Abstract
Objective
Lupus nephritis (LN) increases the risks of end-stage renal disease (ESRD) and death, but these risks are difficult to estimate. Since complement factors play an essential role in the pathogenesis and are deposited in the kidneys as C1q and C3, we studied whether these deposits predict ESRD and death in patients with LN.
Methods
We collected demographic, clinical and pathological data from 183 adult patients with LN classes II–V diagnosed with a first native kidney biopsy. Pathological data included the localization and intensity of immunofluorescence staining of C1q and C3. We obtained dates of incident ESRD and death from the United States Renal Data System and National Death Index, respectively, and evaluated survival curves and hazard ratios for ESRD and death as a composite outcome and as separate outcomes.
Results
The presence and intensity of deposits of C1q and C3 in glomeruli, tubular walls and vascular walls differed between classes and were associated with known unfavourable prognostic factors, such as hypertension, hypoalbuminemia and hypocomplementemia. However, over a median follow-up of 7.5 years, their presence and intensity were associated with neither survival free of ESRD and death nor hazard ratios for ESRD and death.
Conclusion
Renal deposits of complement factors did not predict ESRD and death in patients with LN.
Keywords: biomarker, biopsy, histopathology, immunology, prognosis, systemic lupus erythematosus
Rheumatology key messages
Deposits of complement factors in kidneys play a role in the pathogenesis of lupus nephritis.
The relevance of such deposits for prognosis and treatment has been little studied.
Such deposits did not predict end-stage renal disease and death in this large prospective cohort.
Introduction
Lupus nephritis (LN) occurs in up to two-thirds of patients with SLE and causes increased risks of end-stage renal disease (ESRD) and death [1–3]. These risks can be estimated based on clinical and pathological factors, including race/ethnicity, hypertension, proteinuria, anaemia, serum levels of creatinine, complement factors, anti-dsDNA antibodies, the pattern of glomerular damage, the extent of tubulointerstitial inflammation and the extent of interstitial fibrosis and tubular atrophy, but with variable certainty [3–8]. The effect of treatment to reduce these risks, which often remains disappointing, is also poorly estimated with these factors [3, 7, 9]. Thus a prognosis is difficult to establish.
The complement system plays an essential role in the pathogenesis of LN. Immune complexes are deposited or formed in the kidneys and bind complement factor C1q. Due to activation by C1q and other unknown mechanisms, complement factor C3 is deposited as well. The co-deposition of Igs, C1q and C3 is characteristic of LN. Circulating C3 and C4 are often depressed due to their consumption and associated with disease activity [5, 7, 10]. Complement activation is also associated with subclinical and flaring disease [7, 11, 12]. Some cases have been successfully treated with the complement inhibitor eculizumab; other complement inhibitors are currently being developed for treatment [3, 7, 9, 10].
The relevance of renal deposition of complement factors for the prognosis and treatment of LN has, nonetheless, been little studied. Such a role has been demonstrated in other renal diseases with dysregulation of the complement system. In IgA nephropathy, for example, mesangial deposits of C3, C4 and their end-product C5b-9 are associated with progression of disease to ESRD, independent of glomerular filtration rate (GFR) and proteinuria [13]. Therefore we studied whether renal deposits of complement factors predict ESRD and death in patients with LN.
Methods
Study population
The study population included patients with LN who were ≥18 years of age and had a first native kidney biopsy at Brigham and Women’s Hospital from March 1990 through December 2016. We excluded patients with minimal mesangial or advanced sclerosing LN [6]. For the present study, we additionally excluded 16 patients whose biopsies did not contain non-sclerosed glomeruli assessed with immunofluorescence microscopy. The study complied with the Helsinki Declaration and was approved by Brigham and Women’s Hospital’s Institutional Review Board. Informed consent was not obtained from the patients for this study, as approved by the Institutional Review Board.
Demographic and clinical data
Demographic and clinical data were derived from Brigham and Women’s Hospital’s Lupus Registry and medical records. We systematically defined current hypertension as two or more measurements of systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg within 1 month before or after the biopsy. We recorded the use of medication within 1 month before, but not after, the biopsy. We quantified organ damage using the SLE SLICC/ACR damage index. We excluded parameters reflecting renal damage from the calculation of the SLICC/ACR damage index. We recorded laboratory values within 1 month before the biopsy and the serum level of creatinine 1 year after the biopsy. We recorded the presence of aPL antibodies, systematically defined as the presence of aCL antibodies, anti-β2-glycoprotein antibodies or lupus anticoagulant measured at least twice, any time before the biopsy. We calculated the estimated GFR (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. We systematically defined proteinuria as a urine protein:creatinine ratio of ≥1 g/g.
Pathological data
The biopsies were assessed following standardized criteria with light, immunofluorescence and electron microscopy. Based on assessment of the entire cortical area, biopsies were classified according to the World Health Organization/International Society of Nephrology/Renal Pathology Society (WHO/ISN/RPS) classes. We analysed biopsies with LN class II and V as being class V, class III and V as being class III and class IV and V as being class IV, reflecting similar reclassification when designing treatment [3, 6]. The extent of glomerulosclerosis was quantified as the percentage of segmentally and/or globally sclerosed glomeruli. The extent of tubulointerstitial inflammation, reflecting acute damage, and the extent of interstitial fibrosis and tubular atrophy, reflecting chronic damage, were quantified as the percentages involving the cortical area. Vascular damage due to medial and subintimal sclerosis was systematically defined and quantified as moderate to severe if the lumen was narrowed by ≥25%. Features of active and chronic nephritis were quantified using the National Institutes of Health (NIH) activity and chronicity indices. Immunofluorescence staining was performed for IgG, IgA and IgM, kappa and lambda light chains, C1q and C3, fibrin and albumin. It was used to localize deposits of C1q and C3 in the mesangium, glomerular capillary walls, tubular walls and/or extraglomerular vascular walls and to score the intensity on a scale of 0–4, with trace intensity scored as 0.5. We categorized deposits as absent when the intensity was ≤0.5 and as present when the intensity was ≥1. Glomerular deposits included the highest intensity of deposits in the mesangium and/or capillary walls. We excluded non-specific deposits from our analyses, including segmental glomerular deposits of only IgM, segmental glomerular deposits of IgM and C3 and/or C1q in sclerotic areas and linear deposits of IgG with only albumin along all basement membranes.
ESRD and death
Dates of incident ESRD through December 2016 were retrieved from the United States Renal Data System (USRDS), a national record of patients who need renal replacement therapy [14]. Medical records were linked to the USRDS using unique identifiers following a data use agreement with the USRDS. Dates of death through December 2016 were retrieved from the National Death Index by linkage to medical records using unique identifiers. We used the first occurrence of either ESRD or death as a composite outcome.
Statistical analyses
We evaluated differences in continuous variables with the Wilcoxon or Kruskal–Wallis rank-sum test, differences in categorical variables with Pearson’s chi-squared test and correlations with Spearman’s coefficient. We calculated the change in eGFR and the odds ratio for a decline in eGFR over 1 year after the biopsy with linear and logistic regression, respectively. We drew curves for survival free of ESRD and death and evaluated differences with the log-rank test. We calculated hazard ratios for the composite outcome of ESRD and death and for the single outcome of death with Cox’s proportional hazards model and for the single outcome of ESRD with Fine and Gray’s proportional subhazards model accounting for death as a competing risk. We repeated these calculations with adjustment for demographic covariates, including age, sex, race/ethnicity and the calendar year in which the biopsy was performed, with adjustment for clinical covariates, including current hypertension, the duration of SLE before the biopsy, the SLICC/ACR damage index, the serum level of haemoglobin, the serum level of albumin, the presence of proteinuria and the presence of anti-dsDNA antibodies, or with adjustment for pathological covariates, including the extent of glomerulosclerosis, extent of tubulointerstitial inflammation, extent of interstitial fibrosis and tubular atrophy, presence of moderate to severe vascular damage and the pathological activity and chronicity indices, while excluding patients with missing values of these covariates.
Results
Demographic, clinical and pathological characteristics
Table 1 displays the characteristics of the study population, for all patients and for patients stratified by WHO/ISN/RPS class.
Table 1.
Characteristics of the study population
| Characteristics | All | Class II | Class III | Class IV | Class V |
|---|---|---|---|---|---|
| Number of patients, n | 183 | 21 | 47 | 69 | 46 |
| Age, median (IQR), years | 36 (26–45) | 36 (27–47) | 31 (26–44) | 35 (25–44) | 40 (26–46) |
| Female, % | 83.6 | 76.2 | 76.6 | 87.0 | 89.1 |
| Race/ethnicity, % | |||||
| White | 36.1 | 33.3 | 38.3 | 39.1 | 30.4 |
| Black | 31.7 | 42.9 | 23.4 | 34.8 | 30.4 |
| Hispanic | 18.0 | 9.5 | 27.7 | 14.5 | 17.4 |
| Asian | 11.5 | 14.3 | 8.5 | 8.7 | 17.4 |
| Other or unknown | 2.7 | 0.0 | 2.1 | 2.9 | 4.4 |
| Past medical history, % | |||||
| Hypertension | 58.5 | 71.4 | 53.2 | 62.3 | 52.2 |
| Diabetes mellitus | 4.9 | 4.8 | 6.4 | 2.9 | 6.5 |
| Current hypertension, % | 29.6 | 15.0 | 31.0 | 40.7 | 18.4 |
| Duration of SLE, median (IQR), years | 3.6 (0.5–10.7) | 5.8 (1.7–10.7) | 4.3 (0.3–12.0) | 3.5 (0.4–9.4) | 2.0 (0.7–9.7) |
| SLICC/ACR damage index, median (IQR)a | 2 (1–4) | 2 (1–6) | 2 (1–4) | 2 (1–5) | 1 (0–4) |
| Medication, % | |||||
| ACE inhibitors | 28.3 | 15.0 | 27.9 | 33.3 | 27.9 |
| NSAIDs | 23.5 | 25.0 | 20.9 | 28.3 | 18.6 |
| HCQ | 51.2 | 70.0 | 48.8 | 50.0 | 46.5 |
| High-dose corticosteroidsb | 30.7 | 35.0 | 34.9 | 33.3 | 20.9 |
| Immunosuppressivesc | 21.1 | 25.0 | 25.6 | 16.7 | 20.9 |
| Haemoglobin, median (IQR), g/dl | 10.8 (9.3–12.1) | 11.8 (11.0–12.9) | 10.8 (9.5–11.6) | 10.1 (8.7–10.9) | 11.9 (10.5–12.6) |
| Serum albumin, median (IQR), g/dl | 3.2 (2.7–3.5) | 3.7 (2.9–4.1) | 3.4 (2.9–3.7) | 2.9 (2.4–3.3) | 3.2 (2.6–3.6) |
| Serum creatinine, median (IQR), mg/dl | 0.9 (0.7–1.3) | 0.8 (0.7–1.2) | 0.9 (0.6–1.1) | 1.1 (0.8–1.8) | 0.8 (0.6–1.0) |
| eGFR, median (IQR), ml/min/1.73 m2 | 93 (53–119) | 112 (63–130) | 104 (67–122) | 77 (39–106) | 97 (75–125) |
| Proteinuria, %d | 73.3 | 42.1 | 60.5 | 87.1 | 79.0 |
| Serum C3, median (IQR), mg/dl | 59 (43–83) | 76 (50–116) | 66 (44–97) | 48 (35–60) | 73 (57–97) |
| Serum C4, median (IQR), mg/dl | 9 (6–15) | 11 (7–16) | 10 (7–15) | 7 (5–11) | 12 (8–19) |
| Anti-dsDNA antibodies, % | 81.1 | 79.0 | 80.5 | 95.1 | 60.5 |
| aPL antibodies, %e | 35.9 | 41.7 | 31.8 | 36.1 | 36.4 |
| Glomerulosclerosis, median (IQR), % | 5 (0–22) | 7 (0–23) | 4 (0–14) | 7 (0–28) | 7 (0–15) |
| IF/TA, median (IQR), % | 10 (0–20) | 5 (0–10) | 10 (0–15) | 10 (0–30) | 10 (0–10) |
| Any tubulointerstitial inflammation, % | 14.3 | 10.0 | 19.2 | 17.4 | 6.5 |
| Moderate–severe vascular damage, % | 35.5 | 38.1 | 36.2 | 33.3 | 37.0 |
| Pathological activity index, median (IQR)f | 6 (2–11) | 1 (1–2) | 6 (4–9) | 11 (9–13) | 1 (1–2) |
| Pathological chronicity index, median (IQR)g | 2 (1–3) | 1 (0–3) | 2 (1–3) | 3 (1–5) | 1 (0–3) |
Values were missing for current hypertension in 24 patients, the duration of SLE in 4, the SLICC/ACR damage index in 11, use of medication in 17, haemoglobin in 14, serum albumin in 18, serum creatinine and eGFR in 12, serum C3 in 31, serum C4 in 33, proteinuria in 26, anti-dsDNA antibodies in 24, aPL antibodies in 91, the extent of glomerulosclerosis in 1 and the pathological activity and chronicity indices in 60.
Excluding the parameters reflecting renal damage, maximum score of 37.
Prednisone ≥20 mg/day or equivalent.
Including azathioprine, cyclophosphamide, mycophenolate mofetil, methotrexate and rituximab.
Protein:creatinine ratio ≥1 g/g.
aCL antibodies, anti-β2-glycoprotein antibodies or lupus anticoagulant detected at least twice.
Maximum score of 24.
Maximum score of 12.
Quantified on a scale of 0–4.
ACE: angiotensin-converting enzyme; IF/TA: interstitial fibrosis and tubular atrophy; IQR: interquartile range.
Table 2 describes the presence and intensity of deposits of C1q and C3. Glomerular C1q was more often present in classes III (P = 0.04), IV (P = 0.009) and V (P = 0.03) than in class II and was more intense in classes III (P = 0.006), IV (P < 0.001) and V (P = 0.008) than in class II. Glomerular C3 was more often present in classes IV (P = 0.009) and V (P = 0.005) than in class II, was more intense in classes III (P = 0.03), IV (P < 0.001) and V (P = 0.03) than in class II and was more intense in class IV than in classes III (P = 0.03) and V (P = 0.04). Tubular C1q was more often present in class IV than in classes II (P = 0.02) and V (P = 0.006). Tubular C3 was more often present in classes III (P = 0.03), IV (P < 0.001) and V (P < 0.001) than in class II and more often present in classes IV (P = 0.02) and V (P = 0.03) than in class III. Vascular C3 was more often present in classes IV (P = 0.04) and V (P = 0.03) than in class II.
Table 2.
Renal deposits of complement factors
| Complement factors | All | Class II | Class III | Class IV | Class V |
|---|---|---|---|---|---|
| Glomerular deposits | |||||
| Presence of C1q, % | 79.8 | 57.1 | 80.9 | 84.1 | 82.6 |
| Presence of C3, % | 90.2 | 71.4 | 89.4 | 92.8 | 95.7 |
| Intensity of C1q, median (IQR)a | 2 (1–3) | 1 (0–2) | 2 (1–3) | 3 (2–3) | 2 (1–3) |
| Intensity of C3, median (IQR)a | 2 (2–3) | 2 (0.5–2) | 2 (2–3) | 3 (2–3) | 2 (2–3) |
| Presence of C1q with or without C3, %b | 86.4 | 75.0 | 90.5 | 87.9 | 84.4 |
| Presence of C3 only, % | 13.6 | 25.0 | 9.5 | 12.1 | 15.6 |
| Tubular deposits | |||||
| Presence of C1q, % | 19.7 | 4.8 | 21.3 | 30.4 | 8.7 |
| Presence of C3, % | 45.4 | 9.5 | 34.0 | 56.5 | 56.5 |
| Presence of C1q with or without C3, %c | 41.4 | 33.3 | 55.6 | 52.5 | 15.4 |
| Presence of C3 only, % | 58.6 | 66.7 | 44.4 | 47.5 | 84.6 |
| Vascular deposits | |||||
| Presence of C1q, % | 20.8 | 14.3 | 21.3 | 26.1 | 15.2 |
| Presence of C3, % | 71.6 | 52.4 | 68.1 | 75.4 | 78.3 |
| Presence of C1q with or without C3, %d | 28.4 | 27.3 | 30.3 | 34.0 | 18.9 |
| Presence of C3 only, % | 71.6 | 72.7 | 69.7 | 66.0 | 81.1 |
Quantified on a scale of 0–4.
Presence of C1q without C3 occurred in 4 patients.
Presence of C1q without C3 occurred in 4 patients.
Presence of C1q without C3 occurred in 3 patients.
Glomerular deposits of C1q were most often present with glomerular deposits of C3, reflecting activation of the classical complement pathway. Tubular deposits of C1q with or without C3 were about as often present as tubular deposits of C3 only, the latter reflecting activation of the alternative complement pathway. Vascular deposits of C1q with or without vascular deposits of C3 were less often present than vascular deposits of C3 only. There were no differences between the WHO/ISN/RPS classes, except that the presence of tubular deposits of C1q with or without C3 was less frequent and that the presence of tubular deposits of C3 only was more frequent in class V than in class III (P = 0.005) and class IV (P = 0.002) (Table 2).
The presence and intensity of deposits of C1q and C3 were associated with known prognostic factors, such as lower haemoglobin;, lower serum levels of albumin, C3 and C4; and a higher frequency of anti-dsDNA antibodies, except for vascular deposits of C3. The intensity, but not the presence, of glomerular deposits of C1q and C3 was negatively associated with the SLICC/ACR damage index, excluding the parameters reflecting renal damage, and with the presence of aPL antibodies. The presence of tubular C1q and C3 was associated with a higher frequency of hypertension, whereas the presence of glomerular C3 was associated with a lower frequency of hypertension. The presence and intensity of C1q and C3 were also associated with a higher pathological activity index and/or a lower pathological chronicity index. The presence and intensity of deposits were not associated with the use of medication; the serum level of creatinine; eGFR; the presence of aPL antibodies; the extents of glomerulosclerosis, interstitial fibrosis and tubular atrophy; and tubulointerstitial inflammation (Supplementary Tables S1–S3, available at Rheumatology online).
Change in kidney function
As a secondary analysis among 135 patients with available values of eGFR before and a median of 1.0 year after the kidney biopsy who did not develop ESRD, eGFR increased overall with a median 1.4 ml/min/1.73 m2, but declined in 66 patients (48.9%). The presence and intensity of glomerular C1q and C3 were associated with an increase in eGFR in all patients and patients with class III only and with lower odds for a decline in eGFR in all patients only. The presence of tubular C1q with or without C3 was associated with an increase in eGFR and a lower odds for a decline in eGFR in all patients and in patients with class IV, while the presence of tubular C3 only was naturally associated with the reverse. Otherwise the presence and intensity of deposits trended towards associations with an increase in eGFR and lower odds for a decline in eGFR without statistical significance (Supplementary Table S4, available at Rheumatology online). Adjustment for demographic, clinical or pathological covariates did not substantially change these associations.
ESRD and death
Over a median follow-up of 7.5 years, 49 of all patients (26.8%) reached the composite outcome of ESRD and death, of whom 45 (24.6%) developed ESRD and 14 (7.7%) died with or without having developed ESRD. The composite outcome was more common in class IV than in patients with classes II (P = 0.03), III (P = 0.04) and V (P = 0.03), while the follow-up period was similar across classes (P = 0.50, Supplementary Table S5, available at Rheumatology online).
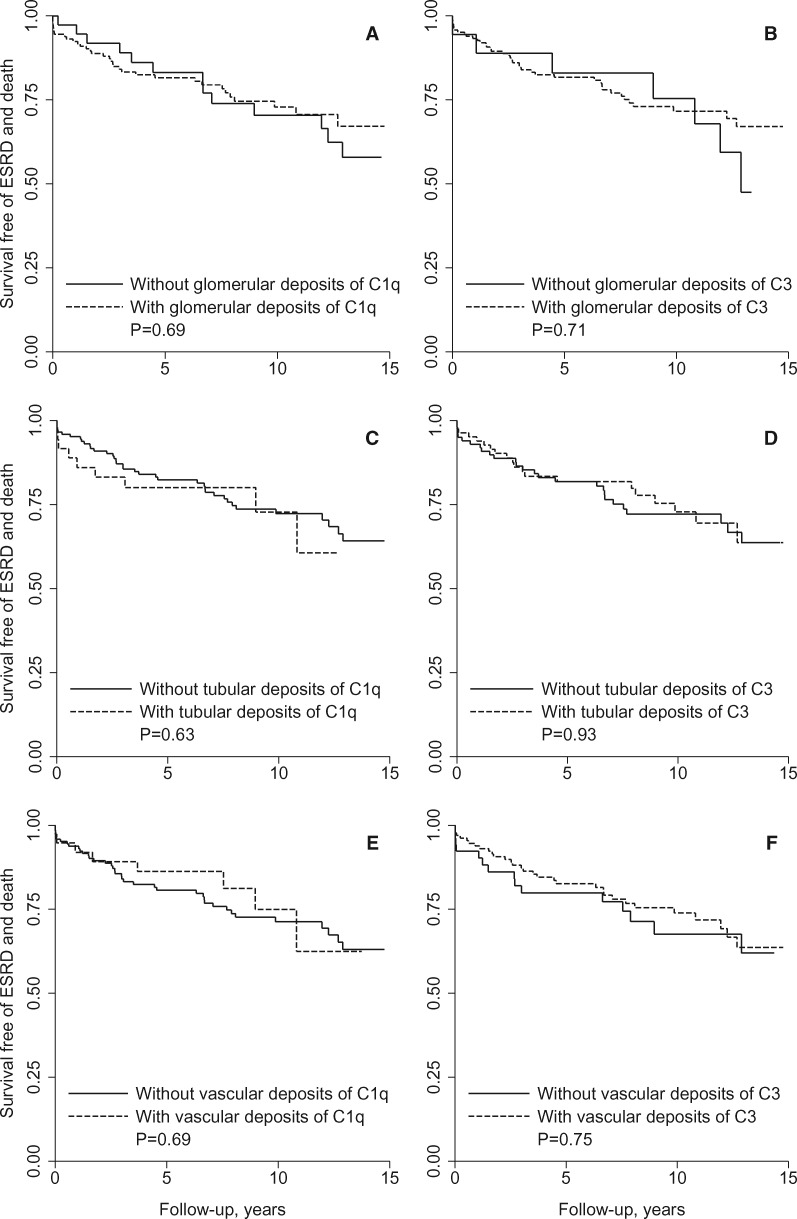
Shown in Fig. 1, survival free of ESRD and death did not differ between patients with or without deposits of C1q or C3. Survival was not dependent on the intensity of C1q (P = 0.73) or C3 (P = 0.25) or on the presence of glomerular, tubular or vascular deposits of C1q with or without C3 or of C3 only (P > 0.53).
Fig. 1.
Renal deposits of complement factors and survival free of ESRD and death
Curves of survival free of the composite outcome of ESRD and death are shown for patients with and without glomerular deposits of (A) C1q and (B) C3, tubular deposits of (C) C1q and (D) C3 and extraglomerular vascular deposits of (E) C1q and (F) C3 with P-values for differences between the curves. Curves are shown up to 15 years after the kidney biopsy, as <10% of the patients had a longer follow-up.
When stratified by WHO/ISN/RPS class, the presence and intensity of glomerular C3 was associated with a lower hazard ratio for the composite outcome of ESRD and death in patients with class IV only, but this association disappeared when adjusting for demographic, clinical or pathological covariates. Otherwise the presence and intensity of deposits were not associated with ESRD and/or death, without or with adjustments (Supplementary Table S5, available at Rheumatology online).
Separate analyses of glomerular deposits localized in the mesangium or capillary walls did not change these results.
Discussion
In this large population of patients with LN, renal deposits of complement factors C1q and C3 were associated with unfavourable clinical and pathological prognostic factors but did not predict ESRD and death. The rationale for our study was the lack of consistent findings from large populations with LN.
Two previous studies showed that the presence of glomerular and vascular deposits of C1q did not predict treatment effect, doubling of creatinine and ESRD [15, 16]. Glomerular C1q was also not associated with prognostic factors. In one of these studies, interestingly, the serum level of creatinine doubled less often when glomerular C1q had resolved in a second biopsy than when it persisted [15].
A recent study showed that glomerular deposits of C3 were associated with active LN, unfavourable prognostic factors and a beneficial treatment effect, but only if stained with an antibody against C3c rather than against C3d, possibly because C3c is cleared faster than C3d [9]. In our study, deposits were stained with an antibody against generic C3, including the long-lasting C3d, which may explain why they were not associated with prognosis.
In two other small studies, the presence of glomerular deposits of C4d was independent of C1q and C3 and associated with more severe proteinuria and a higher pathological activity index in one, but not the other [17, 18]. Follow-up was available only for the former: all patients who developed ESRD exhibited these deposits, but none of those who did not develop ESRD, which reached borderline statistical significance [17].
Activation and deposition of C1q, C3 and C4d leads to the formation and deposition of C5b-9, which lyses cell membranes [5, 10]. The presence and intensity of glomerular C5b-9 were recently reported to be similar in active and chronic LN and not associated with prognostic factors, likely because C5b-9 is cleared slowly. Still, a higher intensity predicted a lack of treatment effect. The intensity of tubular C5b-9 was associated with the extent of interstitial fibrosis and tubular atrophy, reflecting chronic damage [9].
Tubular deposits were reported to be associated with unfavourable prognostic factors and to predict doubling of the serum level of creatinine, but only in a very small population of patients with non-proliferative LN, without specification of the deposits’ composition, and not after adjustment for sex, the serum level of creatinine and the pathological activity and chronicity indices [19]. Immunoelectron microscopy appeared more sensitive to detect deposits than immunofluorescence microscopy. Deposits detected by the latter were associated less with prognostic factors.
The inconsistent findings may be explained by the ambiguous role of complement factors in the pathogenesis of LN. In addition to inducing inflammation and cell damage, they also help to clear immune complexes and cellular debris. Deficiency of a complement factor therefore increases the risk of LN [3, 7, 10]. Our finding that deposits of complement factors were more frequent in the more proliferative classes of LN could be either a cause or a consequence of the inflammation and cell damage in these classes. A comparison with patients who have SLE, but no or only subclinical LN, would help to unravel the causal pathway, but these patients go without a biopsy and cannot be included in studies like ours. However, the absence of an association between the duration of SLE before the biopsy and deposits of complement factors in our study suggests that the deposits may also be unrelated to the extents of inflammation and cell damage.
Now that complement inhibitors are being developed and tested for treatment of LN, it is essential to identify patients who are likely to benefit. Insofar as renal deposits of complement factors do not predict prognosis, it remains uncertain whether they are suitable to guide treatment. Deposits of C5b-9 are regarded as a prerequisite of treatment with eculizumab, which inhibits the formation of C5b-9, but they have only been incidentally studied and seem not to predict prognosis in patients with LN [9]. Since both the classical and alternative complement pathways can lead to the formation and deposition of C5b-9 in the kidney, the effect of eculizumab is unlikely to be predicted by patterns that reflect activation of the classical or the alternative pathway, respectively, characterized by deposits of both C1q and C3, as found most often in glomeruli, or by deposits of C3 only, as found most often in vascular walls. In line with this presumption, our finding that neither pattern was associated with prognosis suggested similar effectiveness of selective inhibition of either pathway upstream of the formation of C5b-9.
In our study we recorded treatment before but not after the biopsy, when the diagnosis of LN was established. This may explain the relatively frequent use of NSAIDs and the relatively infrequent use of HCQ, high-dose corticosteroids and other immunosuppressive medications. While treatment influences prognosis, it was not associated with deposits of complement factors. We could not determine whether treatment before the biopsy permitted or prevented their deposition.
Other limitations of our study relate to the discussions above and provide research questions for future studies. Staining of C4 was not performed, but can help to clarify previous conflicting findings on the prognostic relevance of deposits of C4. Immunoelectron microscopy and mass spectrometry were not available, yet may better detect deposits that are associated with prognosis [19]. Serum levels of anti-dsDNA could not be compared between patients due to changes in the assay over time and anti-C1q antibodies could not be measured, although both are associated with prognosis [3, 6, 7]. Second kidney biopsies were not included, while clearance rates seem to differ between complement factors [9], hinting at differences in the prognostic relevance of complement factors deposited in a first and second biopsy [15].
The assets of our study included a large population of patients with LN for whom kidney biopsies and long-term prospective follow-up were available. It helps to clarify conflicting results from previous smaller studies, suggests that renal deposits of complement factors should not be added to prediction models and challenges their relevance to guide treatment with complement inhibitors.
Supplementary Material
Acknowledgements
The authors thank Dr José A. Gómez-Puerta and Dr Paul J. Hoover for their help in the acquisition of data and Dr Hongshu Guan and Emma Stevens for their help in the technical review of the manuscript.
Funding: This work was supported by a Niels Stensen Fellowship and a travel grant from the Dutch National Association for Lupus, Antiphospholipid Syndrome, Scleroderma and Mixed Connective Tissue Disease, both to J.J.E.K., and by the National Institutes of Health (R01 AR057327, K24 AR066109) to K.H.C.
Disclosure statement: The authors have declared no conflicts of interest. Part of the data reported here was supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Tektonidou MG, Dasgupta A, Ward MM.. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015. Arthritis Rheumatol 2016;68:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jorge A, Wallace ZS, Zhang Y. et al. All-cause and cause-specific mortality trends of end-stage renal disease due to lupus nephritis from 1995 to 2014. Arthritis Rheumatol 2019;71:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almaani S, Meara A, Rovin BH.. Update on lupus nephritis. Clin J Am Soc Nephrol 2017;12:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dall’Era M, Cisternas MG, Smilek DE. et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis Cohort. Arthritis Rheumatol 2015;67:1305–13. [DOI] [PubMed] [Google Scholar]
- 5. Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol 2016;12:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leatherwood C, Speyer CB, Feldman CH. et al. Clinical characteristics and renal prognosis associated with interstitial fibrosis and tubular atrophy (IFTA) and vascular injury in lupus nephritis biopsies. Semin Arthritis Rheum 2019;49:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arriens C, Wren JD, Munroe ME, Mohan C.. Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology 2017;56:i32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsieh C, Chang A, Brandt D. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011;63:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson HR, Medjeral-Thomas NR, Gilmore AC. et al. Glomerular membrane attack complex is not a reliable marker of ongoing C5 activation in lupus nephritis. Kidney Int 2019;95:655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birmingham DJ, Hebert LA.. The complement system in lupus nephritis. Semin Nephrol 2015;35:444–54. [DOI] [PubMed] [Google Scholar]
- 11. Ishizaki J, Saito K, Nawata M. et al. Low complements and high titre of anti-Sm antibody as predictors of histopathologically proven silent lupus nephritis without abnormal urinalysis in patients with systemic lupus erythematosus. Rheumatology 2015;54:405–12. [DOI] [PubMed] [Google Scholar]
- 12. Parikh SV, Malvar A, Song H. et al. Characterising the immune profile of the kidney biopsy at lupus nephritis flare differentiates early treatment responders from non-responders. Lupus Sci Med 2015;2:e000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rizk DV, Maillard N, Julian BA. et al. The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol 2019;10:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. [Google Scholar]
- 15. Alsuwaida A, Husain S, Al Ghonaim M. et al. Prognostic significance of C1q deposition in serial biopsies for predicating the long-term outcome in patients with proliferative lupus nephritis. Saudi J Kidney Dis Transpl 2016;27:305–11. [DOI] [PubMed] [Google Scholar]
- 16. Tan Y, Song D, Wu LH, Yu F, Zhao MH.. Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritis. BMC Nephrol 2013;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahin OZ, Gurses S, Tasli F. et al. Glomerular C4d staining can be an indicator of disease activity in lupus nephritis. Ren Fail 2013;35:222–5. [DOI] [PubMed] [Google Scholar]
- 18. Kim MK, Maeng YI, Lee SJ. et al. Pathogenesis and significance of glomerular C4d deposition in lupus nephritis: activation of classical and lectin pathways. Int J Clin Exp Pathol 2013;6:2157–67. [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Xu J, Zhang X. et al. Tubular basement membrane immune complex deposition is associated with activity and progression of lupus nephritis: a large multicenter Chinese study. Lupus 2018;27:545–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.