Abstract
Objective
Nab-paclitaxel plus gemcitabine (nab-P/G) has been established as a standard first-line treatment in metastatic pancreatic ductal adenocarcinoma (PDAC). S-1, as an oral fluoropyrimidine derivative, demonstrated effective for PDAC. This study aimed to evaluate the efficacy and safety of first-line chemotherapy with nab-paclitaxel plus S-1 (nab-P/S) versus nab-P/G in patients with advanced PDAC.
Methods
Patients with advanced PDAC receiving nab-P/S (n = 65) or nab-P/G (n = 45) as first-line chemotherapy between November 2013 and June 2019 were reviewed.
Results
The objective response rate (ORR) and disease control rate were numerically higher with nab-P/S than with nab-P/G (38.5% vs 28.9%, P = 0.30, 73.8% vs 66.7%, P = 0.42, respectively). ORRs of the primary lesion were similar for both groups (30.8% and 22.2%, P = 0.32). The median progression-free survival and overall survival were comparable between the two groups (5.5 vs 5.7 months, P = 0.34, 10.2 vs 11.3 months, P = 0.74, respectively). Nab-P/S was associated with a numerically lower risk of adverse events, especially hematologic adverse events.
Conclusion
Nab-P/S could be a convenient alternative with similar efficacy and a favorable safety profile compared with nab-P/G as first-line chemotherapy for advanced PDAC, as well as an option for neoadjuvant therapy.
Keywords: advanced pancreatic ductal adenocarcinoma, nab-paclitaxel, S-1, gemcitabine, objective response rate
Introduction
Pancreatic cancer is an extremely serious disease with a poor prognosis, and the 5-year survival rate can be as low as 6%.1 It is estimated that 90,100 Chinese people were newly diagnosed with pancreatic cancer, and 79,400 died from it in 2015.2 Only 20% of patients are eligible for initial resection when diagnosed with pancreatic cancer.1 Chemotherapy options for patients with advanced pancreatic ductal adenocarcinoma (PDAC) are scarce.
Gemcitabine showed a modest benefit against 5-fluorouracil (5-FU) for patients with locally advanced or metastatic PDAC in a pivotal phase III study.3 The addition of erlotinib to gemcitabine prolonged the marginal survival by 2 weeks.4 Currently, fluorouracil, leucovorin and irinotecan plus oxaliplatin (FOLFIRINOX), and nab-paclitaxel plus gemcitabine (nab-P/G) are two regimens widely used for advanced PDAC. Both regimens demonstrated a significant survival advantage over gemcitabine. However, the objective response rate (ORR) was limited to 23–31.6% with increased grade 3 or 4 myelosuppression and peripheral neuropathy, resulting in rigorous patient selection criteria and low dose intensity with each agent.5,6 S-1, as an oral fluoropyrimidine derivative, was proved to be an indication of pancreatic cancer in Japan in 2006. It showed good response rates ranging from 21.1% to 37.5% in the phase II studies for metastatic pancreatic cancers.7,8 Monotherapy with S-1 is non-inferior to gemcitabine and superior to gemcitabine with respect to overall survival (OS) for advanced and postoperative disease, respectively.9,10 It was associated with fewer hematologic adverse events, particularly in neutropenia, and presented a convenient oral alternative.9,10 A synergetic effect with nab-paclitaxel plus S-1 (nab-P/S) was confirmed from preclinical models.11,12 In two phase II studies in China, the new combination of nab-paclitaxel plus S-1 showed a remarkable ORR of 50.0–53.1% with good OS and favorable safety profiles as first-line therapy for advanced PDAC.13,14
Hence, we conducted this retrospective study to evaluate the efficacy and safety of first-line chemotherapy with nab-P/S versus nab-P/G in patients with advanced PDAC.
Methods
Patients
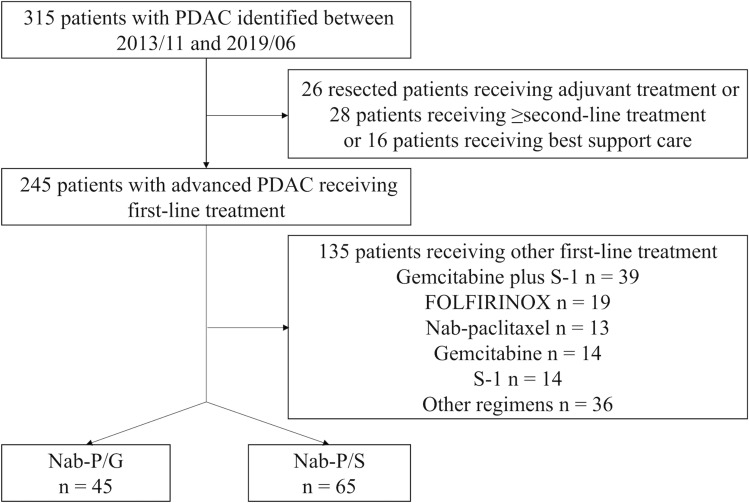
As the positive results of nab-P/G versus gemcitabine were published in October 2013, we identified patients with PDAC treated in our institution between November 2013 and June 2019 (Figure 1). We excluded 205 patients, but identified 110 eligible patients who met the following criteria: (1) histologically or cytologically confirmed PDAC, (2) had locally advanced or metastatic disease, and (3) received first-line chemotherapy of nab-P/S or nab-P/G. Baseline characteristics and clinical outcomes were retrieved from medical records. By reviewing medical records of eligible patients in our institution, we found the information on performance status for each patient may be inaccurate because they had been written by different residents depending on their subjective judgments over 6 years. We have discussed this problem and decided to use BMI as a replacement to objectively reflect the performance status, to some degree, at diagnosis.
Figure 1.
Study flow diagram.
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of Peking University Cancer Hospital & Institute and informed consent was taken from all the patients.
Treatment
Treatment options depended on the senior author’s anecdotal clinical experiences or patients’ decisions. In the nab-P/S group, patients received nab-paclitaxel at a dose of 125 mg/m2 intravenously (IV) on day 1 plus S-1 orally twice daily at a dose according to the body surface area (BSA) (80 mg/d for BSA < 1.25 m2; 100 mg/d for BSA between 1.25 m2 and 1.5 m2; 120 mg/d for BSA ≥ 1.5 m2) on day 1 through 7 of a 14-day cycle. In the nab-P/G group, patients received nab-paclitaxel at a dose of 125 mg/m2 IV plus gemcitabine at a dose of 1000 mg/m2 IV on days 1 and 8 of a 21-day cycle. We used the modified regimen of nab-P/G in our institution. The dose levels of nab-paclitaxel and gemcitabine used were based on the maximum-tolerated dose recommended in a previous phase I/II study,15 and the Chinese Society of Clinical Oncology diagnosis and treatment guidelines for Chinese patients with pancreatic cancer. Treatments continued until halted by disease progression, unacceptable toxicity, or the discretion of the investigator or patient.
Response and Toxicity Assessment
The primary endpoint was the ORR. The secondary endpoints included the ORR of the primary lesion, disease control rate (DCR), progression-free survival (PFS), OS, and safety. All patients were evaluated by computed tomography (CT) at baseline and every 6 weeks for tumor responses, including complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The tumor response was defined as inevaluable when patients had no response evaluation. The ORR and the ORR of primary lesions were assessed by the corresponding author and other senior authors according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. PFS was defined as the date of the initiation of chemotherapy until the date of disease progression determined using imaging or clinical examination or death. OS was defined as the date of the initiation of chemotherapy until the date of death or last follow-up. The last follow-up date was May 31, 2020. Adverse events were assessed and graded according to CTCAE version 4.0. Serum Carbohydrate antigen 19–9 (CA19-9) level was measured on day 1 of each cycle for both groups.
Statistical Analysis
Patients’ baseline characteristics were shown through descriptive analysis. Categorical variables were compared using a Chi-square test or Fisher’s exact test. Continuous variables were described as means (±SD) and compared using Student’s t-test. PFS and OS were evaluated using the Kaplan–Meier method and compared with the Log-rank test. Variables with a P < 0.1 and treatment group were included in the Cox proportional hazards regression model for multivariate analysis.
Statistical tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25 (IBM, Chicago, IL) and GraphPad Prism 7 (La Jolla, Calif).
Results
Baseline Characteristics
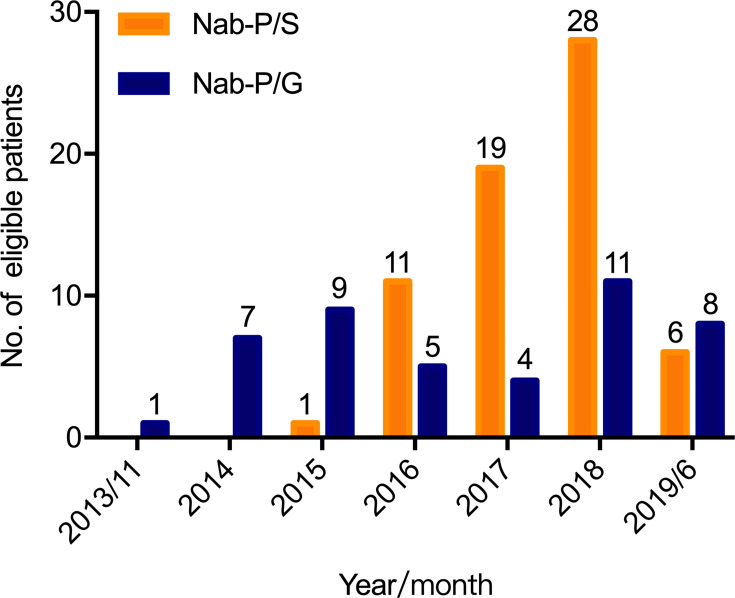
From November 2013 to June 2019, a total of 110 patients (nab-P/S group n = 65, nab-P/G group n = 45) with locally advanced (12.7%) and metastatic (87.3%) PDACs were identified. The distribution of patients for each treatment during each year is shown in Figure 2. The median age was 62 years (range 36–72), and 58.2% (n = 64) were male. The details of the baseline characteristics are summarized in Table 1, with no significant difference between the two groups.
Figure 2.
Distribution of patients for each treatment during each year.
Table 1.
Baseline Characteristics
| Characteristics, n (%) | Nab-P/S n = 65 | Nab-P/G n = 45 |
|---|---|---|
| Age, years | ||
| Mean ± SD | 59.4±7.6 | 57.3±8.9 |
| ≤65 | 50 (76.9) | 40 (88.9) |
| >65 | 15 (23.1) | 5 (11.1) |
| Gender | ||
| Male | 41 (63.1) | 23 (51.1) |
| Female | 24 (36.9) | 22 (48.9) |
| BMI, kg/m2 | ||
| <18.5 | 5 (7.7) | 6 (13.3) |
| 18.5–23.9 | 43 (66.2) | 30 (66.7) |
| ≥24 | 17 (26.2) | 9 (20.0) |
| Tumor site of pancreas | ||
| Head | 22 (33.8) | 18 (40.0) |
| Body/Tail | 42 (66.2) | 27 (60.0) |
| Overlapping lesion | 1 (1.5) | 0 |
| Baseline CA19-9, U/mL | ||
| Mean ± SD | 11,321.9±27,734.0 | 19,006.1±60,792.7 |
| Normal (0–37) | 11 (16.9) | 5 (11.1) |
| Elevated (>37) | 54 (83.1) | 40 (88.9) |
| Disease status | ||
| Locally advanced (stage III) | 9 (13.8) | 5 (11.1) |
| Metastatic (stage IV) | 56 (86.2) | 40 (88.9) |
| Metastatic site | ||
| Liver | 43 (66.2) | 30 (66.7) |
| Peritoneum | 12 (18.5) | 12 (26.7) |
| Lung | 4 (6.2) | 4 (8.9) |
| Number of metastatic sites | ||
| 1 | 28 (43.1) | 21 (46.7) |
| 2 | 15 (23.1) | 11 (24.4) |
| ≥3 | 13 (20.0) | 8 (17.8) |
Abbreviations: BMI, body mass index; CA19-9, carbohydrate antigen 19-9; Nab-P/S, nab-paclitaxel plus S-1; Nab-P/G,nab-paclitaxel plus gemcitabine.
Study Treatment
The median duration of treatment was 2.8 months (range 0.2–9.4) in the nab-P/S group and 2.6 months (range 0.2–8.2) in the nab-P/G group. The reasons for treatment discontinuation and subsequent therapy in nab-P/S versus nab-P/G group are shown in Table 2, including disease progression (55.4% vs 53.3%), investigator discretion (16.9% vs 15.6%), patient discretion (6.2% vs 13.3%), and loss to follow-up (21.5% vs 17.8%). In the nab-P/S group, 20 (30.8%) of 65 patients received second-line treatment, 13 (20.0%) received gemcitabine-based regimens and five (7.7%) received FOLFIRINOX. In the nab-P/G group, 16 (35.6%) of 45 patients received second-line treatment, seven (15.6%) received S-1-based regimens, and three (6.7%) received FOLFIRINOX.
Table 2.
Reasons for Discontinuation and Subsequent Therapy by Treatment Group
| N (%) | Nab-P/S n = 65 | Nab-P/G n = 45 |
|---|---|---|
| Disease progression | 36 (55.4) | 24 (53.3) |
| FOLFIRINOX | 5 (7.7) | 3 (6.7) |
| Nab-paclitaxel plus gemcitabine | 3 (4.6) | – |
| Nab-paclitaxel plus S-1 | – | 2 (4.4) |
| Gemcitabine plus oxaliplatin | 6 (9.2) | 0 |
| S1 plus oxaliplatin | 0 | 2 (4.4) |
| Gemcitabine plus cisplatin | 1a (5.0) | 0 |
| Irinotecan plus S1 | 0 | 2 (4.4) |
| Gemcitabine | 3 (4.6) | 0 |
| S1 | 0 | 1 (2.2) |
| Irinotecan plus trastuzumab | 1b (1.5) | 0 |
| Erlotinib | 1 (1.5) | 0 |
| Anlotinib | 0 | 1 (2.2) |
| Radiotherapy | 0 | 3 (6.7) |
| TACE | 0 | 2 (4.4) |
| Supportive care | 16 (24.6) | 8 (17.8) |
| Doctor discretionc | 11 (16.9) | 7 (15.6) |
| Followed by radiotherapy | 11 (16.9) | 5 (11.1) |
| Followed by surgery | 0 | 2 (4.4) |
| Patient discretiond | 4 (6.2) | 6 (13.3) |
| Other anti-tumor therapy | 2 (3.1) | 2 (4.4) |
| Supportive care | 2 (3.1) | 4 (8.9) |
| Loss to follow-up | 14 (21.5) | 8 (17.8) |
Notes: aA patient with germline BRCA mutation. bA patient with HER2 gene amplification. cWhen patients achieved PR or SD. dWithout evidence of progressive disease.
Abbreviations: Nab-P/S, nab-paclitaxel plus S-1; Nab-P/G, nab-paclitaxel plus gemcitabine; TACE, transarterial chemoembolization.
Efficacy
The median follow-up duration was 16.4 months (range 0.5–26.1 months) as of May 31, 2020. Responses by treatment groups are shown in Table 3. No patient in either group achieved the best response of complete response. Among all patients, the ORR was 38.5% with nab-P/S and 28.9% with nab-P/G (P = 0.299). DCR was similar for both groups (73.8% vs 66.7%, P = 0.415). Among evaluated patients (nab-P/S n = 56, nab-P/G n = 37), nab-P/S still yielded a numerically higher ORR than nab-P/G (44.6% vs 35.1%, P = 0.361). Similarly, the ORR of the primary lesions with nab-P/S was slightly higher than that with nab-P/G (overall patients 30.8% vs 22.2%, P = 0.322; evaluated patients 39.2% vs 32.3%, P = 0.526, respectively).
Table 3.
Response by Treatment Groups
| Nab-P/S n = 65 | Nab-P/G n = 45 | P value | |
|---|---|---|---|
| Target Lesions per RECIST | |||
| Best response, n (%) | |||
| CR | 0 | 0 | |
| PR | 25 (38.5) | 13 (28.9) | |
| SD | 23 (35.4) | 17 (37.8) | |
| PD | 8 (12.3) | 7 (15.6) | |
| NE | 9a (13.8) | 8b (17.8) | |
| ORR | |||
| Overall patients | 38.5% (25/65) | 28.9% (13/45) | 0.299 |
| Evaluated patients | 44.6% (25/56) | 35.1% (13/37) | 0.361 |
| DCR | |||
| Overall patients | 73.8% (48/65) | 66.7% (30/45) | 0.415 |
| Evaluated patients | 85.7% (48/56) | 81.1% (30/37) | 0.552 |
| Primary Lesion | |||
| Best response, n (%) | |||
| CR | 0 | 0 | |
| PR | 20 (30.8) | 10 (22.2) | |
| SD | 28 (43.1) | 19 (42.2) | |
| PD | 3 (4.6) | 2 (4.4) | |
| NE | 14c (21.5) | 14d (31.1) | |
| ORR | |||
| Overall patients | 30.8% (20/65) | 22.2% (10/45) | 0.322 |
| Evaluated patients | 39.2% (20/51) | 32.3% (10/31) | 0.526 |
Notes: aPatients were inevaluable for target lesions per RECIST: loss to follow-up n = 9. bPatients were inevaluable for target lesions per RECIST: loss to follow-up n = 8. cPatients were inevaluable for primary target lesion: loss to follow-up n = 9, without primary lesions after surgery n = 3, lack of specific data of the longest diameter of the primary lesion n = 2. dPatients were inevaluable for primary target lesion: loss to follow-up n = 8, without primary lesions after surgery n = 1, lack of specific data of the longest diameter of the primary lesion n = 5.
Abbreviations: CR, complete response; DCR, disease control rate; Nab-P/S, nab-paclitaxel plus S-1; Nab-P/G, nab-paclitaxel plus gemcitabine; NE, inevaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Among patients with elevated CA19-9 at baseline (nab-P/S n = 54, nab-P/G n = 40), 32 (59.3%) with nab-P/S-1 and 23 (57.5%) with nab-P/G had a ≥50% decline of CA19-9 (P = 0.864).
Survival
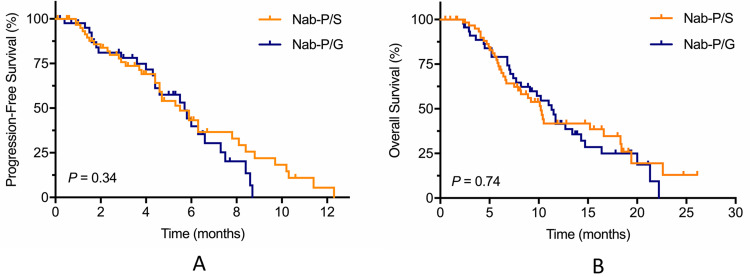
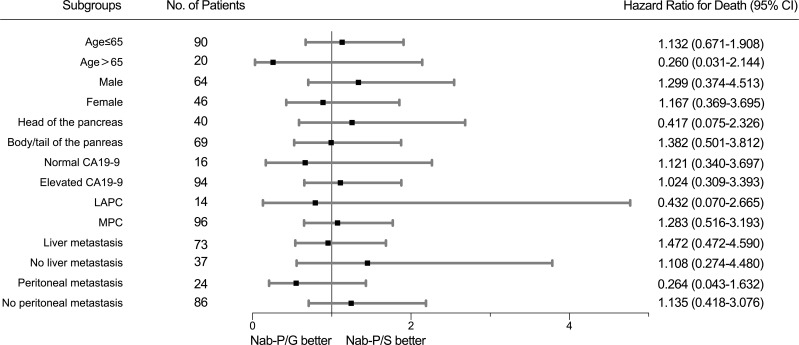
The median PFS was 5.5 months in the nab-P/S group and 5.7 months in the nab-P/G (P = 0.34), with a 6-month PFS rate of 43.2% versus 39.8%, respectively (Figure 3A). The median OS was 10.2 months with nab-P/S as compared with 11.3 months with nab-P/G (P = 0.74) (Figure 3B). Subgroup analyses of overall survival according to stratification factors showed no significant interaction between nab-P/S and nab-P/G in any subgroup (Figure 4).
Figure 3.
Kaplan–Meier estimates for (A) progression-free survival and (B) overall survival.
Figure 4.
The forest plot for overall survival.
Univariate and Multivariate Analysis
In the univariate analysis of prognostic factors for OS, liver metastasis (P < 0.0001), best response (P = 0.039), decline of CA19-9 (P = 0.038), and radiotherapy (P < 0.0001) were significantly associated with OS. Multivariate analysis revealed that no radiotherapy (HR = 8.447, 95% CI, 2.514–28.384, P = 0.001) brought a higher risk for death, whereas tumors located on the body or tail of the pancreas (HR = 0.487, 95% CI 0.256–0.928, P = 0.029) was an independent risk factor of good OS (Table 4).
Table 4.
Univariate and Multivariate Analysis of Overall Survival
| Variables | N | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Median OS, m (95% CI) | P valueb | Hazard Ratio (95% CI) | P valuec | ||
| Treatment group | |||||
| Nab-P/S | 65 | 10.2 (8.5–12.0) | 0.721 | 0.552 | |
| Nab-P/G | 45 | 11.3 (9.5–13.1) | |||
| Age, years | |||||
| ≤65 | 90 | 10.3 (8.9–11.7) | 0.521 | ||
| >65 | 20 | 18.3 (3.8–32.8) | |||
| Gender | |||||
| Male | 64 | 10.2 (8.8–11.6) | 0.426 | ||
| Female | 46 | 11.5 (9.7–13.3) | |||
| BMI, kg/m2 | |||||
| <18.5 | 7 | ||||
| 18.5–23.9 | 43 | ||||
| ≥24 | 17 | ||||
| Tumor site of pancreas | |||||
| Head | 40 | 9.1 (7.1–11.1) | 0.054 | 1 | 0.029 |
| Body/Tail | 69 | 12.7 (9.2–16.2) | 0.487 (0.256–0.928) | ||
| Disease status | |||||
| Locally advanced | 14 | - | 0.052 | 0.306 | |
| Metastatic | 96 | 10.3 (8.2–12.4) | |||
| Baseline CA19-9, U/mL | |||||
| Normal (0–37) | 16 | 11.5 (5.5–17.5) | 0.914 | ||
| Elevated (>37) | 94 | 10.2 (8.2–12.2) | |||
| Liver metastasis | |||||
| Yes | 73 | 8.9 (6.2–11.6) | < 0.0001 | 0.127 | |
| No | 37 | 21.3 (5.9–36.7) | |||
| No. of metastatic sites | |||||
| 1 | 49 | 10.1 (6.0–14.2) | 0.856 | ||
| ≥2 | 47 | 10.4 (8.3–12.5) | |||
| Best response | |||||
| CR+PR | 38 | 13.4 (9.3–14.2) | 0.039 | 0.528 | |
| SD+PD | 55 | 8.9 (7.3–10.5) | |||
| Decline of CA19-9a | |||||
| ≥50% | 55 | 11.7 (8.9–14.5) | 0.038 | 0.320 | |
| <50% | 27 | 6.8 (2.6–11.0) | |||
| Grade 3–4 leukopenia/neutropenia | |||||
| Yes | 25 | 11.7 (10.0–13.4) | 0.302 | ||
| No | 80 | 10.2 (7.8–12.6) | |||
| Radiotherapy | |||||
| Yes | 16 | - | < 0.0001 | 1 | 0.001 |
| No | 94 | 9.8 (7.6–12.0) | 8.447 (2.514–28.384) | ||
Notes: aAmong patients with elevated CA19-9 at baseline. bVariables with bolded P values were included in the Cox proportional hazards regression model. cBolded P values are significant.
Abbreviations: CA19–9, carbohydrate antigen 19–9; CI, confidence interval; CR, complete response; Nab-P/S, nab-paclitaxel plus S-1; Nab-P/G, nab-paclitaxel plus gemcitabine; PD, progressive disease; PR, partial response; SD, stable disease.
In the univariate analysis of prognostic factors for PFS, liver metastasis (P = 0.006), number of metastatic sites (P = 0.031), best response (P < 0.0001), and decline of CA19-9 (P = 0.021) were significantly associated with PFS. However, none of them was the independent prognostic factor for PFS.
Safety
Adverse events (AE) between the two groups are listed in Table 5. No treatment-related death occurred. Patients in the nab-P/S group had numerically higher incidences of all-grade AEs, including hematologic and nonhematologic toxicities. The most frequent AEs in the nab-P/S group were fatigue (78.5%), neutropenia/leukopenia (56.9%), and anemia (50.8%). The most frequent AEs in the nab-P/G group were fatigue (88.9%), neutropenia/leukopenia (66.7%), and nausea/vomiting (64.4%).
Table 5.
Adverse Events
| Events, n (%) | All Grades | Grade 3/4 | P value (Grade 3/4) | ||
|---|---|---|---|---|---|
| Nab-P/S n = 65 | Nab-P/G n = 45 | Nab-P/S n = 65 | Nab-P/G n = 45 | ||
| Hematologic | |||||
| Neutropenia/Leukopenia | 37 (56.9) | 30 (66.7) | 15 (23.1) | 11 (24.4) | 0.868 |
| Febrile neutropenia | 1 (1.5) | 3 (6.7) | 1 (1.5) | 3 (6.7) | 0.303 |
| Thrombocytopenia | 9 (13.8) | 11 (24.4) | 0 | 3 (6.7) | 0.066 |
| Anemia | 33 (50.8) | 28 (62.2) | 2 (3.1) | 1 (2.2) | 1 |
| Nonhematologic | |||||
| Elevated AST/ALT | 15 (23.1) | 17 (37.8) | 0 | 2 (4.4) | 0.165 |
| Fatigue | 51 (78.5) | 40 (88.9) | 1 (1.5) | 3 (6.7) | 0.303 |
| Peripheral neuropathy | 29 (44.6) | 24 (53.3) | 0 | 2 (4.4) | 0.165 |
| Hand-foot syndrome | 7 (10.8) | 5 (11.1) | 0 | 0 | - |
| Diarrhea | 10 (15.4) | 9 (20.0) | 3 (4.6) | 0 | 0.268 |
| Rash | 15 (23.1) | 19 (42.2) | 0 | 2 (4.4) | 0.165 |
| Nausea/Vomiting | 26 (40.0) | 29 (64.4) | 0 | 1 (2.2) | 0.409 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; Nab-P/S, nab-paclitaxel plus S-1; Nab-P/G, nab-paclitaxel plus gemcitabine.
The incidences of grade 3 or 4 neutropenia/leukopenia, febrile neutropenia, and thrombocytopenia were numerically lower in the nab-P/S group than in the nab-P/G group. One patient with grade 3 anemia in the nab-P/S group was evaluated as grade 2 anemia at baseline. However, nab-P/S had a trend toward a higher risk of grade 3 or 4 diarrhea.
Discussion
This is a single-center retrospective study to compare nab-P/S with nab-P/G as first-line chemotherapy in patients with advanced PDAC. The ORR, DCR, and ORR of primary lesion with nab-P/S were slightly higher than those with nab-P/G, respectively. OS and PFS were similar between the two groups. Nab-P/S could be a comparable and convenient alternative with a more favorable safety profile. We have reported similar results with smaller sample size before.16
By reviewing landmark phase III trials establishing first-line chemotherapy for PDAC, gemcitabine has been the standard of care since the 1990s.3 It was shown to be superior to 5-FU with a modest survival advantage (5.65 vs 4.41 months, P = 0.0025) and clinical benefit responses such as pain relief (23.8% vs 4.8%, P = 0.0022). Unfortunately, several combinations of gemcitabine with cytotoxic or target agents failed to show improvements in survival. Moore et al demonstrated the combination of gemcitabine plus erlotinib, an EGFR inhibitor, prolonged median survival by only 10 days versus gemcitabine alone (6.24 vs 5.91 months, P = 0.038).4 In 2011, the results of the phase III PRODIGE 4/ACCORD 11 trial brought a significant breakthrough in patients with good performance. FOLIFRINOX resulted in an 11.1-month median survival compared with 6.8 months with gemcitabine alone (P < 0.001). The ORR was also significantly higher with FOLIRINOX than with gemcitabine (31.6% vs 9.4%, P < 0.001).5 Von Hoff et al showed an improved response rate of 23% with the combination of nab-paclitaxel plus gemcitabine versus 7% with gemcitabine alone (P < 0.001) in the phase III MPACT trial.6 Both median OS and PFS were significantly prolonged with nab-P/G versus gemcitabine (median OS, 8.7 vs 6.6 months, P < 0.001; median PFS, 5.5 vs 3.7 months, P < 0.001). Nab-paclitaxel, as the only drug achieving success among all gemcitabine-based regimens, was considered to deplete collagen and dilate blood vessels, which enabled the delivery of gemcitabine to tumors.17 However, FOLFIRINOX, as well as nab-P/G, correlated with increased toxicity compared with gemcitabine, such as grade 3 or 4 myelosuppression and peripheral neuropathy.5,6
S-1 (an oral drug containing tegafur, gimeracil, and oteracil potassium) has shown promising activity and convenience of administration as first-line therapy in the previous trials for metastatic PDAC in Japan.7,8 In the phase III GEST study, gemcitabine plus S-1 (GS) did not show superiority to gemcitabine in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan.9 However, S-1 demonstrated non-inferiority to gemcitabine in terms of OS (P < 0.001 for non-inferiority). The ORR was 21.0% with S-1 and 13.3% with gemcitabine (P = 0.02). The superiority of S-1 to gemcitabine (P < 0.0001 for superiority) as adjuvant chemotherapy was reported for Japanese patients with resected pancreatic cancer in the phase III JASPAC 01 study.10 As a useful oral alternative, S-1 developed a lower incidence of grade 3 or worse hematologic adverse events and elevated AST/ALT levels when compared with gemcitabine, but a higher risk of diarrhea.9,10
Based on the results of phase III trials above, efforts have been made to develop a new combination of nab-paclitaxel and S-1 recently. Two single-arm phase II trials in China reported by Shi et al and Zhang et al showed promising survival (median OS, 9.4–13.6 months) and high ORR (50.0–53.1%) with first-line nab-P/S in patients with advanced PDAC.13,14 The remarkable results of ORR also indicated the possible role of nab-P/S in the neoadjuvant setting for LAPC. The triweekly regimen of nab-P/S was used in these two trials. Patients in the nab-P/S group were treated with nab-paclitaxel at a dose of 120 mg/m2 IV on days 1 and 8 plus S-1 orally twice daily at a dose according to BSA (80 mg/d for BSA < 1.25 m2, 100 mg/d for BSA between 1.25 m2 and 1.5 m2, 120 mg/d for BSA ≥ 1.5 m2) on days 1–14 every 3 weeks. Nab-P/S was observed with a favorable safety profile.
In the present study, we reported a real-world experience of a single center for nab-P/S or nab-P/G as first-line therapy in advanced PDAC. In our series, the ORR with nab-P/S was seemingly better than nab-P/G but did not reach 50.0% (nab-P/S vs nab-P/G, 38.5% vs 28.9%, P = 0.30). On the one hand, these results were based on the retrospective data with inherent bias. On the other hand, we used the biweekly regimen of nab-P/S in our institution. The dose intensity decreased by 30.6% for nab-paclitaxel and 33.3% for S-1 in our biweekly regimen compared with the triweekly regimen. However, it also indicated that dosage reduction did not weaken the antitumor activity. The survival time with nab-P/S (10.2 months) was similar to those in the previous phase II studies, and the adverse events were controllable.
Nab-paclitaxel in combination with fluoropyrimidine showed good clinical outcomes. A recent non-comparative, randomized phase II trial in France assessed the activity and safety of a new regimen of nab-paclitaxel plus simplified leucovorin and 5-FU, as well as a standard treatment regimen of nab-P/G. At 4 months, 56% (40/72) of patients in the leucovorin and 5-FU group were free from disease progression, while 54% (21/39) in the nab-P/G group.18 Favorable median PFS (5.9 vs 4.9 months), median OS (11.4 vs 9.2 months), and safety profiles were reported in the leucovorin and 5-FU group. Nab-paclitaxel plus capecitabine resulted in a good ORR of 41.4% with good tolerance in a phase II trial.19
There are no standard second-line treatments. From our experience, we commonly use fluoropyrimidine-based regimens combined with irinotecan or oxaliplatin (FOLIRINOX, FOLFIRI, and FOLFOX) or gemcitabine-based regimens combined with platinum drugs. Further investigation on the optimal regimens after the failure of nab-P/S is warranted. After nab-P/G failure, fluoropyrimidine-based regimens were also suggested by Pointet et al as second-line therapy, which has a manageable toxicity profile and promising clinical outcomes.20 A platinum-based regimen was observed with a clear trend for longer survival time than irinotecan or nal-IRI-based regimen after nab-P/G.21 Clinical trials are always preferred for all patients with disease progression.
There were limitations to the present study. First, this was a retrospective study with its inherent bias. We conducted a phase II, randomized study at nearly the same time, and the preliminary analysis was presented at the 2019 Gastrointestinal Cancers Symposium.22 Second, we only enrolled patients from a single center, and the sample size was small. Third, the dose of the triweekly regimen of gemcitabine plus nab-paclitaxel was modified for Chinese patients. A phase I/II study recommended nab-paclitaxel (120 mg/m2) followed by gemcitabine (1000 mg/m2) administered on days 1 and 8 every 3 weeks for Chinese patients with untreated PDAC. The ORR was 41.67%, and the median PFS and OS were 5.23 months and 12.17 months, respectively.15 In the Chinese Society of Clinical Oncology Diagnosis and Treatment Guidelines for Chinese Patients with Pancreatic Cancer, the MPACT regimen of nab-P/G was recommended and can be adjusted into a triweekly regimen (nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2 on days 1 and 8 every 3 weeks) when necessary. Fourth, S-1 is widely used in Asian countries. The pharmacokinetics and pharmacodynamics of S-1 might be different between Western and East Asian patients.23 The optimal dosage and role of S-1 should be re-assessed in non-Asian patients.
Conclusion
Nab-paclitaxel plus S-1 could be a comparable and convenient alternative with a favorable safety profile as first-line chemotherapy for advanced PDAC, as well as an option for neoadjuvant treatment.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article. This paper was presented at 2019 ASCO as an abstract presentation with interim findings (doi: 10.1200/JCO.2019.37.15_suppl.e15743).
Funding Statement
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi: 10.1016/S0140-6736(16)00141-0 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno H, Okusaka T, Ikeda M, et al. An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology. 2005;68(2–3):171–178. doi: 10.1159/000086771 [DOI] [PubMed] [Google Scholar]
- 8.Okusaka T, Funakoshi A, Furuse J, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61(4):615–621. doi: 10.1007/s00280-007-0514-8 [DOI] [PubMed] [Google Scholar]
- 9.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31(13):1640–1648. doi: 10.1200/JCO.2012.43.3680 [DOI] [PubMed] [Google Scholar]
- 10.Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a Phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–257. doi: 10.1016/S0140-6736(16)30583-9 [DOI] [PubMed] [Google Scholar]
- 11.Suenaga M, Yamada S, Fujii T, et al. S-1 plus nab-paclitaxel is a promising regimen for pancreatic cancer in a preclinical model. J Surg Oncol. 2016;113(4):413–419. doi: 10.1002/jso.24147 [DOI] [PubMed] [Google Scholar]
- 12.Li JA, Xu XF, Han X, et al. Nab-paclitaxel plus S-1 shows increased antitumor activity in patient-derived pancreatic cancer xenograft mouse models. Pancreas. 2016;45(3):425–433. doi: 10.1097/MPA.0000000000000501 [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Zhang S, Han Q, et al. Nab-paclitaxel plus S-1 in advanced pancreatic adenocarcinoma (NPSPAC): a single arm, single center, phase II trial. Oncotarget. 2017;8(54):92401–92410. doi: 10.18632/oncotarget.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Du C, Sun Y, et al. Nab-paclitaxel plus S-1 as first-line followed by S-1 maintenance for advanced pancreatic adenocarcinoma: a single-arm phase II trial. Cancer Chemother Pharmacol. 2018;82(4):655–660. doi: 10.1007/s00280-018-3650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DS, Wang DS, Wang ZQ, et al. Phase I/II study of albumin-bound nab-paclitaxel plus gemcitabine administered to Chinese patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2013;71(4):1065–1072. doi: 10.1007/s00280-013-2102-4 [DOI] [PubMed] [Google Scholar]
- 16.Zong Y, Peng Z, Ming L, et al. Nab-paclitaxel/S-1(AS) versus nab-paclitaxel/gemcitabine (AG) for first-line chemotherapy in advanced pancreatic ductal adenocarcinoma (aPDAC): a retrospective analysis of efficacy and safety. J Clin Oncol. 2019;37(15_suppl). doi: 10.1200/JCO.2019.37.15_suppl.e15743 [DOI] [Google Scholar]
- 17.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. doi: 10.1200/JCO.2011.36.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachet JB, Hammel P, Desramé J, et al. Nab-paclitaxel plus either gemcitabine or simplified leucovorin and fluorouracil as first-line therapy for metastatic pancreatic adenocarcinoma (AFUGEM GERCOR): a non-comparative, multicentre, open-label, randomised Phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2(5):337–346. doi: 10.1016/S2468-1253(17)30046-8 [DOI] [PubMed] [Google Scholar]
- 19.Scheithauer W, Kornek G, Prager G, et al. Phase II trial of capecitabine plus nab-paclitaxel in patients with metastatic pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7(2):234–238. doi: 10.3978/j.issn.2078-6891.2015.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pointet AL, Tougeron D, Pernot S, et al. Three fluoropyrimidine-based regimens in routine clinical practice after nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: an AGEO multicenter study. Clin Res Hepatol Gastroenterol. 2020;44(3):295–301. doi: 10.1016/j.clinre.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Merz V, Cavaliere A, Messina C, et al. Multicenter retrospective analysis of second-line therapy after gemcitabine plus nab-paclitaxel in advanced pancreatic cancer patients. Cancers (Basel). 2020;12(5):1131. doi: 10.3390/cancers12051131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zong Y, Peng Z, Wang X, et al. Efficacy and safety of nab-paclitaxel plus S-1(nab-P/S-1) versus nab-paclitaxel plus gemcitabine (nab-P/Gem) for first-line chemotherapy in advanced pancreatic ductal adenocarcinoma (aPDAC): a randomized study. J Clin Oncol. 2020;38(4_suppl):717. doi: 10.1200/JCO.2020.38.4_suppl.717 [DOI] [Google Scholar]
- 23.Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011;102(2):478–483. doi: 10.1111/j.1349-7006.2010.01793.x [DOI] [PubMed] [Google Scholar]