Abstract
Objectives
To evaluate the occurrence and progression of facet joint ankylosis in the whole spine using low-dose CT (ldCT) in radiographic axial spondyloarthritis (r-axSpA) and compare progression of facet joint ankylosis and syndesmophytes.
Methods
Patients with r-axSpA from the Sensitive Imaging in Ankylosing Spondylitis (SIAS) cohort underwent ldCT at baseline (n = 60) and 2 years (n = 53). Facet joints (right and left, levels C2-S1) were scored as ankylosed, not ankylosed or unable to assess. Joints that were frequently poorly visible (>15% missing), were excluded. Inter-reader reliability on the patient level was assessed with intraclass correlation coefficients (ICCs) and smallest detectable change (SDC). Ankylosis was assessed at joint level and patient level for both timepoints. Syndesmophytes were assessed with CT syndesmophyte score.
Results
Levels C5-T2 were difficult to assess and excluded from all further analyses. Facet joint ICCs were good to excellent for status scores (0.72–0.93) and poor to excellent for progression scores (0.10–0.91). Facet joint ankylosis was detected at every level but most frequently in the thoracic joints. In total, 48% of patients showed 2-year progression. Most progression occurred in the thoracic segment. Using SDCs as cutoff, 18% of patients had progression of facet joint ankylosis only, whereas 20% of patients had progression of syndesmophytes only.
Conclusion
This is the first study evaluating facet joints in the whole spine by ldCT in r-axSpA. Facet joint ankylosis was detected most often in the thoracic spine. Assessing facet joints in addition to syndesmophytes detected substantially more patients with damage progression over two years.
Keywords: spondyloarthritis, ankylosing spondylitis, outcomes research, low-dose computed tomography
Rheumatology key messages
Facet joint ankylosis occurred in every spinal segment but most in the thoracic spine.
Low-dose CT detected facet joint ankylosis progression independent of syndesmophyte progression over 2 years.
Introduction
Radiographic axial spondyloarthritis (r-axSpA), also known as ankylosing spondylitis, is an inflammatory disease of the spine and sacroiliac joints that can result in ossification of the spine [1]. Structural damage is therefore an important outcome of studies assessing interventions [2]. Structural changes like bone proliferation in the form of syndesmophytes are traditionally imaged using conventional radiography (CR) [3]. Currently, the modified Stoke Ankylosing Spondylitis Score (mSASSS) is the most validated and widely used method for assessing radiographic damage and progression on CR. A downside of CR, however, is that the thoracic spine that constitutes half of the vertebrae cannot be reliably assessed due to overprojection of the ribs [4, 5]. Studies using cross-sectional imaging (e.g. CT and MRI) have found syndesmophytes to occur most often in the thoracic spine [3, 6]; thus, using CR can lead to an underestimation of the extent of structural damage in an individual. Whilst most studies assessed structural damage and ossification of the vertebrae, facet joints have also been reported to ankylose in patients with r-axSpA [7–10]. Facet joints, also known as (zyg)apophyseal joints, are synovial joints that lie posterior to the vertebrae. Facet joints are not included in scoring methods such as the mSASSS, and have not been studied as thoroughly as syndesmophytes. Only a few studies have assessed the occurrence and progression of facet joint ankylosis and its relation with syndesmophytes, and only in parts of the spine. Maas et al. [8] used CR to assess facet joint ankylosis in part of the cervical spine (C2-C6) in r-axSpA patients on TNF inhibitors. They reported that, although commonly present together, facet joint ankylosis progression in r-axSpA could be detected without syndesmophyte progression. Tan et al. [11] used full dose CT to assess a part of the thoracolumbar spine (T10–L4) and reported a high correlation between syndesmophytes and facet joint ankylosis. As most damage and progression of damage are found in the thoracic spine, it would be interesting to study facet joints in this segment. However, to our knowledge no data are available on facet joint ankylosis in the whole spine, nor on the complete thoracic segment.
CT is an alternative method to image bone proliferations in the spine and, contrary to CR, is able to also visualize the thoracic segment. CT does, however, have a relatively high radiation dose which poses extra risks to the patients and makes it unsuitable for repeated use in the same patient [2]. However, by reducing the radiation dose of a CT scan to a level tolerable for repeated imaging of the whole spine, structural lesions can be studied over time on all spinal levels. Such a CT scan, low-dose CT (ldCT), was recently used by our group to image syndesmophyte progression in r-axSpA patients in the whole spine [12, 13]. Compared with CR, assessed using the mSASSS, whole spine ldCT assessed using the novel CT Syndesmophyte Score (CTSS) detected more progression in the form of new and growing syndesmophytes in r-axSpA patients, with most progression occurring in the thoracic spine [13].
In the current study, we aimed to develop a scoring system for facet joint ankylosis to assess facet joint ankylosis in all segments of the spine using whole spine ldCT. We first assessed the reliability of ldCT to detect facet joint ankylosis and thereafter assessed ankylosis progression over 2 years. Finally, we compared the detection of 2-year facet joint ankylosis progression and syndesmophyte progression in the same patients.
Methods
Study population
Data of the Sensitive Imaging in Ankylosing Spondylitis (SIAS) cohort were used, comprising n = 60 r-axSpA patients of which n = 30 were from Leiden, the Netherlands and n = 30 from Herne, Germany. Inclusion criteria were age ≥18 years, clinical diagnosis of r-axSpA, fulfillment of the modified New York criteria, between 1 and 18 syndesmophytes on CR of lateral cervical and lumbar spine and ≥1 inflammatory lesion on spinal MRI [12, 13]. Patients were followed for 2 years, during which time all treatments were allowed. No specific level of disease activity was required. Exclusion criteria were routine MRI contraindications, pregnancy and circumstances that would invalidate informed consent. Guidelines for Good Clinical Practice were followed and all patients signed an informed consent form before inclusion. The study was approved by the Medical Ethical Committees of Leiden and Herne.
Scoring procedure
Whole-spine ldCT were performed at baseline and at 2-year follow-up. Images were acquired on a 64-section CT scanner (in Leiden: Aquilion 64, Toshiba Medical Systems, Otawara, Japan and in Herne: Somatom Emotion 16, Siemens, Erlangen, Germany); axial 1 and 3 mm slice from C2 to S1 and sagittal and coronal reconstructions (2 mm slice) were made. The effective radiation dose per ldCT was ∼4 mSv. Further image specifications are reported in a previous publication on this cohort [12].
Images in the axial plane with 1 mm slice were assessed by two trained central readers independently, paired per patient and blinded for time order and patient information. Both readers were clinical PhD students in rheumatology on the topic of axSpA. Both were experienced in performing clinical research and in scoring different modalities. Training was overseen by the head radiologist of the musculoskeletal radiology department (MR). Left and right facet joints from C2 to S1 were assessed on a 3-point scale as normal, irregular and ankylosed.
Joints were scored as ankylosed if at least a part of the joint was ankylosed. Joints that could not be assessed due to poor visibility were recorded as missing. Irregularity was, however, hard to assess on the ldCT images. For analysis, scores were therefore dichotomized as 0 (no ankylosis) and 1 (ankylosed), with ‘irregular’ coded as 0. For examples of images that were used for scoring, see Supplementary Fig. S1, available at Rheumatology online.
The CTSS was used to assess syndesmophytes, which were scored by the same readers and on the same images on which the facet joints were assessed [12]. Vertebral units, consisting of the bottom half of a vertebra, the intervertebral disc space (IDS) and the top half of the next vertebra, were assessed from C2 to S1 in the sagittal and coronal plane. Syndesmophytes were assessed in eight quadrants per vertebral units and recorded as 0 (no syndesmophyte), 1 (syndesmophyte not reaching 50% of the IDS), 2 (syndesmophyte reaching or exceeding 50% of the IDS) and 3 (syndesmophyte bridges the IDS) with a maximum score range per patient of 0–552.
Analyses
The readability of the facet joints on the ldCT images was assessed first. Heatmaps were made of the percentages of patients with a missing joint score on each level. Arbitrarily, levels on which ≥15% of patients had a missing score at baseline or at 2 years for at least one reader were excluded from analyses due to poor visibility and difficulty to assess.
Facet joints were assessed first. Inter-reader reliability was assessed with intraclass correlations coefficients (ICCs) (two-way average, absolute agreement) on patients’ total sum scores for the whole spine and the cervical, thoracic and lumbar segments separately, and by calculating the smallest detectable change (SDC) [14]. The percentages of patients with ankylosed facet joints at baseline and follow-up were presented per left and right joint and per reader. To assess the prevalence of ankylosis on group level, status scores were calculated for the whole spine and per segment by patient per timepoint. Status scores were averaged to a mean score per reader to provide the average number of ankylosed facet joints per patient.
Progression scores were calculated as change from baseline to 2-year follow-up per joint per reader and summed for the whole spine and each segment. Analyses were performed for the whole spine and for each segment. Per analysis, patients with >25% of missing progression scores were excluded. For patients with <25% missing scores, their remaining missing scores were imputed using the patient’s mean segment progression score. Progression scores were averaged to a mean score per reader. Multiple cutoffs were used to define progression per patient. Per reader, progression was defined with a cutoff of >0. In case we used the mean progression score of both readers, a cut-off of >0.5 was applied and also the SDC to show progression beyond measurement error [15]. Except for the progression data per reader, all analyses were based on the mean scores of the readers.
Net progression at the group level was calculated as the number of patients with positive progression (>0.5) minus the number of patients with negative progression (<–0.5) divided by the total number of patients [16].
Next, facet joint ankylosis progression was compared with syndesmophyte progression as assessed with CTSS. Patients were included in this analysis if both CTSS and facet joint ankylosis scores from both readers at both time points were available, with <80% ankylosed facet joints and <80% bridging syndesmophytes on CTSS at baseline. Moreover, only patients with <25% missing progression scores for CTSS and facet joint ankylosis were analysed.
To compare progression of facet joint ankylosis and syndesmophytes, progression was defined with the cutoffs of >0.5 and >SDC and presented in a two-by-two table. All analyses were performed with STATA 14 (StataCorp. 2015, Stata Statistical Software: Release 14, StataCorp LP, College Station, TX, USA).
Patient and public involvement
The Rheumatology department of the Leiden University Medical Centre has structural patient participation in all her research projects. To this end, a patient council of 10 patients has been established. One of the subjects patients have been putting forward in our research meeting and questionnaires is a wish to prevent development and progression of irreversible structural damage in axSpA. Patients were not directly involved in the design or conducting of the study.
Results
Clinical data and ldCT facet joint scores were available for 60 r-axSpA patients at baseline [mean age 48.3 (s.d. 9.6), 85% male, 80% HLA-B27+, mean number of syndesmophytes 6.9 (s.d. 5.2) for reader 1 and 6.2 (s.d. 4.7) for reader 2], of which 53 patients also had data at 2-year follow-up [mean age 49 (s.d. 9.9), 85% male, 79% HLA-B27+]. Baseline demographics are presented in Supplementary Table S1. For the comparison of syndesmophyte progression and facet joint ankylosis progression, 44 patients were included [mean age 49.4 (s.d. 10), 84% male, 79% HLA-B27+].
Regarding facet joint scores, heatmaps presenting percentages of patients with missing joint scores for both readers are given in Supplementary Fig. S2, available at Rheumatology online. The largest percentages of missing scores are seen in the lower cervical and upper thoracic segments, ranging from 8% to 18% for reader 1 and 15% to 52% for reader 2 considering both baseline and follow-up. In both baseline and follow-up, ≥15% of missing scores for at least one reader are found in levels C5-T2. These levels were excluded from all subsequent analyses. Maximum score range then became 0–38 for the whole spine, and 0–6, 0–22 and 0–10 for the cervical, thoracic and lumbar segments, respectively.
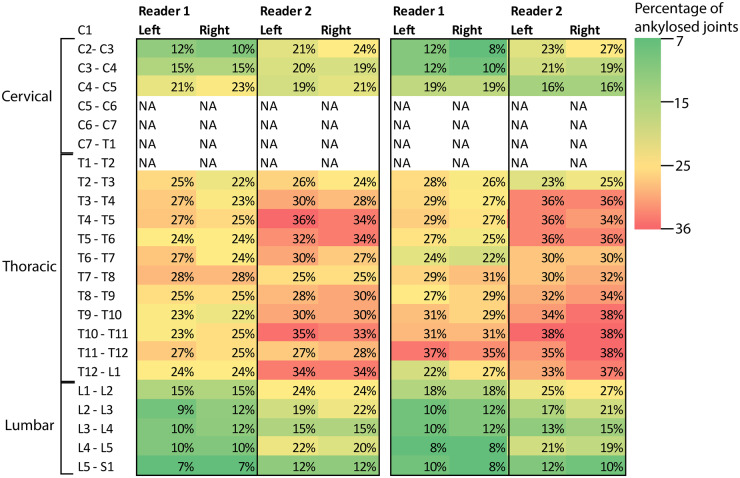
Fig. 1 shows the percentages of patients with ankylosed facet joints at baseline and follow-up per site, per level and per reader. Both readers reported most ankylosed facet joints in the thoracic segment, ranging from 22% to 28% and 22% to 37% in the thoracic segment for reader 1 for baseline and follow-up, respectively. For reader 2, this was 24% to 36% and 23% to 38%.
Fig. 1.
Percentages of patients with ankylosed facet joints
Percentages are given per joint level for the left and right joint, per reader, for baseline (left) and 2-year follow-up (right).
Baseline ICCs were 0.93, 0.84, 0.91 and 0.81 for the whole spine and cervical, thoracic and lumbar segments, respectively (Table 1). ICCs at follow-up were 0.91, 0.72, 0.9 and 0.85 for the whole spine and cervical, thoracic and lumbar segments, respectively. Progression score ICCs were 0.56, 0.91, 0.56 and 0.1 for the whole spine and cervical, thoracic and lumber segments, respectively.
Table 1.
Status scores, progression scores, ICCs and SDCs of facet joints
| Reader 1 | Reader 2 | ICC (95% CI) | SDC | |
|---|---|---|---|---|
| Mean (s.d.) range | Mean (s.d.) range | |||
| Whole spinea max score 38 | ||||
| Baseline | 7.3 (10) 0–34 | 9.4 (10.9) 0–37 | 0.93 (0.87, 0.96) | |
| Follow-up | 7.8 (9.7) 0–34 | 10.3 (10.9) 0–34 | 0.91 (0.82, 0.95) | |
| Progression score | 1.0 (2.8) −8.4–11.9 | 1.1 (2.6) −4.2–11.2 | 0.56 (0.22, 0.75) | 1.98 |
| Cervical segmentb max score 6 | ||||
| Baseline | 0.9 (1.8) 0–6 | 1.1 (2) 0–6 | 0.84 (0.73, 0.90) | |
| Follow-up | 0.7 (1.6) 0–6 | 1.2 (1.9) 0–6 | 0.72 (0.52, 0.84) | |
| Progression score | 0.0 (0.4) −2–1 | 0.0 (0.4) −2–1 | 0.91 (0.83, 0.95) | 0.22 |
| Thoracic segmentc max score 22 | ||||
| Baseline | 5.4 (7.4) 0–22 | 6.5 (7.6) 0–22 | 0.91 (0.85, 0.95) | |
| Follow-up | 6.0 (7.2) 0–22 | 7.3 (7.9) 0–22 | 0.90 (0.82, 0.94) | |
| Progression score | 0.9 (2.5) −8.8–7 | 0.9 (2.3) −4.4–11 | 0.56 (0.21, 0.75) | 2.67 |
| Lumbar segment max score 10 | ||||
| Baseline | 1.0 (2.5) 0–10 | 1.8 (3.1) 0–10 | 0.81 (0.67, 0.89) | |
| Follow-up | 1.1 (2.5) 0–10 | 1.8 (3.0) 0–10 | 0.85 (0.73, 0.92) | |
| Progression score | 0.0 (0.8) −2–3 | 0.2 (0.9) −2–5 | 0.10 (−0.58, 0.48) | 1.14 |
Joint levels C2–C5 and T2–S1.
Joint levels C2–C5.
Joint levels T2–L1.
Baseline: n = 60. Follow-up: n = 52 (r1), n = 53 (r2). Whole spine progression score: n = 50 (r1), n = 52 (r2), n = 50 (ICC, SDC). Cervical progression score: n = 44 (r1), n = 45 (r2), n = 40 (ICC, SDC). Thoracic progression score: n = 50 (r1), n = 52 (r2), n = 50 (ICC, SDC). Lumbar progression score: n = 51 (r1), n = 52 (r2), n = 51 (ICC, SDC).
ICC: intraclass correlation coefficients; SDC: smallest detectable change.
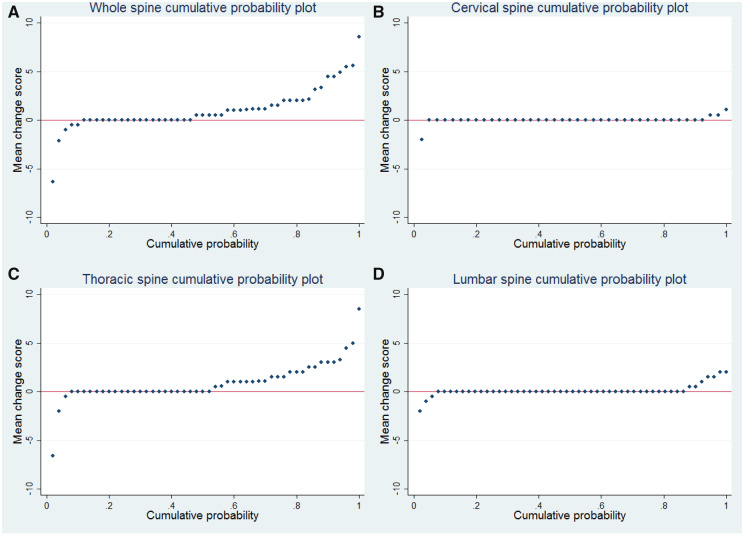
Baseline whole spine status scores were 7.3 and 9.4 for reader 1 and 2, respectively, and 7.8 and 10.3 for follow-up for reader 1 and 2 respectively. Almost the whole range from 0 to 38 ankylosed joints was spanned for both timepoints for both readers, reflecting great variability in the number of ankylosed joints between patients. The mean 2-year whole spine progression was 1.0 ankylosed joint for reader 1 and 1.1 ankylosed joint for reader 2 (Table 1/Fig. 2). Fig. 2 shows that just over half of the patients showed 0 or negative whole-spine progression (Fig. 2A) and that most progression occurred in the thoracic segment (Fig. 2C). Using a cutoff of >0.5, 48% of patients had 2-year whole spine facet joint ankylosis progression (Table 2).
Fig. 2.
Cumulative probability plots of progression of facet joint ankylosis
Plots are shown for the whole spine (A) and cervical (B), thoracic (C) and lumbar (D) segments
Table 2.
Number of r-axSpA patients with progression of facet joint ankylosis over 2-year follow-up
| Reader 1 n (%) | Reader 2 n (%) | |
|---|---|---|
| Whole spine change >0 | 15/50 (30%) | 22/52 (42%) |
| Cervical segment change >0 | 2/44 (4%) | 3/45 (7%) |
| Thoracic segment change >0 | 15/50 (30%) | 17/52 (33%) |
| Lumbar segment change >0 | 3/51 (6%) | 6/52 (12%) |
| Average of 2 readers n (%) | ||
| Whole spine | ||
| Change >0.5 | 24/50 (48%) | |
| Change >SDC (1.98) | 13/50 (26%) | |
| Net progression (>0.5) | 21/50 (42%) | |
| Cervical segment | ||
| Change >0.5 | 1/40 (2.5%) | |
| Change >SDC (0.22) | 3/40 (7.5%) | |
| Net progression | 0/40 (0%) | |
| Thoracic segment | ||
| Change >0.5 | 23/50 (46%) | |
| Change >SDC (2.67) | 7/50 (14%) | |
| Net progression | 21/50 (42%) | |
| Lumbar segment | ||
| Change >0.5 | 5/51 (10%) | |
| Change >SDC (1.14) | 4/51 (8%) | |
| Net progression | 3/51 (6%) | |
Whole spine: n = 50 (r1), n = 52 (r2), n = 50 (average 2 readers).
Cervical segment: n = 44 (r1), n = 45 (r2), n = 40 (average 2 readers).
Thoracic segment: n = 50 (r1), n = 52 (r2), n = 50 (average 2 readers).
Lumbar segment: n = 51 (r1), n = 52 (r2), n = 51 (average 2 readers).
Net progression: number of patients with progression >0.5 – number of patients with progression <−0.5 / total number of patients × 100.
r-axSpA: radiographic axial spondyloarthritis; SDC: smallest detectable change.
When using the SDC (1.98) as cutoff, 26% of patients had 2-year whole spine progression. Net progression was 42%. Fig. 3 plots the syndesmophyte progression against the facet joint ankylosis progression for all 44 patients. The trend was that higher progression scores in facet joint ankylosis were generally found in patients with higher syndesmophyte progression scores. However, progression of bone formation was not always present in both sites. Comparing progression (>0.5) of facet joint ankylosis and syndesmophytes, 17 (39%) of the 44 patients had progression of both syndesmophytes and facet joint ankylosis, four patients (9%) had progression of facet joints only, 22 patients (50%) had progression of syndesmophytes only and one patient (2%) had no progression. When using the SDCs as cutoff (1.98 for facet joint ankylosis, 14.4 for CTSS), four patients (9%) had progression of both syndesmophytes and facet joint ankylosis, eight patients (18%) had progression of facet joint ankylosis only, nine patients (20%) had progression of syndesmophytes only and 23 patients (52%) had no progression.
Fig. 3.
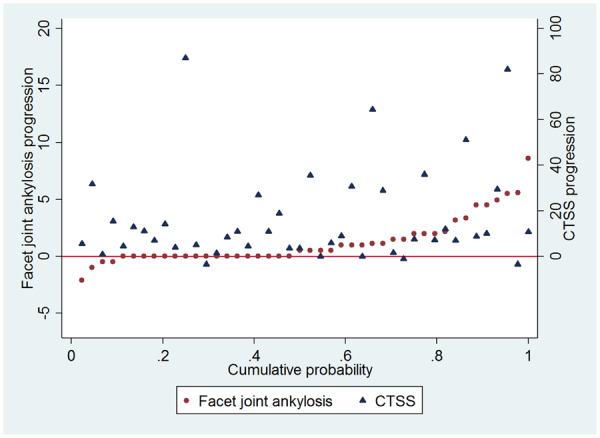
Cumulative probability plot showing 2-year progression of syndesmophytes and facet joint ankylosis
Syndesmophytes were scored with the Computed Tomography Syndesmophyte Score (CTSS). Facet joint ankylosis scores are mean progression scores of the two readers. The plot is ordered by the facet joint ankylosis progression scores. Dots and triangles on the same location on the X-axis belong to the same patient. The scale on the left side of the figure belongs to the facet joint ankylosis progression scores, the scale on the right belongs to the CTSS progression scores.
Discussion
This study assessed the occurrence and progression of structural damage in the facet joints of patients with r-axSpA using whole-spine ldCT. Facet joint ankylosis and new development of ankylosis over two years was detected in all spinal segments but was most common in the thoracic segment (T2-L1). Previous studies have examined facet joint ankylosis in parts of the spine. In comparison to our findings, they reported slightly higher percentages of cervical and lumbar ankylosed facet joints on CR [7, 9], lower percentages of ankylosed thoracolumbar facet joints on CT [11] and fewer patients with progression over 4 years on CR [9] and CT [11]. Although direct comparison of these studies is difficult due to the use of different techniques, the joints assessed and patient selection, all studies reported presence and progression of facet joint ankylosis.
Our finding that most facet joint ankylosis occurs and develops in the thoracic segment is in line with the fact that most syndesmophytes and progression thereof occurs in the thoracic segment [3, 6, 12, 13]. A striking finding in our population is that in a subset of patients (9% for a cutoff of >0.5 and 18% when using the SDC), facet joint ankylosis progression was detected without syndesmophyte progression. Detection of more progression of spinal damage by assessing both facet joint ankylosis and syndesmophytes in the whole spine could potentially be useful in intervention trials. With higher sensitivity of an outcome, treatment effects can be detected using a smaller number of patients or with a shorter follow-up. More experience is needed before applying the technique to clinical trials however, for instance by applying ldCT in patients with less spinal damage at baseline and ideally by showing progression over a shorter period of time (e.g. one year).
Apart from the fact that ankylosis occurred and progressed most in the thoracic segment, this segment showed good reliability. In contrast, reliability of readings in the lumbar segment was poor, which may be due to the low progression, which may reduce the level of ICCs even in case of good agreement [17]. Reliability of the cervical segment was excellent, but rates of facet joint ankylosis and facet joint ankylosis progression were low, while the bottom half of the cervical segment could not be assessed. Net progression scores were positive for the whole spine and the thoracic and lumbar segments, reflecting that there was progression of facet joint ankylosis when correcting for regression due to measurement error [16]. Future studies may consider scoring facet joint ankylosis in the thoracic segment only.
An important question for understanding the pathophysiology of axSpA is whether syndesmophyte formation precedes facet joint ankylosis or vice versa. In general, studies reported that facet joint ankylosis and syndesmophytes are highly correlated. Tan et al. [11] reported that facet joint ankylosis without syndesmophytes at the same vertebral level was rare in T10-L4. De Vlam and Mielants [7] reported that about two-thirds of levels with facet joint ankylosis did not have bridging syndesmophytes, but that bridging syndesmophytes rarely occurred without facet joint ankylosis on CR. However, all studies included patients with longstanding r-axSpA with generally extensive spinal damage. Ideally, the possible longitudinal association between facet joint ankylosis and syndesmophyte formation should be studied in a prospective cohort of recent-onset axial SpA. Another interesting topic of research would be to test if facet joint ankylosis or syndesmophytes have the most impact on physical functioning and spinal mobility.
This study has several limitations. The study was performed in r-axSpA patients with already extensive spinal damage at baseline with signs of active spinal inflammation on MRI. Therefore, there is a need for caution when extrapolating these results to other r-axSpA patient populations.
LdCT allowed us to visualize the whole spine at a reduced radiation exposure compared with CT. However, this influenced image quality: beam hardening artefacts at the cervicothoracic level and a lower spatial resolution to detect the facet joints. To minimize scoring errors, readers scored the joints as missing in case of doubt due to poor visibility. Despite the fact that ldCT uses less radiation than CT, it is still significantly more than CR. Therefore, we would like to stress that the use of ldCT is intended for clinical research and not daily clinical practice where, over many years, repeated imaging may be needed to study disease progression. Moreover, the current method assessed the facet joints solely on ankylosis. Possible degenerative changes, such as joint space narrowing and signs of diffuse idiopathic skeletal hyperostosis (DISH) were not considered and not controlled for. However, a broad assessment of structural changes in the facet joints and their specificity for axSpA was not the scope of this article. Whether ldCT is fit to assess degenerative changes and whether the extent to which we found facet joint ankylosis is specific for axSpA requires additional studies.
In summary, we developed and tested a scoring system for facet joint ankylosis on whole-spine ldCT in patients with r-axSpA. Facet joint ankylosis occurred in every segment of the spine but was most prevalent and prone to progress in the thoracic spine. In a subset of patients, progression of facet joint ankylosis was detected without progression of syndesmophytes.
Supplementary Material
Acknowledgements
Funding: This work was supported by the Dutch Rheumatism Association. Reumafonds, 9–1-301.
Disclosure statement: F.vG. reports grants from Reuma Nederland during the conduct of the study and grants from Reuma Nederland, Stichting Vrienden van Sole Mio, MSD, AbbVie and Novartis outside the submitted work; A.S. reports personal fees from NOVARTIS, outside the submitted work; D.vdH. reports personal fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daichii, Eisai, Elly-Lilly, Galapagos, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB Pharma outside the submitted work and is Director of Imaging Rheumatology BV; the other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 2. van der Heijde D, Braun J, Deodhar A. et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology 2019;58:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braun J, Baraliakos X, Buehring B, Kitlz U, Fruth M.. Imaging of axial spondyloarthritis. New aspects and differential diagnoses. Clin Exp Rheumatol 2018;36 (Supp 114):S35–S42. [PubMed] [Google Scholar]
- 4. Averns HL, Oxtoby J, Taylor HG. et al. Radiological outcome in ankylosing spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J Rheumatol 1996;35:373–6. [DOI] [PubMed] [Google Scholar]
- 5. MacKay K, Mack C, Brophy S, Calin A.. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum 1998;41:2263–70. [DOI] [PubMed] [Google Scholar]
- 6. Baraliakos X, Landewé R, Hermann K. et al. Inflammation in ankylosing spondylitis: a systematic description of the extent and frequency of acute spinal changes using magnetic resonance imaging. Ann Rheum Dis 2005;64:730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vlam K, Mielants H, Veys EM.. Involvement of the zygapophyseal joint in ankylosing spondylitis: relation to the bridging syndesmophyte. J Rheumatol 1999;26:1738–45. [PubMed] [Google Scholar]
- 8. Maas F, Arends S, Brouwer E. et al. Incorporating assessment of the cervical facet joints in the modified Stoke ankylosing spondylitis spine score is of additional value in the evaluation of spinal radiographic outcome in ankylosing spondylitis. Arthritis Res Ther 2017;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maas F, Spoorenberg A, Brouwer E. et al. Radiographic damage and progression of the cervical spine in ankylosing spondylitis patients treated with TNF-α inhibitors: facet joints vs. vertebral bodies. Semin Arthritis Rheum 2017;46:562–8. [DOI] [PubMed] [Google Scholar]
- 10. Simkin PA, Downey DJ, Kilcoyne RF.. Apophyseal arthritis limits lumbar motion in patients with ankylosing spondylitis. Arthritis Rheum 1988;31:789–802. [DOI] [PubMed] [Google Scholar]
- 11. Tan S, Yao J, Flynn JA, Yao L, Ward MM.. Zygapophyseal joint fusion in ankylosing spondylitis assessed by computed tomography: associations with syndesmophytes and spinal motion. J Rheumatol 2017;44:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Bruin F, de Koning A, van den Berg R. et al. Development of the CT Syndesmophyte Score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort. Ann Rheum Dis 2018;77:371–7. [DOI] [PubMed] [Google Scholar]
- 13. de Koning A, de Bruin F, van den Berg R. et al. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis 2018;77:293–9. [DOI] [PubMed] [Google Scholar]
- 14. Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D.. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Heijde D, Simon L, Smolen J. et al. How to report radiographic data in randomized trials in rheumatoid arthritis: guidelines from a roundtable discussion. Arthritis Rheum 2002;47:215–8. [DOI] [PubMed] [Google Scholar]
- 16. Sepriano A, Ramiro S, Landewé R, Dougados M, van der Heijde D.. Percentage of progressors in imaging: can we ignore regressors? RMD Open 2019;5:e000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lukas C, Braun J, van der Heijde D. et al. Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 2007;34:862–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.