Abstract
Importance:
Parenting interventions have been found to normalize children’s cortisol regulation among high-risk children early in development; it is important to investigate sustainability of these effects and their mechanisms, given the maladaptive outcomes associated with cortisol dysregulation.
Objective:
To determine whether the Attachment and Biobehavioral Catch-up (ABC) intervention, implemented in infancy, predicts cortisol regulation in middle childhood via changes in parental sensitivity.
Design:
Double blind randomized clinical trial design; started January 2006, with the follow-up for this project concluded March 2016
Setting:
Parents of children under age 2 referred from child protective services agencies in a large, mid-Atlantic city
Participants:
103 parent-child dyads (45.6% female children) with histories of child protective services involvement, randomly assigned to receive ABC (n = 45) or a control intervention (n = 58); in infancy, the children’s ages ranged from 1.60 to 25.30 months (M = 9.87 months); at the middle childhood follow-up, they ranged from 8.0 to 11.0 years old (M = 8.52 years).
Interventions:
Both conditions included 10-week, in-home, manualized interventions. The experimental condition, ABC, has 3 primary targets for parents: increasing nurturance to child distress, increasing following the child’s lead, and decreasing frightening behavior. The control intervention, Developmental Education for Families (DEF), is an adaptation of a program focused on enhancing cognitive and language development.
Main Outcomes and Measures:
Parental sensitivity was coded from a semi-structured interaction task between the parent and child in early childhood. Middle childhood diurnal cortisol slopes were modeled by collecting salivary cortisol samples from children at wake-up and bedtime over the course of 3 consecutive days.
Results:
ABC participation in infancy was associated with increased parental sensitivity post-intervention, β = 0.28, p = .004, and this increased sensitivity predicted steeper decline across the day in children’s cortisol concentration in middle childhood, β = −.53, p= .002. The indirect effect of ABC on cortisol regulation via sensitivity was significant, β = −0.15, p = .038.
Conclusions and Relevance:
ABC has an indirect effect on middle childhood diurnal cortisol regulation via parental sensitivity; future research should seek to determine how this enhanced neurobiological regulation relates to children’s behavioral, socioemotional, and psychological outcomes.
Trial Registration:
clinicaltrials.gov Identifier: NCT02093052
Keywords: Parental sensitivity, cortisol, intervention, early adversity
Children facing early life adversity often show dysregulated functioning of the hypothalamic pituitary adrenal (HPA) axis in the form of blunted diurnal cortisol rhythms. Attachment and Biobehavioral Catch-up (ABC) is an intervention developed to enhance biological and behavioral regulation in children exposed to early adversity by targeting parental sensitivity. Implemented in infancy, ABC has been found to have lasting effects on children’s diurnal cortisol production into early childhood. The present study explored the enduring impact of ABC on cortisol regulation in middle childhood and examined the role of parental sensitivity as a mediator. Participants were 103 Child Protective Services referred children and their parents who participated in a randomized clinical trial of ABC. At post-intervention (M age = 20.66 [5.23] months), parental sensitivity was coded from a semi-structured interaction task. In middle childhood, parents collected saliva samples from their child (M age = 8.52 [0.67] years) at wake-up and bedtime for 3 consecutive days. Samples were assayed to determine cortisol concentration and the diurnal cortisol slope was estimated as a latent change score. Parents who were randomized to ABC were rated higher in parental sensitivity during their children’s infancy than parents in the control group, β = 0.28, p = .004, and this increased sensitivity predicted a steeper, more normative decline in cortisol concentration from wake-up to bedtime in middle childhood, β = −.53, p = .002. Further, the indirect effect of ABC on children’s diurnal cortisol slope via post-intervention sensitivity was significant, β = −0.15, p = .038, suggesting that parental sensitivity mediated the association between ABC and children’s diurnal cortisol slope in middle childhood.
1. Introduction
Children facing early life adversity, such as child maltreatment, are vulnerable to dysregulation of the hypothalamic pituitary adrenal (HPA) axis.1, 2 This dysregulation can take the form of atypical diurnal patterns in the body’s production of cortisol, an end product of the HPA axis. However, there is evidence that high quality parenting can contribute to adaptive HPA axis functioning.3,4 Interventions, such as Attachment and Biobehavioral Catch-up (ABC), have been developed to facilitate sensitive caregiving in an effort to support positive development in children following experiences of maltreatment. ABC, which is implemented in infancy or toddlerhood, has been found to have lasting effects on children’s diurnal cortisol rhythm into early childhood.5 The present study explores the enduring impact of ABC on HPA axis regulation into middle childhood.
1.1. The Impact of Maltreatment on the Regulation of the HPA Axis
The HPA axis has several important and orthogonal functions, among them mounting a stress response and maintaining a diurnal pattern of cortisol production. These processes involve the hypothalamus, which releases corticotrophin releasing factor (CRH), a hormone that targets the anterior portion of the pituitary gland. In response, the anterior pituitary gland releases adrenocorticotropic hormone (ACTH), which targets the cortex of the adrenal glands. This initiates the release of glucocorticoids (i.e., cortisol in humans). The diurnal pattern of cortisol is characterized by a rise in values prior to waking, with a peak at 30 minutes after wake-up, followed by a steep decline, then a gradual decrease in concentration across the day to its nadir at bedtime. In young children, this pattern typically emerges by three months of age6, 7 and mirrors that of adults by age two.8 Thus, the first two years of life may be a sensitive period for the development of healthy diurnal cortisol regulation.
Risk factors, such as extreme poverty,9 institutional care,10 and child maltreatment,11 can create a context of stress that leads to diurnal cortisol dysregulation. For children who have experienced maltreatment, this perturbation often takes the form of low morning cortisol values which translate to blunted, or flattened, rates of decline over the course of the day.1, 2 Child maltreatment has been shown to have negative effects on HPA axis regulation into adolescence12 and adulthood.13 The diurnal rhythm of cortisol is integral to the regulation of the immune system response, metabolic systems, and cardiovascular functioning, which may explain why dysregulation of this system has been associated with a host of negative physical outcomes across the life span.14 In addition, cortisol dysregulation is associated with maladaptive behavioral,15, 16 social-emotional,17 and psychological18 outcomes for children.
1.2. Intervening to Regulate HPA Axis Functioning
Although child maltreatment has been shown to relate to dysregulation of HPA axis functioning, there is evidence that high quality, sensitive parenting can serve as a buffer against environmental adversity. Parental sensitivity, which is characterized by timely and appropriate responses to a child’s social signals,19, 20 has been found to have positive benefits for children’s cortisol regulation. In a cross-sectional study with a non-maltreated sample, higher quality parenting was found to predict a steeper diurnal cortisol slope than lower quality parenting in both young children and adolescents.3 Furthermore, prospective studies have found that parental sensitivity experienced in early childhood is predictive of later cortisol regulation, such that children with more sensitive caregivers in early childhood exhibited more normative cortisol production when they were adolescents.4 Taken together, these findings highlight that enhancing early parental sensitivity could have positive effects on cortisol production through adolescence, which suggests that parental sensitivity may be an important target for interventions to improve child outcomes.
Intervening to enhance the caregiving environment of high-risk children has been shown to promote adaptive regulation of the HPA axis in children.5, 21, 22 Bernard and colleagues2 found that children placed in foster care showed more normative cortisol levels than children who remained with neglecting birth parents following Child Protective Services (CPS) involvement. Although this study is limited by its cross-sectional and correlational design, findings may suggest that a positive shift in caregiving environment is associated with improvements in cortisol regulation. Experimental evidence from randomized clinical trials further support the association between enhanced caregiving and cortisol regulation. Fisher and colleagues23 investigated the effects of a family-based therapeutic intervention, the Multidimensional Treatment Foster Care for Preschoolers (MTFC-P), which trains foster parents to respond in contingent, predictable ways to children’s behavior. They found that the cortisol profiles of preschool-aged children whose foster parents received the intervention resembled those of non-maltreated controls, with higher morning values and a steeper decline in cortisol across the day than children in standard foster care placement.23
Interventions that enhance parenting behavior among CPS-involved birth parents have also been found to enhance children’s regulation of cortisol. Cicchetti and colleagues24 investigated the impact of parental and relational interventions on cortisol regulation in a sample of maltreated toddlers living with their birth parents. Following the interventions, children who experienced maltreatment were found to have cortisol profiles that were comparable to those of non-maltreated controls, whereas the children who were maltreated and received no intervention exhibited increased dysregulation of cortisol.24 This effect held one year post-intervention, as maltreated children whose parents completed an intervention continued to have more normative cortisol regulation than children with a history of maltreatment whose parents had not participated.24 In a randomized clinical trial investigating the efficacy of Attachment and Biobehavioral Catch-up (ABC), CPS-involved children whose parents received ABC exhibited more normalized cortisol production, characterized by higher wake-up values and a steeper slope from wake-up to bedtime, than children participating in a control intervention.21 Similar to Cicchetti and colleagues24 who found sustained effects one year post-intervention, ABC has also been found to have lasting effects on children’s HPA axis functioning in high-risk children three years post-intervention.5 Specifically, in a follow-up study of the same sample, ABC children continued to exhibit more normative cortisol production in early childhood than children in the control intervention.5
1.3. Present Study
Exposure to chronic early life adversity, such as child maltreatment, is associated with the down-regulation of HPA axis activity, often in the form of blunted diurnal cortisol slopes.1, 2 High quality parenting has been associated with healthy cortisol regulation among children3, 4 and interventions that target parental sensitivity in infancy, such as ABC, have been shown to have lasting effects on normalizing HPA axis activity of high-risk children into early childhood.5, 24 What is unclear is the extent to which these positive effects on HPA axis function are sustained into later developmental periods, and the mechanisms that account for the association between ABC intervention participation and HPA axis regulation. The present study sought to investigate the enduring effects of ABC participation on cortisol regulation into middle childhood, as well as examine whether parental sensitivity serves as a mediator of the association between ABC and HPA axis regulation.
2. Methods
2.1. Participants
The current sample included 103 parent-child dyads who were involved with CPS when the children were infants due to risk for child neglect. At the start of intervention, the children’s ages ranged from 1.60 to 25.30 months (M = 9.87 [5.86] months) and at the time of the middle childhood measure of cortisol regulation, children’s ages ranged from 8.0 to 11.0 years old (M = 8.52 [0.67] years). Demographic information for the sample is provided in Table 1. Written informed consent was obtained from parents and verbal assent was obtained from children in middle childhood. The study was approved by the University of Delaware Institutional Review Board.
Table 1.
Sample Demographic Characteristics. SD: standard deviation
| Characteristic | ABC Intervention n = 45 | DEF Control Intervention n = 58 |
|---|---|---|
| Child Gender, n (%) | ||
| Male | 26 (57.8) | 30 (51.7) |
| Female | 19 (42.2) | 28 (48.3) |
| Child Ethnicity, n (%) | ||
| White | 3 (6.7) | 6 (10.3) |
| African-American | 27 (60.0) | 38 (65.5) |
| Hispanic | 3 (6.7) | 10 (17.2) |
| Biracial | 12 (26.7) | 4 (6.9) |
| Child Age, mean (SD) | ||
| Pre-Intervention (months) | 10.32 (5.42) | 9.55 (6.18) |
| Range | 2.5–2.41 | 1.6–25.3 |
| Post-Intervention (months) | 20.62 (5.10) | 20.70 (5.39) |
| Range | 9.05–32.80 | 11.80–33.00 |
| Middle childhood (years) | 8.6 (0.81) | 8.45 (.55) |
| Range | 8.00–11.00 | 8.00–10.00 |
| Parent Income, mean (SD) | 24, 731 (16,362) | 23, 502 (23,974) |
| Parent Education, years, mean (SD) | 10.73 (11.43) | 11.42 (2.14) |
| Parental Sensitivity, mean (SD) | ||
| Pre-intervention | 2.13 (0.88) | 2.20 (1.10) |
| Range | 1.00–4.00 | 1.00–5.00 |
| Post-intervention | 2.48 (1.09) | 1.95 (0.77) |
| Range | 1.00–5.00 | 1.00–4.00 |
2.2. Procedure
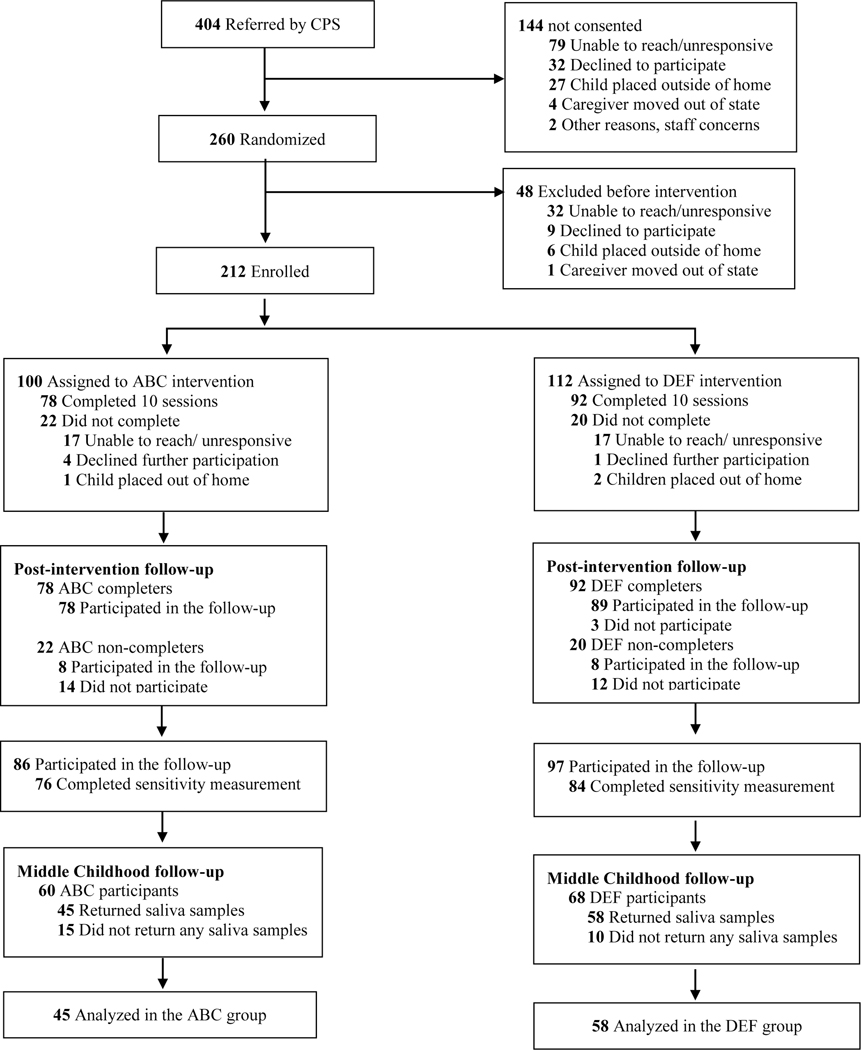
Starting in January 2006, parents of children between birth and 2 years of age were referred to the study by CPS caseworkers as part of a city-level program in a large mid-Atlantic city that was designed to divert children from entering foster care. The only inclusion criteria for referral to the study were that the target child was under the age of 2 and living with their birth parents. Children were excluded if they had serious medical conditions that interfered with locomotion, such as cerebral palsy. Study access to formal CPS records was restricted; however, based on parent report and agency referral sources, the conditions that most frequently led to participation in the diversion program were homelessness, domestic violence, maltreatment of other children, and parental substance abuse. Following referral, a project coordinator contacted families for recruitment, obtained consent from interested parents during an initial home visit, and randomly assigned families to the experimental or control group using a randomly generated sequence of numbers. Parents and research staff were blind to experimental condition. Families completed research visits before and after their intervention, and annually thereafter until children were 4 years old. The CONSORT flow diagram is displayed in Figure 1. Of the families that were randomized, 183 (86.3%) participated in at least one post-intervention follow-up visit during this initial study period of early childhood, which concluded in July 2012. The present study also includes data collected during the study’s second phase, which followed children into middle childhood. Families were contacted around the target child’s 8th birthday and completed middle childhood follow-up lab visits at the University of Delaware. This portion of the follow-up project ran from June 2014 and concluded in March 2016. Of the original sample, 128 participants (60.3%) were retained at the middle childhood follow-up. This subsample did not differ significantly in terms of race/ethnicity (χ2(3, N = 201) = 4.76, p = .190), child sex (χ2(1, N = 206) = .028, p = .867), income (t(101) = −.403, p = .688), or parental education (t(176) = −.455, p = .664) from the sample that did not participate in the middle childhood follow-up. Saliva samples were collected from 103 children (described below) for analyses of cortisol concentration, while the remaining children (n = 25) lacked cortisol data because the families did not return the samples.
Figure 1.
Consort flow diagram. CPS: Child Protective Services; ABC: Attachment and Biobehavioral Catch-up; DEF: Developmental Education for Families
2.3. Interventions.
Both the experimental and control treatments consisted of 10 weekly sessions and were implemented by trained parent coaches in the families’ homes.
2.3.1. Experimental intervention: Attachment and Biobehavioral Catch-up.
The ABC intervention has 3 primary targets: increasing parental nurturing behavior when children are distressed (parental nurturance to distress), increasing parental following the lead when children are not distressed (parental sensitivity), and decreasing frightening, harsh, and intrusive parent behavior. These targets were selected based on parental behaviors that are theoretically and empirically linked with child attachment as well as biological and behavioral regulation. Specifically, the importance of nurturance was included as a target because we found that children who were placed into foster care were especially likely to develop disorganized attachments unless their foster parents were nurturing.41 We reasoned that nurturance was critical for children who had experienced adversity if they were to develop organized attachments. The second target, following the lead, was included in response to our finding that children living with neglecting parents showed flat diurnal patterns of cortisol production.2 Although we could not find experimental evidence, correlational evidence suggested that the children of sensitive, responsive parents developed better self-regulatory capabilities than children of unresponsive parents.26 Third, we observed anecdotally in parents’ homes that some parents were frightening and intrusive in their interactions. We were aware of the evidence that frightening behavior would undermine children’s self-regulation and their ability to rely on parents27 – even if parents were nurturing and responsive. In addition to the delivery of the manualized content about the rationale for each intervention target, parents were provided with specific feedback about their behaviors that relate to intervention targets during the sessions via in-the-moment commenting and the use of video-recordings. In studies that have examined the effectiveness of ABC, in-the-moment commenting has been found to be a key component of the intervention.28 ABC has been shown to increase parental sensitivity,29, 30 as well as enhance attachment quality,31 and help regulate behavior,32 affect,33 and HPA axis function5,.21 in children.
2.3.2. Control intervention: Developmental Education for Families (DEF).
The Developmental Education for Families (DEF) intervention is an adaptation of a home visiting program focused on educating parents regarding their child’s cognitive, motor, and language development.34–36 The parent coaches engage in a range of activities with parents and children that support development in these domains.
2.4. Measures
2.4.1. Parental sensitivity.
To assess for parental sensitivity, parents and children were video-recorded completing a semi-structured interaction task pre-intervention, approximately 1-month post-intervention, and when children were 1 and 2 years old; if children were between 1 to 2 years old at the time of the post-intervention visit, this annual age-based visit was combined with the 1-month post visit. Parent behaviors were coded for sensitivity to non-distress using established protocols.29, 30 Of the videos, 40% were double coded (ICC = .70) and scores were averaged across coders. For children who had multiple assessments of parental sensitivity after the intervention, scores were averaged for analyses.
2.4.2. Saliva sampling and assay.
At the middle childhood follow-up visit, research staff trained parents to collect and store saliva samples in their homes. For three consecutive days, parents helped their child collect saliva samples via passive drool into pre-labeled vials within 30 minutes of the child waking up and immediately before bedtime. Parents also completed a daily diary to provide information on saliva sampling date and time, child health status, medication usage, and whether the child had eaten prior to collecting the sample. Parents were also instructed to not collect samples while their child was sick. Table 2 provides the descriptive statistics for the saliva samples. Samples were collected from the parents and stored in a - 20°C freezer prior to being assayed in duplicate using a high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, LLC). The duplicate samples were assayed on the same plate to minimize variability. The intra-assay and inter-assay coefficients of variation were below 7% and 11%, respectively.
Table 2.
Saliva sampling descriptive statistics
| Mean (SD) [Range] | ||||
|---|---|---|---|---|
| Intervention | n | Time of Sample | Cortisol (μg/dL) | Log transformed Cortisol (μg/dL*) |
| ABC intervention | ||||
| Waking | ||||
| Day 1 | 40 | 7:48 (1:47) | 0.14 (0.12) | −1.08 (0.56) |
| [12:00–10:40] | [0.004–0.46] | [−2.40 to −.34] | ||
| Day 2 | 43 | 7:59 (1:26) | 0.14 (0.13) | −1.0 (0.56) |
| [5:30– 1:14] | [0.004–0.55] | [−2.40 to −.26] | ||
| Day 3 | 43 | 8:03 (1:23) | 0.15 (0.12) | −1.10 (0.65) |
| [5:30–1:16] | [0.004–0.53] | [−2.70 to −.28] | ||
| Bedtime | ||||
| Day 1 | 38 | 9:02 (1:02) | 0.07 (0.08) | −1.50 (0.64) |
| [7:00–11:18] | [0.002–0.27] | [−2.70 to −.57] | ||
| Day 2 | 40 | 9:16 (1:03) | 0.08 (0.10) | −1.50 (0.64) |
| [7:00–11:47] | [0.004–0.32] | [−2.40 to −.50] | ||
| Day 3 | 45 | 9:18 (1:01) | 0.13 (0.15) | −1.31 (0.72) |
| [7:00–11:27] | [0.004–0.52] | [−2.40 to −.28] | ||
| DEF control intervention | ||||
| Waking | ||||
| Day 1 | 55 | 8:18 (1:17) | 0.12 (0.12) | −1.24 (0.66) |
| [6:00–11:34] | [0.001–0.54] | [−3.00 to −.27] | ||
| Day 2 | 56 | 8:00 (1:10) | 0.11 (0.11) | −1.23 (0.62) |
| [5:50–11:40] | [0.003–0.51] | [−2.52 to −.29] | ||
| Day 3 | 54 | 8:05 (1:41) | 0.12 (0.13) | −1.16 (0.55) |
| [12:30a-11:30] | [0.003–0.65] | [−2.52 to −.19] | ||
| Bedtime | ||||
| Day 1 | 53 | 8:25 (2:56) | 0.06 (0.06) | −1.56 (0.59) |
| [6:00–11:30] | [0.003–0.25] | [−2.52 to −.60] | ||
| Day 2 | 57 | 8:33 (2:58) | 0.06 (0.07) | −1.57 (0.58) |
| [12:00a-11:30] | [0.001–0.31] | [−3.00 to −.51] | ||
| Day 3 | 54 | 8:55 (1:04) | 0.06 (0.10) | −1.59 (0.68) |
| [4:46–10:57] | [0.001–0.43] | [−3.00 to −.36] | ||
(to convert cortisol values from micrograms per deciliter to nanomoles per liter, multiply by 27.59). ABC: Attachment and Biobehavioral Catch-up; DEF: Developmental Education for Families; SD: standard deviation
2.4.2.1. Cortisol data preparation.
As indicated above, each child could have provided as many as six saliva samples, for a total of 618 possible samples. Of the total, 578 samples (93.53%) were included in analyses, with 24 (3.88%) either not collected by the family or removed due to insufficient saliva volume and 16 (2.59%) removed as outliers. Outliers were removed from analyses using established procedures;21 biologically implausible values (those > 2.0 μg/dL: 4 values) and values > 3 SDs above the mean (12 values) were excluded. In addition, 14% of samples had cortisol concentration below the detectable limit, so their values were replaced with .004 μg/dL. To normalize the positively skewed distribution of the cortisol values, the values were log transformed. Table 2 displays the descriptive statistics of the cortisol values.
2.5. Analytic Approach
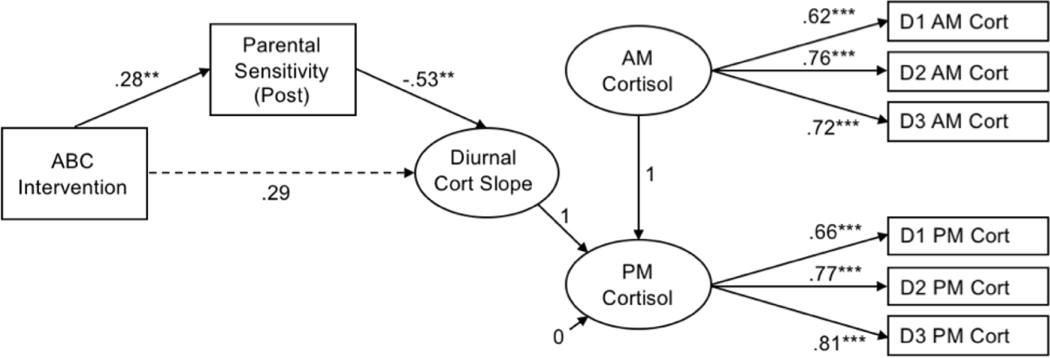
Intervention groups were compared on key demographic variables. We estimated power for the direct effect of ABC on cortisol using data from an earlier study demonstrating that ABC had a medium effect on diurnal cortisol (d = −.43) at a preschool follow-up time point.5 To detect a within-between interaction effect of this magnitude using a repeated measures ANOVA (using an alpha error probability of .05 and a correlation between wake-up and bedtime cortisol of r = .58), our sample size of N = 103 afforded >.80 power. Primary analyses were conducted in MPlus 8.0.37 Wake-up (AM) and bedtime (PM) cortisol values were defined as latent factors, each with three indicators (i.e., log-transformed cortisol values from three samples). Child diurnal cortisol slope was specified as a latent change score,15, 38, 39 representing the change in cortisol from wake-up to bedtime (i.e., PM cort – AM cort); a more negative latent change score reflected a steeper (i.e., more normative) decline in cortisol across the day. Sample time was included as a time-varying covariate by regressing each cortisol indicator on the standardized time of sample collection. To determine whether parental sensitivity mediated the association between intervention group and children’s diurnal cortisol slope, a series of regression pathways was modeled (See Figure 2): the outcome (i.e., latent change score for cortisol) was regressed on the predictor (i.e., intervention group) and on the mediator (post-intervention sensitivity); post-intervention sensitivity was regressed on intervention group and pre-intervention sensitivity. In order to test for mediation, we estimated the indirect effect of intervention group on middle childhood cortisol via post-intervention sensitivity. Child sex and racial/ethnic minority status were included as covariates. The model was estimated using maximum likelihood estimation and absolute model fit was assessed with the chi-square test of model fit, the root mean square error of approximation (RMSEA), and the comparative fit index (CFI).
Figure 2.
Path diagram with standardized coefficients for full model of ABC assignment predicting children’s diurnal cortisol slope via parental sensitivity. D1 AM Cort-D3 AM Cort and D1 PM Cort-D3 PM Cort represent log-transformed wake-up cortisol indicators and bedtime cortisol indicators for Days 1 through 3, respectively. Covariates (pre-intervention sensitivity, child sex, racial/ethnic minority status, sampling time) were included in the model, but are not depicted for simplicity. Model fit statistics indicated good fit: χ2/df = 1.22, RMSEA = .05, CFI = 0.92. **p <.01, *** p <.001. ABC: Attachment and Biobehavioral Catch-up
3. Results
ABC and DEF groups did not differ with regard to children’s sex, minority status, parental education, or income. For primary analyses, the model fit statistics demonstrated good fit to the data. The chi-square test of model fit was non-significant, χ2(85) = 103.63, p = .082, which indicates acceptable model fit.40, 41 Similarly, the RMSEA = .05 and CFI = 0.92 met criteria for good fit between the estimated model and observed data, based on respective cutoffs of ≤ .08 and ≥ .90.42. 43 Figure 1 shows the model with standardized coefficients and significance levels, with detailed information about estimated parameters presented in Table 3.
Table 3.
Model estimated parameters for the full model. C.I.: 95% confidence interval; SE: standard error; est.: standardized estimate; ABC: Attachment and Biobehavioral Catch-up.
| Effect | Standardized Estimate, β [C.I.] | SE | Est./SE | p |
|---|---|---|---|---|
| Measurement model factor loadings | ||||
| AM cortisol | ||||
| Day 1 wake-up | 0.62 [0.49, 0.75] | 0.07 | 9.03 | 0.000 |
| Day 2 wake-up | 0.76 [0.64, 0.89] | 0.06 | 12.24 | 0.000 |
| Day 3 wake-up | 0.72 [0.59, 0.86] | 0.07 | 10.54 | 0.000 |
| PM cortisol | ||||
| Day 1 bedtime | 0.66 [0.54, 0.78] | 0.06 | 10.88 | 0.000 |
| Day 2 bedtime | 0.77 [0.64, 0.89] | 0.06 | 12.21 | 0.000 |
| Day 3 bedtime | 0.81 [0.70, 0.91] | 0.05 | 15.03 | 0.000 |
| Path model: Direct effects | ||||
| Diurnal cortisol slope regressed ON | ||||
| Post-intervention sensitivity | −0.53 [−0.87, −0.19] | 0.17 | −3.04 | 0.002 |
| ABC intervention | 0.29 [−0.01, 0.60] | 0.16 | −1.87 | 0.061 |
| Child female sex | 0.02 [−0.29, 0.32] | 0.16 | 0.11 | 0.916 |
| Racial/ethnic minority status | 0.06 [−.023, 0.36] | 0.15 | 0.43 | 0.668 |
| Post-intervention sensitivity ON | ||||
| ABC intervention | 0.28 [0.09, 0.47] | 0.10 | −2.90 | 0.004 |
| Pre-intervention sensitivity | 0.23 [−0.05, 0.51] | 0.14 | 1.61 | 0.108 |
| Child female sex | 0.20 [0.01, 0.40] | 0.10 | 2.02 | 0.043 |
| Racial/ethnic minority status | −0.09 [−0.29, 0.11] | 0.10 | −0.86 | 0.391 |
| Path model: Indirect effects | ||||
| ABC intervention to cortisol slope, via sensitivity | −0.15 [−0.29, −0.01] | 0.07 | 2.08 | 0.038 |
Controlling for parental sensitivity at pre-intervention, assignment to ABC was associated with parental sensitivity at post-intervention, β = 0.28, p = .004, 95% CI [0.09, 0.47]. Parents who were assigned to ABC had higher ratings of parental sensitivity (M = 2.48, SD = 1.09) at post-intervention than parents in the control condition (M = 1.95, SD = 0.77). In addition, there was a significant association between parental sensitivity at post-intervention and children’s diurnal cortisol slope in middle childhood, controlling for pre-intervention sensitivity, child sex, and child minority status, β = −0.53, p = .002, 95% CI [−0.87, −0.19]. Children with more sensitive parents had steeper declines in their cortisol from wake-up to bedtime than children with less sensitive parents. Further, the indirect effect of ABC participation on children’s diurnal cortisol slope via post-intervention sensitivity was significant, β = −0.15, p = .038, 95% CI [−0.29, −0.01]. This indicated that parental sensitivity mediated the association between ABC participation on children’s diurnal cortisol slope. ABC participation did not have a significant direct effect on children’s diurnal cortisol slope before (β = 0.29, p = .061, 95% CI [−0.01, 0.60]) or after (β = −0.14, p = .36, 95% CI [−0.17, 0.45]) including parental sensitivity in the model as a mediator.
4. Discussion
The present study examined the enduring effects of the ABC intervention on child cortisol regulation in middle childhood and explored the role of early parental sensitivity as a potential mediator of this association. Parents assigned to ABC when their children were infants exhibited higher levels of post-intervention parental sensitivity than parents assigned to a control intervention. Higher post-intervention levels of parental sensitivity during infancy predicted a greater decline in cortisol concentration across the day in middle childhood, as evidenced by steeper diurnal cortisol slopes, than lower levels of sensitivity. Although ABC did not have a significant direct effect on middle childhood diurnal cortisol regulation, post-intervention sensitivity mediated the association between ABC and middle childhood diurnal cortisol slopes. This study emphasizes the importance of early interventions to support long term benefits for children’s regulatory abilities following experiences of maltreatment.
ABC targets several aspects of parenting behavior, including nurturance to distress, following the lead, and frightening behavior; the decision to specifically test parental sensitivity (i.e., following the lead) as a mediator of intervention effects on cortisol regulation was influenced by previous literature. Past research has suggested that sensitive parenting that involves contingent responsiveness to children’s cues supports children’s regulatory capacities generally26 and cortisol regulation,4, 44 in particular. Consistent with these correlational studies, the present study offers experimental evidence that following the lead serves as one mechanism by which ABC influences children’s cortisol regulation. Nevertheless, it will be important to explore the extent to which improvements in the other parenting dimensions (i.e., nurturance, frightening behavior) also explain intervention effects on HPA axis functioning, as well as whether each parenting target predicts distinct outcomes from the others. Such evidence of unique effects of each target on outcomes may inform the development of new interventions, as well as inform approaches for personalizing existing interventions for different parents.
Given that our findings demonstrated that increased parental sensitivity during infancy led to more normative cortisol regulation in middle childhood, it is important to consider processes involved in sustaining these effects over time. Although diurnal cortisol slopes are dynamic and susceptible to contextual factors, there is evidence that this metric of cortisol regulation also exhibits trait-like stability across childhood.45 Altered profiles of HPA axis regulation following early life stress have been found to be sustained over time.46 Given that ABC has been found to predict more normative cortisol regulation than the control condition immediately after21 and 3 years post-intervention,5 it is plausible that this experience with more sensitive parenting early in life may have helped set a trajectory for normative cortisol regulation across childhood. Although early caregiving experiences have been found to be more predictive of later cortisol regulation than concurrent measures of caregiving,4 it is possible that sustained changes in caregiving quality may have also supported sustained changes in cortisol regulation. Future studies should examine the trajectory of post-intervention parenting behavior over time and its subsequent influence on cortisol regulation over time. It will also be important to investigate whether the neurobiogical benefits associated with the enhanced parental sensitivity early in life extend beyond middle childhood, into adolescence and adulthood.
The findings of the present study have important implications, given that cortisol dysregulation has been associated with increased instances of mental and physical health disorders across the lifespan.14 Moreover, HPA axis dysregulation has been identified as a candidate mechanism by which early adverse experiences confer risk for psychopathology47 and physical health putcomes.48 Therefore, regulation of cortisol following experiences of maltreatment is key to aid in the prevention and possible amelioration of negative health outcomes for children in high-risk environments. Given the importance of cortisol regulation to subsequent health outcomes, it will be critical to examine whether the enhanced diurnal cortisol regulation associated with increased parental sensitivity impacts future mental and physical health for children.
The present study had a number of methodological strengths, including its randomized design and use of a control intervention with the same structure and duration as ABC. The longitudinal nature of the study allowed for the examination of prospective associations between ABC assignment in infancy, parental sensitivity in early childhood, and children’s diurnal cortisol regulation in middle childhood. In addition, observing parental sensitivity at multiple time-points allowed us to account for normative within-person fluctuations in sensitivity. Despite these strengths, the study also had limitations. In particular, access to participant CPS records was limited; thus, information on the specific types, duration, and severity of maltreatment experienced by the children in the sample was not available to the researchers. The collection of only wake-up and bedtime samples of cortisol is a limitation to the findings; the sampling of multiple timepoints in the day may have provided a more robust picture of the regulation of cortisol across the day. Another limitation of this study was the reliance on parents to accurately collect saliva samples immediately following wake-up and prior to bedtime, as well as accurately record the sampling times. Although this was a limitation of the present study, it should be noted that with proper training and resampling, maltreating families exhibit saliva sampling adherence comparable to demographically similar non-maltreating families.49 In addition, we did not control for all variables that can influence cortisol levels, such as quality and quantity of sleep, daily experiences with acute stress, and contextual risk factors, such as neighborhood, home environment, and parent/child psychopathology. Despite these limitations, the present study provides additional support for the utility of ABC as a change agent in the quality of parental behavior in infancy, which subsequently enhances children’s ability to regulate neurobiology over time.
4.1. Conclusion
In conclusion, the present study provides experimental evidence of the sustained effect of ABC on middle childhood diurnal cortisol regulation via early childhood parental sensitivity. This finding emphasizes the importance of early intervention in supporting children’s regulatory abilities following experiences of maltreatment. Future research should seek to determine the enduring impact of ABC participation beyond this developmental period, as well as examine how this enhanced neurobiological regulation relates to children’s behavioral, psychological, and physical health outcomes.
Highlights.
Attachment and Biobehavioral Catch-up (ABC) intervention predicted increases in parental sensitivity
Parental sensitivity in infancy predicted steeper diurnal cortisol slopes in middle childhood
Parental sensitivity mediates the association between ABC and diurnal cortisol regulation in middle childhood.
Parental sensitivity is a key target of ABC implicated in the sustained regulation of cortisol into middle childhood.
5. Acknowledgements
5.1 Source of support: This work was supported by grant R01MH074374 (to Dozier) and R01 MH119310 (to Bernard) from the National Institutes of Health.
5.2 Role of the Funder/Sponsor: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declarations of interest: none
Conflict of interest declaration: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dozier M, Manni M, Gordon MK, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreat. 2006;11(2):189–197. doi: 10.1177/1077559505285779 [DOI] [PubMed] [Google Scholar]
- 2.Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Arch Pediatr Adolesc Med. 2010;164(5):438–443. doi: 10.1001/archpediatrics.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31(3):218–231. doi: 10.1177/0165025407074634 [DOI] [Google Scholar]
- 4.Roisman GI, Susman E, Barnett Walker K, et al. Early family and child care antecedents of awakening cortisol levels in adolescence. Child Dev. 2009;80(3):907–920. doi: 10.1111/j.1467-8624.2009.01305.x [DOI] [PubMed] [Google Scholar]
- 5.Bernard K, Hostinar C, Dozier M. Intervention effects on diurnal cortisol rhythms of CPS-referred infants persist into early childhood: Preschool follow-up results of a randomized clinical trial. JAMA Pediatr. 2015;169(2):112–119. doi: 10.1001/jamapediatrics.2014.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Dev Psychobiol. 1998;33(4):327–337. doi: [DOI] [PubMed] [Google Scholar]
- 7.Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch Dis Child. 1983;58(6):454–456. doi: 10.1136/adc.58.6.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1):199–220. doi: 10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- 9.Doom JR, Cook SH, Sturza J, et al. Family conflict, chaos, and negative life events predict cortisol activity in low income children. Dev Psychobiol. 2018;60(4):364–379. 10.1002/dev.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koss KJ, Hostinar CE, Donzella B, Gunnar MR. Social deprivation and the HPA axis in early development. Psychoneuroendocrinology. 2014;50:1–13. doi: 10.1016/j.psyneuen.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51(1):14–23. doi: 10.1002/dev.20333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575 [DOI] [PubMed] [Google Scholar]
- 14.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopesand mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard K, Zwerling J, Dozier M. Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Dev Psychobiol. 2015;57(8):935–947. 10.1002/dev.21324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin CG, Kim HK, Bruce J, Fisher PA. Child diurnal cortisol rhythms, parenting quality, and externalizing behaviors in preadolescence. Psychoneuroendocrinology. 2014;40:170–180. doi: 10.1016/j.psyneuen.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alink LRA, Cicchetti D, Kim J, Rogosch FA. Longitudinal associations among child maltreatment,social functioning, and cortisol regulation. Dev Psychol. 2012;48(1):224–236. doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm Behav. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth MDS. Maternal sensitivity scales. Power. 1969;6:1379–1388. [Google Scholar]
- 20.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Erlbaum; 1978 [Google Scholar]
- 21.Bernard K, Dozier M, Bick J, Gordon MK. Intervening to enhance cortisol regulation among children at risk for neglect: results of a randomized clinical trial. Dev Psychopathol. 2015;27(3):829–841. doi: 10.1017/S095457941400073X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: a systematic review. Pediatrics. 2014;133(2):312–326. doi: 10.1542/peds.2013-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23(3):789–800. doi: 10.1017/S0954579411000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dozier M, Stovall KC, Albus KE, Bates B. Attachment for infants in foster care: The role ofcaregiver state of mind. Child Dev. 2001;72(5):1467–1477. doi: 10.1111/1467-8624.00360 [DOI] [PubMed] [Google Scholar]
- 26.Raver CC. Relations between social contingency in mother child interaction and 2-year-olds’ social competence. Dev Psychol. 1996; 32(5):850–859. doi: 10.1037/0012-1649.32.5.850. [DOI] [Google Scholar]
- 27.Schuengel C, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Frightening maternal behaviorlinking unresolved loss and disorganized infant attachment. J Consult Clin Psychol. 1999; 67(1):54–63. doi: 10.1037//0022-006x.67.1.54 [DOI] [PubMed] [Google Scholar]
- 28.Caron EB, Bernard K, Dozier M. In vivo feedback predicts parent behavior change in the Attachment and Biobehavioral Catch-up intervention. J Clin Child Adolesc Psychol. 2016;47(sup1):1–12. doi: 10.1080/15374416.2016.1141359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bick J, Dozier M. The effectiveness of an attachment based intervention in promoting foster mothers’ sensitivity toward foster infants. Infant Ment Health J. 2013;34(2):95–103. doi: 10.1002/imhj.21373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernard K, Simons R, Dozier M. Effects of an attachment-based intervention on Child Protective Services-referred mothers’ event-related potentials to children’s emotions. Child Dev. 2015;86(6):1673–1684. doi: 10.1111/cdev.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E. Enhancing attachment organization among maltreated children: Results of a randomized clinical Trial. Child Dev. 2012;83(2):623–636. doi: 10.1111/j.1467-8624.2011.01712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dozier M, Peloso E, Lindhiem O, et al. Developing evidence based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. J Soc Issues. 2006;62(4):767–785. doi: 10.1111/j.1540-4560.2006.00486.x [DOI] [Google Scholar]
- 33.Lind T, Bernard K, Ross E, Dozier M. Intervention effects on negative affect of CPS-referred children: Results of a randomized clinical trial. Child Abuse Negl. 2014;38(9):1459–1467. doi: 10.1016/j.chiabu.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks-Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low-birthweight,premature infants: Changes in cognition and behavior over the first three years. Child Dev. 1993;64(3):736–753. doi: 10.1111/j.1467-8624.1993.tb02940.x [DOI] [PubMed] [Google Scholar]
- 35.Ramey CT, Yeates KO, Short EJ. The plasticity of intellectual development: insights frompreventative intervention. Child Dev. 1984;55(5):1913–1925. doi: 10.2307/1129938 [DOI] [PubMed] [Google Scholar]
- 36.Ramey CT, McGinness GD, Cross L, Collier AM, Barrie-Blackley S. The Abecedarian approach tosocial competence: Cognitive and linguistic intervention for disadvantaged preschoolers In Borman K K, ed. The social life of children in a changing society. Hillsdale, NJ: Erlbaum; 1982:145–174. [Google Scholar]
- 37.Muthén L, Muthén B. Mplus, version 8 [computer software]. Los Angeles, CA: Author; 2017. [Google Scholar]
- 38.Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Dev Psychopathol. 2008;20(2):473–491. doi: 10.1017/S0954579408000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data In Collins LM, Sayer AG, eds. Decade of behavior: New methods for the analysis of change. Washington, DC: American Psychological Association; 2001:139–175. [Google Scholar]
- 40.Byrne BM. A primer of LISREL: Basic applications and programming for confirmatory factor analytic models. New York, New York: Springer-Verlag; 1989. [Google Scholar]
- 41.Carmines EG, McIver JP. Analyzing models with unobserved variables In Bohrnstedt GW, Borgatta EF, eds. Social measurement: Current issues. Beverly Hills, CA: Sage Publications; 1981:65–115. [Google Scholar]
- 42.Browne MW, Cudeck R. Alternative ways of assessing model fit In Bollen KA, Long JS, eds. Testing structural equation models. London: Sage Ltd; 1993:136–162. [Google Scholar]
- 43.Chen FF, Sousa KH, West SG. Teacher’s corner: Testing measurement invariance of second-order factor models. Struct Equ Modeling. 2005;12(3):471–492. doi: 10.1207/s15328007sem1203_7 [DOI] [Google Scholar]
- 44.DePasquale CE, Raby KL, Hoye J, Dozier M. Parenting predicts Strange Situation cortisol reactivity among children adopted internationally. Psychoneuroendocrinology. 2018;89:86–91. doi: 10.1016/j.psyneuen.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2012;54(5):493–502. doi: 10.1002/dev.20607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Essex MJ, Shirtcliff EA, Burk LR, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23(4):1039–1058. doi: 10.1017/S0954579411000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koss KJ, Gunnar MR. Annual research review: Early adversity, the hypothalamic-pituitaryadrenocortical axis, and child psychopathology. J Child Psychol Psychiatry. 2018;59(4):327–346. doi: 10.1111/jcpp.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berens AE, Jensen SKG, Nelson CA. Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 2017;15(1): 35–147. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentino K, De Alba A, Hibel LC, Fondren K, McDonnell CG. Adherence to diurnal cortisol sampling among mother–child dyads from maltreating and non-maltreating families. Child Maltreat. 2017;22(4):286–294. doi: 10.1177/1077559517725208 [DOI] [PMC free article] [PubMed] [Google Scholar]