induced by ozone through activation of HO-1 play an important role in neuroprotection and suppression of neuro-inflammation in neurodegenerative diseases (Sagai and Bocci, 2011).
(f) Skin and mucosal infections: Ozone is used in diabetic foot, ischemic ulcers, necrosis, decubitus, abscesses, anal fissures, fistulae, aphthous stomatitis, osteomyelitis, stomatitis, vulvovaginitis and onychomycosis (Sagai and Bocci, 2011).
(g) Backache and orthopedic diseases: when 30–35 g/ml of ozone is directly administered into the center of intersomatic space corresponding to disc herniation, it dissolves in the water of nucleus pulposus and reacts with macromolecular glycoproteins composed of carbohydrates and polypeptide chains (proteoglycans, collagen II and IV). This generates OH which reabsorbs hydrolytic products and free water, thus causing shrinkage of nucleus and disappearance of herniated material (Sagai and Bocci, 2011).
3.3. Mechanism of ozone in killing the viruses
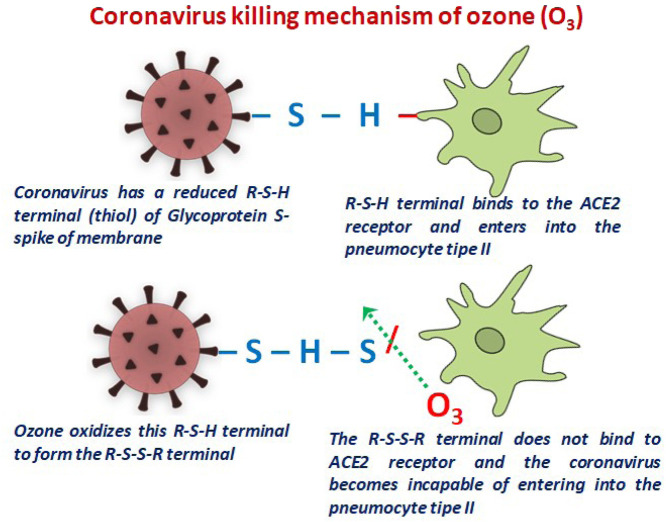
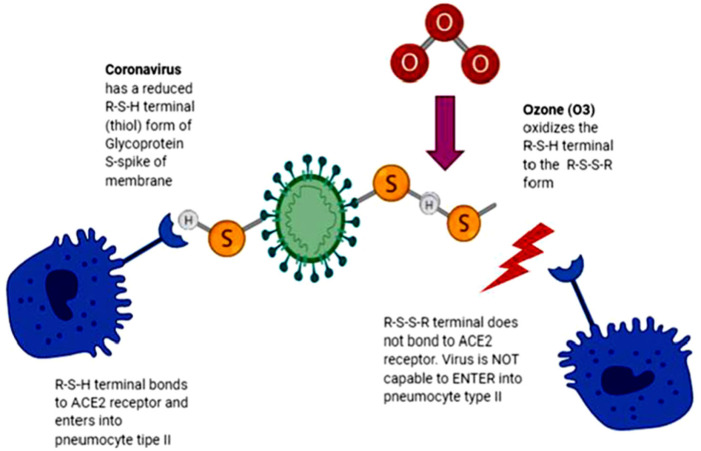
Ozone disrupts viral proteins, lipoproteins, lipids, glycolipids, or glycoproteins, making them susceptible to oxidizing effect and participates in redox reactions. It also damages the viral envelope glycoproteins and peplomers, which are essential for the attachment of virus to host receptors. Coronaviruses contain cysteine residues in their spike and envelope proteins (Rowen and Robins, 2020). These cysteine residues are made of sulfhydryl groups called thiol groups (R-SH). These thiol groups in a reduced state in the virus play a pivotal role in the viral entry and viral membrane fusion with the host cell membrane as described in the viral structure. Hence, when ozone reacts with these thiol groups/sulfhydryl groups, it oxidizes them into to sulphonic acid residues (R-SO3-H) as shown in Fig. 3 (Fernández-Cuadros et al., 2020). This process can be enhanced at higher temperatures. Ozone by altering these cysteine residues decreases the growth of the virus by 2 log lower than the wild type virus (Rowen and Robins, 2020). In addition, coronaviruses have a cysteine dependent papain (a cysteine dependent protein enzyme) which is oxidized and degraded to sulfonate/sulphonic acid.
Fig. 3.
Mechanism of ozone in preventing the binding of S-spike protein residues to the ACE2 receptor (Fernández-Cuadros et al., 2020).
The spike proteins also have tryptophan which can be degraded by ozone (Rowen and Robins, 2020, Fernández-Cuadros et al., 2020). Ozone incorporation into the serum of the human body produces lipid and protein peroxides which have antiviral effects. Ozone also alters viral envelope and viral genome significantly enough resulting in nonpathogenic dysfunctional viruses. These attenuated viruses help in evoking a unique immunological response to various strains of a particular virus creating a host-specific auto vaccine (de Wit et al., 2016, Bocci, 1992). Besides the antibodies produced in the body to respond to any virus itself, it produces many highly active forms of ozone. Therefore, the addition of ozone essentially enhances the antigen–antibody response against viral infections in the host (Di Paolo et al., 2004).
3.4. Mechanism of therapeutic actions of ozone
In blood, ozone combines and gets dissolved in the plasma propagating the decomposition of the gas into a variety of reactive oxygen species like superoxide anion (), hydrogen peroxide (HO2) and hydroxyl radical (OH) ions (Di Paolo et al., 2004, Elvis and Ekta, 2011). Moreover, the peroxidation of plasma lipids results in formation of Lipid Oxidation Products (LOPS) (Di Paolo et al., 2004). The above two mechanisms mainly help in enhancing the defense mechanisms (Di Paolo et al., 2004, Azuma et al., 2014). Ozone activates cytoplasmic transcription factors by secondary messengers like (a) hypoxia-inducible factor 1- (HIF-1), (b) Nuclear factor kappa beta (NF- ) and (c) Nuclear factor erythroid – 2 – related factor 2 (Nrf2) (Hernández et al., 2020).
In addition to the above, there is also production of hyper soluble antioxidants (Di Paolo et al., 2004) and ozonides (Rowen and Robins, 2020). The hydrogen peroxide produced by oxidizing effect of ozone mainly results in shifting the hemoglobin dissociation curve in the RBCs to the right and releasing oxygen, inducing glycolysis which increase ATP, thus reducing hypoxia (Rowen and Robins, 2020, Hernández et al., 2020). They also cause production of interleukins (IL-6), interferon gamma (antiviral action) (Rowen and Robins, 2020), TNF, TGF, nitrogen oxide in the leukocytes, release of growth factors in platelets, and propagates the antioxidant effect in all cells (Di Paolo et al., 2004, Bocci, 1992). Ozone also stimulates Kreb’s cycle enhancing the oxidation carboxylation of pyruvate to produce ATP, reduces the amount of NADH, thus promoting the oxidation of cytochrome C, production of a vasodilator and prostacycline, activation of glutathione peroxide, catalase and superoxide dismutase (Rowen and Robins, 2020, Elvis and Ekta, 2011).
Ozone also activates the macrophages and neutrophils, accelerates the manifestation of major histocompatibility complex (MHC) Class I and II on diseased cells, lymphocytes and monocytes, and is also responsible for the improvement of cyto-toxicity either antibody-mediated or MHC-inhibited, or via natural killer (NK) cells enhancing the immune responses against many micro-organisms including viruses (Bocci, 1992). Ozone by activating H2O2 in neutrophils and lymphocytes causes activation of tyrosine kinase which phosphorylates NF- , thus activating cytokines (Hernández et al., 2020). Effect of ozone on lung tissue can be varied. Ozone can increase specific and mean airway resistance and respiratory rate, and also reduce the maximal trans-pulmonary pressure and tidal volume (Elvis and Ekta, 2011). Ozone when given at appropriate dosage levels, mainly adds on to the preexisting oxidative stress in the body thus reaching the calculated dosage necessary to combat the infections. It is thus said to have a precise therapeutic window (Di Paolo et al., 2004). Therefore, very low doses are not effective as they are masked by the existing antioxidants and very high doses can be toxic mainly to the pulmonary system. In terms of atherosclerosis, chronic viral infections, neurodegenerative diseases, tumor growth and aging; ozone administration can actually balance and maintain the antioxidant levels in the body, while the endogenous ROS go down during chronic oxidative stresses. Furthermore, it can also slow down the process of depletion of endogenous ROS and hence a long term calculated therapy with ozone can restore their levels in the body in the above mentioned conditions (Di Paolo et al., 2004). If the period of stress continues, ozone therapy can enhance the production of existing heat-shock proteins (HSP), glucose-regulated proteins (GRP) and oxidative shock proteins (OSP) (Di Paolo et al., 2004). - acts on nitric oxide and iNOS signaling pathways. NO inhibits palmitoylation of S (spike) protein of SARS CoV2, hence prevents the binding of S protein to ACE2 receptor primarily in lung tissue (Franzini et al., 2020).
Ozone therapy (OT) utilizes around 1 to 5% ozone in 95 to 99% trioxygen as a gas (around 10 to 70 mcg ozone/cc gas). This blend is called “medical ozone” (Rowen and Robins, 2020). It is suggested that ozone in the form of autohemotherapy can combat infections (de Wit et al., 2016, Di Paolo et al., 2004), where the minor ozone autohemotherapy and major ozone autohemotherapy involves the use of 5 to 10 mL and 200 to 250 mL of blood respectively as described by Wolff in 1974. The process is relatively straightforward: first, blood will be collected in a glass having sodium citrate of heparin, kept in vicinity of mixture at concentrations varying from 15 to 80 g/mL for around 5 to 10 min and then reinfused into the intend patients. This is typically carried out two times a week for about 7 to 8 weeks. Besides being used in various vascular and cardiovascular diseases, it is found to be effective in chronic viral infections like Herpes Zoster, Hepatitis, Herpes I and II, Acinetobacterbaumanni, C.difficile, MRSA (Elvis and Ekta, 2011) and also in fungal and bacterial infections (Di Paolo et al., 2004, Rowen and Robins, 2020). It is also seen that activation of immunity occurs at 30–55 g/cc (Elvis and Ekta, 2011).
Ozone is also said to have anti-hypoxic effects wherein, (i) it improves the transportation of oxygen and in cellular metabolism like glycolysis, Krebs cycle, (ii) it induces negative charge in RBCs increasing the blood cell elasticity and (iii) it improves the metabolism of inflamed tissues by increasing their oxygenation and reduces local inflammatory processes (Gupta and Mansi, 2012). It also increases the synthesis of ribosomes, and mitochondria in the cells thus enhancing aerobic respiration and enhances the protein anabolism. Ozone is also known for the increased production of NO which helps in vasodilation of arterioles and veins and also in angiogenesis (Gupta and Mansi, 2012).
3.5. Proposed dosage of ozone and related evidences
Ozone is not toxic when it is given at concentrations of 0.05 ppm for a continuous period of 8 h (Gupta and Mansi, 2012). Ozone is found to play a major role in the field of endodontics and prosthodontics. In a study conducted by Komali (2012), ozone was used as oil to lubricate, and irrigate the canals of the teeth, and was later used at a concentration of 30 ml to slowly insufflate the canals for 45 to 60 s This resulted in activation of fibroblasts and increased the activity of healing. In another study by the same author, the gram positive and gram negative bacteria were killed by 0.5–4 mg/l of ozonized water in the oral mucosa (Komali, 2012). The study also states that using 20 mg/h of ozone reduces the Methicillin-resistant Staphylococcus aureus (MRSA) and E-coli T1 phage virus bacteria from 3.1 10(3) CFU/mL to 1.0 10(0) CFU/mL in 10 min later during the oral activity (Komali, 2012).
4. Case studies
4.1. Ozone gas as a virus decontaminating agent
Hudson et al. (2009) have developed an ozone gas-based portable apparatus to decontaminate the buildings such as the rooms in health-care facilities, hotels, etc using the anti-viral properties of ozone. They tested against 12 viruses, which include influenza, strain H3N2, etc., and including the mouse coronavirus (MCV) in DBT cells. They observed that at the peak ozone gas concentration of 20–25 ppm, it required only a short-period of high-humidity (relatively humidity of 90%) to reach the maximum anti-viral efficacy.
Furthermore, their experiment also emphasized that the nature of surface on which the samples containing the inoculum was placed did not affect the result. Interestingly, the materials like plastics, glass, cotton, stainless steel and fabrics showed the same level of inactivation. The study also highlighted that the size of the inoculum, degree of dilution of virus, and presence of any cellular debris also did not influence the anti-viral efficacies. In addition, the presence of human blood, tissues and corpses were also proven not to alter the degree of inactivation of viruses. This study also showed that when specific amounts of virus were sprayed in 2 chambers with and without ozone gas, 99% of viruses were inactivated in the chamber sprayed with ozone gas. They also highlighted that there was not even a single virus which was resistant to ozone. Finally, they also demonstrated the removal of ozone from the environment using a built-in catalytic converter equipped with the apparatus.
4.2. Ozone therapy as a novel treatment for “conventionally untreatable” viral illnesses
Rowen and Robins (2020) have proposed the use of ozone on treating the coronavirus in the context that the coronaviruses are abundance in cysteine, which must not be damaged for effective viral properties. However, the sulfhydryl functional groups are susceptible to oxidation and the therapy can make use of this vulnerability to deactivate the virus. They have highlighted that ozone therapy could assist the human system in deactivating the thiols in viruses in tissues and in blood by creating more oxidized environment. Notably, the spike protein of coronavirus is also abundance in tryptophan (Broer et al., 2006), which is vulnerable to oxidation (Virender and Nigel, 2010) next to cysteine.
Further, in this report they have mentioned that how ozone was used to treat patients infected with Ebola epidemic (dated back in October 2014), where they employed the Directly Administered Oxygen/Ozone gas Intravenously (DIV) using ozone of concentrations of 30–55 mcg of ozone/cc in a quantity of 20 cc and administered it to 5 patients on board over few min and found that all the 5 patients survived the attack of the virus. They mentioned that the treatment required an ozone generator, which could be run on car battery, medical-grade compressed , a syringe (and butterfly-type needle for the DIV technique). However, this method was found to cause few side effects including transient chest tightness, cough and irritation of veins. Nevertheless this method carried negligible cost and produced low waste materials in comparison to the autohaemotherapy and could be effectively employed during epidemics.
4.3. A cohort study on ozone therapy for SARS-CoV-2 pneumonia patients
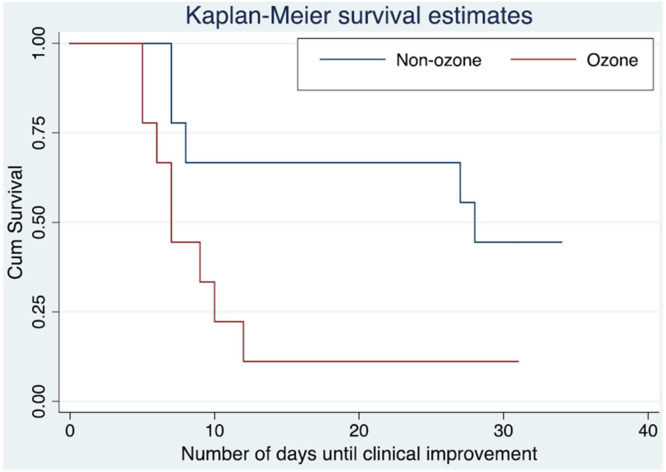
Hernandez et al. (2020) have conducted cohort study on 18 COVID-19 confirmed patients with severe pneumonia. The mean age of the patients was 68 years with SD of 15 years, and 72.2% (n 13) of the patients were male. Out of 18 patients, the ozonated autohemotherapy was given to 9 patients (50%). In the study, they observed that in an unadjusted comparison, the ozonated autohemotherapy required significantly shorter time to achieve the clinical improvement with higher proportion of patients, who achieved clinical improvements in 14 days. Similarly, in risk adjusted studies, it required shorter mean time to achieve the clinical improvement (Fig. 4).
Fig. 4.
Kaplan–Meier survival curves (Hernandez et al., 2020).
Notably, the following were highlighted as limitations of this study; small sample size, wide adjusted estimates of 95% Cls, possibility of unmeasured residuals, requires external validity. On the other hand, the key strengths of the study were considered as its observations on the real world COVID-19 patients and the use of regression modeling for the primary clinical outcome and risk-adjustments analyses, where it is claimed to provide new data denoting to the effective contribution of ozonated-autohaemotherapy towards treating severe COVID-19 pneumonia.
4.4. Cytoprotective properties of ozone therapy in treating the COVID-19
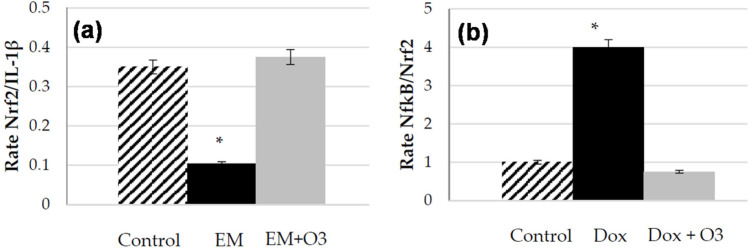
The report by Martínez-Sánchez et al. (2020) highlights that the action mechanism of ozone involves the modulation of as the Keap1/Nrf2/ARE pathways and the reduction of IL-6 and IL-1. In such process, as the homeostasis of free radicals and antioxidant balance are associated with the modulation (typically known as the ozone therapy), it can induce the cytoprotective effect which can be effectively exploited to treat the clinical conditions caused by SARS-CoV/COVID-19 (Fig. 5(a)–(b)). It has also been highlighted that the underlying principles and action mechanisms of cytoprotective activity of ozone therapy have been demonstrated clinically with other viruses and therefore future clinical studies are highly needed to validate and establish the potential use of ozone therapy in treating COVID-19.
Fig. 5.
(a) Nrf2/IL-1 as biomarkers of balance Nrf2/NF-B pathway activation after and before treatment [Control-healthy volunteers; EM-Multiple sclerosis; EM+O3-Multiple sclerosis treated with O2/O3], (b) Rate of the fold change values of NF-B/Nrf2 as index of balance NF-B/Nrf2 pathway activation with and without treatment [Control-Cardiomyocytes culture; DoX-cells plus doxorubicin; DoX+3- cell treated with doxorubicin (100 nM) and ozone] (Martínez-Sánchez et al., 2020).
4.5. Ozone therapy-major autohemotherapy (MAH): A preliminary evaluation
Study 1Zheng et al. (2020) have demonstrated their experience in treating two patients confirmed with COVID-19 by ozone therapy called major autohemotherapy (MAH). In this treatment, the MAH was given to two patients (two males of 53 and 66 years old) once on a daily basis consequently for 7 days. In each turn, 100 mL of venous blood was taken and blended with ozone gas at 1:1 ratio of to blood volume, with the end-concentration of being 20 g/mL. Upon the MAH-treatment, their symptoms disappeared rapidly and started recovering. It was observed that as compared to the baseline chest-imaging, the subsequent serial CT-scans exhibited progressively absorbed bilateral lung lesions. Later, the patients were discharged after meeting the necessary various criteria. Further, no adverse effects associated with MAH-treatment were observed and reported. In addition to this, they also highlighted that these two results were compared with two other cases of similar age and illness without subjecting to MAH-treatment, where they realized that the patients without MAH required extended duration of viral disinfection and treatment. They concluded that the ozone therapy-MAH may be a promising modality in treating and limiting the COVID-19 infections and further clinical studies are needed to evaluate the parameters such as dosage, effectiveness and duration of treatment. Notably, this clinical study was later approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (WDRY2020-K020).
Study 2: In a study conducted by Franzini et al. (2020) 78 positive elderly patients were considered. Among them many were also suffering from ARDS and interstitial pneumonia. O2– treatment was given to all these patients and their lab parameters were drawn and subjected to analysis. It was observed that there was a major reduction in inflammation burst (CRP 30%, IL-6 25%), a reduction in D-dimer (35%) and an elevation in pulmonary function indicated by O saturation% 10%, and PaO2/FiO2 6%. Ozone was delivered to these patients by withdrawing 200 ml of blood from patients, then they were collected in a CE certified SANO3 bag, treated with 45 g/ml of O2– mixture and then introduced into patient’s blood. This autohaemotherapy reported 0.7 adverse events for every 100,000 treatments. The treatment lasted for 30 min and was also found to be cost-effective. This treatment lasted from 5–5 cycles with 100-200 ml of O2– once a day for 5 consecutive days (dosage: 45 g/ml). Reduction was observed in inflammatory parameters like pro-calcitonin, PCT and fasting glucose. The greatest variability was seen with respect to serum lactate (mean: −8.220% 39.91SD) and PaO2/FiO2 (mean: +13.812% 58.897SD). In the same study patients having aortic dissection were also studied, in whom an increase in PO2 and SatO2 were observed with ozone concentrations of 40 g/ml, 80 g/ml and 160 g/ml and hence concluded that ozone does not harm erythrocytes (Franzini et al., 2020).
4.6. Intravenous ozonated saline: A novel therapy for COVID-19
Among many adjunctive ozone therapies for COVID-19, one of the recognized mechanisms is to improve the oxygenation of blood/tissue to avert the failures in multi-organ due to hypoxia. In this direction, Thorp et al. (2020) have examined the effect of ozonated-saline, which administered intravenously, on the oxygenation in human body. The obtained results showed that the intravenously administered ozonated-saline improved the oxygenation of blood/tissues and extended the ability of person to stay in a hypoxic-environment.
4.7. Ozone as a non-drug therapy
Wang et al. (2020a) have proposed non-drug therapy based techniques using light, thermal and gas-phase sources to combat the coronaviruses. Their proposal includes the following three techniques; (i) A new ventilator based on thermal and micro ozone, (ii) A treatment cabin based on far-infrared (IR), thermal-oxygen, thermal-air and thermal-micro ozone and (iii) Cold plasma physical treatment based on the pulse plasma to produce micro ozone to be inhaled to kill the viruses. However, these treatment plans are still in infant state and they have to be optimized and tested before the human use. It has been highlighted that as compared with the drug-based therapies, these physical techniques are safer as they would not cause any side effects, user-friendly and cost-effective.
4.8. Ozone to combat COVID-19 in environment
Apart from the possible applications of ozone-based therapies to combat coronaviruses in human, ozone has also been demonstrated to combat the coronaviruses in the environment through the mechanisms such as face masks, Lee et al. (2020) hand sanitizers (Anon, 2020) and disinfectants to inactivate the viruses in food industries (Roberto et al., 2020).
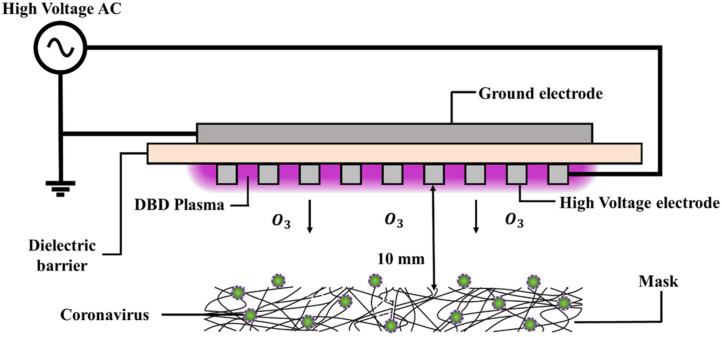
Lee et al. (2020) have demonstrated the loss of infectivity of virus to the human cell line (MRC-5) when introduced to ozone gas even for a short-period of time (1 min). Notably, in this investigation the human-coronavirus (HCoV-229E) was used instead of SARS-CoV-2 and the gas was generated via a system called dielectric barrier-discharge plasma (BDP) generator. Interestingly, the study also demonstrated that there was no functional and structural decline occurred in the face-masks even after their exposure to gas for 5 min. in this mechanism, the augmentation of HCoV-229E-RNA, as observed through reverse-transcription polymerase chain reaction, indicated that the loss of viral activity by ozone could be primarily due to the damage of envelop-proteins or viral-envelopes (Fig. 6).
Fig. 6.
Schematic-diagram describing the disinfection of a face mask contaminated by a coronavirus using ozone produced by a dielectric barrier discharge plasma generator (Lee et al., 2020).
Very recently, Samir K. Pal and his group (Anon, 2020) have developed a long lasting hand sanitizer called “nano-sanitizer” consisting of alcohol encapsulated by the ozone-impregnated peroxidized lipid layers. Apparently, the ozone-impregnated peroxidized lipid layer protects the alcohol molecules and keep them long lasting on the surface on which it is sprayed. This nano sanitizer overcomes the existing problems with typical hand sanitizers such as dehydration of skin owing to frequent-use and instant anti-microbial activity without any protective action. Thus, this nano sanitizer ensures the hygienic and convenient sanitization for durable period of time.
5. Challenges in ozone treatment
One of the major drawbacks is with respect to airway hyperactivity and inflammation, because ozone must never be inhaled. Ozone reacts with Polyunsaturated Fatty Acids (PUFA) found in lipids of alveolar lining layer as mentioned before to form Lipid Ozonation Products. In addition to this, ozone in presence of water forms hydrogen peroxide and many aldehydes. This results in activation of specific lipases like phospholipase A2/phospholipase C which produces arachidonic acid. Arachidonic acid finally produces prostaglandins, platelet activating factor via cyclooxygenases and lipoxygenases enzymes. This interaction of ozone with various biomolecules also releases 4-hydroxynonenal, lipoperoxyl radicals, malonylaldehyde, isoprastanes, and ozonide in addition to hydrogen peroxide, of which hydrogen peroxide and 4-hydroxynonenal are most toxic and activate signal transduction pathways like NF- , NO-synthase, protein kinases to enhance the release of TNF, IL-1, IL-8, IFN , and TGF 1. This enhances the production of ROS and hypochlorous acid with high inflow of neutrophils and activated macrophages, thus creating a vicious cycle (Elvis and Ekta, 2011, Sagai and Bocci, 2011).
Ozone can also form 3-hydroxy-5-oxo-5,6-secocholestan-6-al which can cause pulmonary toxicity, Alzheimer’s disease and atherosclerosis. Furthermore, the excess peroxidation can alter the membrane permeability, where the loss of functional groups can inactivate enzymes causing cell injury and cell death. Ozone can combine with NO2 to form photochemical smog, hence can damage lung alveoli. It has been stated that antioxidants vitamin C, E are both useful in nullifying the oxidant effect of ozone (Elvis and Ekta, 2011). Hence the above mentioned reactions can be prevented when mixed with human blood since ozone equilibrates with extracellular and intraerythrocytic water and then to hemoglobin to get completely oxidized (pO2 increases from 40 to 400 mmHg]. Hence it is proposed that the dosage of ozone must be 25% of potent antioxidant capacity of plasma (Sagai and Bocci, 2011).
6. Conclusion
COVID-19 is an unprecedented pandemic situation in the world. In pursuit of various treatment modalities, the drug-based therapy is considered to be more effective in all levels. However, the non-drug based approaches such as ozone () therapy could be useful in combating coronaviruses and other deadly viruses in human body as well as in environment (Valdenassi et al., 2020). There are multiple mechanisms proposed for anti-viral properties of ozone, which include (i) veridical properties, (ii) inhibition of viral-replication, (iii) oxygenation of blood and tissues, (iv) inhibition of inflammatory mediators, (v) activation of immune defenses via endogenous antioxidant, (vi) up regulation of HO-1 in endothelial cells, (vii) resisting the formation of micro-thrombus, (viii) delivery of more oxygen to tissues by stimulating the 2–3 diphosphoglycerate, (ix) promoting of immune activity through nuclear-factor and (x) cytoprotective activity (Rowen, 2019, Toon, 2020, Simonetti et al., 2019, Kahle et al., 2015). The major drawback of this therapy is considered to be its dosage, which could turn adverse upon if an optimum dosage is not administered as required by the patient. The other targets for controlling the infection include (i) spike protein based vaccine, (ii) Inhibition of transmembrane protease serine 2 (TMPRSS2) activity, (iii) blocking ACE2 receptor, and (iv) delivering excessive soluble form of ACE2 all of which requires further investigation and research. Accordingly, the meticulous and comprehensive research along with extensive clinical trials, ozone therapy can be effectively deployed across the world, even to the people below poverty line. Ozone therapy can be brought forefront with technological developments in all levels and promising to treat coronavirus and other deadly viruses in the world (Rubio-Romero et al., 2020, Coccia, 2017, Sterpetti, 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anon,, 2020, https://www.researchgate.net/publication/343127569_Active_respirator_mask_nano-sanitiser_developed_by_SNBNCBS_can_help_in_combatting_COVID_19.
- Azuma K., Mori T., Kawamoto K., Kuroda K., Tsuka T., Imagawa T., Osaki T., Itoh F., Minami S., Okamoto Y. Anti-inflammatory effects of ozonated water in an experimental mouse model. Biomed. Rep. 2014;2(671) doi: 10.3892/br.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V. Ozonization of blood for the therapy of viral diseases and immunodeficiencies. A hypothesis. Med. Hypotheses. 1992;39:30. doi: 10.1016/0306-9877(92)90136-z. [DOI] [PubMed] [Google Scholar]
- Broer R., Boson B., Spaan W., Cosset F.L., Corver J. Important role for the transmembrane domain of severe acute respiratory syndrome coronavirus spike protein during entry. J. Virol. 2006;80(1302) doi: 10.1128/JVI.80.3.1302-1310.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Therapy. 2020;5(84) doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaolin H., Yeming W., Xingwang L., Lili R., Jianping Z., Yi H., Li Z., Guohui F., Jiuyang X., Xiaoying G., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Sources of technological innovation: Radical and incremental innovation problem-driven to support competitive advantage of firms. Tech. Anal. Strateg. Manage. 2017;29(1048) [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. An index to quantify environmental risk of exposure to future epidemics of the COVID-19 and similar viral agents: Theory and practice. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynthia L., Qiongqiong Z., Yingzhu L., Linda V.G., Steve P.W., Linda J.C., Jeffrey S., Anne C.G., Angela D.D., Susan J., Dana A. Research and development on therapeutic agents and vaccines for covid-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(315) doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo N., Bocci V., Gaggiotti E. Ozone therapy. Int. J. Artif. Organ. 2004;27(168) doi: 10.1177/039139880402700303. [DOI] [PubMed] [Google Scholar]
- Elvis A.M., Ekta J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011;2(66) doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282(1) doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cuadros M.E., Albaladejo-Florín M.J., Peña Lora D., Álava-Rabasa S., Pérez-Moro O.S. SN Comprehensive Clinical Medicine 1-9. 2020. Ozone () and SARS-CoV-2: Physiological bases and their therapeutic possibilities according to covid-19 evolutionary stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini M., Valdenassi L., Ricevuti G., Chirumbolo S., Depfenhart M., Bertossi D., Tirelli U. Oxygen-ozone (O2-) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G., Mansi B. Ozone therapy in periodontics. J. Med. Life. 2012;5(59) [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., Megawati D., Hayati Z., Wagner A.L., Mudatsir M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health. 2020;13(667) doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A., Papadakos P.J., Torres A., González D.A., Vives M., Ferrando C., Baeza J. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2020;67(245) doi: 10.1016/j.redar.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A., Viñals M., Pablos A., Vilas F., Papadakos P., Wijeysundera D., Vives M. 2020. Ozone therapy for patients with SARS-COV-2 pneumonia: a single-center prospective cohort study (Preprint) [DOI] [Google Scholar]
- Hudson J.B., Manju S., Selvarani V. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone: Sci. Eng. 2009;31(216) [Google Scholar]
- Kahle J.J., Neas L.M., Devlin R.B., Case M.W., Schmitt M.T., Madden M.C., Diaz-Sanchez D. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: A crossover study of healthy young volunteers. Environ. Health Persp. 2015;123:310. doi: 10.1289/ehp.1307986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komali G. Ozone therapy-a revolutionary noninvasive therapy in dentistry. Open Access Sci. Rep. 2012;1(473) [Google Scholar]
- Lee J., Bong C., Bae P., Abafog A., Baek S., Shin Y.B., Park M., Park S. 2020. Fast and easy disinfection of coronavirus-contaminated face masks using ozone gas produced by a dielectric barrier discharge plasma generator. [medRxiv 2020.04.26.20080317] [DOI] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Ann. Rev. Virol. 2016;3:237. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sánchez G., Schwartz A., Di Donna V. Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidants. 2020;9(389) doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto Q.L., Jose Miguel B.M., Teofilo E.T., Betty R., Ivan B., Ociel M. Inactivation of Coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Sci. Agropecu. 2020;11:257. [Google Scholar]
- Rowen R.J. Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: A review of current knowledge and experience. Med. Gas Res. 2019;9(232) doi: 10.4103/2045-9912.273962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen R.J., Robins H. A plausible penny costing effective treatment for corona virus - ozone therapy. J. Infect. Dis. Epidemiol. 2020;6(113) [Google Scholar]
- Rubio-Romero J.C., del Carmen Pardo-Ferreira M., García J.A.T., Calero-Castro S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020;129 doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagai M., Bocci V. Mechanisms of action involved in ozone therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011;1(29) doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti V., Quagliariello V., Franzini M., Iaffaioli R.V., Maurea N., Valdenassi L. Ozone exerts cytoprotective and anti-inflammatory effects in cardiomyocytes and skin fibroblasts after incubation with doxorubicin. Evid Based Complement Alternat. Med. 2019;2019 doi: 10.1155/2019/2169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpetti A.V. Lessons learned during the COVID-19 virus pandemic. J. Am. Coll. Surg. 2020;230(1092) doi: 10.1016/j.jamcollsurg.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp J.A., Hollonbeck S.A., Viglione D.D., Green P.C., Hodge J.R., Tamburro J.A., Tran T.N., Glassman D.S. Novel therapy for COVID-19 does intravenous ozonated-saline affect blood and tissue oxygenation? J. Gynecol. Res. Obstet. 2020;6:046. [Google Scholar]
- Tizaoui C. Ozone: A potential oxidant for COVID-19 Virus (SARS-CoV-2) Ozone: Sci. Eng. 2020;42:378–385. [Google Scholar]
- Toon J. Ozone disinfection could safely allow reuse of personal protective equipment. Biomed. Saf. Stand. 2020;50(121) [Google Scholar]
- Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (-) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharm. Sci. 2020;24(4059) doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- Virender K.S., Nigel J.D.G. Oxidation of amino acids, peptides and proteins by ozone: A Review. Ozone: Sci. Eng. 2010;32:81. [Google Scholar]
- Wang S.G., Liu W., Song Y.H., Xia T., Lu X.Q., Song L., Li Q.W., Sun J., Yin X.M., Feng X.Q., Yang Y.J., Sun T. Non-drug therapy to combat coronavirus. Open J. Regen. Med. 2020;9(65) [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollution. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(635) doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(523) doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(586) doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Dong M., Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26040. doi: 10.1002/jmv.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]