Abstract
Objective
The objective of this study was to investigate whether diacerein has comparable efficacy with celecoxib in pain reduction for treatment in symptomatic knee OA patients.
Methods
This randomized double-blind multicentre non-inferiority trial evaluated diacerein vs celecoxib treatment in patients with Kellgren–Lawrence grade 2–3 and pain scoring ≥4 (10-cm VAS). Patients were randomized to 6 months of treatment with diacerein 50 mg (n = 187) once daily for 1 month and twice daily thereafter, or celecoxib 200 mg (n = 193) once daily. The primary outcome was the change in WOMAC pain score (0–50 cm) at 6 months, and the secondary outcomes were WOMAC sub-scores, VAS pain score, and the OMERACT–OARSI responder rate.
Results
In the per protocol population, the adjusted mean change from baseline in the WOMAC pain score was –11.1 ( 0.9) with diacerein (n = 140) and –11.8 (0.9) with celecoxib (n = 148). The intergroup difference was 0.7 (95% CI: −1.8, 3.2; P = 0.597), meeting the non-inferiority margin. Supportive analysis of the intention-to-treat population gave similar results. Other outcomes showed no significant difference between treatment groups. The incidence of treatment-related adverse events was low and balanced between groups, but a greater incidence of diarrhoea occurred with diacerein (10.2% vs 3.7%). Diarrhoea was considered mild-to-moderate in all but one case with complete resolution.
Conclusions
Diacerein was non-inferior to celecoxib in reducing knee OA pain and improving physical function. Diacerein also demonstrated a good safety profile.
Trial registration
A multicentre study on the effect of DIacerein on Structure and Symptoms vs Celecoxib in Osteoarthritis is a National Institutes of Health (NCT02688400) and European Clinical Trial Database (2015–002933-23) registered phase III (Canada) or IV (Europe) study.
Keywords: diacerein, celecoxib, osteoarthritis, non-inferiority trial, SYSADOA
Rheumatology key messages
Diacerein has comparable efficacy with celecoxib, regarding pain reduction after 6 months of treatment for knee OA.
No new safety issue has been identified; DISSCO confirms the positive benefit–risk ratio of diacerein treatment for knee OA.
Diacerein is an alternative to the use of COX-2 inhibitors in knee OA treatment.
Introduction
OA is one of the most common debilitating chronic diseases, particularly in the older population [1, 2], contributing to a marked reduction of quality of life and a gradual loss of function [3, 4]. It is also associated with an increased risk of cardiovascular disease and death [5, 6].
To date, OA treatment remains mainly symptomatic. Among the pharmacological interventions, NSAIDs, cyclooxygenase-2 (COX-2) inhibitors, and analgesics such as paracetamol (acetaminophen), which have fairly rapid onset of action, have been commonly used and recommended [7–11]. Although these drugs are popular for acute flare-ups of the disease, their use for an extended period could lead to significant adverse events (AEs) such as cardiovascular diseases, renal and liver toxicity [12–15].
Symptomatic slow-acting drugs for OA (SYSADOAs) are a group of drugs that includes diacerein, glucosamine, and chondroitin sulfate (CS), which have been used for many years for the non-acute treatment of OA symptoms. SYSADOAs have been recommended alone and in combination with analgesics/NSAIDs for long-term management of OA [16–18]. Moreover, the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) guidelines have suggested that they could be used as first line (background) treatment for chronic therapy [16, 18, 19].
Diacerein is an anthraquinone derivative, which has been successfully used for the treatment of OA symptoms and it has a positive benefit-to-risk ratio [17, 20–23].
Studies have clearly established the efficacy of diacerein compared with placebo in knee and hip OA patients [17, 20–23], and a similar level of efficacy in relieving OA symptoms and improving function compared with conventional non-selective NSAIDs [17, 23]. Importantly, diacerein demonstrated a marked carry-over effect, with efficacy retained for weeks or months after treatment discontinuation [17, 20–23]. In contrast to NSAIDs, diacerein does not affect the synthesis of prostaglandins [24]. In addition, diacerein’s main mechanism of action is the inhibition of IL-1β and its signalling pathway, and it has been demonstrated to have an anticatabolic effect on OA tissues [25]. This alternative mechanism of action likely explains the absence of upper gastrointestinal (GI) tract [26] and cardiovascular toxicity [27] as compared with NSAIDs.
Due to a better GI safety profile, COX-2 selective inhibitors such as celecoxib have become one of the most commonly used and recommended NSAIDs for the symptomatic treatment of OA [7–11, 13, 18, 19]. However, no study has yet explored the comparative efficacy and safety of diacerein vs celecoxib. Thus, the main outcome of the DISSCO study (a multicentre study on the effect of DIacerein on Structure and Symptoms vsCelecoxib in Osteoarthritis) was to investigate whether diacerein has comparable efficacy with that of celecoxib after 6 months of treatment in symptomatic knee OA patients with moderate-to-severe disease. The study is a non-inferiority trial: as such, the reference treatment’s efficacy must be established or widely used, as is the case for celecoxib, which has also proven to be superior to placebo [28–30]; therefore, a placebo or untreated control group would be deemed inappropriate from a methodological point of view [31].
Methods
Study design
DISSCO is a National Institutes of Health (NIH; NCT02688400) and European Clinical Trial Database (EudraCT 2015–002933-23) registered phase III (Canada) or IV (Europe) international, multicentre, double-blind, randomized, controlled, parallel-group, non-inferiority, symptom-modifying clinical trial comparing diacerein (50 mg twice daily) with celecoxib (200 mg once daily). Patients were enrolled at public hospitals or clinics in Canada and in Europe (Spain, Austria, Belgium and Czech Republic). The study was conducted from 12 May 2016 to 26 June 2018. The trial was performed according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice. Institutional or central ethics approval was obtained from all participating centres, and all patients gave their written informed consent to participate.
Patient selection
Men and women ≥50 years of age, with primary and symptomatic knee OA complying with the classification criteria of knee OA established by the ACR [32], with OA of Kellgren–Lawrence grade 2 or 3 [33] and moderate-to-severe pain [Visual Analogue Scale (VAS) pain score (0–10 cm) while walking on a flat surface ≥4 cm] at inclusion were selected for the study. In patients with bilateral disease, the most symptomatic knee (VAS score ≥4 cm) at screening was selected as the target knee, if it met the inclusion criteria. Patients were excluded if they had concurrent medical or arthritic conditions that could confound the evaluation of the index joint, or coexisting disease that could preclude successful completion of the trial, such as history of cardiovascular or GI events. The list of selection criteria is fully detailed in Supplementary Table S1, available at Rheumatology online.
Treatment regimens and randomization
Subjects were assigned sequentially in a 1:1 ratio using a computer-generated randomization list prepared by an independent biostatistician (Inferential, Paris, France) using the PROC PLAN SAS System (version 9.2) software. Treatment allocation depended on the time sequence in which patients entered the study, thus minimizing the selection bias.
Included patients were randomized to receive either diacerein 50 mg taken once a day for 1 month and diacerein 50 mg twice daily thereafter (Artrodar/Verboril, TRB Chemedica, Geneva, Switzerland) or celecoxib 200 mg (Celebrex, Pfizer Canada, Kirkland, Québec, Canada; Celecoxib, Apotex, Toronto, Ontario, Canada) once daily. Patients in both groups were taking the same number of capsules daily. (For further details refer to Supplementary Methods, available at Rheumatology online.) Patients in the diacerein group took one capsule of diacerein and one capsule of matched placebo for 1 month and then two capsules of diacerein, while patients in the celecoxib group took one capsule of celecoxib and one capsule of matched placebo. Capsules of celecoxib available on the market were over-encapsulated to visually and physically match the capsules used for diacerein, providing identical appearance to allow for a double-blind design.
Patients were allowed to take acetaminophen 500 mg tablets up to 2 g/day (500 mg four times daily) as rescue medication, except during the 48 h before clinical evaluation. Compliance with the study treatment was assessed by the investigative centres.
Patient compliance with study medication intake was assessed at each study visit (through patient interview and accountability of drug dispensed/retrieved) and recorded in the electronic case report form. Patients were regarded as compliant if the calculated compliance was at least 75% of the study medication required to be taken during the study, unless a dose was withheld due to AEs or other unavoidable reasons (which required approval by the investigator). Patients deemed to be non-compliant were withdrawn from the study and an End of Study form was completed.
Outcomes
The primary objective was to show that diacerein is non-inferior to celecoxib in terms of pain reduction (Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] pain subscale) after 6 months (182 days) of treatment in symptomatic knee OA patients.
Safety outcomes included incidence of AEs, changes in various laboratory tests and vital signs.
Statistical analysis
The sample size calculation was established to test the non-inferiority of diacerein vs celecoxib in the assessment of the change from baseline in WOMAC pain at day 182 (6 months). It was deemed that 144 patients per treatment group were sufficient to claim non-inferiority with a power of 90% at a one-sided significance level of 2.5%, assuming a similar mean decrease in WOMAC pain score at day 182 in the diacerein and celecoxib groups, and a common standard deviation (s.d.) of 26 in the two treatment groups [28–30, 34–36]. A delta of ten units (scale 0–100; same as a delta of 5 in the original range 0–50) was used in the study. Such a margin was selected as, according to Ehrich et al. [37], the minimal perceptible clinical improvement (MPCI) in patients with knee OA with the WOMAC questionnaire was determined to be 9.7 for WOMAC pain and 11.1 mm for WOMAC pain question 1 (pain walking on a flat surface). This margin is also well in line with previous similar published randomized clinical trials [28, 34].
The main analyses were performed using the per protocol (PP) population defined as all randomized patients who received at least one dose of the study medication, who had an efficacy measurement at inclusion and at least one corresponding post-inclusion efficacy measurement (for the primary efficacy variable) and who did not present any major deviation of the protocol over the 6-month follow-up period. In non-inferiority trials, the PP is used in the primary analysis as it is the most conservative approach [38, 39]. The intention-to-treat (ITT) population included all randomized patients who received at least one dose of the study medication, had an efficacy measurement at inclusion and at least one corresponding post-inclusion efficacy measurement (for the primary efficacy variable), while the safety population consisted of all patients who took at least one dose of the study medication. All analyses were performed according to the randomization group, regardless of the study medication actually received.
Non-inferiority of diacerein vs celecoxib was assessed by computing the difference in the adjusted mean change from baseline in WOMAC pain subscale score after 182 days of treatment between diacerein and celecoxib groups from a Mixed Model for Repeated Measurements (MMRM). MMRM was adjusted for treatment group, time and treatment using time as fixed factor and the subject and error terms as random factors. The response variable was the absolute change from baseline in WOMAC pain score, and the baseline value of the WOMAC pain score was used as a covariate. The within-patient variance covariance matrix was assumed to be unstructured.
The treatment comparisons were carried out by means of the contrasts on the treatment factor by time effect. Treatment effects were estimated by means of Least Square Means (LSM) and their standard error (s.e.) and 95% confidence interval (CI). Differences between treatments were the differences between LSM and their s.e. and 95% CI. For the non-inferiority claim, the upper bound of the 95% CI of the difference in the adjusted mean change between diacerein and celecoxib at day 182 had to be inferior to 5 (scale 0–50) on the PP population.
Supportive analyses of the primary efficacy criterion were also conducted. The primary analysis was repeated on the ITT population and using the Last Observation Carried Forward (LOCF) approach to test the robustness of the results.
Quantitative data were analysed with the Student’s t test (Gaussian variable) or the Mann–Whitney test (non-Gaussian variable), and the qualitative data were analysed with a χ2 test.
All secondary end points were analysed on the ITT at day 182/early termination.
Statistical analyses were performed using SAS® software version 9.2 (SAS Institute, Cary, NC, USA). For all analyses, statistical tests were set two-sided at 5% significance level.
Results
Patient disposition and characteristics
A total of 527 patients were screened, and 380 underwent randomization. Of these, 376 were included in the safety population, 370 in the ITT analysis, and 288 in the PP analysis. The completion rate and the reasons for withdrawal were similar in the two treatment groups (Fig. 1).
Fig. 1.
Flow diagram of patients
ITT: intention-to-treat; PP: per protocol
The baseline and clinical characteristics of the participants analysed and those with discontinued treatment were identical (data not shown). Patients in the two treatment groups of the PP population (primary analysis) had similar demographics and baseline characteristics (Table 1), and the groups were well balanced at baseline. The mean WOMAC pain score at baseline was for diacerein 28.7 (9.1) and for celecoxib 27.0 (8.2); the VAS pain intensity was 6.5 (1.3) and 6.4 (1.2), respectively. The characteristics of the ITT population (secondary outcomes) were similar to those of the PP population (Supplementary Table S2, available at Rheumatology online).
Table 1.
Baseline demographic and clinical characteristics of the PP population
| Diacerein (n = 140) | Celecoxib (n = 148) | |
|---|---|---|
| Age – years | 63.7 (6.1)a | 64.1 (6.5) |
| Women – n (%) | 102 (72.9) | 111 (75.0) |
| Body mass index – kg/m2 | 31.5 (5.8) | 30.1 (5.1) |
| Identification of studied knee (right) – n (%) | 62 (44.3) | 74 (50.0) |
| Most common analgesics before study inclusion – n (%) | ||
| Paracetamol | 28 (20.0) | 36 (24.3) |
| Ibuprofen | 11 (7.9) | 6 (4.1) |
| Celecoxib | 9 (6.4) | 4 (2.7) |
| Diclofenac | 8 (5.7) | 6 (4.1) |
| WOMAC | ||
| Total score (0–240 cm) | 136.6 (44.2) | 129.6 (38.3) |
| Pain score (0–50 cm) | 28.7 (9.1) | 27.0 (8.2) |
| Stiffness score (0–20 cm) | 11.8 (4.3) | 11.7 (3.9) |
| Physical function score (0–170 cm) | 96.1 (33.7) | 90.9 (28.8) |
| VAS pain score (0–10 cm) | 6.5 (1.3) | 6.4 (1.2) |
| Joint swelling and effusion (yes) – n (%) | 30 (21.6) | 35 (23.8) |
| Patient’s global assessment of disease activity (0–10 cm) | 6.1 (1.9) | 5.8 (1.8) |
| Investigator’s global assessment of disease activity (0–10 cm) | 5.9 (1.3) | 5.9 (1.4) |
| Quality of life (SF-36) (0–100) | ||
| Physical component summary | 34.8 (7.1) | 34.1 (6.6) |
| Mental component summary | 48.7 (10.3) | 50.3 (9.6) |
Data are mean (s.d.) unless otherwise indicated.
n: number of patients in the treatment group; PP: per protocol; SF-36: 36-Item Short Form Health Survey; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Primary efficacy analysis
In the PP population, the adjusted absolute mean (s.e.) change from baseline in the WOMAC pain score at day 182 was −11.1 ( 0.9) in the diacerein group vs –11.8 (0.9) in the celecoxib group (Table 2). The adjusted mean intergroup difference (diacerein – celecoxib) was 0.7 (95% CI: −1.8, 3.2; P = 0.597). Non-inferiority of diacerein to celecoxib was proven, since the upper bound of the 95% CI of the intergroup difference was lower than five. The supportive analyses performed on the ITT population confirmed the conclusion of the primary analysis (Table 2).
Table 2.
Primary efficacy analysis: absolute change in WOMAC pain score
| Diacerein | Celecoxib | P-value | Treatment differences | |
|---|---|---|---|---|
| Primary analysis | ||||
| PP | (n = 140) | (n = 148) | ||
| −11.1 (0.9)a | −11.8 (0.9)a | 0.597b | 0.7 (−1.8, 3.2)c | |
| Supportive analysis | ||||
| ITT | (n = 183) | (n = 187) | ||
| −9.6 (0.8)a | −10.0 (0.8)a | 0.712b | 0.4 (−1.9, 2.7)c | |
| ITT (LOCF)d | (n = 183) | (n = 187) | ||
| −9.5 (0.8)a | −10.0 (0.8)a | 0.657b | 0.5 (−1.7, 2.8)c | |
WOMAC pain score scale 0–50.
Absolute change from baseline to day 182, MMRM model adjusted mean (s.e.).
MMRM model, P-value of treatment effect.
MMRM model adjusted means (95% CI).
MMRM model where missing data were imputed by the LOCF approach.
ITT: intention-to-treat; LOCF: last observation carried forward; MMRM: mixed models for repeated measurement; n: number of patients in the treatment group; PP: per protocol; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Secondary outcomes
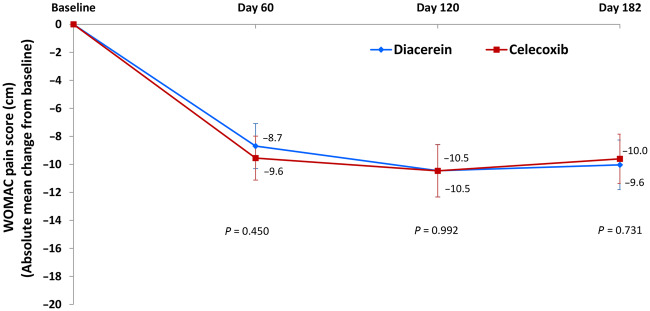
The results of the secondary efficacy criteria (ITT population) are summarized in Table 3. There was no statistically significant difference between the two treatment groups at day 182 in any of the WOMAC scores or VAS pain (Table 3). Both treatments led to a rapid decrease in WOMAC subscores, as well as VAS pain by day 60, which was sustained over time with no significant intergroup difference at any timepoint (Fig. 2; Supplementary Figs S1 and S2, available at Rheumatology online). The rate of responders based on Outcome Measures in Rheumatology Clinical Trials and Osteoarthritis Research Society International (OMERACT–OARSI) criteria [40] was similar in both groups (P > 0.05) and sustained over time (Supplementary Fig. S3, available at Rheumatology online).
Table 3.
Secondary efficacy analyses in the ITT population
| Outcome | Diacerein (n = 183) | Celecoxib (n = 187) | P-value |
|---|---|---|---|
| WOMAC total score (0–240 cm) | |||
| Baseline | 135.5 (44.3) | 130.1 (38.0) | – |
| Day 182 | 94.5 (55.3) | 87.1 (56.6) | – |
| Change | −41.0 (53.1) | −42.9 (55.0) | 0.813a |
| WOMAC stiffness score (0–20 cm) | |||
| Baseline | 11.8 (4.2) | 11.7 (4.0) | – |
| Day 182 | 8.2 (5.0) | 7.7 (5.0) | – |
| Change | −3.6 (5.0) | −4.0 (5.3) | 0.477a |
| WOMAC function score (0–170 cm) | |||
| Baseline | 95.2 (33.7) | 91.6 (28.8) | – |
| Day 182 | 68.1 (40.3) | 62.3 (40.8) | – |
| Change | −27.2 (39.0) | −29.3 (39.8) | 0.714a |
| VAS pain score (0–10 cm) | |||
| Baseline | 6.6 (1.3) | 6.4 (1.3) | – |
| Day 182 | 4.2 (2.6) | 3.9 (2.6) | – |
| Change | −2.3 (2.6) | −2.5 (2.6) | 0.686a |
| OMERACT-OARSI responder at Day 182 – n (%) | 99 (55.6) | 97 (53.3) | 0.658b |
| Joint swelling and effusion at Day 182 – n (%) | 19 (10.7) | 23 (12.5) | 0.601b |
| Consumption of rescue medication (tablets per day; days 0–182) | 1.1 (1.8) | 0.9 (1.0) | 0.330a |
| Patient’s global assessment of disease activity (0–10 cm) | |||
| Baseline | 5.9 (1.9) | 5.8 (1.8) | – |
| Day 182 | 4.2 (2.5) | 3.8 (2.5) | – |
| Change | −1.8 (2.8) | −2.0 (3.0) | 0.592a |
| Investigator’s global assessment of disease activity (0–10 cm) | |||
| Baseline | 5.9 (1.3) | 5.9 (1.4) | – |
| Day 182 | 3.9 (2.4) | 3.2 (2.3) | – |
| Change | −2.0 (2.6) | −2.7 (2.6) | 0.011a |
| Patient’s global assessment of response to therapy at Day 182 (0–10) | 3.9 (2.6) | 3.6 (2.5) | 0.381a |
| Investigator’s global assessment of response to therapy at Day 182 (0–10) | 3.9 (2.5) | 3.4 (2.4) | 0.057a |
| Quality of life (SF-36) (0–100) | |||
| Physical component summary | |||
| Baseline | 34.7 (7.0) | 34.0 (6.4) | – |
| Day 182 | 37.2 (7.8) | 38.6 (8.3) | – |
| Change | 2.5 (6.7) | 4.6 (8.1) | 0.008a |
| Mental component summary | |||
| Baseline | 49.2 (10.1) | 49.4 (10.1) | – |
| Day 182 | 51.0 (8.8) | 49.7 (9.4) | – |
| Change | 1.6 (8.3) | −0.1 (8.9) | 0.042a |
Data are mean (s.d.) or as indicated.
For continuous variables, between-group comparisons were done using a Student’s t test or Mann–Whitney test.
Between-group comparisons using a χ2 test.
n: number of patients in the treatment group; ITT: intention-to-treat; OARSI: Osteoarthritis Research Society International; OMERACT: Outcome Measures in Rheumatology Clinical Trials; SF-36: 36-Item Short Form Health Survey; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Ostoearthritis Index.
Fig. 2.
Absolute change from baseline in WOMAC pain score – comparisons between treatment groups (ITT populations)
Data are mean (95% CI). Between-group comparisons were done using a Student’s t-test. ITT: intention-to-treat; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
There was a marked gradual decrease of about 50% in the percentage of patients with joint swelling and/or effusion over time, without statistically significant differences between groups (Supplementary Fig. S4, available at Rheumatology online).
In addition, there was a small but sustained improvement in the patient’s global assessment of disease activity, without significant intergroup difference at any time (Table 3). The investigator’s change in the global assessment of disease activity at day 182 was slightly but significantly higher (P = 0.011) in the celecoxib than in the diacerein group. No statistically significant intergroup difference was, however, identified at the other visits (data not shown). There was a numerical trend favouring diacerein (P = 0.057) for the investigator’s global assessment of response to therapy at day 182. Improvement in the Short Form 36 (SF-36) quality of life questionnaire at day 182 was slightly higher in the celecoxib group for Physical Component Summary (PCS) (P = 0.008) and in the diacerein group for Mental Component Summary (MCS) (P = 0.042).
The consumption of rescue medication (number of acetaminophen tablets per day) over the study period was low and similar between the groups (Table 3). The overall treatment compliance, defined as patients having taken ≥75% of the medication during the 6-month study period, was the same in the two treatment groups (diacerein, 91.3%; celecoxib, 93.6%). The mean duration of treatment was 155.9 ( 52.8) and 159.4 (52.7) days with diacerein and celecoxib, respectively.
Safety
Overall, both products were well tolerated during the study. No new safety issue arose regarding treatments in terms of AEs, blood tests, or physical examination.
The number of patients with emergent AEs considered by the investigators as related to the study treatment was higher in the diacerein group (n = 49; 26.3%) than in the celecoxib group (n = 33, 17.4%), and this finding was mainly due to an increase in diarrhoea in the former (10.2% vs 3.7%) (Supplementary Table S3, available at Rheumatology online).
Of the 19 patients in the diacerein group who had diarrhoea, nine were considered mild (4.8%), nine moderate (4.8%) and one (0.5%) experienced severe diarrhoea. In the celecoxib group, of the seven patients with diarrhoea, it was considered mild in five patients (2.6%) and moderate in two (1.1%). Diarrhoea led to permanent study drug discontinuation in nine (4.8%) patients in the diacerein vs three (1.6%) in the celecoxib group.
Three SAEs were considered by the investigators as possibly related to the study drug in the diacerein group and none in the celecoxib group. The three SAEs (abdominal pain, elevated transaminase and gamma-glutamyl transferase) all occurred in a single patient and resolved spontaneously following drug withdrawal. Other SAEs in both groups were considered as unrelated to the study drug by the investigators.
Discussion
This study demonstrated that diacerein is non-inferior to celecoxib in patients with primary knee OA with moderate-to-severe pain in terms of pain reduction (WOMAC pain subscale) after 6 months of treatment. Overall, the treatment effect on OA symptoms was similar in both treatment groups. The results confirm the efficacy of diacerein [17] and confirm the findings from previous studies in patients with moderate-to-severe knee and hip OA pain [41–45].
The effect of treatment on pain and/or symptoms was already observed at day 60 and was maintained for the entire duration of the study. A slightly more rapid onset of action was observed with celecoxib at day 60, which is in line with findings from previous studies [17, 41, 43, 45]. A possible explanation includes the fact that celecoxib, but not diacerein, inhibits prostaglandin synthesis, a mode of action known to induce a rapid reduction in signs and symptoms of OA [14, 23]. The step-up diacerein regimen (50 mg daily dose for the first month to 100 mg daily thereafter) might also explain the slight delay in response to therapy. However, one must note that no significant differences have been identified between the two treatments at any time regarding the extent of improvement of disease symptoms. However, it should also be acknowledged that the first observation time of the effect of treatment on symptoms was done at day 60, which may have underestimated the more rapid time to response to therapy of celecoxib vs diacerein. This is one of the reasons why in the early phase of treatment of OA symptoms with SYSADOAs, the combined use of NSAIDs and SYSADOAs could be most useful [16, 18, 19, 41, 45]. Importantly, the compliance to treatment and use of rescue medications were similar for both treatments and therefore did not interfere with the response to treatment.
The results of the OMERACT–OARSI responder analysis also support the excellent clinical response to both treatments: ∼60% of patients were considered as responders at days 120 and 182. These results confirm the efficacy of diacerein at reducing OA pain and improving physical function and patient global assessment. Our data also support previous findings that diacerein efficacy on OA pain is comparable with that reported for NSAIDs [17, 41, 43] and other SYSADOAs such as CS [46, 47].
For both treatments, the gradual decrease over time in the percentage of patients with joint swelling/effusion likely reflects the significant anti-inflammatory action of the drugs and is in line with previous data comparing celecoxib with CS [46], another SYSADOA.
One may question the results of the investigator’s global assessment of disease activity as well as SF-36 improvement (physical and mental components) showing differences between the treatment groups. Albeit statistically significant, they represent a very small fraction of difference, which is certainly not perceptible by patients, hence considered not clinically relevant.
The incidence of AEs related to drug treatment was balanced between groups, except for GI side effects (diarrhoea) in the diacerein group. These were considered to be generally mild to moderate, and accounted for only a low incidence of permanent discontinuation in the diacerein group, which was in line with previous reports [17]. The incidence of diarrhoea, the main side effect known to be associated with diacerein treatment, was lower in this study than was previously reported in some other studies [17, 20, 42]. This could be explained first by the patient selection, which excluded those with significant GI diseases, and second by the step-up diacerein regimen in this trial. Finally, the drug formulation used in the present study may also have had an impact on the low incidence of diarrhoea [48].
The good safety profile of diacerein was also demonstrated by the absence of any treatment-related SAEs, except for one case of minor and transient increase in liver transaminases with rapid spontaneous resolution upon cessation of diacerein. Of note, the concomitant intake of fibrate provided an alternative possible cause for altered liver function tests [49].
The absence of major safety issues with diacerein in this trial supports the latest European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) guidelines [18, 19], which propose diacerein as a safe alternative over NSAIDs or celecoxib, especially for individuals at cardiovascular or GI risk who have contraindications for treatment with such drugs.
It should be noted that patients with past or present history of upper GI, renal and/or cardiovascular conditions were excluded from the study. As part of any study design using an NSAID comparator, these exclusion criteria probably favoured per patient selection having a significantly better long-term celecoxib safety profile than one may expect in a real-world scenario. Moreover, patients included in the study were those at low risk of cardiovascular disease, which may have impacted the results by ‘protecting’ the celecoxib cohort from the occurrence of such events. Similarly, patients with chronic kidney disease, another well-known contraindication for NSAID usage, were excluded from the study, which may explain the low incidence of worsening high blood pressure.
In addition to this selection bias, another limitation of this study relates to the duration of the study and the choice of the clinical outcomes. All end points evaluated at day 182 were patient-reported outcomes. As such, they might be influenced not only by the study treatment but also by additional factors such as staff and participant expectations, personal perception of pain, or perceived invasiveness of the treatment [50]. However, according to Ehrich et al. [37], the MPCI is about 10 for WOMAC knee pain (scale 0–100), while such a margin was easily surpassed by both diacerein and celecoxib groups at 6 months, demonstrating the usefulness of both drugs, yet no significant differences between these two interventions was seen.
Although there is a well-known placebo effect in OA, we did not include a placebo group as the study design is a non-inferiority trial. Moreover, it would have been unethical to use a placebo treatment arm, particularly while studying patients with moderate-to-severe pain for a 6-month period [31], and in view of the fact that diacerein has already been shown to have a beneficial effect on pain in several placebo-controlled clinical trials in knee OA [17, 21, 23]. The washout period for the rescue analgesic before evaluations was long enough not to have impact on the studied pain outcomes.
Another limitation pertains to the preselected (as per MPCI) 10-point non-inferiority margin of diacerein vs celecoxib for WOMAC knee pain (scale 0–100). This represents roughly a 50% preserved fraction of the effect size of celecoxib over placebo, which may be conservative. However, the results of our trial yielded an even tighter lower boundary of non-inferiority for WOMAC pain score (3.2 on a scale of 0–50), maintaining even more of the preserved fraction (closer to 70%) than originally planned.
Since this study was powered to primarily assess the efficacy of both treatments, another limitation is the impossibility to ascertain, per patient number, due to careful selection and the relatively short-term study duration, the occurrence of SAEs such as major cardiac, GI or renal side effects.
These results demonstrate that diacerein is non-inferior to celecoxib in reducing pain. No differences were found for stiffness or functional limitations after 6 months of treatment in patients with moderate-to-severe pain from knee OA, and diacerein demonstrated a good safety profile and tolerability. DISSCO therefore confirms the positive benefit–risk ratio of diacerein in knee OA and re-establishes diacerein as a therapeutic option to avoid the use of COX-2 inhibitors in this indication.
Supplementary Material
Acknowledgements
JPP, JPR and JMP contributed equally to manuscript preparation. All authors had full access to all of the study data, take responsibility for the integrity of the data and the accuracy of the data analysis, and have approved the submitted version of the work. JPP, JPR, PP, MD and JMP were responsible for the study concept and design. JPP, JPR, PP, MD, LB, ED, FM, KP and JMP were responsible for the acquisition and/or analysis and interpretation of the data, and drafting of the manuscript. JPP, JPR, MD and JMP were responsible for critical revision of the manuscript with respect to intellectual content. JPP, JPR, PP, MD and JMP were responsible for the statistical analysis. The authors would like to thank the following people from the DISSCO Trial Investigator Group: Asuncion Acosta Pereira (Barcelona, Spain), Pierre Arsenault (Sherbrooke, Canada), Francisco J. Blanco (A Coruna, Spain), Murray Baron (Montréal, Canada), Jean-Pierre Brasseur (Yvoir, Belgium), Martin Cohen (Montréal, Canada), Stephan Domayers (Zicksee, Austria), Julio Fernandes (Montréal, Canada), André Fréchette (Québec, Canada), Suneil Kapur (Ottawa, Canada), Burkhard F. Leeb (Stockerau, Austria), Marc Leon (Hornu, Belgium), Ingrid Möller (Barcelona, Spain), Jordi Monfort Faure (Barcelona, Spain), Petr Němec (Brno, Czech Republic), Cristobal Orellana (Sabadell, Spain), Juan Povedano (Seville, Spain), Montserrat Romera Baures (Barcelona, Spain), Frank Raeman (Merksem, Belgium), Pieter Reynders (Brussels, Belgium), Jan Rosa (Prague, Czech Republic), Serge Schreiber (La Louvière, Belgium), Jan Schulz (Montréal, Canada), Jacqueline Uson (Madrid, Spain), Pascale Verret (Montréal, Canada). The authors also thank Santa Fiori and Jacqueline Brunet for their assistance with preparation of the manuscript. Publicly available data can be gathered from the NIH (https://clinicaltrials.gov/ct2/show/NCT02688400? term=02688400&rank=1) or EudraCT (https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-002933-23/results) clinical trial databases. Additional unpublished data may be obtained upon reasonable request from TRB Chemedica (scientific@trbchemedica.com) as long as the request is evaluated as scientifically relevant.
Funding: This work was supported by TRB Chemedica International SA. The sponsor provided study medications free of charge and met the expenses that arose during the course of the study. The sponsor also participated in the design of the study.
Disclosure statement: JPP has received a study grant and speaker fees from TRB Chemedica, speaker fees from Mylan, and is a shareholder in ArthroLab Inc. JPR and MD are consultants for ArthroLab Inc. KP has received honoraria for lectures and/or consultation from AbbVie, BMS, Egis, Fidia, Medac, MSD, Pfizer, TRB Chemedica and UCB. JMP has received a study grant from TRB Chemedica, and is a shareholder in ArthroLab Inc. PP is an employee of ArthroLab Inc. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Woolf AD, Pfleger B.. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R. et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 3. Hiligsmann M, Cooper C, Arden N. et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2013;43:303–13. [DOI] [PubMed] [Google Scholar]
- 4. Litwic A, Edwards MH, Dennison EM, Cooper C.. Epidemiology and burden of osteoarthritis. Br Med Bull 2013;105:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang H, Bai J, He B, Hu X, Liu D.. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep 2016;6:39672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO.. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:938–46. [DOI] [PubMed] [Google Scholar]
- 7. Jordan KM, Arden NK, Doherty M. et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang W, Nuki G, Moskowitz RW. et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–99. [DOI] [PubMed] [Google Scholar]
- 9. Hochberg MC, Altman RD, April KT. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- 10. McAlindon TE, Bannuru RR, Sullivan MC. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management. In: NICE clinical guideline [CG177] 2014. https://www.nice.org.uk/guidance/cg177 (14 November 2018, date last accessed). [PubMed]
- 12.FDA. Drug Safety Communication 13 January 2011: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit: Boxed Warning Will Highlight Potential for Severe Liver Failure. 2011. https://wayback.archive-it.org/7993/20170111224110/http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm (21 February 2020, date last accessed).
- 13. Bhala N, Emberson J, Merhi A, Abramson S. et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C.. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016;45(4 Suppl):S22–7. [DOI] [PubMed] [Google Scholar]
- 15. Roberts E, Delgado Nunes V, Buckner S. et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2016;75:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruyere O, Cooper C, Pelletier JP. et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253–63. [DOI] [PubMed] [Google Scholar]
- 17. Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moca Trevisani V.. Diacerein for osteoarthritis. Cochrane Database Syst Rev 2014;2:CD005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruyere O, Cooper C, Pelletier JP. et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016;45(4 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- 19. Bruyère O, Honvo G, Veronese N. et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum 2019;49:337–50. [DOI] [PubMed] [Google Scholar]
- 20. Rintelen B, Neumann K, Leeb BF.. A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch Intern Med 2006;166:1899–906. [DOI] [PubMed] [Google Scholar]
- 21. Bartels EM, Bliddal H, Schondorff PK. et al. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage 2010;18:289–96. [DOI] [PubMed] [Google Scholar]
- 22. Panova E, Jones G.. Benefit–risk assessment of diacerein in the treatment of osteoarthritis. Drug Saf 2015;38:245–52. [DOI] [PubMed] [Google Scholar]
- 23. Pavelka K, Bruyere O, Cooper C. et al. Diacerein: benefits, risks and place in the management of osteoarthritis. An opinion-based report from the ESCEO. Drugs Aging 2016;33:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franchi-Micheli S, Lavacchi L, Friedmann CA, Zilletti L.. The influence of rhein on the biosynthesis of prostaglandin-like substances in vitro. J Pharm Pharmacol 1983;35:262–4. [DOI] [PubMed] [Google Scholar]
- 25. Martel-Pelletier J, Pelletier JP.. Effects of diacerein at the molecular level in the osteoarthritis disease process. Ther Adv Musculoskelet Dis 2010;2:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrillo M, Montrone F, Ardizzone S, Caruso I, Porro GB.. Endoscopic evaluation of diacetylrhein-induced gastric-mucosal lesions. Curr Ther Res Clin Exp 1991;49:10–5. [Google Scholar]
- 27. Mattei E, Marzoli GA, Oberto G, Brunetti MM. Diacerein effects on the cardiovascular function of the conscious dog following repeated oral administration. RTC Study No. 70600. RTC Research Toxicology Centre. 2009.
- 28. Bingham CO, Sebba AI, Rubin BR. et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology 2007;46:496–507. [DOI] [PubMed] [Google Scholar]
- 29. Rother M, Lavins BJ, Kneer W. et al. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis 2007;66:1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bingham CO III, Bird SR, Smugar SS, Xu X, Tershakovec AM.. Responder analysis and correlation of outcome measures: pooled results from two identical studies comparing etoricoxib, celecoxib, and placebo in osteoarthritis. Osteoarthritis Cartilage 2008;16:1289–93. [DOI] [PubMed] [Google Scholar]
- 31. Piaggio G, Elbourne DR, Altman DG. et al. Reporting of noninferiority and equivalence randomized trials. JAMA 2006;295:1152–60. [DOI] [PubMed] [Google Scholar]
- 32. Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 33. Kellgren JH, Lawrence JS.. Radiological assessment of osteoarthosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Birbara C, Ruoff G, Sheldon E. et al. Efficacy and safety of rofecoxib 12.5 mg and celecoxib 200 mg in two similarly designed osteoarthritis studies. Curr Med Res Opin 2006;22:199–210. [DOI] [PubMed] [Google Scholar]
- 35. Bingham CO III, Smugar SS, Wang H, Tershakovec AM.. Early response to COX-2 inhibitors as a predictor of overall response in osteoarthritis: pooled results from two identical trials comparing etoricoxib, celecoxib and placebo. Rheumatology 2009;48:1122–7. [DOI] [PubMed] [Google Scholar]
- 36. Schnitzer TJ, Kivitz A, Frayssinet H, Duquesroix B.. Efficacy and safety of naproxcinod in the treatment of patients with osteoarthritis of the knee: a 13-week prospective, randomized, multicenter study. Osteoarthritis Cartilage 2010;18:629–39. [DOI] [PubMed] [Google Scholar]
- 37. Ehrich EW, Davies GM, Watson DJ. et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27:2635–41. [PubMed] [Google Scholar]
- 38.International Conference on Harmonization (ICH) E9 Expert Working Group. ICH Harmonised Tripartite Guideline Topic E9: Statistical principles for clinical trials. Stat Med 1999;18:1905–42. [PubMed] [Google Scholar]
- 39.Committee for Proprietary Medicinal Products (CPMP). CPMP/EWP/482/99 Final. Points to consider on switching between superiority and non-inferiority. London: European Medicines Agency, 2000. https://www.ema.europa.eu/en/documents/scientific-guideline/points-consider-switching-between-superiority-non-inferiority_en.pdf (21 February 2020, date last accessed).
- 40. Pham T, Van Der Heijde D, Lassere M. et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol 2003;30:1648–54. [PubMed] [Google Scholar]
- 41. Nguyen M, Dougados M, Berdah L, Amor B.. Diacerhein in the treatment of osteoarthritis of the hip. Arthritis Rheum 1994;37:529–36. [DOI] [PubMed] [Google Scholar]
- 42. Pelletier JP, Yaron M, Haraoui B. et al. Efficacy and safety of diacerein in osteoarthritis of the knee: a double-blind, placebo-controlled trial. The Diacerein Study Group. Arthritis Rheum 2000;43:2339–48. [DOI] [PubMed] [Google Scholar]
- 43. Zheng WJ, Tang FL, Li J. et al. Efficacy and safety of diacerein in osteoarthritis of the knee: a randomized, multicenter, double-dummy, diclofenac-controlled trial in China. APLAR J Rheumatol 2006;9:64–9. [Google Scholar]
- 44. Brahmachari B, Chatterjee S, Ghosh A.. Efficacy and safety of diacerein in early knee osteoarthritis: a randomized placebo-controlled trial. Clin Rheumatol 2009;28:1193–8. [DOI] [PubMed] [Google Scholar]
- 45. Singh K, Sharma R, Rai J.. Diacerein as adjuvant to diclofenac sodium in osteoarthritis knee. Int J Rheum Dis 2012;15:69–77. [DOI] [PubMed] [Google Scholar]
- 46. Hochberg MC, Martel-Pelletier J, Monfort J. et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis 2016;75:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reginster JY, Dudler J, Blicharski T, Pavelka K.. Pharmaceutical-grade chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann Rheum Dis 2017;76:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pelletier JP, Martel-Pelletier J.. Diacerein-containing products: same risk of diarrhoea? Aging Clin Exp Res 2018;30:411–2. [DOI] [PubMed] [Google Scholar]
- 49. Newman CB, Preiss D, Tobert JA. et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2019;39:e38–e81. [DOI] [PubMed] [Google Scholar]
- 50. McAlindon TE, Driban JB, Henrotin Y. et al. OARSI Clinical Trials Recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage 2015;23:747–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.