Significance
Despite being ubiquitous in aquatic environments, amphipods are rarely targeted by seals or toothed whales, likely because they contain too little energy to warrant catching individually. We report a remarkable case of Baikal seals in Lake Baikal, among the least studied seal species. Seals individually hunted tiny (<0.1 g) endemic amphipods (the world’s only freshwater planktonic species) at the highest rates ever recorded for aquatic mammals, primarily by following diel depth changes of amphipod swarms. Baikal seals have specialized comb-like cheek teeth, allowing them to expel water that enters the mouth during high-speed foraging. Thus, even tiny organisms can be important foods for aquatic mammals that catch prey individually, if the environment and predators’ adaptations allow high foraging rates.
Keywords: biologging, endemic species, feeding morphology, foraging behavior
Abstract
Understanding what, how, and how often apex predators hunt is important due to their disproportionately large effects on ecosystems. In Lake Baikal with rich endemic fauna, Baikal seals appear to eat, in addition to fishes, a tiny (<0.1 g) endemic amphipod Macrohectopus branickii (the world’s only freshwater planktonic species). Yet, its importance as prey to seals is unclear. Globally, amphipods are rarely targeted by single-prey feeding (i.e., nonfilter-feeding) mammals, presumably due to their small size. If M. branickii is energetically important prey, Baikal seals would exhibit exceptionally high foraging rates, potentially with behavioral and morphological specializations. Here, we used animal-borne accelerometers and video cameras to record Baikal seal foraging behavior. Unlike the prevailing view that they predominantly eat fishes, they also hunted M. branickii at the highest rates (mean, 57 individuals per dive) ever recorded for single-prey feeding aquatic mammals, leading to thousands of catches per day. These rates were achieved by gradual changes in dive depth following the diel vertical migration of M. branickii swarms. Examining museum specimens revealed that Baikal seals have the most specialized comb-like postcanine teeth in the subfamily Phocinae, allowing them to expel water while retaining prey during high-speed foraging. Our findings show unique mammal–amphipod interactions in an ancient lake, demonstrating that organisms even smaller than krill can be important prey for single-prey feeding aquatic mammals if the environment and predators’ adaptations allow high foraging rates. Further, our finding that Baikal seals directly eat macroplankton may explain why they are so abundant in this ultraoligotrophic lake.
The world’s deepest ancient lake, Lake Baikal in Russia, has a diverse endemic fauna, including Baikal seals Pusa sibirica, the apex predator of the lake and the only pinniped species inhabiting exclusively freshwater systems. The prevailing view is that Baikal seals predominantly eat fishes (pelagic sculpins, Comephorus spp. and Cottocomephorus spp.) (1–3). An endemic amphipod Macrohectopus branickii, the main diet of pelagic sculpins (4, 5), can also be found in the stomach contents of seals (6). Yet, its small size (<0.1 g) and the lack of indigestible body parts (e.g., otolith in fishes) make quantitative assessments of its contribution difficult. Amphipods in Lake Baikal have rapidly evolved from a few immigrant groups to >340 endemic species, representing a textbook example of adaptive radiation (7, 8). Among them, M. branickii exhibits an extreme adaptation with a distinct slender body and fully pelagic lifestyle (Fig. 1A), unlike many other benthic species with more stout bodies. This unusual amphipod is the dominant macroplankton in the lake. It forms dense aggregations and exhibits diel vertical migration (i.e., staying deep during the day and migrating to shallow depths at night) (9), similar to other macroplanktons in different systems. Therefore, M. branickii could potentially be important prey readily accessible at night for Baikal seals, much like Antarctic krill for some Antarctic seals (10). However, to what extent Baikal seals eat M. branickii in addition to fishes, let alone how they catch them, are currently unclear.
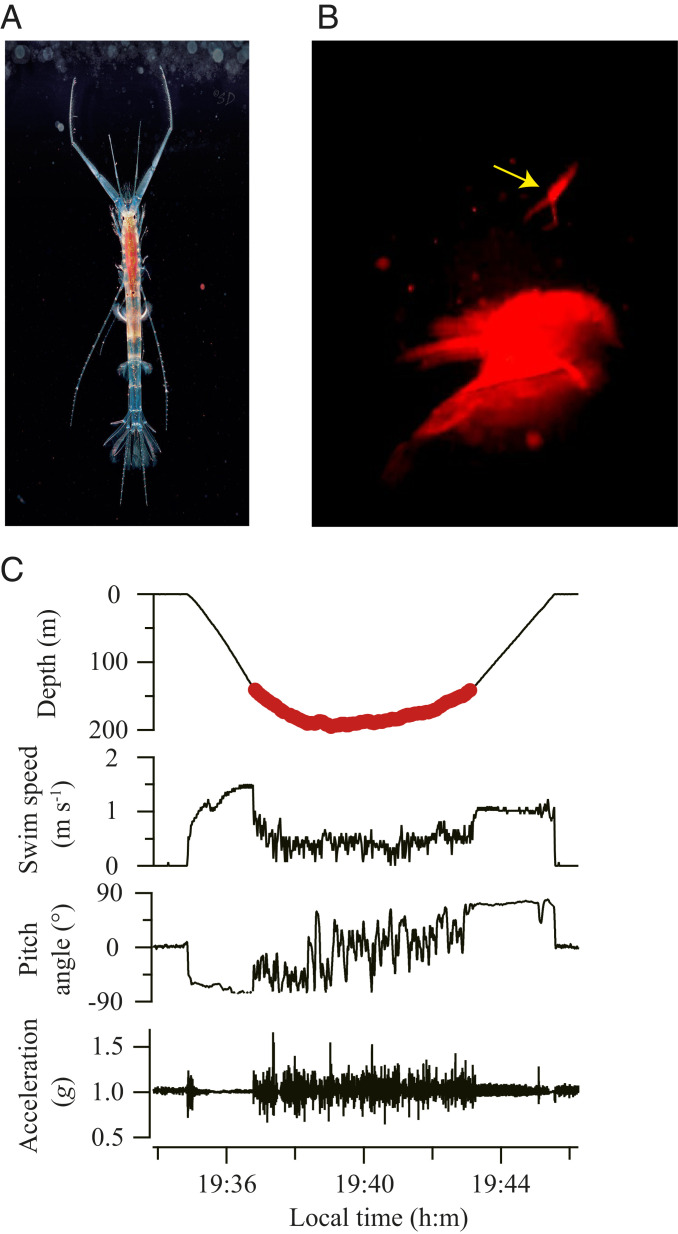
Fig. 1.
Baikal seals hunt endemic amphipods in Lake Baikal. (A) M. branickii, the world’s only freshwater amphipod with a fully pelagic lifestyle (Photo credit: S. Didorenko). (B) Image from animal-borne video footage, showing the seal about to hunt a M. branickii (yellow arrow) by stretching its neck. M. branickii is in an upside-down position with its paired antennae visible. (C) A foraging dive on M. branickii by a seal, showing depth, swim speed, pitch angle (with positive and negative values indicating upward and downward attitude, respectively), and body acceleration (i.e., the vectorial sum of triaxial accelerations). The simultaneously recorded video footage showed that the seal encountered and hunted M. branickii nearly continuously (total, 154 individuals) during the bottom phase (denoted by many red markers that appear as a line).
Globally, amphipods are rarely targeted by aquatic mammals, except for a few filter-feeding baleen whales (11–13) and local populations of Arctic seals (14, 15), despite their high diversity and wide distribution. Amphipods are typically much smaller than Antarctic krill, a preferred crustacean prey of many aquatic mammals, including pinnipeds (10). Gaining an energy surplus by hunting small amphipods is thus likely difficult for aquatic mammals, especially those that catch prey individually (i.e., pinnipeds and toothed whales). If Baikal seals eat M. branickii as part of their main diet, they would exhibit exceptionally high foraging rates with specialized foraging strategies, so that this small crustacean becomes energetically profitable. Moreover, if M. branickii is part of their diet, Baikal seals might also exhibit morphological adaptations on their feeding apparatus (i.e., teeth). Because water inevitably enters the mouth while animals forage during dives, high foraging rates could lead to excessive water intake unless they can expel water effectively. Crabeater and leopard seals, the two krill-feeding phocid seals in the Southern Ocean, have specialized postcanine teeth with developed cusps, allowing them to expel water through the cusp spacing while retaining krill (16, 17). Baikal seals also have cusped postcanine teeth, which are “like a comb” (1); however, the possibility that their teeth represent adaptations for feeding amphipods has never been explored.
Here, we used modern electronic tags (i.e., accelerometers and video cameras) to show that Baikal seals individually hunt thousands of tiny (<0.1 g) M. branickii per day, primarily by tracking the diel vertical migrations of its swarms. We also show that Baikal seals have the most developed cusped teeth in the subfamily Phocinae (“northern true seals”), suggesting the function of their teeth as a filter for expelling water while retaining prey during high-speed foraging. Our findings reveal the unique predator–prey interaction driven by the adaptive radiation of amphipods along with the behavioral and morphological specializations of seals in an ancient lake. Our results also have important implications for the function of the Lake Baikal ecosystem.
Results
The video footage recorded by seal-borne cameras while seals were diving at night (n = 3 seals, 2 h for each individual) confirmed that they encountered and individually hunted M. branickii and fishes (Comephorus spp., Cottocomephorus spp, and unidentifiable fish larvae) (Movies S1 and S2). M. branickii was readily identified by its slender body and, in some cases, paired long antennae (Fig. 1B). Most M. branickii seen in the footage were in a vertical (upright or upside-down) position, and either displayed no response or bent its body quickly (a presumable escape response) at the timing of hunting action by the seals. No unsuccessful hunting attempts on M. branickii were observed, although a few hunting attempts on fishes were apparently unsuccessful. During foraging dives, seals encountered and hunted 78 ± 45 (mean ± SD) individual M. branickii per dive (n = 17 dives, excluding data from Seal 1 where relatively dark footage precluded us from detecting all events). The maximum foraging rate was 154 individual M. branickii per dive (see Movie S3 for a sequence of frequent feeding events). Fishes were hunted less frequently (2.3 ± 3.2 individuals per dive, n = 17 dives). We selected footage that clearly showed M. branickii in a stretched position in front of the seal head (SI Appendix, Fig. S1). The mean estimated body length of M. branickii based on the footage (Materials and Methods) was 23 ± 4 mm (range, 14–35 mm; n = 32), which corresponds to 91 mg in weight (18).
During a total of 556-h accelerometer data (n = 8 seals), parts of which overlapped with video footage, seals exhibited dive bouts (repeated cycles of a dive and the postdive surface period), prolonged surfacing events between dive bouts, and haul-out behavior on the shore (SI Appendix, Table S1). Seals tended to haul out during the daytime (SI Appendix, Fig. S2). They also rested during dives, as evident from characteristic drift dives (19) and U-shaped dives with minimal activities (based on swim speed and body acceleration) during the bottom phase. The M. branickii feeding dives confirmed by the video footage were characterized by decreased swim speed, frequent changes in pitch angles, and increased body accelerations during the bottom phase (Fig. 1C). These dives were part of long dive bouts observed at night, during which dive depth gradually decreased from dusk to midnight and then increased until dawn (Fig. 2 A and B). Gradual changes in depth were also evident over a finer time scale, that is, during the bottom phase of each dive (Fig. 2 C and D). Based on the behavioral characteristics during dives, 1,036 foraging dives targeting M. branickii were detected from all accelerometer data. These dives occurred primarily at night (SI Appendix, Fig. S3). Dives indicative of active chasing of fishes, during which seals made multiple up-and-down movements in the bottom phase (20), were also observed primarily during the daytime; however, we had no video evidence for that type of dives.
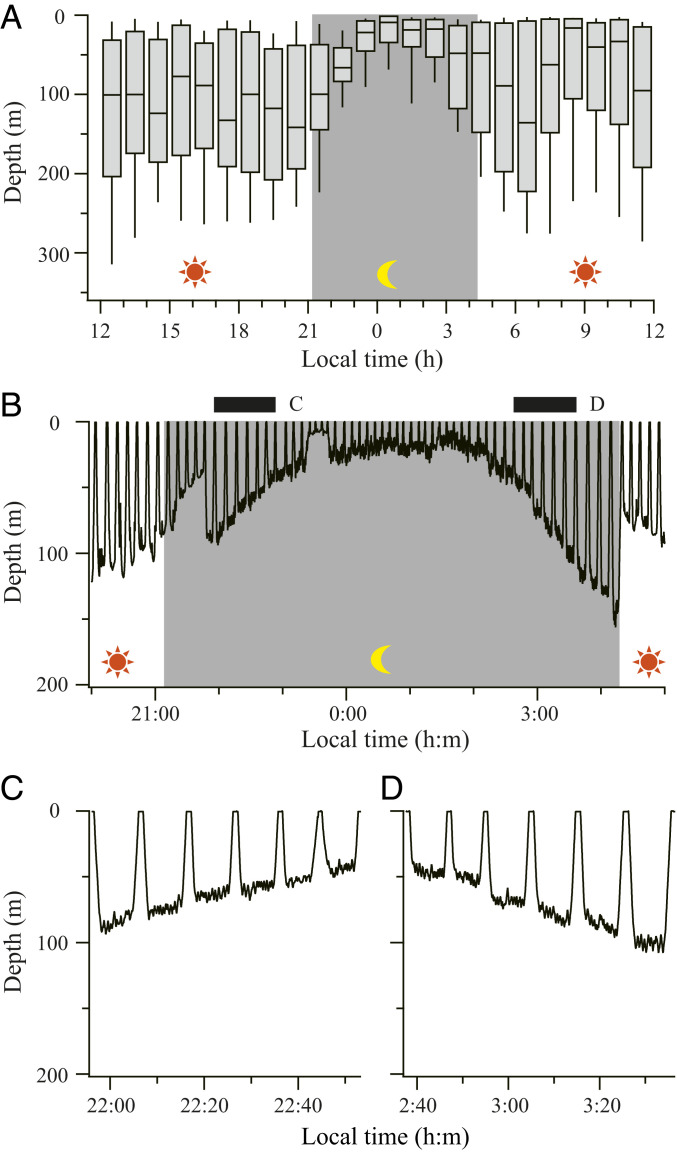
Fig. 2.
Gradual changes in dive depth at night. (A) Dive depth plotted against time of day for all eight Baikal seals. For each hour, the 25th percentile, the median, and the 75th percentile are shown by boxes, and the 9th and 91st percentiles are shown by error bars. Background color represents the day (white) and night (gray) based on the local sunset and sunrise time. (B) An example of time-series depth data. Black bars on top represent the periods shown in detail in C and D. (C and D) Enlarged views of time-series depth data, showing that the seal gradually changed depth not only during a series of dives but also during the bottom phase of each dive.
The number of feeding events on M. branickii per dive confirmed by the video footage was linearly related to the number of acceleration signals (Materials and Methods) per dive (Fig. 3). Therefore, foraging rates on M. branickii could be estimated from accelerometer data alone. We applied the acceleration signal analyses to the M. branickii feeding dives detected from all accelerometer data. The mean foraging rate per dive was 57 individual M. branickii (n = 1,036 dives; the range of mean for individual seals, 42–89) (SI Appendix, Table S2). This rate was exceptionally high for the mean dive duration (10.1 min) among pinnipeds and toothed whales (Fig. 4). The mean foraging rate per 24-h active period (i.e., excluding periods of haul-out events, prolonged surfacing events, and resting dives) was 4,278 ± 2,124 individual M. branickii (the range for individual seals, 1,520–7,892) (SI Appendix, Table S2). The mean foraging rate per hour during dive bouts targeting M. branickii was 320 individual M. branickii (the range of mean for individual seals, 197–602).
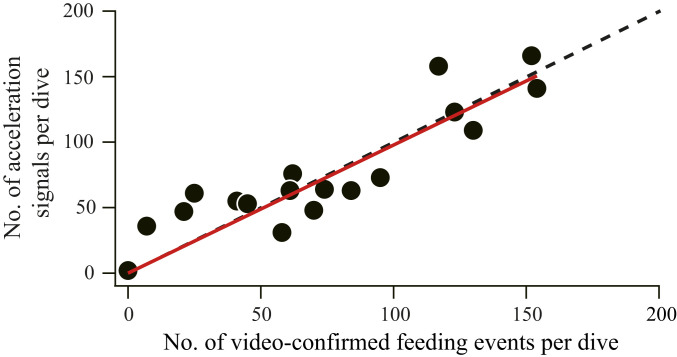
Fig. 3.
Acceleration signals as indicators of foraging rates. Relationship between the number of amphipod (M. branickii) feeding events per dive confirmed by the video footage and the number of acceleration signals per dive. The linear regression through the origin (Y = 0.99 × X, R2 = 0.94, n = 22 dives) is shown in red. The broken, diagonal line represents an ideal case where acceleration signal rates are equal to video-confirmed foraging rates.
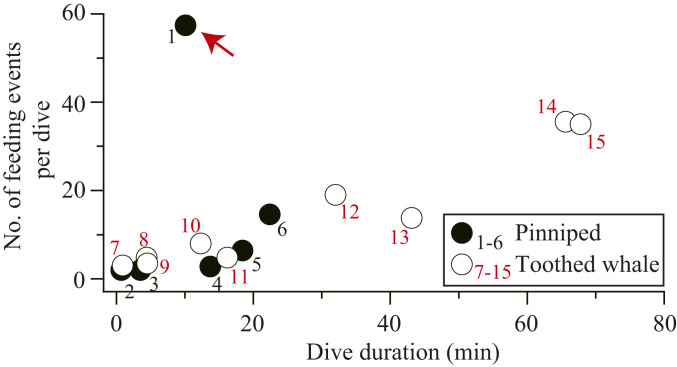
Fig. 4.
Exceptionally high foraging rate of Baikal seals. The average number of feeding events per dive plotted against dive duration for Baikal seals targeting amphipods (M. branickii) (1, red arrow) and other aquatic mammals that catch prey individually (pinnipeds and toothed whales). Other species shown are Antarctic fur seal (2) (38), Australian fur seal (3) (39), Weddell seal (4) (40), southern elephant seal (5) (41), northern elephant seal (6) (42), harbor porpoise (7) (43), Risso’s dolphin (8) (43), killer whale (9) (43), long-finned pilot whale (10) (43), short-finned pilot whale (11) (43), Blainville’s beaked whale (12) (43), sperm whale (13) (43), Cuvier’s beaked whale (14) (43), and Baird’s beaked whale (15) (43).
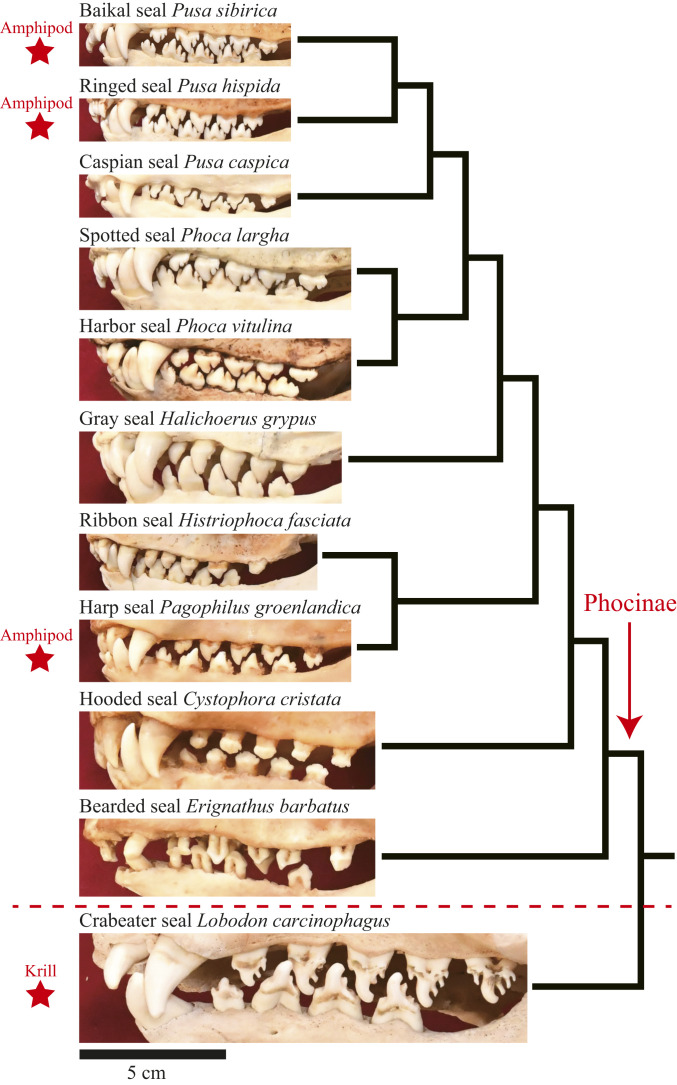
Examining a museum collection of seal skulls revealed that Baikal seals had the most specialized postcanine teeth with developed cusps among all 10 extant species of the subfamily Phocinae (Fig. 5). As known for krill-feeding crabeater seals, Baikal seal teeth formed a filter when the mouth was closed; however, cusp spacing was smaller in Baikal seals. Similar, but less developed cusped teeth were found in ringed and harp seals, both of which consume, at least in some areas, large amounts of marine pelagic amphipod Themisto libellula (14, 15).
Fig. 5.
Tooth shape associated with pelagic crustacean feeding in seals. All 10 extant species of the subfamily Phocinae (above the broken red line) and the crabeater seal, a krill-feeding species outside Phocinae, are shown with a phylogenetic relationship among species (44). The species known to consume large amounts of pelagic amphipods or krill are marked by stars. Mirror images are used for the bearded and crabeater seal due to poor conditions on one side of the skulls.
Discussion
Amphipods as Food for Baikal Seals.
Unlike the prevailing view that Baikal seals predominantly eat pelagic sculpins that eat M. branickii (1–3), we provided video evidence that Baikal seals also directly hunt M. branickii at night. Although our video footage is limited to three individuals with 2-h video per animal, we were able to link the video-based foraging rates to acceleration signal rates (Fig. 3) to extend recording durations effectively (21). By doing so, we showed that all eight individuals (total recording duration, 556 h) exhibited similar foraging behaviors and hunted thousands of M. branickii per 24-h active period. Fishes are also important, as evident from occasional fish-feeding events observed in the video footage at night and dives indicative of active chasing of fishes recorded primarily during the daytime. Based on our body size estimates of M. branickii (mean, 23 mm in length and 91 mg in weight), the seals may have collected 389 ± 193 g (range, 138–718 g) M. branickii per 24-h active period. This estimate is conservative because some feeding events may not have been visible in the video footage, potentially leading to underestimates of video- and acceleration-based foraging rates. Baikal seals in captivity eat fish at 3.7% body mass (corresponding to 1.4–2.6 kg for the tagged seals) per day (22). M. branickii is thus an energetically important component of their diets, as previously suggested by stable isotope analyses (4). On a calorie basis, M. branickii may have met 20 ± 9% (range, 8–39%) of the seals’ daily energy requirements; however, this estimate is sensitive to input parameters and should be treated with caution (Materials and Methods). Moreover, seals tended to haul out during the daytime, when M. branickii stays deeper (9) and foraging dives targeting M. branickii occurred only occasionally (SI Appendix, Figs. S2 and S3). It further suggests the importance of M. branickii as prey to Baikal seals. Although diet composition may vary seasonally and regionally, the seals previously tagged in a different area (southern basins) and different season (October) all exhibited diving behavior indicative of M. branickii feeding (19, 20). Our results are thus robust at least during ice-free seasons (May–October).
With its small size (<0.1 g), M. branickii is an important dietary item for Baikal seals only because the seals’ foraging rates are exceptionally high (Fig. 4). In a remarkable case, we observed in the video footage that a seal encountered and hunted 154 individual M. branickii during the 390-s bottom phase of a dive, meaning that it hunted one prey every 2.5 s. Given that foraging rates are evaluated as rates per hour, our records (197–602 individual M. branickii per hour) are comparable to “ultrahigh foraging rates” of harbor porpoises (23), which exhibited up to 550 capture attempts on small fishes per hour. Our estimates of Baikal seal foraging rates broadly agree with a report of the stomach contents of three young individuals sampled in September (6). They contained 300–700 g of M. branickii and no fishes, corresponding to 3,300–7,700 individual M. branickii given an individual weight of 91 mg.
Factors Underlying High-Speed Foraging on Tiny Prey.
How do Baikal seals achieve such high foraging rates? Seals decreased swim speed during the bottom phase of dives when they hunted M. branickii (Fig. 1C). They also changed dive depth gradually throughout the night (Fig. 2), a pattern consistent with the diel vertical migration of M. branickii (9). Our results indicate that Baikal seals forage in dense swarms of M. branickii and track their vertical movement at night. As an estimate of M. branickii density, 570–4,094 individuals·m−2 were reported by vertical net tows (24). The swarms of M. branickii ascend and descend as fast as 4 m·min−1 (9). Accordingly, the seals tagged in this study apparently tracked them not only during a series of dives but also during the bottom phase of each dive (Fig. 2 C and D). Such fine-scale tracking of vertically migrating prey has never been reported for other mammalian or avian divers.
A potential drawback of high foraging rates is that water inevitably enters the mouth each time the mouth is opened. Swallowing prey and water together would require extra muscular works and slow down foraging rates, although drinking freshwater (i.e., the case of Baikal seals) causes less osmoregulatory stress on animals than seawater. We found that Baikal seals have the most specialized comb-like postcanine teeth among the subfamily Phocinae (Fig. 5), suggesting that they can expel water while retaining prey during high-speed foraging. Although direct observations on captive animals are needed to confirm how they use their teeth, several lines of evidence further support our suggestion. First, comb-like teeth appear to have convergently evolved at least twice in Phocinae. The three species possessing that type of teeth (i.e., Baikal, ringed, and harp seals) are the only pinniped species known to consume large amounts of pelagic amphipods (M. branickii for Baikal and T. libellula for ringed and harp seals) (14, 15). Second, outside Phocinae, crabeater and leopard seals, the two krill-feeding phocid species in the Southern Ocean, also have specialized cusped teeth (17). The usage of their teeth as a filter was observed for a captive crabeater seal (16). The cusp spacing is smaller in Baikal than crabeater seals, presumably reflecting the difference in size between amphipods and krill. Whereas crabeater seals may capture multiple krill at a time (17), we observed that Baikal seals always hunt amphipods individually. We suggest that they expel water from the mouth through the cusp spacing after each foraging event, and by doing so, achieve high foraging rates without drinking excessive water. Baikal seals may also be able to such in prey at a distance, as known for crabeater seals (16).
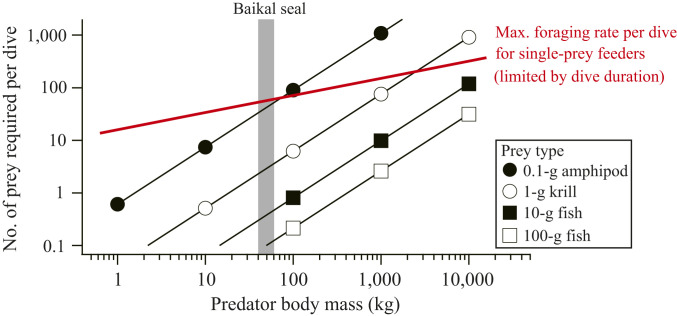
Lastly, Baikal seals (∼50 kg in weight) are among the smallest phocid seals, which is an important factor underlying their unusual prey selection of tiny (<0.1 g) amphipods. To illustrate this point, we modeled the relationships among predator body size, prey body size, and the number of prey a predator needs to consume per dive to replenish the energy expended during a dive cycle (a dive and the postdive surface period) for endothermic divers (Fig. 6). We further assumed that the number of prey collectible in a dive is ultimately limited by dive duration in single-prey feeders (red line in Fig. 6), as observed for our Baikal seals (Fig. 1C). Our simple model based on the allometry of diving behavior (25) and metabolic rates (26) indicates that, as predator body mass increases, the number of prey required per dive increases more rapidly (scaling exponent, 1.08) than dive duration does (scaling exponent, 0.33) for a given type of prey. This pattern holds for the range of scaling exponents (0.67–0.75) reported for endotherm metabolic rates (27). Larger single-prey feeders are thus busier during dives if the prey is small, and there is a critical body mass above which they cannot maintain energy balance with small prey. For example, to maintain energy balance by eating 0.1-g amphipods, a 10-kg animal would need to catch seven individuals during its 1.3-min dive duration (one prey every 11 s), whereas a 1,000-kg animal would need to catch 1,090 individuals during its 5.6-min dive duration (one prey every 0.3 s), a foraging rate that is too high to achieve for single-prey feeders. Therefore, the small body size of Baikal seals helps them to gain an energy surplus by hunting tiny amphipods at physically possible foraging rates. A major limitation of this simple model is that the allometry of diving behavior (25) can predict the dive duration of a given species only roughly. Nevertheless, the model explains why amphipods are important food for various diving seabirds (<3 kg in body mass) (28), but not for aquatic mammals, excluding a few small seals (14, 15) and giant filter-feeding (i.e., multiple-prey feeding) baleen whales (11–13).
Fig. 6.
A modeled relationship (on a log-log scale) among predator body size, prey body size, and the number of prey required per dive in endothermic divers. The last parameter is the minimum requirement for sustainable activities, defined as the number of prey a predator needs to consume to replenish the energy expended during a dive cycle (dive and the postdive surface period). Different symbols represent different prey types. Red line is the putative, maximum sustainable foraging rate per dive in single-prey feeders, which was assumed to be proportional to dive duration. See Materials and Methods for details.
Ecosystem Implication.
Our finding has an important implication for the function of the Lake Baikal ecosystem. Lake Baikal is an ultraoligotrophic system characterized by high water transparency and low chlorophyll-a concentrations (29), indicating its limited capacity for supporting energy-demanding apex predators. Moreover, aquatic mammals in freshwater systems are globally threatened by anthropogenic environmental changes, with some species having undergone local or global extinction (30). Nevertheless, Baikal seals remain highly abundant with an estimate of 82,500–115,000 individuals over the lake (31,720 km2) and are categorized as Least Concern by the International Union for Conservation of Nature (IUCN) Red List (31). Their overall population density (2.6–3.6 individuals·km−2) is much higher than other seal populations in closed systems, including Caspian seals (104,000–168,000 individuals in 371,000 km2, 0.28–0.45 individuals·km−2), Ladoga ringed seals (6,000–9,000 individuals in 17,700 km2, 0.34–0.51 individuals·km−2), and Saimaa ringed seals (320 individuals in 4,400 km2, 0.07 individuals·km−2) (31). This apparent paradox could partly be resolved by our finding that Baikal seals directly eat extremely abundant (estimated total biomass, 110,000 tons; ref. 18) macroplankton (M. branickii) in addition to fishes. When energy is transferred from a trophic level to an upper level, substantial portions are lost (32). Therefore, shortcutting trophic chains by apex predators eating macroplankton (i.e., the prey of fishes) rather than fishes themselves leads to higher energy transfer efficiency from phytoplankton to apex predators. It can, consequently, lead to a higher capacity of the ecosystem for supporting apex predators for a given level of primary production. However, more detailed food web analyses and consideration of other factors (e.g., human disturbance level) are warranted to draw firm conclusions.
In conclusion, we found a unique interaction between endemic freshwater seals and endemic pelagic amphipods, driven by the adaptive radiation of amphipods in Lake Baikal. To gain an energetic profit from the small (<0.1 g) amphipods, Baikal seals hunt them individually at exceptionally high rates (>50 individuals per dive and thousands of individuals per day). This strategy is enabled by 1) the dense aggregation and predictable, diel vertical migration of amphipods; 2) the comb-like postcanine teeth of Baikal seals, allowing them to hunt amphipods frequently without drinking excessive water; and 3) the small body size of Baikal seals, allowing them to gain an energy surplus by hunting amphipods at physically possible foraging rates. This study demonstrates that organisms even smaller than krill can be important prey for single-prey feeding aquatic mammals if the environment and predators’ adaptations allow high foraging rates. Further, our finding that Baikal seals shortcut the food chain of the lake by directly eating macroplankton may explain why this species is so abundant in this ultraoligotrophic system.
Materials and Methods
Fieldwork and Instruments.
Fieldwork was conducted at Ushkany Islands (53.85°N, 108.65°E) in Lake Baikal, Russia, in June 2018 under a permit from the Federal Agency for Fisheries of the Ministry of Agriculture of the Russian Federation (032018030101). Eight seals hauling out on the shore were manually captured and transported to a boat. They were immobilized by using a custom-made stretcher, measured, weighed, and instrumented before they were released from the shore (SI Appendix, Table S1). For each seal, an alumina plate was glued to the front part of the back. Then, a biologging package composed of an accelerometer (ORI1300-3MPD3GT, Little Leonardo), video camera (DVL2000M130-SW or DVL400M, Little Leonardo), float, time-scheduled release system (Little Leonardo), satellite transmitter (Wildlife Computers), and radio transmitter (Advanced Telemetry Systems) was attached to the plate (20). The packages detached from the seals 2–4 d after the deployment and floated to the surface. They were located using signals from satellite and radio transmitters and recovered using a boat. A package weighed 215 g, representing 0.4% of the average body mass of the seals (51.3 kg).
The ORI1300-3MPD3GT accelerometer recorded depth, temperature, and swim speed at 1-s intervals, and triaxial acceleration and geomagnetism at 1/20-s intervals for the full deployment period (2–4 d). The DVL2000M130-SW camera recorded images of 640 × 480 pixels at 30 frames per second with near red flash for ∼2 h. This camera was programmed to start filming at night and used for four seals; however, one seal (Seal 5) was hauling out during the recording period, and useful footage was obtained only for three seals (SI Appendix, Table S1). The DVL400M camera had no flash and was used for four seals. All footage recorded by this camera during dives were too dark to detect any feeding events.
Data Analyses.
The timing of hunting events and the type of prey were visually checked from the footage filmed by the camera with flash. To estimate the size of M. branickii hunted by the seals, the footage that clearly showed M. branickii in a stretched position in front of the seal head were selected (SI Appendix, Fig. S1). The length of M. branickii (excluding antennae) relative to the distance between the paired, inner eye whiskers of the seal was measured on screen. It was converted into the absolute length based on the measured, whisker-to-whisker length of seals (20 mm). The range of our length estimates (14–35 mm, n = 32) was reasonable for female M. branickii (18) (males are much smaller in this species). The mean length (23 mm) was larger than the peak length (17 mm) reported in a net sampling study (9). It suggests either that seals selectively foraged in the swarms of large individuals or that net sampling was biased toward smaller individuals due to better escaping capabilities of larger individuals (33).
Accelerometer data were analyzed using the software Igor Pro (WaveMetrics). A dive was defined as any excursion below the surface to a depth of >2 m. Haul-out events were detected as prolonged periods when depth was zero and temperature was higher than 4 °C (the highest water temperature). Higher foraging rates on M. branickii by the seals confirmed by the video footage were apparently associated with higher fluctuations of body acceleration (recorded on the back of the animals) during dives (SI Appendix, Fig. S4). We thus examined how foraging rates could be estimated from acceleration data alone. Visual inspections found that the video-confirmed feeding events did not always correspond to specific body acceleration signatures, presumably because the head movements associated with feeding events are buffered by the neck movements. That is, body acceleration data contained the information on prey pursuits by the seals, but not on the hunting action or prey ingestion during each foraging event. As such, we took a correlation approach, where we sought an acceleration signal that gives the best correlation (i.e., the highest possible R2 value) between the acceleration-based signal rates and the video-confirmed foraging rates on the dive-by-dive basis. The signals produced by the following procedure provided a highly linear relationship (through the origin) with an R2 value of 0.94 and a slope close to 1 (Fig. 3). First, the vectorial sum of triaxial accelerations [(x2 + y2 + z2)0.5] was high-pass filtered to remove low-frequency noise stemming from changes in body postures and flipper stroking activities. Second, the filtered accelerations (recorded at 20 Hz) were downsampled to 5 Hz to smooth the data. Third, the periods when the absolute values of the filtered accelerations exceeded a threshold (0.031 g) were extracted as signals. This signal analysis was applied to all M. branickii feeding dives detected from the accelerometer data (based on the behavioral characteristics during dives, such as decreased swim speed; Fig. 1C). By doing so, the number of M. branickii hunted during each foraging dive was estimated.
Tooth Shape.
The collection of seal skulls of the National Museum of Nature and Science, Japan, was examined and photographed. The IDs of the specimen shown in Fig. 5 are NSMT-M29687 (Baikal), NSMT-M30083 (ringed), NSMT-M30058 (Caspian), NSMT-M28358 (spotted), NSMT-M19766 (harbor), NSMT-M29091 (gray), NSMT-M14988 (ribbon), NSMT-M24778 (harp), NSMT-M37905 (hooded), NSMT-M24777 (bearded), and NSMT-M28438 (crabeater).
Energetics.
The energetic contribution of M. branickii to the daily energy requirement of Baikal seals was estimated for each seal tagged. It was the total weight of M. branickii consumed per 24-h active period (estimated from tag data) multiplied by its energy content and digestive efficiency, divided by the seal’s energy expenditure per day. The energy content of M. branickii is unavailable and was set at 4.5 kJ·g−1 based on the water content (78%) and energy content per dry mass (20.5 kJ·g−1) of a different marine pelagic amphipod Themisto libellula (34). Digestive efficiency, the proportion of energy digested by animals to the energy contained in prey, was set at 84% (35). Daily energy expenditure (in kilojoules) of free-swimming seals was set at 1.3 times the Kleiber’s Equation (26) based on a study on northern elephant seals (36), and calculated as 367.73 × BM0.756, where BM is body mass in kilograms. Although the result gave an idea for the importance of M. branickii as prey to Baikal seals, it is sensitive to parameter inputs and should be treated with caution. Our estimate of the average size of M. branickii based on the video footage (23 mm in length, corresponding to 91 mg in weight) may have errors. Assuming, for example, that the true body length is 20 mm and 26 mm (i.e., 3 mm shorter and longer, respectively, than our estimate), the weight of individual M. branickii would decrease to 60 mg (by 34%) and increase to 128 mg (by 41%), respectively (18). The estimate of the total weight of M. branickii consumed by the seals and its energetic contribution would change by the same proportions. Similarly, there are uncertainties about the energy content of M. branickii and the daily energy expenditure of free-swimming Baikal seals, both of which are influential parameters.
The relationship among predator body size, prey body size, and the number of prey a predator needs to consume per dive to replenish the energy expended during a dive cycle (dive and the postdive surface period) was modeled for endothermic (mammalian and avian) divers (Fig. 6). Energy expenditure during a dive cycle was modeled as a function of BM based on the allometry of dive duration (in seconds, 35.5 × BM0.326) (25), surface duration (in seconds, 18.8 × BM0.331) (25), and the energy expenditure per day (in kilojoules, 367.73 × BM0.756) (36). The number of prey required per dive was calculated for the following four prey types: 0.1-g amphipod, 1-g krill, 10-g fish, and 100-g fish. Energy contents were set at 4.5 kJ·g−1 for amphipod (see above), 6.5 kJ·g−1 for krill (calculated from a water content of 74% and an energy content per dry mass of 25.1 kJ·g−1; ref. 34), and 5.0 kJ·g−1 for fish (37). Digestive efficiency was set at 84% (35). The modeled number of prey required per dive scaled as BM1.08 with different intercepts depending on prey types (solid lines in Fig. 6). It was compared with the putative, maximum sustainable foraging rate per dive for single-prey feeders, which was assumed to be proportional to dive duration and, thus, scales as BM0.33 (25) (red line in Fig. 6). Its intercept was determined by assuming that the mean foraging rate on M. branickii by Baikal seals recorded in this study (57 individuals per dive) is the maximum sustainable rate for a 50-kg animal. This assumption is not critical, given that our objective of this modeling was to illustrate how and why the body size of endothermic divers could limit the body size of potential prey, rather than to predict the maximum possible body mass of predators that can survive off each type of prey.
Supplementary Material
Acknowledgments
We thank M. Ovdin and V. Peterfeld for their help in obtaining the permit; S. Balhanov, N. Matashin, E. Petrov, and V. Tkachev for their help in the fieldwork; Y. Tajima for the access to museum specimens; L. Yampolsky for the information on Lake Baikal amphipods; S. Didorenko for the photo in Fig. 1A; and Y. Papastamatiou, K. Shiomi, A. Takahashi, and two anonymous reviewers for the comments on the draft. This work was funded by the National Geographic Society Grant NGS-KOR-293R-18.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014021117/-/DCSupplemental.
Data Availability.
Data on Baikal seal diving behavior and for Fig. 4 have been deposited in the Arctic Data archive System (ADS) of the National Institute of Polar Research (https://ads.nipr.ac.jp/dataset/A20201027-001).
References
- 1.Thomas J., Pastukhov V., Elsner R., Petrov E., Phoca sibirica. Mamm. Species 188, 1–6 (1982). [Google Scholar]
- 2.Silow E. A., Gurman V. J., Stom D. J., Rosenraukh D. M., Baturin V. I., Mathematical models of lake Baikal ecosystem. Ecol. Modell. 82, 27–39 (1995). [Google Scholar]
- 3.Moore M. V., et al. , Climate change and the world’s “sacred sea”—Lake Baikal, Siberia. Bioscience 59, 405–417 (2009). [Google Scholar]
- 4.Yoshii K., et al. , Stable isotope analyses of the pelagic food web in Lake Baikal. Limnol. Oceanogr. 44, 502–511 (1999). [Google Scholar]
- 5.Miyasaka H., et al. , Feeding ecology of two planktonic sculpins, Comephorus baicalensis and Comephorus dybowskii (Comephoridae), in Lake Baikal. Ichthyol. Res. 53, 419–422 (2006). [Google Scholar]
- 6.Pastukhov V., Seal of Baikal (Nauka, Novosibirsk, 1993) (in Russian). [Google Scholar]
- 7.Sherbakov D. Y., Molecular phylogenetic studies on the origin of biodiversity in Lake Baikal. Trends Ecol. Evol. 14, 92–95 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Naumenko S. A., et al. , Transcriptome-based phylogeny of endemic Lake Baikal amphipod species flock: Fast speciation accompanied by frequent episodes of positive selection. Mol. Ecol. 26, 536–553 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Melnik N. G., Timoshkin O. A., Sideleva V. G., Pushkin S. V., Mamylov V. S., Hydroacoustic measurement of the density of the Baikal macrozooplankter Macrohectopus branickii. Limnol. Oceanogr. 38, 425–434 (1993). [Google Scholar]
- 10.Bengtson J. L., Stewart B. S., Diving and haulout behavior of crabeater seals in the Weddell sea, Antarctica, during March 1986. Polar Biol. 12, 635–644 (1992). [Google Scholar]
- 11.Kawamura A., Food of sei whale taken by Japanese whaling expeditions in the Antarctic season 1967/68. Sci. Rep. Whales Res. Inst. 22, 127–152 (1970). [Google Scholar]
- 12.Hazard K. W., Lowry L. F., Benthic prey in a bowhead whale from the northern Bering Sea. Arctic 37, 166–168 (1984). [Google Scholar]
- 13.Dunham J. S., Duffus D. A., Diet of gray whales (Eschrichtius robustus) in clayoquot sound, British columbia, Canada. Mar. Mamm. Sci. 18, 419–437 (2002). [Google Scholar]
- 14.Lydersen C., Angantyr L. A., Wiig Ø., Øritsland T., Feeding habits of Northeast Atlantic harp seals (Phoca groenlandica) along the summer ice edge of the Barents Sea. Can. J. Fish. Aquat. Sci. 48, 2180–2183 (1991). [Google Scholar]
- 15.Holst M., Stirling I., Hobson K. A., Diet of ringed seals (Phoca hispida) on the east and west sides of the North Water Polynya, northern Baffin Bay. Mar. Mamm. Sci. 17, 888–908 (2001). [Google Scholar]
- 16.Klages N. T. W., Cockcroft V. G., Feeding behaviour of a captive crabeater seal. Polar Biol. 10, 403–404 (1990). [Google Scholar]
- 17.Davis R. W., Marine Mammals: Adaptations for an Aquatic Life (Springer Nature, 2019). [Google Scholar]
- 18.Bekman M. Y., Afanasyeva E. L., “Distribution and production of Macrohectopus” in Biological Productivity of the Baikal Pelagic Region and its Dynamics, Votintsev K. K., Ed. (Nauka, Novosibirsk, 1977), pp. 76–98 (in Russian). [Google Scholar]
- 19.Watanabe Y. Y., Baranov E. A., Miyazaki N., Drift dives and prolonged surfacing periods in Baikal seals: resting strategies in open waters? J. Exp. Biol. 218, 2793–2798 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Watanabe Y., Baranov E. A., Sato K., Naito Y., Miyazaki N., Foraging tactics of Baikal seals differ between day and night. Mar. Ecol. Prog. Ser. 279, 283–289 (2004). [Google Scholar]
- 21.Watanabe Y. Y., Takahashi A., Linking animal-borne video to accelerometers reveals prey capture variability. Proc. Natl. Acad. Sci. U.S.A. 110, 2199–2204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov K. B., Baranov E. A., “Standard energy exchange in Baikal seal (Phoca sibirica)” in Marine Mammals of Holarctic (Baikal, Russia, 2002), pp. 123–124. [Google Scholar]
- 23.Wisniewska D. M., et al. , Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 26, 1441–1446 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Naumova E. Y., Zaidykov I. Y., Makarov M. M., Recent quantitative values of Macrohectopus branickii (Dyb.)(amphipoda) from Lake Baikal. J. Great Lakes Res. 46, 48–52 (2020). [Google Scholar]
- 25.Halsey L. G., Butler P. J., Blackburn T. M., A phylogenetic analysis of the allometry of diving. Am. Nat. 167, 276–287 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Kleiber M., The fire of Life. An Introduction to Animal Energetics (John Wiley & Sons, 1961). [Google Scholar]
- 27.White C. R., Cassey P., Blackburn T. M., Allometric exponents do not support a universal metabolic allometry. Ecology 88, 315–323 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Bocher P., et al. , Amphipod-based food web: Themisto gaudichaudii caught in nets and by seabirds in Kerguelen waters, southern Indian Ocean. Mar. Ecol. Prog. Ser. 223, 261–276 (2001). [Google Scholar]
- 29.Hampton S. E., et al. , Sixty years of environmental change in the world’s largest freshwater lake–Lake Baikal, Siberia. Glob. Change Biol. 14, 1947–1958 (2008). [Google Scholar]
- 30.May-Collado L. J., Agnarsson I., Phylogenetic analysis of conservation priorities for aquatic mammals and their terrestrial relatives, with a comparison of methods. PLoS One 6, e22562 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IUCN, The IUCN Red List of Threatened Species (Version 2020-1, 2020). https://www.iucnredlist.org. Accessed 1 July 2020.
- 32.Hairston N. G. Jr, Hairston N. G. Sr, Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am. Nat. 142, 379–411 (1993). [Google Scholar]
- 33.Hill H. J., Trathan P. N., Croxall J. P., Watkins J. L., A comparison of Antarctic krill Euphausia superba caught by nets and taken by macaroni penguins Eudyptes chrysolophus: Evidence for selection? Mar. Ecol. Prog. Ser. 140, 1–11 (1996). [Google Scholar]
- 34.Percy J. A., Fife F. J., The biochemical composition and energy content of Arctic marine macrozooplankton. Arctic 34, 307–313 (1981). [Google Scholar]
- 35.Mårtensson P.-E., Nordøy E. S., Blix A. S., Digestibility of krill (Euphausia superba and Thysanoessa sp.) in minke whales (Balaenoptera acutorostrata) and crabeater seals (Lobodon carcinophagus). Br. J. Nutr. 72, 713–716 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Maresh J. L., et al. , Free-swimming northern elephant seals have low field metabolic rates that are sensitive to an increased cost of transport. J. Exp. Biol. 217, 1485–1495 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Boyd I. L., Estimating food consumption of marine predators: Antarctic fur seals and macaroni penguins. J. Appl. Ecol. 39, 103–119 (2002). [Google Scholar]
- 38.Jeanniard-du-Dot T., Trites A. W., Arnould J. P. Y., Guinet C., Reproductive success is energetically linked to foraging efficiency in Antarctic fur seals. PLoS One 12, e0174001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foo D., et al. , Testing optimal foraging theory models on benthic divers. Anim. Behav. 112, 127–138 (2016). [Google Scholar]
- 40.Liebsch N., Wilson R. P., Bornemann H., Adelung D., Plötz J., Mouthing off about fish capture: Jaw movement in pinnipeds reveals the real secrets of ingestion. Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 256–269 (2007). [Google Scholar]
- 41.Le Bras Y., Jouma’a J., Picard B., Guinet C., How elephant seals (Mirounga leonina) adjust their fine scale horizontal movement and diving behaviour in relation to prey encounter rate. PLoS One 11, e0167226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naito Y., et al. , Unravelling the mysteries of a mesopelagic diet: A large apex predator specializes on small prey. Funct. Ecol. 27, 710–717 (2013). [Google Scholar]
- 43.Goldbogen J. A., et al. , Why whales are big but not bigger: Physiological drivers and ecological limits in the age of ocean giants. Science 366, 1367–1372 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Fulton T. L., Strobeck C., Multiple markers and multiple individuals refine true seal phylogeny and bring molecules and morphology back in line. Proc. Biol. Sci. 277, 1065–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on Baikal seal diving behavior and for Fig. 4 have been deposited in the Arctic Data archive System (ADS) of the National Institute of Polar Research (https://ads.nipr.ac.jp/dataset/A20201027-001).