Abstract
Objectives
To compare colour duplex ultrasonography (CDU) findings with axillary 18F-fluorodeoxyglucose (FDG) PET/CT findings and to compare the diagnostic performance of temporal and axillary artery CDU with temporal artery CDU alone.
Methods
Patients suspected of GCA were retrospectively included. Presence of a halo or occlusion was considered a positive CDU finding. FDG-PET/CT-assessed axillary artery involvement was defined as axillary artery FDG uptake higher than liver uptake. The reference was the clinical diagnosis after 6 months, which was based on symptomatology and additional diagnostic tests, with the exception of CDU.
Results
Of the 113 included patients, GCA was diagnosed in 41. Twenty-eight out of 41 GCA patients underwent a FDG-PET/CT. FDG-PET-assessed extra-cranial GCA was present in 20/41 patients, of which 13 showed axillary involvement on FDG-PET/CT. An axillary halo was found in eight of these 13 patients. Six out of the 20 patients with FDG-PET-assessed GCA showed no axillary involvement on CDU or FDG-PET/CT. Five of them had single artery involvement on FDG-PET/CT (two aorta; three vertebral artery). One patient had an axillary occlusion on CDU, consistent with FDG-PET/CT results. Overall, sensitivity and specificity of temporal artery CDU was 52% (95% CI: 35, 67) and 93% (95% CI: 84, 97), respectively. Adding axillary artery results improved sensitivity to 71% (95% CI: 55, 84), while specificity did not change.
Conclusion
Presence of an axillary halo or occlusion on CDU is consistent with axillary artery FDG-PET/CT results, but a negative CDU does not rule out axillary involvement. Adding axillary artery assessment to temporal artery assessment may substantially increase the diagnostic performance of CDU.
Keywords: giant cell arteritis, ultrasound, diagnostic imaging
Rheumatology key messages
In GCA axillary artery involvement on CDU is consistent with axillary artery involvement on FDG-PET/CT.
A negative axillary artery CDU does not rule out extra-cranial GCA.
Adding axillary artery assessment to temporal artery CDU improves the diagnostic performance for GCA.
Introduction
GCA is a systemic autoimmune disease characterized by inflammation of medium- and large- sized arteries. The best-known form of GCA involves the cranial arteries, but the aorta and its branches (extra-cranial arteries) can in many patients be affected as well [1]. Due to the heterogenic disease presentation and the importance of early treatment, fast diagnostic testing is strongly advised in order to confirm or exclude GCA [2].
Temporal artery biopsy (TAB) has long been regarded as the gold standard for diagnosing GCA [3]. However, recent data showed that colour duplex ultrasonography (CDU) of the temporal artery has a higher sensitivity than TAB for the diagnosis of GCA [4]. Furthermore, CDU is patient-friendly and more cost-effective than TAB. Therefore, CDU is now recommended as first imaging modality in centres with high expertise in patients presenting with predominantly cranial symptoms [5].
CDU can potentially also be used for the assessment of extra-cranial artery inflammation [1, 6]. However, the value of CDU in diagnosing extra-cranial GCA is unclear [7]. First, limited data are available on the relation between CDU and 18F-fluorodeoxyglucose (FDG) PET/CT, which has been proven a sensitive and accurate tool for diagnosing extra-cranial GCA [8–10]. Second, the added value of extra-cranial CDU to temporal artery CDU in an overall GCA-suspected population is unclear, because most studies predominantly included patients with cranial symptoms [1, 4, 11].
In our hospital CDU examination of both cranial and extra-cranial arteries has been part of the diagnostic work-up for patients with suspected GCA since 2013. Furthermore, FDG-PET/CT imaging has been performed in many patients suspected of GCA with extra-cranial involvement.
The overall aim of this retrospective study was to evaluate the performance of axillary artery CDU in GCA. We therefore compared axillary CDU findings with axillary 18F-FDG PET/CT findings, which we considered the reference standard for detecting extra-cranial involvement. Furthermore, we determined the added diagnostic value of temporal and axillary CDU for GCA, when compared with temporal artery CDU alone.
Methods
Study design
This retrospective descriptive study was conducted at the University Medical Center Groningen (UMCG). The ethics committee of the UMCG reviewed the study (METc 2018/316) and concluded that the study does not fall under the scope of the Medical Research Involving Human Subjects Act and that written informed consent was not mandatory.
Patients
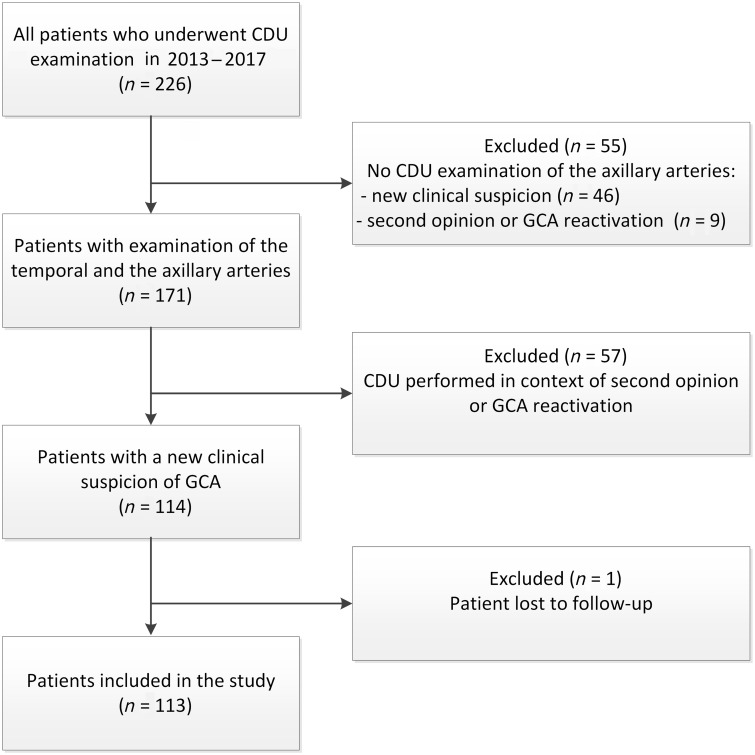
In our hospital CDU examination has been incorporated in the routine diagnostic work flow of patients suspected of GCA. When CDU was introduced as a diagnostic tool in 2010, the temporal arteries were the primary focus of examination. Since 2013, the CDU examination has been expanded with routine assessment of the axillary arteries by trained technicians. The subclavian and carotid arteries are also evaluated when sufficient time is available. We searched in the database of our vascular laboratory for all CDU examinations performed between January 2013 and November 2017. Patients were included when both the temporal and the axillary arteries had been investigated. Only patients with a CDU examination of a new clinical GCA suspicion were included. Assessments in tertiary referral GCA patients or suspected GCA relapses were excluded (Fig. 1).
Fig. 1.
Flowchart of patient inclusion
CDU: colour duplex ultrasonography.
Data on excluded patients with a new suspicion of GCA but without extra-cranial CDU (n = 46) can be found in Supplementary Table S1, available at Rheumatology online. TAB and FDG-PET/CT were performed to the same extent in included and excluded patients, indicating no differences in clinical suspicion. Furthermore, the proportion of patients with the reference diagnosis of GCA was comparable between groups.
Data collection
Data on presenting symptoms, medication use, physical examination findings, and laboratory findings were obtained from medical records. Additional diagnostic tests (TAB, MRI or FDG-PET/CT) were performed at the discretion of the responsible physicians. All the performed diagnostic tests were registered and reviewed, as described in detail below.
CDU assessment
All CDU examinations in our study had been routinely performed by two experienced, trained medical technicians. All CDU examinations were performed using the same colour duplex ultrasound system (ACUSON S2000 Ultrasound System, Siemens Healthineers, Erlangen, Germany) with an 18L6 high-density transducer operating at a B-mode frequency of 16 MHz for the temporal artery and a B-mode frequency of 9 MHz for the extra-cranial arteries. Patients were examined when in a supine position. Presence of any occlusion, stenosis or halo was reported in the medical record and key images of every examination were saved. In this study, we registered the conclusion of the CDU report as a dichotomous variable: halo sign or occlusion was present or not present. Presence of any abnormality, either unilateral or bilateral, was considered to be consistent with GCA.
In our clinical practice, the clinician decides whether the CDU is positive or negative. However, for the current study we wanted to assess the accuracy purely of the CDU in the hands of the vascular technicians, unbiased by the clinical suspicion. Therefore, in this study, the biomedical engineer of our vascular laboratory decided whether the images were positive or negative in cases with an inconclusive report.
In our hospital a halo was defined as a hypoechoic, homogeneous circumferential vessel wall thickening with an intima–media thickness (IMT) ≥ than the predefined cut-off value. Although our data collection started 5 years prior to publication of the OMERACT criteria, our definition of the CDU appearance of a halo is in line with this worldwide consensus [12]. Since 2013 the IMT cut-off value for a halo of the temporal artery has been 1.0 mm in prednisolone-naïve patients and 0.7 mm in patients using prednisolone in our hospital. However, in 10 treatment-naïve patients a temporal hypoechoic, circumferential wall thickening between 0.7 and 1.0 mm was described by the sonographer. Taking into account the axial resolution of our ultrasound system (0.2 mm) and the recent suggestion to further lower temporal artery cut-offs, we decided to register this vessel wall thickening as a halo [13].
For the extra-cranial arteries an IMT cut-off value of 1.5 mm has been applied in our hospital. However, in the medical record of three patients, an axial hypoechoic wall thickening was described between 1.0 and 1.2 mm without signs of atherosclerosis. Based on recent findings, we decided to include hypoechoic wall thickening ≥1.0 mm as a halo as well [14].
FDG-PET/CT scan assessment
All 18F-FDG PET/CT scans were performed using a Biograph mCT camera system (Siemens Medical Systems, Knoxville, TN, USA). The image acquisition was performed according to a standardized protocol [15]. In short, whole-body (from head to knees) or total-body (from head to toes) PET scans were acquired after at least 4 h of fasting. A dose of 3 MBq/kg 18F-FDG was injected 60 (±5) minutes prior to the start of the PET scan. A low-dose CT scan was acquired for attenuation correction and anatomical localization.
All FDG-PET/CT scans were assessed by two experienced nuclear medicine physicians (R.S. and A.G.), who were blinded for the CDU findings and the complete medical history. An overall expert opinion-based interpretation of the whole FDG-PET/CT scan (i.e. gestalt) was made and was registered as a dichotomous variable: consistent with GCA or not consistent with GCA [16]. The assessor agreement was 100%.
In order to compare CDU findings with FDG-PET/CT findings, the qualitative 18F-FDG uptake in the aorta and in three aortic branches (carotid, axillary and subclavian arteries) was also registered. Previous work of our group, in which FDG-PET/CT scans were compared between GCA patients and controls with atherosclerosis, showed that a vascular FDG-uptake higher than the liver FDG-uptake is highly specific for GCA, especially when the FDG pattern is diffuse [16]. Therefore, in this study, a diffuse vascular FDG pattern with a FDG uptake higher than the liver FDG uptake was considered positive for GCA [16, 17]. In four cases the assessors disagreed on the quantitative FDG uptake (κ: 0.93) and consensus was reached afterwards.
Temporal artery biopsy
In this study biopsy results were reported as positive or negative for GCA or inconclusive in order to assess the clinical diagnosis. All biopsy results were reported by an experienced pathologist. A positive biopsy was defined as a biopsy showing vasculitis characterized by a predominance of mononuclear cell infiltration or granulomatous inflammation, usually with multinucleated giant cells [18].
Clinical diagnosis
Two clinical experts (D.M. and E.B.) independently assessed whether or not GCA was the final clinical diagnosis after at least 6 months in all included patients. They reviewed the complete history of the patients, including cranial and systemic symptoms, laboratory findings, findings at additional diagnostic tests (TAB, FDG-PET/CT or MRI) and response to glucocorticoids, but not the CDU data. In five cases the assessors disagreed (κ: 0.90). In three of them consensus was reached afterwards and in two of them an independent third expert (M.S.) made the final diagnosis. The final diagnosis was in 98% of the included patients in agreement with the diagnosis of the treating clinician.
Outcome measures
The primary outcome was the comparison of axillary CDU with FDG-PET/CT findings and the diagnostic value of adding axillary artery assessment to temporal artery CDU. This was also investigated for the subclavian and carotid arteries (secondary outcomes). Furthermore, the influence of prednisolone on the diagnostic performance of CDU was investigated.
Statistical analyses
Data are presented as numbers and percentages, mean and standard deviation, or median with the 25th and 75th percentile. Normality of data was visually tested with Q–Q and P–P plots. Differences between patients with and without FDG-PET-proven extra-cranial GCA were tested with the Mann–Whitney U-test or Student’s t-test, whichever was appropriate. In cases of categorical variables, the χ2 or Fisher’s exact test was used. The sensitivity and specificity (with 95% CIs) of CDU was calculated with the clinical diagnosis as reference standard. An α of 5% was defined as statistically significant in all tests. All data was analysed with SPSS Software 23.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of included patients
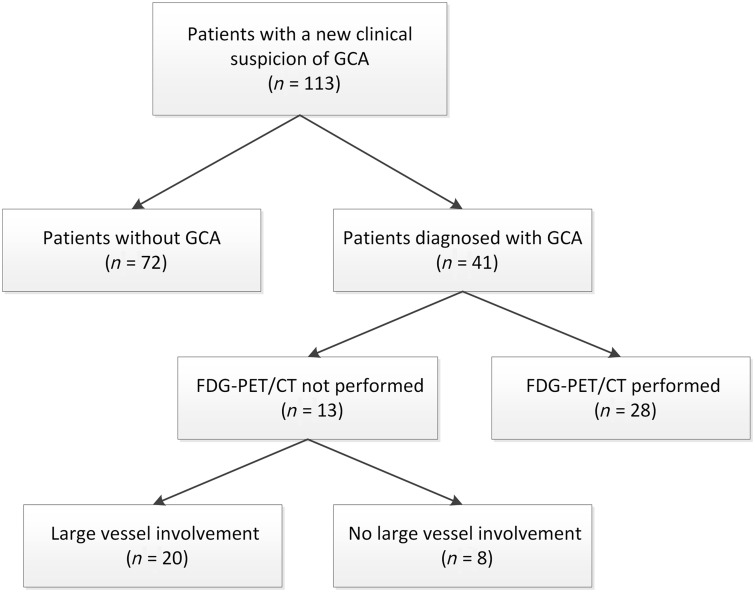
We included 113 patients; 41 (36%) of them had the clinical diagnosis of GCA (Fig. 2). Baseline characteristics of patient with and without the clinical diagnosis of GCA are shown in Table 1. Among the 72 patients in whom the clinical diagnosis of GCA was rejected, 17 had PMR, 10 had visual symptoms due to atherosclerosis, eight had tension headache, nine had other auto-immune disease (five RA, one sarcoidosis, one Sjögren’s syndrome, one granulomatosis with polyangiitis, one adult-onset Still’s disease), five had an aneurysm, one had listeria aortitis and seven had infection.
Fig. 2.
Flowchart of clinical diagnosis and PET proven large-vessel involvement
A total of 113 patients underwent CDU of the temporal and axillary arteries. Twenty patients with the final diagnosis of GCA had FDG-PET/CT-proven large vessel involvement. CDU and FDG-PET/CT results were compared between these 20 patients. CDU: colour duplex ultrasonography; FDG: fluorodeoxyglucose.
Table 1.
Characteristics of study population and comparison between patients with and without FDG-PET-positive findings for extra-cranial GCA
| Characteristic | Patients without positive FDG-PET/CT findings for extra-cranial GCA (n = 21) | Patients with positive FDG-PET/CT findings for extra-cranial GCA (n = 20) | Patients without GCA (n = 72) | P-value |
|---|---|---|---|---|
| Age, mean (s.d.), years | 73 (8) | 66 (7) | 68 (11) | 0.079 |
| Female, n (%) | 15 (71) | 7 (35) | 47 (65) | 0.019 |
| Clinical characteristics, n (%) | ||||
| Previous PMR diagnosis | 6 (29) | 3 (15) | 10 (14) | 0.454 |
| New headache | 14 (68) | 10 (50) | 33 (46) | 0.279 |
| Scalp tenderness | 8 (38) | 6 (30) | 18 (25) | 0.585 |
| Jaw claudication | 12 (57) | 5 (25) | 11 (15) | 0.037 |
| Visual symptoms | 9 (43) | 5 (25) | 18 (25) | 0.228 |
| Temporal artery abnormalitiesa | 15 (71) | 6 (30) | 18 (25) | 0.008 |
| Temperature ≥38°C | 4 (19) | 2 (10) | 9 (13) | 0.663 |
| Nights sweats | 5 (24) | 9 (45) | 6 (8) | 0.089 |
| Weight loss ≥2 kg | 8 (38) | 9 (45) | 14 (19) | 0.654 |
| Laboratory findings | ||||
| ESR, median((IQR), mm/hb | 49 (39–91) | 92 (50–105) | 34 (15–60) | 0.041 |
| ESR ≥50 mm/h, n (%)b | 10 (48) | 15 (75) | 25 (35) | 0.031 |
| CRP, median (IQR), mg/lc | 52 (11–97) | 49 (27–122) | 11 (3–29) | 0.348 |
| CRP ≥25 mg/l, n (%) | 11 (52) | 16 (80) | 21 (29) | 0.062 |
| WBC, median (IQR), 109/l | 10 (9–14) | 10 (8–12) | 9 (7–10) | 0.390 |
| TAB, n (%) | ||||
| Positive | 12 (57) | 5 (25) | — | — |
| Negative | 5 (24) | 3 (15) | 20 (28) | — |
| Inconclusive | 3 (14) | — | 1 (1) | — |
| Fulfilment of 1990 ACR criteria, n (%) | 17 (81) | 11 (55) | 3 (4) | 0.074 |
| PET/CT scan, n (%) | ||||
| Positive | — | 20 (100) | 1 (1) | — |
| Negative | 8 (38) | — | 30 (42) | — |
| MRI scan, n (%) | ||||
| Positive | 2 (10) | — | — | — |
| Negative | — | — | 4 (6) | — |
Tenderness, pain or decreased pulsations.
Six missing.
Two missing. P-value: patients without PET-proven extra-cranial GCA vs patients with PET-proven extra-cranial involvement. FDG: fluorodeoxyglucose; IQR: interquartile range; TAB: temporal artery biopsy; WBC: white blood count.
The remaining 15 patients had other diagnosis: muscle complaints (three), gout (two), trigeminal neuralgia (three), ocular disease (one), jaw dysfunction syndrome (two), malignancy (two), fever of unknown origin (one) and idiopathic intracranial hypertension (one).
A total of 59 out of 113 (52%) patients underwent a FDG-PET/CT scan (Table 1). In the other 54 patients the treating clinician had ordered a temporal biopsy (n = 21) or a MRI scan (n = 3) instead or no additional diagnostic test was performed (n = 30). None of these 30 patients had the clinical diagnosis of GCA at 6 months.
Of the 41 GCA patients, 20 had FDG-PET positive findings for extra-cranial GCA (Fig. 2). In the other 21 GCA patients, no FDG-PET/CT was performed (n = 13) or the FDG-PET was negative (n = 8). In line with the literature, patients with extra-cranial involvement were younger and classical cranial symptoms were less prevalent than in patients without extra-cranial involvement, although this was only significant for jaw claudication and temporal artery abnormalities (Table 1).
Axillary CDU compared with FDG-PET/CT in extra-cranial GCA
In the subgroup of patients with FDG-PET positive findings for extra-cranial GCA (n = 20), the axillary artery was PET positive in 13/20 patients. An axillary artery halo was found in eight of these 13 patients (for further details see Supplementary Table S2, available at Rheumatology online).
Of the remaining 5/13 patients, FDG-PET/CT showed axillary artery involvement, but axillary artery CDU was negative. One out of five was on prednisolone (see section below); the four other patients were treatment naïve.
Furthermore, 6/20 patients showed no axillary involvement on both FDG-PET/CT and CDU. Five of them had isolated extra-cranial artery involvement on FDG-PET/CT: two of the aorta and three of the vertebral artery. Finally, one patient had an axillary occlusion on CDU, consistent with low FDG uptake on PET.
With respect to the GCA patients without a FDG-PET/CT scan (n = 13), two showed an axillary halo. Of the GCA patients with a negative FDG-PET/CT scan (n = 8), none had a positive extra-cranial CDU.
Added value of axillary CDU for overall diagnostic performance
For the second aim of this study, we focused on the whole included study population (n = 113). CDU showed a halo sign in at least one temporal artery in 26 patients: 21 with GCA and five without GCA (Table 2). In 87 patients no temporal halos were found: 67 without GCA and 20 with GCA. This resulted in a sensitivity of 52% (95% CI: 35, 67) and a specificity of 93% (95% CI: 84, 97) for the reference standard, i.e. the clinical diagnosis of GCA.
Table 2.
CDU abnormalities in patient with and without GCA
| Patients with GCA (n = 41) | Patients without GCA (n = 72) | |
|---|---|---|
| Any abnormality | 31 (76) | 5 (7) |
| Solely in the temporal artery | 18 (44) | 5 (7) |
| Solely in extra-cranial arteries | 10 (24) | — |
| Axillary artery | 8 | |
| Subclavian artery | 2 | |
| Carotid artery | 0 | |
| Temporal and extra-cranial arteries | 3 (7) | — |
Data presented as n (%). CDU: colour duplex ultrasonography.
Eight patients showed an abnormality in the axillary arteries only (i.e. no temporal artery abnormalities), all true positives (Table 2). Thus, CDU of the axillary arteries identified an extra eight patients who did have GCA, but would have been missed with temporal artery CDU only. Adding CDU results of axillary arteries improved sensitivity to 71% (95% CI: 55, 84), while specificity did not change [93% (95% CI: 84, 97)].
Subclavian and carotid CDU compared with FDG-PET/CT in extra-cranial GCA
CDU of the subclavian arteries was performed in 12 out of 20 PET-positive patients (60%). In four of them a halo was found. PET-assessed subclavian artery involvement was demonstrated in two out of these four. In the remaining two patients, one had isolated aortic involvement on FDG-PET/CT (Supplementary Table S2, available at Rheumatology online). The added diagnostic value of subclavian artery CDU for the reference standard was not calculated due to missing values.
CDU of the carotid artery was performed in 17 out of 20 patients (85%). A carotid artery halo was found in six patients, while PET-assessed carotid artery involvement was found in only four of them. The other way around, PET-assessed carotid involvement was found in five patients, who did not have a halo on CDU. A halo at the carotid artery was never the only extra-cranial abnormality on CDU, so adding the results of the carotid arteries did not further improve the diagnostic performance.
Influence of prednisolone
In patient in whom prednisolone was started after presentation (n = 17), CDU examination was performed within 3 days, with the exception of one patient (10 days).
Twenty-three patients were using prednisolone at presentation. In 10 of them prednisolone was continued during CDU, mostly in a lower dosage. The indication for prednisolone was a history of PMR (n = 8), an unspecified autoimmune disease (n = 1) and post-transplantation immunosuppression (n = 1). The dosage varied between 5 and 30 mg/day. In the other 13 patients, prednisolone was discontinued prior to imaging. The time between the day of the last prednisolone dose and CDU ranged from 4 to 37 days. The indication was PMR in all 13 patients. The dosage varied between 5 and 30 mg/day.
Excluding all patients using prednisolone during CDU (n = 27) did not change the diagnostic performance of temporal CDU [sensitivity 56% (95% CI: 35, 76), specificity 92% (82–97)] or the performance of temporal CDU extended with the axillary arteries [sensitivity 76% (95% CI: 55, 91), specificity 92% (95% CI: 82, 97)] (for numbers, see Supplementary Table S3, available at Rheumatology online).
Excluding all patients using prednisolone in the month before CDU (n = 40) did also not change the diagnostic performance of temporal CDU [sensitivity 55% (95% CI: 32, 76), specificity 94% (84–99)] or the performance of temporal CDU extended with the axillary arteries [sensitivity 77% (95% CI: 55, 92), specificity 94% (95% CI: 84, 99)] (for numbers, see Supplementary Table S4, available at Rheumatology online).
Discussion
CDU is recommended by EULAR as first choice diagnostic imaging modality in patients with predominant cranial symptoms [5]. However, the value of CDU in extra-cranial GCA is not yet fully established. Therefore, in this retrospective study we explored the diagnostic value of extra-cranial CDU. We found that the presence of a halo sign at the axillary artery corresponds with increased axillary 18F-FDG uptake, but FDG-PET/CT more frequently detects axillary involvement. Furthermore, we found that examination of the axillary arteries, in addition to the temporal artery, increased the sensitivity of CDU for the clinical diagnosis of GCA, while the specificity remained high.
Our finding that the presence of a halo sign at the axillary artery corresponds with an increased axillary 18F-FDG uptake is in agreement with the findings of Löffler et al. and Nielsen et al. [10, 19]. They compared extra-cranial CDU results with FDG-PET/CT results in 30 patients with large vessel vasculitis and 20 controls. Interestingly, in our study as well as in the study of Löffler et al., no axillary CDU abnormalities were found in a few treatment naïve patients with PET-proven axillary involvement (4/20 in our study and 4/30 in study of Löffler et al.). Nielsen et al. prospectively evaluated the accuracy of axillary artery CDU in 46 patients with FDG-PET/CT-proven large vessel involvement. They also found that most, but not all, PET positive axillary arteries were CDU positive (20/73 axillary arteries were PET positive and CDU negative).
In most recent literature a cut-off value of 1.0 mm was chosen for a halo of the axillary arteries [14, 19]. Although the formal cut-off value for the extra-cranial arteries in our hospital was 1.5 mm, in clinical practice our sonographers registered extra-cranial halos up to 1.0 mm. In the study of Nielsen et al., in which a halo was also defined according to visual CDU appearance, the mean IMT in PET-positive axillary arteries was 1.32 mm and in PET-negative axillary arteries 0.64 mm. Based on the receiver operating characteristic curve, Nielsen et al. found that a cut-off of 1.0 mm had the highest accuracy for axillary artery involvement. Nevertheless, even with this cut-off value the sensitivity for axillary artery involvement was only 70% using FDG-PET/CT as reference.
A potential explanation for these negative CDU and positive PET results might be vessel wall inflammation, causing an increased 18F-FDG signal, without severe intima–media thickening or at least not large enough to cause a clear halo sign on CDU. FDG-PET/CT detects early inflammation, while CDU detects morphological changes that occur in a latter phase [20].
In a previous study of Schmidt et al., CDU results of the temporal, axillary, subclavian and proximal brachial arteries in 176 GCA patients are described [1]. As in our study, they found that a substantial part of the included extra-cranial GCA patients (20 out of 53) had no temporal artery abnormalities on CDU. The authors therefore conclude that performing axillary artery CDU increases the diagnostic yield of CDU, although no formal sensitivity and specificity calculations could be performed due to the inclusion of only GCA patients. Our study, in which all patients who underwent CDU were included irrespective of the final diagnosis, confirms this conclusion by showing that the sensitivity increases, while specificity remains high.
Contrary to our findings, Diamantopoulus et al. showed that adding axillary artery CDU to temporal artery CDU improved the sensitivity with only 2%, while this was 19% in our study [11]. Furthermore, in the TABUL study of Luqmani et al. only 9 out of 381 (2.4%) suspected GCA patients had axillary involvement, indicating that the role of axillary CDU would be limited [4]. However, both studies predominantly included patients with cranial symptoms. In our study all GCA-suspected patients were included, irrespective of the symptoms (cranial or extra-cranial). Furthermore, the characteristics and performed diagnostic tests between the excluded patients (n = 46) and the included study population (n = 113) were similar, indicating that the presence of a diagnostic suspicion bias is unlikely.
We found that only a few participants had single subclavian involvement, as was found by Schmidt et al. [1]. However, in our study the subclavian artery was also infrequently investigated due to technical issues and time constraints. Therefore, no conclusions can be drawn on the added value of subclavian investigation. With respect to the carotid arteries, we found that a halo at the carotid arteries was never the only abnormality on CDU, supporting that examination of the carotids does not further improve the diagnostic yield of CDU [21].
For the temporal artery an IMT cut-off value of even 0.3–0.4 mm has recently been suggested, while in our study only temporal artery halos up to 0.7 mm were registered [14]. Halos smaller than 0.7 mm cannot be reliably visualized with ultrasound systems with lower frequency probes, including the one in our hospital, due to a limited resolution. The inability to detect small halos might have contributed to the relatively low sensitivity of CDU in our study. Schäfer et al. demonstrated that 100% sensitivity and high specificity can be reached using advanced CDU equipment and low IMT cut-off values [14].
Besides cut-off value, two other factors should be mentioned that might have influenced the sensitivity of CDU in our study. First, differences in vessel involvement in the included population. Some patients in our study had only aortic or vertebral artery involvement on FDG-PET/CT. These patients were classified as having GCA. However, their CDU exam of the temporal and axillary arteries was negative, which contributed to a lower overall sensitivity of CDU. This underlines the limited usefulness of CDU in isolated aortic involvement and lowers CDU sensitivity [5]. Rather than excluding cases of isolated artery involvement afterwards, we aimed to reflect the clinical practice by including every patient suspected of GCA irrespective of the final diagnosis or vessels involved.
The second factor is awareness of the clinical suspicion. In our study CDU was performed by experienced vascular technicians who are unaware of the degree of clinical suspicion. This might have influenced the real-life interpretation of CDU and therefore also sensitivity of CDU.
The high specificity of CDU in our study is in agreement with the high specificity reported in a recently published meta-analysis of eight studies, which showed a pooled specificity of 96% for the clinical diagnosis of cranial GCA [7]. Although the overall specificity was very high, none of the individual studies included in the meta-analyses reached a 100% specificity. This means that in every study at least one false-positive halo was found, as was the case in our study. In literature various case reports have been published of patients with a halo sign and a diagnosis other than GCA [22]. Vessel wall swelling can also occur in other pathological conditions, such as amyloidosis, but the exact mechanisms needs to be further investigated [23].
Excluding all patients on prednisolone did not change the diagnostic performance of CDU in this study. This can be explained by the fact that most patient on prednisolone treatment in our study are patients with a history of PMR, treated with low dose prednisolone, who develop GCA symptoms during prednisolone tapering. The fact that GCA symptoms appear may suggest clinically relevant vascular involvement. Furthermore, our clinicians are highly aware of the influence of prednisolone on CDU results. In almost all patients in whom prednisolone treatment was started for the first time, CDU examination was performed within 3 days. It has been shown that a halo sign can disappear in 1–2 weeks after prednisolone therapy [24–26]. Rather than excluding all patients on prednisolone, we performed sub-analyses in order to evaluate the influence of prednisolone in our population.
This study has some limitations. First, the outcome of the FDG-PET/CT scan was known by the clinicians and was therefore used to diagnose GCA. In this study the clinical diagnosis after 6 months was also the reference standard. Consequently, the diagnostic performance of the FDG-PET/CT scan could not be independently determined. We therefore used the FDG-PET/CT scan only as gold standard for extra-cranial involvement. Nevertheless, a FDG‐PET/CT scan was not available for every GCA patient. Therefore, the number of false-negative extra-cranial CDU exams might be higher than reported.
Second, we were not able to reliably reconstruct the a priori clinical suspicion because of the retrospective study design. The diagnostic performance of CDU might be different depending on the level of clinical suspicion. Further research is required to prospectively investigate the value of extended CDU in patients with a low, intermediate and high level of GCA suspicion. Third, since this was a retrospective analysis, we could not study the inter-observer agreement between both sonographers.
We conclude that in patients with new onset GCA the presence of an axillary halo is consistent with axillary artery involvement on FDG-PET/CT. These findings suggest that in cases of an axillary artery halo, no 18F-FDG PET/CT scanning is required to diagnose extra-cranial GCA. However, our retrospective data show that FDG-PET/CT more frequently detects axillary involvement and a negative axillary CDU does not rule out extra-cranial GCA in the setting of our study. Besides, isolated aorta or vertebral artery involvement is a potential pitfall for CDU. Furthermore, we conclude that adding investigation of the axillary arteries to temporal artery CDU improves the diagnostic performance of CDU.
Supplementary Material
Acknowledgements
The authors would like to thank Anne van Gessel and Annet Possel-Nicolai for performing the CDU examinations.
Funding: No specific funding was received for this paper.
Disclosure statement: D.J.M. reports grants from Boehringer Ingelheim, grants from Sanofi and grants from Actelion, outside the submitted work. R.H.J.A.S. reports grants from Siemens and grants from IBA Webinar, outside the submitted work. E.B. reports speaker fees and consulting fees from Roche, outside the submitted work. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A, Natusch A.. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:96–101. [DOI] [PubMed] [Google Scholar]
- 2. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G.. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford) 2016;55:66–70. [DOI] [PubMed] [Google Scholar]
- 3. Monti S, Floris A, Ponte C, Schmidt WA. et al. The use of ultrasound to assess giant cell arteritis: review of the current evidence and practical guide for the rheumatologist. Rheumatology (Oxford) 2018;57:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luqmani R, Lee E, Singh S. et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and Treatment of Giant Cell Arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dejaco C, Ramiro S, Duftner C. et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 6. Czihal M, Zanker S, Rademacher A. et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol 2012;41:231–6. [DOI] [PubMed] [Google Scholar]
- 7. Duftner C, Dejaco C, Sepriano A. et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018;4:e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs M, Briel M, Daikeler T. et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging 2012;39:344–53. [DOI] [PubMed] [Google Scholar]
- 9. Grayson PC, Alehashemi S, Bagheri AA, Civelek AC. et al. 18F-Fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loffler C, Hoffend J, Benck U, Kramer BK, Bergner R.. The value of ultrasound in diagnosing extracranial large-vessel vasculitis compared to FDG-PET/CT: a retrospective study. Clin Rheumatol 2017;36:2079–86. [DOI] [PubMed] [Google Scholar]
- 11. Diamantopoulos AP, Haugeberg G, Hetland H. et al. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken) 2014;66:113–9. [DOI] [PubMed] [Google Scholar]
- 12. Chrysidis S, Duftner C, Dejaco C. et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018;4:e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt WA. Ultrasound in the diagnosis and management of giant cell arteritis. Rheumatology (Oxford) 2018;57(Suppl_2):ii22–ii31. [DOI] [PubMed] [Google Scholar]
- 14. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA.. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology (Oxford) 2017;56:1479–83. [DOI] [PubMed] [Google Scholar]
- 15. Boellaard R, Delgado-Bolton R, Oyen WJ. et al. FDG PET/CT: eANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stellingwerff MD, Brouwer E, Lensen KJ. et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore) 2015;94:e1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slart R; Writing group; Reviewer group et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging 2018;45:1250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunder GG, Bloch DA, Michel BA. et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 2010;33:1122–8. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen BD, Hansen IT, Keller KK. et al. Diagnostic accuracy of ultrasound for detecting large-vessel giant cell arteritis using FDG PET/CT as the reference. Rheumatology (Oxford) 2020;59:2062–73. [DOI] [PubMed] [Google Scholar]
- 20. Jiemy WF, Heeringa P, Kamps J. et al. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging of macrophages in large vessel vasculitis: current status and future prospects. Autoimmun Rev 2018;17:715–26. [DOI] [PubMed] [Google Scholar]
- 21. Aschwanden M, Kesten F, Stern M. et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2 × 11 arterial regions. Ann Rheum Dis 2010;69:1356–9. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt WA. The ultrasound halo sign of temporal arteries: is it always giant cell arteritis? Rheumatology (Oxford) 2019;58:1898–9. [DOI] [PubMed] [Google Scholar]
- 23. Molina Collada J, Ruiz Bravo-Burguillos E, Monjo I. et al. Positive ultrasound halo sign of temporal arteries due to amyloidosis. Rheumatology (Oxford) 2019;58:2067–9. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ.. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42. [DOI] [PubMed] [Google Scholar]
- 25. Hauenstein C, Reinhard M, Geiger J. et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012;51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt WA, Moll A, Seifert A. et al. Prognosis of large-vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:1406–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.