Abstract
Complement is integral to a healthy functioning immune system and orchestrates various innate and adaptive responses against viruses and other pathogens. Despite its importance, the potential beneficial role of complement in immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been overshadowed by reports of extensive complement activation in severe coronavirus disease 2019 (COVID-19) patients. Here, we hypothesize that complement may also have a protective role and could function to enhance virus neutralization by antibodies, promote virus phagocytosis by immune cells, and lysis of virus. These functions might be exploited in the development of effective therapeutics and vaccines against SARS-CoV-2.
Highlights
Complement has been implicated in playing some role in severe COVID-19 pathogenesis. However, the evidence to support this is largely inferred from case–control studies.
The potential protective role of complement has been largely ignored, which might contribute to innate and adaptive immunity against SARS-CoV-2 infection.
Immunity to many pathogens relies on complement to enhance antibody-mediated neutralization and mediate phagocytosis and lysis. These mechanisms might also contribute to immunity against SARS-CoV-2 infection, and complement might be potentially exploited in antibody-based therapeutics and vaccines.
Careful selection of vaccine adjuvants and epitopes included in vaccine constructs can influence whether vaccine-induced antibodies activate complement.
Mutations in monoclonal antibodies can be used to promote hexamer formation between antibodies, which can significantly improve complement binding and activation.
Beyond Directly Neutralizing Antibodies
The COVID-19 pandemic is a major global concern as there is no pre-existing immunity to the novel causative agent, SARS-CoV-2, and severe disease often has a poor prognosis. Considerable efforts are underway to develop effective interventions including vaccines and passive immunization therapies using purified immunoglobulins and recombinant monoclonal antibodies (MAbs). These strategies largely focus on the virus spike (S) protein (see Glossary), which interacts via the receptor-binding domain (RBD) with host angiotensin-converting enzyme 2 (ACE2) to facilitate cellular entry and viral replication [1]. This approach aims to elicit neutralizing antibodies, although we know that for other pathogens, neutralizing antibodies are not always sufficient to confer a high degree of protective efficacy and additional immune mechanisms may be needed. This may include antibody-mediated activation of the complement system, which can lead to various immunological outcomes against target pathogens. While several recent studies have implicated complement activity in severe disease, we instead hypothesize that complement may also contribute to protective immunity to SARS-CoV-2, which is a research area that has been largely understudied. Here, we discuss the potential role of complement in innate immune responses and adaptive immune responses, and how complement may be targeted or exploited for the development of therapeutics and vaccines against SARS-CoV-2.
Complement in Innate and Adaptive Immunity
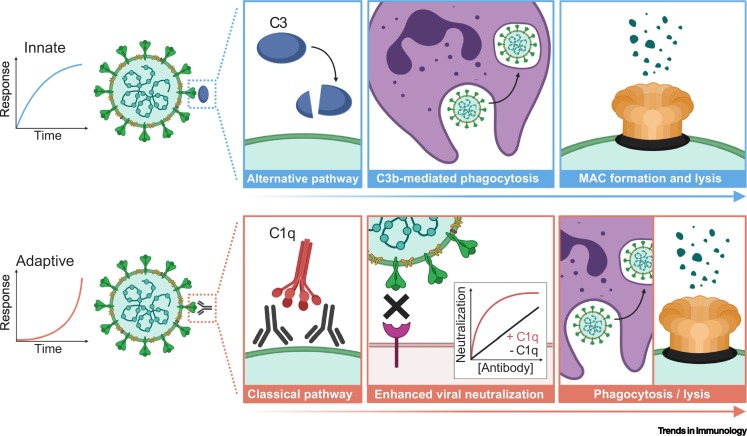
Human complement is an organized system comprising >30 serum proteins; many of which contain protease activity enabling one complement protein to activate another in a sequential cascade [2]. This process can be initiated by three distinct pathways. The classical pathway is an adaptive immune response activated by interactions between complement protein C1q and antibodies bound to antigens (IgM, IgG1, and IgG3 have the greatest activity). The classical pathway can also occur as an innate response activated by natural IgM or preformed autoantibodies [3,4]. The remaining two pathways are innate responses that rapidly activate against pathogens in an antibody-independent manner. These include the mannose-binding lectin (MBL) pathway whereby MBL directly binds to sugar molecules expressed on pathogen surfaces, and the alternative pathway that occurs by spontaneous activation of C3 on target cells (Figure 1 , Key Figure) [2].
Figure 1.
Key Figure. Potential Mechanisms of Innate and Adaptive Complement Activation against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Innate complement activation occurs rapidly against target pathogens via the mannose-binding lectin (not shown) or alternative pathway, the latter initiated by spontaneous C3 activation. Potential mechanisms of innate complement activation against SARS-CoV-2 might include: (i) deposition of C3b that can interact with C3b receptors (CR1, CR3, and CRIg) on phagocytes for clearance and degradation of the virus; and (ii) deposition of C5b and formation of the membrane attack complex (MAC) that creates a pore in the membrane leading to lysis of the virus. Adaptive complement activation is dependent on the acquisition of antigen-specific antibodies, which takes time to develop. Potential mechanisms of adaptive complement activation against SARS-CoV-2 might include: (i) C1q binding to antigen-specific antibody that can significantly enhance antibody-mediated neutralization of the virus, possibly due to a larger antibody–C1q complex more effectively blocking receptor–ligand interactions, or via C1q stabilization or enhancement in the binding of low affinity antibodies, or because C1q might reduce the antibody threshold required for neutralization; (ii) deposition of C3b and phagocytosis; and (iii) C5b deposition, MAC formation and lysis [2]. This figure was created using BioRender (https://biorender.com/).
Initiation by all three pathways leads to C3 protein activation and subsequent C5 activation. This involves protein cleavage into the activated C3a and C5a subunits, which play a major role in proinflammatory responses and recruitment of immune cells. The C3b cleavage product can adhere to pathogens, tagging them for uptake and degradation (phagocytosis) via C3b receptors on immune cells [2]. Pathogen clearance can also be mediated by the C5b fragment, which forms part of the membrane attack complex (MAC) that inserts into the target cell membrane, causing pore formation and lysis [2]. However, sublytic amounts of MAC on the surface of nucleated cells can instead play a role in activation and proliferation, as observed for Schwann cells and oligodendrocytes [5]. Another important role of complement is enhancement of antibody neutralization activity resulting from C1q binding to antibodies, even in the absence of other complement proteins [6,7].
Host cells are protected against complement activation by complement regulatory proteins that target all three complement activation pathways. Various pathogens have also evolved to express proteins that mimic these regulatory functions or bind soluble human regulatory proteins as an evasive mechanism against host complement attack [8]. This evolutionary advantage clearly highlights the key role complement plays in pathogen recognition and clearance.
Role of Complement Activation during COVID-19
There have been several reports on the role of complement in COVID-19 disease in humans, all focused on innate complement activation that occurs during acute infection. These studies generally conclude that excess complement activity can contribute to severe disease pathology (Table 1 ). Indeed, serum concentrations of C3 have been reported to be lower in COVID-19 patients compared to healthy controls, possibly reflective of protein activation and cleavage into the C3a component – found at higher concentrations in patients relative to healthy controls – although this remains to be further assessed [9., 10., 11.]. Downstream complement proteins, C5a and soluble (s)C5b–C9 that forms the MAC, are also elevated in the serum of COVID-19 patients, and are further increased with disease severity relative to controls [10., 11., 12., 13.]. While these data suggest that complement is dysregulated following infection with SARS-CoV-2, this conclusion can be misleading, given that serum concentrations may be within the normal healthy range. For example, three studies reported that C3 concentrations were only below the normal range in 9.7%, 18.6%, and 47% of COVID-19 patients (12%, 2%, and 0% had severe disease, respectively) [14., 15., 16.]. In the same studies, C4 levels (involved in classical and MBL pathways) were below the normal range in 3.2%, 4.3%, and 0% of patients [14., 15., 16.]. While serum concentrations of activated complement subunits are elevated in COVID-19 patients, these amounts are ~10-fold lower than those observed in bacterial sepsis patients [10]. Extensive deposition of complement proteins MASP2 (MBL pathway), C3, C4, and C5b–C9 has also been reported in lung and cutaneous tissue samples from severe COVID-19 patients, but healthy samples were included as experimental controls in only one single study (control data were not shown) [17., 18., 19., 20.]. Moreover, complement may contribute to dysregulated cytokine responses observed in some COVID-19 patients, which has been described as a cytokine storm, although there is some question as to whether this term is misleading given that the concentrations of interleukin-6 in COVID-19 patients have been reported to be up to 40-fold lower than those typically reported for acute respiratory distress syndrome (ARDS) [21]. Therefore, this question needs to be further addressed; additional discussions on immune responses during COVID-19 have been reviewed elsewhere [22,23].
Table 1.
Summary of Serum Complement Concentrations in COVID-19 Patients versus Controls
| Complement | Participants | Concentration | Refs |
|---|---|---|---|
| C1q | Mild patients (n = 32); severe patients (n = 39). | ↓ in severe vs mild (P < 0.001). | [76] |
| MBL | Patients (n = 65); healthy controls (n = 72). | ↑ in patients vs controls (P = 0.018). | [26] |
| Patients with respiratory failure (n = 23) and without respiratory failure (n = 16). | No difference between groups. | [25] | |
| C3 | Patients (n = 33). | Some ↓ (9.7%) than normal range. | [14] |
| Patients (n = 30). | Some ↓ (47%) that normal range. | [15] | |
| Patients (n = 182). | Some ↑ (12%) or ↓ (19%) than the normal range. | [16] | |
| Patients (n = 72); healthy controls (n = 20). | ↓ in patients vs controls (P < 0.01). | [9] | |
| Patient survivors (n = 169) and nonsurvivors (n = 67). | ↓ in nonsurvivors vs survivors (P <0.05). | [27] | |
| Patient survivors (n = 414) and nonsurvivors (n = 125). | ↓ in nonsurvivors vs survivors (P < 0.001). | [77] | |
| C3a | Patients (n = 122); healthy controls (n = 10). | ↑ in patients vs controls (P < 0.001). | [10] |
| Severe patients receiving maintenance hemodialysis (n = 19); hemodialysis controls (n = 10). | ↑ in patients vs controls (P < 0.001). | [11] | |
| C3c | Patients (n = 122); healthy controls (n = 10). | ↑ in patients vs controls (P < 0.05). | [10] |
| C4 | Patients (n = 33). | Some ↑ (12.9%) or ↓ (3.2%) than normal range. | [14] |
| Patients (n = 30). | Within the normal range. | [15] | |
| Patients (n = 182). | Some ↑ (4%) or ↓ (4%) than the normal range. | [16] | |
| Patients (n = 72); healthy controls (n = 20). | ↓ in patients vs controls (P < 0.01). | [9] | |
| Patient survivors (n = 169) and nonsurvivors (n = 67). | No difference between groups. | [27] | |
| Patient survivors (n = 414) and nonsurvivors (n = 125). | ↓ in nonsurvivors vs survivors (p = 0.001); within normal range for the survivor group. | [77] | |
| C4d | Patients with respiratory failure (n = 23) and without respiratory failure (n = 16). | ↑ in patients with respiratory failure (p = 0.034). | [25] |
| C5a | Severe patients (n = 46); healthy controls (n = 27). | ↑ in patients vs controls (P < 0.001). | [24] |
| Patients (n = 17); healthy controls (n = 27). | ↑ in patients vs controls (P < 0.001). | [12] | |
| Mild patients (n = 54); moderate patients (n = 68); severe patients (n = 106). | ↑ with disease severity (P = 0.02). | [78] | |
| Severe patients receiving maintenance hemodialysis (n = 19); hemodialysis controls (n = 10). | ↑ in patients vs controls (P < 0.01). | [11] | |
| Patients with respiratory failure (n = 23) and without respiratory failure (n = 16). | No difference between groups. | [25] | |
| sC5b–C9 | Severe patients (n = 46); healthy controls (n = 27). | ↑ Higher in patients vs controls (P < 0.001). | [24] |
| Patients (n = 122); healthy controls (n = 10). | ↑ in patients vs controls (P < 0.001). | [10] | |
| Patients (n = 17); healthy controls (n = 27). | ↑ in patients vs controls (P < 0.001). | [12] | |
| Patients (n = 25); healthy controls (n = 10). | ↑ in patients vs controls (P < 0.05). | [13] | |
| Patients with respiratory failure (n = 23) and without respiratory failure (n = 16). | ↑ in patients with respiratory failure (P = 0.008). | [25] |
There has been little investigation into the relationship between complement and clinical complications associated with SARS-CoV-2 infection. One study found moderate correlations between sC5b–C9 concentrations and a single marker of endothelial perturbation (von Willebrand factor, Spearman’s correlation coefficient 0.517, P < 0.001) [24]; additional markers were quantified but their correlations were not reported [24]. In hospitalized patients, increased plasma concentration of sC5b–C9 was also associated with an increased odds of respiratory failure (odds ratio 31.9, confidence interval 1.3–746.6, P= 0.03) relative to those with lower concentrations [25]. In critically ill patients, the concentrations of MBL were correlated with markers of coagulopathy (d-dimer, Spearman’s correlation coefficient 0.390, P = 0.002) [26]. One study evaluated the relationship between complement and mortality and found that increased C3 in serum was associated with a reduced odds of mortality (odds ratio 0.073, confidence interval 0.007–0.722, P = 0.025) relative to those with lower concentrations [27].
Hypothetically, local and systemic complement activation during the acute phase of infection, prior to the development of adaptive immunity, may cause disease pathology. Potential mechanisms for complement-mediated pathology may include complement-induced inflammation, leukocyte infiltration, and MAC formation and lysis of virus-infected cells in the lungs. While reasonable, the evidence to support the directionality of this hypothesis is scarce and inferring causality from case–control studies is often problematic, potentially leading to misleading conclusions. For example, neutralizing antibody titers against SARS-CoV-2 can increase with disease severity, yet neutralizing antibodies are also thought to contribute to protective immunity [28]. These differences, however, warrant further investigation. Many of the correlations reported have been from univariate analysis that did not adjust for other covariables that may have been associated with poor clinical outcomes [24., 25., 26.]. There is also a lack of in vitro evidence to support the role of complement in disease pathology. Studies have shown that recombinant SARS-CoV-2 proteins can activate the MBL and alternative pathways, suggesting these mechanisms may contribute to disease pathology, although this remains to be rigorously assessed [29,30]. Indeed, there is currently no direct evidence that complement can lyse SARS-CoV-2-infected human cells.
We instead highlight the possibility that complement might also play a protective role against SARS-CoV-2 infection. Patients with mild disease generally report normal serum concentrations of complement proteins (C3 and C4), which suggests that normally functioning immune mediators may be able to contribute to immunity and reduce disease severity [14., 15., 16.]. In line with this, an examination of >6000 COVID-19 patients found that individuals with a dysregulated complement system were more prone to developing severe disease than those with a healthy immune system [31]. Therefore, for most individuals, complement activation might contribute to reduced disease severity, whereas for a smaller percentage of individuals, complement might be dysregulated and associated with susceptibility to severe disease. This possibility remains to be tested.
Complement mediates protective immunity against many pathogens through activation of the antibody-dependent classical pathway. Currently, little is known about the potential role of antibody-complement interactions in immunity against SARS-CoV-2.
Lessons on Immunity from Other Human Pathogens
In some studies, innate complement activation and inflammation have also been implicated in the pathology of SARS [32., 33., 34., 35., 36.], caused by SARS-CoV-1, and closely related to SARS-CoV-2. This might occur through MBL binding to the virus, which appears to be neutralizing, but could also lead to complement activation and downstream inflammation [32]. Overall, there is no clear consensus on the role of MBL in SARS [32,33], and the evidence that complement is hyperactive during the acute stage of infection is also conflicting [34,35]. The strongest evidence that supports complement in disease pathology comes from murine infection models. Mice deficient in C3 (C3 genetic knockout, C3 tm1Crr), central to all complement pathways, presented less disease pathology and inflammation than control wild-type mice did [36]. While an important observation, murine and human complement and related immune mechanisms are considerably different, which limits direct extrapolation of these findings to human immunity [37]. Furthermore, murine complement may act more potently against a human-specific pathogen than human complement would; indeed, enhanced cross-species activity was observed in a study of human complement against the murine-specific pathogen, Plasmodium berghei [38].
Complement can also be activated by antigen-specific antibodies, and therefore, can contribute to adaptive immune responses. Immunity to many viral and nonviral pathogens relies on antibodies and antibody-mediated neutralization. The binding of C1q alone or additional complement components can significantly enhance antibody neutralization, and in some cases, complement is essential for neutralizing activity. This has been demonstrated in vitro for the human pathogens, West Nile virus [6], Nipah virus [39], vaccinia virus [40], hepatitis C virus [41], and others [8,42], often using a combination of human and nonhuman (mouse, rabbit, and guinea pig) antibodies and complement.
There appears to be some balance between the pathogenic and protective roles of complement observed with various infectious pathogens. For example, severe malaria is associated with innate complement activation and inflammation [43], while protective antimalarial immunity is associated with classical complement activation via antibodies [7,44,45]. Therefore, we hypothesize that under optimal conditions, antibodies and complement might contribute to pathogen neutralization and reduced disease severity. However, when the immune response is suboptimal and cannot effectively clear the infection, complement might instead become hyperactive and cause inflammation-related pathologies. This might also be true for SARS-CoV-2, whereby failure to control infection might lead to a high viral load that triggers multiple pathogenic processes, including those mediated by complement. This concept was supported by a repeat infection study in rabbits. Specifically, rabbits acquired some immunity after primary infection with Middle East respiratory syndrome coronavirus (MERS-CoV), but this was not sufficient to confer protection against re-infection, which instead resulted in increased inflammation. It was only after two infections that an appropriate antibody response was acquired, and when infected for a third time, the infection was controlled, and little immunopathology was observed [46]. Altogether, complement has demonstrated both pathogenic and protective roles in immunity to various pathogens.
Complement-Based Therapeutics
Inhibitors targeting the early stages of complement activation have been used in small case studies to treat COVID-19. These include a case series of a C1-inhibitor in noncritical COVID-19 patients (conestat alfa, n = 5) and a MASP2-inhibitor in severe COVID-19 patients with ARDS (naroplimab, n = 6), whereby all patients had recovered and were discharged from the hospital by days 22 and 91, respectively [47,48]. The C3 inhibitor, AMY-101, was also shown to improve clinical outcomes in COVID-19 patients with ARDS (n = 4), as indicated by reduced inflammatory markers (such as CRP and LDH) and improved respiratory function [49,50]. There have been several reports of individuals (n = 8) already on C5 inhibitors (eculizumab and ravulizumab) for unrelated conditions, at the time of SARS-CoV-2 infection [51., 52., 53., 54.]. While most patients had a standard recovery, two were admitted to the intensive care unit, and one did not recover [52,53]. Other case reports of C5 inhibitors (eculizumab and LFG316) administered to severe/critical COVID-19 patients (n = 22) also found some promising results [50,55., 56., 57.]. Although, two patients acquired bacterial infections and another two developed mechanical ventilation-associated pneumonia, all four died [50,56,57]. Few control studies have been performed; one of these evaluated a combination of C5 (eculizumab) and JAK1/2 (ruxolitinib) inhibitors (treatment n = 7, control n = 10) in COVID-19 patients [58]. A larger study evaluated the experimental treatment of severe patients with a C5 inhibitor (eculizumab; treatment n = 35, control n = 45) [59]. The treatment group presented enhanced survival at day 28 (P = 0.005), although serious adverse events were twofold higher than in controls, and included infectious complications and ventilator-associated pneumonia [59]. The one randomized control, phase 2 trial (NCT04333420)i of a C5 inhibitor administered to severe COVID-19 patients (vilobelimab; treatment n = 15, control n = 15) found no improvement in the primary outcome of respiratory function (PaO2/FiO2) [60], but the authors noted that the study was not sufficiently powered to detect significant differences between groups [60].
While these studies suggest some promise, it is too early to conclude whether complement inhibitors have any clinical benefit in treating severe COVID-19. Several clinical trials of AMY-101 and eculizumab are ongoing, as well as larger clinical studies. We also need to acknowledge that complement inhibitors may not be suitable for all patients, particularly for those with mild disease that display serum concentrations of complement proteins within the normal range [14., 15., 16.]. Complement may contribute to mounting an effective immune response, and so, administration of therapeutics to inhibit complement might potentially be harmful in COVID-19 patients with mild disease who may be mounting an effective immune response. Furthermore, complement deficiencies are known to increase the risk of bacterial infections [61], and several participants treated with eculizumab were reported to have bacterial infections and ventilator-associated pneumonia (some of which did not recover) [50,56,57,59,60]. Overall, larger, randomized controlled clinical trials are required to formally evaluate the therapeutic efficacy of complement inhibitors.
Exploiting Complement for Antibody Therapeutics and Vaccines
Many licensed viral vaccines elicit neutralizing antibodies, and are needed for protective immunity [62]. Complement can significantly enhance antibody neutralization [6], and consequently, we posit that it should be considered when developing antibody-based therapeutics and vaccines against SARS-CoV-2 (Figure 1). Presumably, enhanced neutralization might result from the antibody–C1q complex being larger and more effective at blocking receptor–ligand interactions than antibody alone, or possibly, through the action of C1q, stabilizing or enhancing the binding of low affinity antibodies, although this remains to be tested. For some viruses, such as West Nile virus, C1q binding can reduce the number of IgG molecules required to bind the virus surface and mediate neutralization, overall reducing the antibody titer threshold for neutralization [6]. Therefore, complement might be exploited to reduce the problem of antibody concentrations waning over time – as has been reported for SARS-CoV-2 [28,63] – because antibodies that bind complement can maintain neutralizing activity at low concentrations compared to in the absence of complement. Additionally, C1q binding might reduce the problem of antibody-dependent enhancement that has been reported for some virus infections, such as West Nile virus [6]; however, whether this is an issue with SARS-CoV-2 infection remains to be established. Of note, using an in vitro model, complement was also found to reduce antibody-mediated enhancement of host cell invasion by a different pathogen, Plasmodium falciparum [7]. Complement activation can clear viruses via MAC formation on the virus surface resulting in lysis (such as Zika virus [64]), and through the promotion of phagocytosis via complement receptors on immune cells including neutrophils, monocytes, and macrophages [2]. Antibodies also promote complement-mediated lysis of virus-infected cells, as demonstrated in vitro using human cell lines infected with the influenza virus [65].
Antibody-based therapies are being explored to treat COVID-19, including the use of purified polyclonal immunoglobulins and recombinant MAbs. Purified polyclonal immunoglobulins have been used for decades for prophylaxis and treatment of infections, such as rabies and hepatitis A virus infections, establishing principles that underpin targeted approaches using modern recombinant antibody technologies. While there is substantial potential for the use of MAbs in the treatment and prevention of infectious diseases [66], few are currently available for clinical use. Although some results are preliminary, several MAbs have been reported to neutralize SARS-CoV-2 in vitro [67,68]. One of these MAbs (2-15) was also shown to reduce viral RNA copy numbers in a golden Syrian hamster model of SARS-CoV-2, compared to control animals that received saline solution only [68]. These MAbs have significant potential as candidate therapeutic and preventive agents. In general, recent advances in MAb technologies have identified a point mutation in the Fc region (E430G) of IgG that promotes hexamer formation between neighboring IgG molecules, significantly enhancing C1q binding and complement activation [69]. These hexamer antibodies have been largely explored in cancer therapeutics and demonstrate potent complement activation and complement-mediated lysis of cancer cells (isolated from patient cancer cells and cell lines) in vitro [70]. These approaches might also be potentially valuable for treating infectious diseases, including COVID-19, which certainly merits further investigation. This principle was recently shown with Neisseria gonorrhoeae bacterial infection, whereby a MAb with the hexamerization mutation had greater complement fixation and protective efficacy than the wild-type IgG in a mouse infection model (wild-type BALB/c mice) [71]. Presumably, further enhancing the neutralizing activity of anti-SARS-CoV-2 MAbs by complement binding might be preferable to directly neutralizing MAbs that lack complement activity. We also propose considering combining different therapeutic approaches; for example, it might be possible to use engineered MAbs with enhanced C1q binding for viral neutralization in combination with a C5 inhibitor to prevent terminal complement activation and inflammation in specific clinical syndromes, such as ARDS. However, this remains to be tested.
From another angle, vaccines can also be modified to induce antibodies with enhanced binding to C1q; one approach is to modulate the IgG subclass profile, which can be achieved using appropriate vaccine adjuvants (e.g., QS21, AS01, MF59, and Alum) [72]. In humans, IgG1 and IgG3 can potently activate complement (IgG3 > IgG1), while IgG2 and IgG4 have little to no activity [66]. Epitopes included in vaccine constructs are also important, as antibodies to different epitopes, even of the same antigen, can have considerable differences in the ability to bind C1q [73]. There is also evidence that targeting multiple epitopes might enhance antibody–complement activity overall [45].
The binding affinity of SARS-CoV-2 S protein to the receptor ACE2 is ~4-fold higher than seen for the closely related SARS-CoV-1, raising concerns that achieving highly effective neutralizing antibodies might be challenging with SARS-CoV-2 [67]. We argue that complement fixation by antibodies is worth exploring as a potential strategy to achieve maximal antibody-mediated virus neutralization. The molecular details of antibodies targeting SARS-CoV-2 are beginning to emerge, which can help understand the potential of complement interactions to enhance immunity. Neutralizing MAbs largely target the RBD and sterically block ACE2 receptor binding [74]. Therefore, C1q binding by antibodies might enhance inhibition of receptor binding. In contrast, non-neutralizing MAbs tend to bind outside the RBD [74]. Moreover, antibodies targeting specific epitopes have the potential for intermolecular crosslinking of SARS-CoV2 S proteins, which might increase the effective avidity of IgG and increase antibody-mediated virus neutralization [75]. C1q binding to multiple IgG molecules might achieve a similar effect to increase the effective avidity and enhance neutralization. Collectively, further evaluations of the role of complement and how this cascade might enhance antibody activity might be a promising avenue to maximize the efficacy of antibody-based therapeutics and vaccines, awaiting future research.
Concluding Remarks
Complement has multiple roles in immunity, including innate and adaptive responses. While excess complement activation has been implicated in severe COVID-19 disease pathology, data supporting this role, or the benefit of complement inhibitors, remain limited (see Outstanding Questions). Importantly, the potential involvement of complement factors in protective immunity has been largely ignored for SARS-CoV-2, but has been defined with other viruses, bacteria, and protozoa. Results from small studies of complement inhibitor therapeutics should not overshadow the potential to harness the beneficial activities of complement. The implications for antibody–complement interactions in virus neutralization and immunity need to be investigated and could be applied to antibody-based therapeutics or active vaccination strategies against SARS-CoV-2.
Outstanding Questions.
What is the role of antibodies against SARS-CoV-2 in fixing and activating complement to mediate immunological functions, including enhanced virus neutralization and complement-mediated phagocytosis and lysis of the virus?
What are the specific epitopes of antibodies that can potently bind and activate complement? Does targeting multiple epitopes by antibodies achieve greater complement fixation and activation?
Can C1q binding by antibodies enhance SARS-CoV-2 virus neutralization, as seen for some other viruses and pathogens? Does C1q binding increase receptor blocking effects of antibodies, or can C1q cross-link IgG to increase its effective avidity?
What properties and mutations in monoclonal antibodies promote complement interactions and can these be exploited to increase therapeutic or prophylactic efficacy?
Which vaccine designs, adjuvants, and constructs can effectively induce antibodies that fix and activate complement against SARS-CoV-2?
What role do antibody–complement interactions play in naturally acquired and vaccine-induced protective immunity against SARS-CoV-2?
What is the role of innate complement activation in immunity to SARS-CoV-2? Does it play a role in controlling viremia?
What are the specific mechanisms by which complement contributes to the pathogenesis of severe disease?
Can inhibitors of complement activation be used therapeutically to reduce progression to severe disease, or improve outcomes in severe disease? What are the COVID-19 syndromes or complications for which complement inhibitors have the most clinical benefit?
Alt-text: Outstanding Questions
Acknowledgments
The authors were supported by funding from the National Health and Medical Research Council of Australia (grants 1173046, 1092789). Burnet Institute is supported by the NHMRC Independent Research Institute Infrastructure Support Scheme and a Victorian State Government Operational Infrastructure grant.
Glossary
- Acute infection
initial exposure to pathogen; often characterized by the rapid onset of clinical symptoms.
- Acute respiratory distress syndrome
characterized by acute respiratory failure and often associated with inflammation in the lungs.
- Adaptive immune response
slowly acquired immune response that is highly specific to the target pathogen often characterized by the production of antigen-specific antibodies.
- Angiotensin converting enzyme 2
host ligand for the SARS-CoV-2 spike protein; the former is expressed on lung alveolar epithelial cells and small intestinal epithelial cells, such as in mice and humans.
- Antibody-dependent enhancement
an unusual process where antibody binding to a virus can enhance viral entry into host cells via interactions with antibody Fcγ receptors.
- Innate immune response
rapid immune response that recognizes patterns or specific molecules commonly found on pathogens (and not host cells); often characterized by an inflammatory response.
- Membrane attack complex
represents the terminal phase of complement activation, whereby complement proteins (C5b–C9) form a protein complex that inserts into the target cell membrane and causes lysis.
- Neutralizing antibodies
can effectively bind to a pathogen and inhibit its activity; the most common form of neutralization is the inhibition of cellular entry or invasion.
- Phagocytosis
describes the process of pathogen engulfment and destruction within specialized immune cells; this can be mediated by complement receptors expressed on phagocytes.
- Receptor binding domain
specific domain of the spike protein that interacts with the host ligand, ACE2.
- Spike (S) protein
glycoprotein expressed on the surface of SARS-CoV-2 that interacts with host ACE2 to facilitate cellular invasion.
Resources
ihttps://clinicaltrials.gov/ct2/show/NCT04333420References
- 1.Walls A.C. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merle N.S. Complement system part I - molecular mechanisms of activation and regulation. Front. Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayasekera J.P. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najaoui A. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP) Eur. J. Haematol. 2012;88:167–174. doi: 10.1111/j.1600-0609.2011.01718.x. [DOI] [PubMed] [Google Scholar]
- 5.Tegla C.A. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol. Res. 2011;51:45. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehlhop E. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe. 2009;6:381–391. doi: 10.1016/j.chom.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle M.J. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avirutnan P. Complement and its role in protection and pathogenesis of flavivirus infections. Vaccine. 2008;26:I100–I107. doi: 10.1016/j.vaccine.2008.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L., Chen C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19) Epidemiol. Infect. 2020;148 doi: 10.1017/S095026882000165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Nooijer A.H. Complement activation in the disease course of coronavirus disease 2019 and its effects on clinical outcomes. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa646. Published online October 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prendecki M. Temporal changes in complement activation in haemodialysis patients with Covid-19 as a predictor of disease progression. Clin. Kidney J. 2020;13:889–896. doi: 10.1093/ckj/sfaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cugno M. Complement activation in patients with COVID-19: a novel therapeutic target. J. Allergy Clin. Immunol. 2020;146:215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skendros P. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R. Distinguishable immunologic characteristics of COVID-19 patients with comorbid type 2 diabetes compared with nondiabetic individuals. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/6914878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X.-S. Diarrhea is associated with prolonged symptoms and viral carriage in COVID-19. Clin. Gastroenterol. Hepatol. 2020;18:1753–1759. doi: 10.1016/j.cgh.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du H. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2020 doi: 10.1111/all.14452. Published online June 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magro C. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valtueña J. Vascular obliteration because of endothelial and myointimal growth in COVID-19 patients. Int. J. Dermatol. 2020 doi: 10.1111/ijd.15300. Published online November 12, 2020. [DOI] [PubMed] [Google Scholar]
- 19.Occidental M. Investigating the spectrum of dermatologic manifestations in COVID-19 infection in severely ill patients: a series of four cases. J. Cutan. Pathol. 2020 doi: 10.1111/cup.13867. Published online September 8, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Magro C.M. Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Hum. Pathol. 2020;106:106–116. doi: 10.1016/j.humpath.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha P. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 22.Perico L. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2020 doi: 10.1038/s41581-020-00357-4. Published online October 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cugno M. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Automimmun. 2020 doi: 10.1016/j.jaut.2020.102560. Published online October 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holter J.C. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. 2020;117:25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson O. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically ill COVID-19 patients. Thromb. Haemost. 2020 doi: 10.1055/s-0040-1715835. Published online September 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang S. Decreased complement C3 levels are associated with poor prognosis in patients with COVID-19: A retrospective cohort study. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seow J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5 doi: 10.1038/s41564-020-00813-8. 1598–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao T. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020 doi: 10.1101/2020.03.29.20041962. Published online June 18, 2020. [DOI] [Google Scholar]
- 31.Ramlall V. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26:1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ip W.E. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan F.F. Influence of FcγRIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens. 2005;66:291–296. doi: 10.1111/j.1399-0039.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang R.T. Serum proteomic fingerprints of adult patients with severe acute respiratory syndrome. Clin. Chem. 2006;52:421–429. doi: 10.1373/clinchem.2005.061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao W. Determination of serum acute phase reaction protein in patients with severe acute respiratory syndrome. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:92–93. [Chinese journal of preventive medicine] [PubMed] [Google Scholar]
- 36.Gralinski L.E. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurtovic L. Complement in malaria immunity and vaccines. Immunol. Rev. 2020;293:38–56. doi: 10.1111/imr.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamoto Y. Plasmodium berghei: sporozoites are sensitive to human serum but not susceptible host serum. Exp. Parisitol. 1992;75:361–368. doi: 10.1016/0014-4894(92)90249-a. [DOI] [PubMed] [Google Scholar]
- 39.Johnson J.B. Interactions of human complement with virus particles containing the Nipah virus glycoproteins. J. Virol. 2011;85:5940–5948. doi: 10.1128/JVI.00193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benhnia M.R.-E.-I. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J. Virol. 2009;83:12355–12367. doi: 10.1128/JVI.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer K. Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis C virus E2 chimeric glycoprotein. J. Virol. 2002;76:2150–2158. doi: 10.1128/jvi.76.5.2150-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellors J. Viral evasion of the complement system and its importance for vaccines and therapeutics. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01450. Published online July 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silver K.L. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell. Microbiol. 2010;12:1036–1045. doi: 10.1111/j.1462-5822.2010.01492.x. [DOI] [PubMed] [Google Scholar]
- 44.Kurtovic L. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med. 2018;16:61. doi: 10.1186/s12916-018-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiling L. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat. Commun. 2019;10:610. doi: 10.1038/s41467-019-08528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houser K.V. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urwyler P. Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and Kallikrein-Kinin system. Front. Immunol. 2020;11:2072. doi: 10.3389/fimmu.2020.02072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rambaldi A. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020 doi: 10.1016/j.imbio.2020.152001. Published online November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastaglio S. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mastellos D.C. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulasekararaj A.G. Terminal complement inhibition dampens the inflammation during COVID-19. Br. J. Haematol. 2020;190:e141–e143. doi: 10.1111/bjh.16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trimarchi H. Eculizumab, SARS-CoV-2 and atypical hemolytic uremic syndrome. Clin. Kidney J. 2020;13:739–741. doi: 10.1093/ckj/sfaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pike A. COVID-19 infection in patients on anti-complement therapy: the Leeds National Paroxysmal Nocturnal Haemoglobinuria service experience. Br. J. Haematol. 2020;191:e1. doi: 10.1111/bjh.17097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Araten D.J. Mild clinical course of COVID-19 in 3 patients receiving therapeutic monoclonal antibodies targeting C5 complement for hematologic disorders. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.927418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diurno F. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eu. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 56.Zelek W.M. Complement inhibition with the C5 blocker LFG316 in severe COVID-19. Am. J. Respir. Crit. 2020;202:1304–1308. doi: 10.1164/rccm.202007-2778LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurence J. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin. Immunol. 2020;219 doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giudice V. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front. Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Annane D. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100590. Published online November 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlaar A.P. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30341-6. Published online September 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skattum L. Complement deficiency states and associated infections. Mol. Immunol. 2011;48:1643–1655. doi: 10.1016/j.molimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibarrondo F.J. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiela B. Active human complement reduces the Zika virus load via formation of the membrane-attack complex. Front. Immunol. 2018;9:2177. doi: 10.3389/fimmu.2018.02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irani V. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015;67:171–182. doi: 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- 67.Wan J. Human-IgG-neutralizing monoclonal antibodies block the SARS-CoV-2 infection. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 69.Diebolder C.A. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oostindie S.C. CD20 and CD37 antibodies synergize to activate complement by Fc-mediated clustering. haematologica. 2019;104:1841–1852. doi: 10.3324/haematol.2018.207266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gulati S. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Apostólico J.d.S. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016;2016 doi: 10.1155/2016/1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teeling J.L. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 74.Kreer C. Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell. 2020;182:843–854. doi: 10.1016/j.cell.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnes C.O. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842. doi: 10.1016/j.cell.2020.06.025. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y. Clinical characteristics and immune injury mechanisms in 71 patients with cOVID-19. Msphere. 2020;5 doi: 10.1128/mSphere.00362-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect. Dis. Poverty. 2020;9:1–10. doi: 10.1186/s40249-020-00723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busch M.H. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020;142:1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]