Abstract
Acute inflammation is a protective reaction by the immune system in response to invading pathogens or tissue damage. Ideally, the response should be localized, self-limited, and returning to homeostasis. If not resolved, acute inflammation can result in organ pathologies leading to chronic inflammatory phenotypes. Acute inflammation and inflammation resolution are complex coordinated processes, involving a number of cell types, interacting in space and time. The biomolecular complexity and the fact that several biomedical fields are involved, make a multi- and interdisciplinary approach necessary. The Atlas of Inflammation Resolution (AIR) is a web-based resource capturing an essential part of the state-of-the-art in acute inflammation and inflammation resolution research. The AIR provides an interface for users to search thousands of interactions, arranged in inter-connected multi-layers of process diagrams, covering a wide range of clinically relevant phenotypes. By mapping experimental data onto the AIR, it can be used to elucidate drug action as well as molecular mechanisms underlying different disease phenotypes. For the visualization and exploration of information, the AIR uses the Minerva platform, which is a well-established tool for the presentation of disease maps. The molecular details of the AIR are encoded using international standards. The AIR was created as a freely accessible resource, supporting research and education in the fields of acute inflammation and inflammation resolution. The AIR connects research communities, facilitates clinical decision making, and supports research scientists in the formulation and validation of hypotheses. The AIR is accessible through https://air.bio.informatik.uni-rostock.de
Keywords: Acute inflammation, Inflammation resolution, Molecular interaction map, Systems biology, Disease map, Inflammatory mediators, Pro-resolving mediators, Molecular switches
Abbreviations
- 5 S-HETE
5 S-Hydroxyeicosatetraenoic acid
- a.u.
Arbitrary unit
- AA
Arachidonic acid
- AIR
Atlas of Inflammation Resolution
- APPs
Acute phase proteins
- c.f.u.
Colony forming unit
- DAMPs
Damage-associated molecular patterns
- DC
Dendritic cells
- DHA
Docosahexaenoic acid
- DSS
Dextran sodium sulfate
- EPA
Eicosapentaenoic acid
- FACS
Fluorescence-activated cell sorting
- FC
Fold change
- ILC
Innate lymphoid cells
- LC-MS-MS
Liquid chromatography with tandem mass spectrometry
- lncRNA
Long non-coding RNA
- LTB4
Leukotriene B4
- M1
M1 macrophage
- M2
M2 macrophage
- MIM
Molecular interaction map
- miRNA
Micro RNA
- Mres
Resident macrophages
- NK cell
Natural killer cell
- PAMPs
Pathogen-associated molecular patterns
- PGE2
Prostaglandin E2
- PIM
Pro-inflammatory mediators
- PMN
Polymorphonuclear leukocyte
- PTGS2
Prostaglandin-endoperoxide synthase 2
- RvD1
Resolvin D1
- SBGN
Systems Biology Graphical Notation
- SBML
Systems Biology Markup Language
- SPM
Specialized pro-resolving mediators
- TF
Transcription factors
- Th cell
T helper cell
1. Introduction
The acute inflammatory response is the first protective reaction mounted by the host tissue against invading pathogens, foreign bodies and/or injury (Anthony, 1990). Acute inflammation is a highly coordinated, active, nonlinear spatial-temporal process for the removal of invading pathogens and the repair of damaged tissues to reestablish homeostasis (Bara et al., 2013; Serhan, 2014 ; Serhan and Savill, 2005). If the acute inflammatory response is not resolved, it can contribute to organ pathology and amplify many widely occurring chronic inflammatory clinical phenotypes including arthritis, neurodegenerative diseases, metabolic syndrome, asthma, allergy, diabetes, inflammatory processes of aging and cancers, organ fibrosis, cardiovascular and periodontal diseases (Chiurchiù et al., 2018; Doyle et al., 2018; Fullerton and Gilroy, 2016; Grivennikov et al., 2010; Libby et al., 2014; Medzhitov, 2008; Ortega-Gómez et al., 2013; Perretti et al., 2017; Shimizu, 2009; Van Dyke and Serhan, 2003; Viola and Soehnlein, 2015).
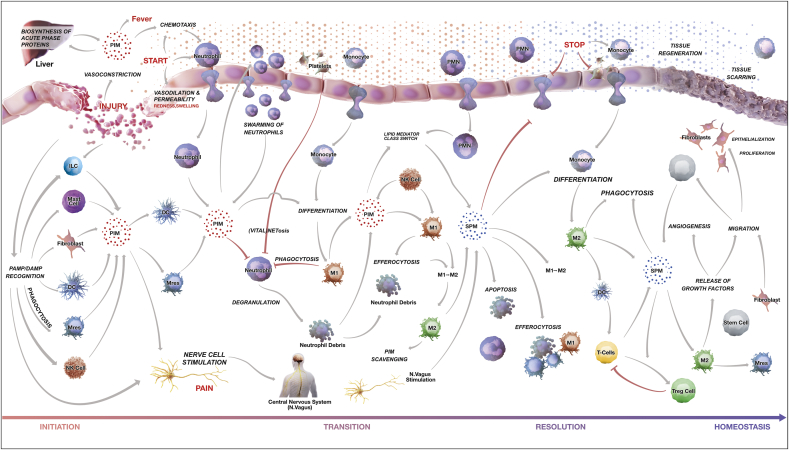
The landscape of the entire acute inflammatory response can be broadly divided into four phases, namely, i) inflammation initiation, ii) transition, iii) resolution and iv) return to a new state of homeostasis. The boundaries of all these phases are appreciated at the cellular and tissue level and are just being defined at the molecular and biomarker levels. The physiological terrain leading to acute inflammatory responses contains a large number of pro-inflammatory mediators (PIM) and specialized pro-resolving mediators (SPM), proteins, peptides, autacoids, varieties of innate immune cells and a vast number of regulators (molecular switches) in the form of feedback and feedforward loops, making the whole system dynamic and complex (Fig. 1).
Fig. 1.
Phenotype level representation of the AIR. The landscape of acute inflammatory response is divided into four overlapping phases: Initiation, transition, resolution and return to tissue homeostasis. Interactions between immune cell types, vascular endothelial cells, mucosal epithelial cells as well as the associated processes and phenotypes are depicted. Arrows indicate information flow. The color of arrows indicates regulation type; gray for activation and red for inhibition. Each process is connected with underlying manually curated and annotated molecular interaction maps. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Understanding the interplay between different regulators is necessary to understand immune cell function and phenotypic adaptations during acute inflammation and its resolution. Perturbation of these chemical mediator networks and molecular switches, e.g. through mutations or dysregulated expression, can lead to a misrouted behavior to clear or eradicate the initiating stimulus and ultimately cause the emergence of chronic diseases (Levy and Serhan, 2014; Libby et al., 2014; Serhan and Levy, 2018; Tabas, 2010).
To conceptually analyze and intuitively visualize interactions in such a vast regulatory network, the construction of a molecular interaction map is the first step in modern biological studies (Kitano et al., 2005; Kohn, 1999). A number of comprehensive regulatory maps have been constructed to help in the understanding of the mechanisms underlying complex biological systems (Calzone et al., 2008; Fujita et al., 2014; Khan et al., 2017; Matsuoka et al., 2013), however, only a handful of attempts have been made so far to develop a mechanistic understanding of complex inflammatory disorders through the investigation of underlying molecular interaction maps (Netea et al., 2017; Novershtern et al., 2008; Wu et al., 2010; Yang et al., 2017).
Research communities working on inflammation and inflammation-resolution are highly diverse, typically focusing on either one immune cell type, a specific mediator, or pathway associated with any specific disease pathophysiology under investigation. Intriguingly, the presence of nonlinear interactions between immune cells and associated pathways renders the prediction of outcomes highly non-intuitive and requires a bird's-eye view of the whole system together with a systems biological analysis. Moreover, to this date, there is no common web platform wherein a community can share their viewpoints on the impact that acute inflammatory phenotypes may have on the emergence of specific disease settings. It would be prudent and apt to design a multi-layered representation of inflammation and inflammation resolution, where manipulation of one or more parameters in one stratum would automatically predict the resulting mechanistic changes in another layer.
We conceptualized the Atlas of Inflammation Resolution (AIR) as a community resource to connect clinicians, scientists, students, and pharmaceutical companies working in the area of acute and chronic inflammation. This can be realized only when the AIR is able to support 1) clinicians in decision making (e.g. patient stratification; predicting response to therapy; therapy personalization; prognosis prejudgment), 2) research scientists in the development of hypotheses for experimental design (e.g. identification of molecular switches, mapping of high-throughput experimental data, understanding mechanisms), and 3) pharmaceutical companies in the identification of new therapeutic targets. The multi-level nature of the systems interacting as presented in the AIR can serve as a starting point for defining and designing acute inflammation and inflammation resolution related dynamic mathematical models. The AIR would naturally be useful for education and training purposes as well. With this in mind, we present the AIR as a novel community resource.
AIR allows users to visualize detailed molecular level events underlying biological processes, pathways and cell/tissue-related phenotypes associated with acute inflammation initiation, transition, resolution and finally return to homeostasis. All the submaps are prepared using standard Systems Biology Markup Language (SBML) (Hucka et al., 2003) to ensure their reusability and are accessible through the MINERVA platform (Hoksza et al., 2019) using a web-browser. The AIR is a portal which enables connection to state-of-the-art publicly available databases, providing information about genes, proteins, lipids, micro RNAs (miRNAs), long non-coding RNAs (lncRNAs), chemicals and drugs. It is furthermore a tool to highlight research gaps and supports the formulation of new hypotheses to address those gaps.
In the following sections, we describe the methods and workflow used for the construction of AIR along with the functionality that users will be provided with when accessing the AIR. The paper also details the use of AIR by clinicians, scientists, students, and pharmaceutical industries.
2. Results
2.1. Entry to the AIR
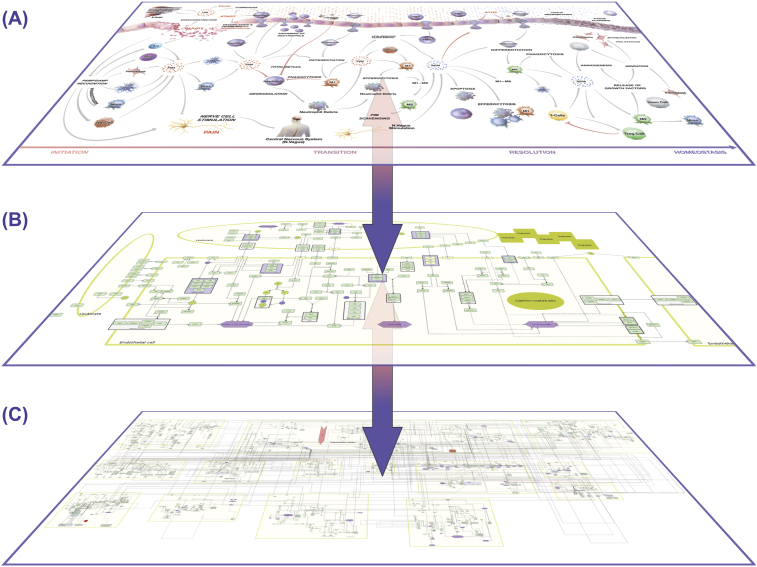
The AIR is a collection of molecular, subcellular, cellular and tissue level maps in the context of acute inflammation and inflammation resolution. Depending on the user's need, the AIR provides various entry points to access all the curated information. AIR is designed with the aim to serve diverse communities including clinicians, research scientists and drug developers from pharmaceutical industry. Clinicians generally rely on the higher-level organization, i.e. at the cellular and tissue level to understand the pathophysiology of disease. They are interested in either classifying patients into various risk groups or in therapy scheduling based on patient's pathological reports. Time-series transcriptomics profiling of patients is getting popular day-by-day in hospitals which motivate clinicians to use the data in therapy personalization without involving themselves in a data analysis pipeline (Delhalle et al., 2018). On the other hand, investigators are interested in generating new hypotheses for the design of experiments related to the phenotype under observation. AIR provides multiple layers to extract all of this multi-level information as shown in Fig. 2.
Fig. 2.
Hierarchical organization of AIR. (A) The top phenotype layer contains immune cell types, cellular processes/phenotypes and tissue level organization. Clinicians are generally interested in connecting their patient data to this layer. (B) Each process in the top layer is connected to a respective signal flow diagram. The process layer describes key molecules/pathways regulating processes in the top layer. This layer is suitable for research scientists to generate new hypotheses on the mechanistic insights of disease phenotype regulation. (C) The lower layer contains a comprehensive Molecular Interaction Map (MIM) where all the processes are merged together at the molecular level. The layer is also enriched with currently available experimentally validated regulatory information. Each layer provides an opportunity to map and analyze specific data (e.g. Top layer: FACS analysis; middle layer: immune signaling; bottom layer: multi-omics data). Due to the communication across multiple layers, the AIR provides a platform to initiate integrative data analysis.
2.2. Description of the various submaps available on AIR
For better navigation and visualization, the AIR is divided into four phases; these are: i) inflammation initiation; ii) transition; iii) resolution and iv) return to a new state of function and homeostasis.
The phase of inflammation initiation starts with the onset of acute inflammation (e.g. invasion by pathogens, tissue damage due to injury or surgery etc.), operated by the innate branch of immunity, where the body recognizes damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) and releases various PIM and acute phase proteins (APPs) to initiate immune responses. The detailed molecular level events are summarized in submaps ‘DAMPs and PAMPs recognition’ and ‘Regulation of APPs’. Selected chemoattractants and APPs trigger the production of various PIMs such as chemokines and others, which in turn are responsible for immediate vasoconstriction followed by vasodilation and increased vascular permeability to facilitate the recruitment of neutrophils to fight against invading pathogens. Various innate immune cells, such as tissue resident macrophages (Mres), dendritic cells (DC), mast cells and fibroblasts also trigger the production of PIM. In the AIR, molecular level events summarizing these processes are shown in the submaps ‘Chemotaxis’, ‘Vasoconstriction, vasodilation and permeability’, ‘Biosynthesis of PIM’, and ‘Leukocyte adhesion and transmigration’.
Acute inflammation is commonly recognized by the five cardinal signs (Redness, Heat, Swelling, Pain and impaired function) known to ancient physicians (Anthony, 1990; Scarborough and Majno, 1977). These cardinal signs are also integrated in various submaps. For the inflammation transition phases, neutrophil swarming was considered as an active process where large number of neutrophils gather around a site of acute inflammation. As detailed molecular mechanisms underlying neutrophil swarming are not known, we provide a separate submap describing this process. The aim here is to strengthen all the submaps and connecting missing links with the help of broad inflammation and inflammation-resolution communities. The myriad of cellular processes associated with neutrophils and other white blood cells, such as neutrophil apoptosis, phagocytosis, efferocytosis etc. are also shown in subsequent submaps.
The AIR describes two important switches (macrophage polarization to the broadly defined functionally distinct cell types transitions (e.g. M1 to M2) and lipid mediator class switch) as detailed submaps which are mainly responsible for initiating the inflammation resolution phase. Specifically, for the lipid mediator class switch, the AIR provides detailed molecular events and reactions associated with the production of PIM and SPM from arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Serhan, 2017; Serhan and Levy, 2018) in separate submaps. Other submaps which are associated with inflammation resolution phase are ‘STOP signals for platelet aggregation’ links to the coagulation cascade (Simon, 2004), ‘STOP signal for neutrophil adhesion’, ‘Monocyte differentiation’ and ‘Th cell signaling cascade, linking resolution to adaptive immunity’.
For the phase of homeostasis and return to tissue function, we included processes, such as ‘Tissue scarring and regeneration’, ‘Epithelialization’, ‘Stem cell recruitment and proliferation’ and ‘Angiogenesis’ in subsequent submaps. Submaps currently available on the AIR are listed in Supplementary Table 1.
In all submaps, genes, proteins and metabolites are represented by their official names. This helps in connecting all the submaps together in the deepest molecular level layer of the AIR (Fig. 2B). Various submaps are also integrated together with regulatory components including transcription factors, miRNAs, lncRNAs, chemicals and drugs molecules.
We have developed a comprehensive Atlas of Inflammation Resolution or AIR, covering more than 30 highly interconnected submaps associated with the acute inflammation onset, transition, resolution and homeostasis at the molecular level. Key points summarizing the AIR are provided in Text Box 1.
Text Box1. The AIR at a glance:
-
•
The AIR is the first comprehensive collection of molecular interaction maps underlying acute inflammation initiation, transition, resolution, repair and return to homeostasis.
-
•
The AIR for acute inflammation and inflammation resolution is prepared by extending disease genes associated with primary clinical indications of acute inflammation and known DAMPs, PAMPs with experimentally validated interacting partners.
-
•
The AIR provides biosynthesis and down-stream signaling cascades of protein mediators (e.g. annexin-2, IL-10, TGF-β, INF-α) and lipid mediators (e.g. prostaglandins, leukotrienes, lipoxins, resolvins, protectins and maresins) along with time-series LC-MS-MS data from selected acute-inflammatory phenotypes which can be used for designing new therapeutics.
-
•
Details of all PIM and SPM including their full chemical names, synonyms, ChEBI ID, precursor molecules, regulatory enzymes, downstream targets and 3D chemical structures can be searched using interactive plugins connected to the AIR.
-
•
Procedure to use the plugins, mapping of experimental data onto the AIR and estimation of phenotype levels are described in details on https://air.bio.informatik.uni-rostock.de
-
•
The AIR is a portal to other databases (e.g. miRTarbase, UniProt, GenBank, PubMed etc).
-
•
Users can type in the name of protein/gene/regulatory molecule/lipid/biological processes to find the associated functional modules.
-
•
Connections to known drugs and chemicals can be searched directly from the AIR with linked databases.
-
•
Molecular interaction maps available on the AIR are both human and machine readable in a standardized SBGN, SBML format to allow their reproducibility and further bioinformatics analyses.
-
•
The AIR can be visualized with several regulatory layers including transcription factors, miRNA, lncRNA, drug candidates.
-
•
The AIR itself becomes a knowledge-base to generate hypotheses around acute inflammation and inflammation resolution.
-
•
The AIR provides a scaffold to understand/hypothesize the drug mode of action in inflammation resolution.
-
•
All the edges present in AIR are annotated with PubMed IDs. Thus, all the information present in the AIR is reliable and transparent.
-
•
With visualization of levels of molecular, processes and cellular phenotypes level visualization, the AIR can be used as a tool to translate results from experimental animals to the clinical settings.
-
•
The AIR is enriched with recurring structural patterns called network motifs including feedback/feedforward loops, which induce non-linear dynamics.
-
•
The AIR provides an interface for the inflammation research community to interact.
Alt-text: Text Box1
3. Discussion
3.1. Acute inflammation and its resolution as a coordinated process
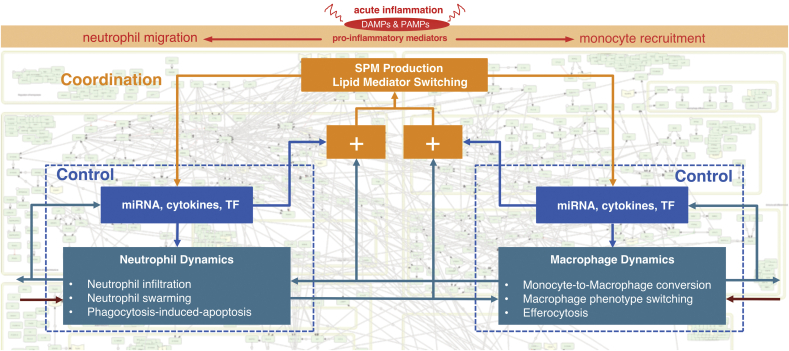
The AIR demonstrates that inflammation resolution is a multilevel spatio-temporal process. This view suggests the application of Interaction Balance Coordination principle from systems theory, originally developed by Mesarovic and colleagues (Mesarovic et al., 2004; Varaiya, 1972), to the field of inflammation resolution. According to this principle, the behavior of complex biological systems with multiple levels of structural and functional organization, is harmonized through the coordination of different interacting subsystems, each of which has a distinct function on its own and contributes in a way that advances the objective of the whole system. As our body responds to the perturbations initiated by damage, injury to the tissues or invasion by pathogens, the main goal is to regain homeostasis through the coordination of several subprocesses each of which is regulated by large numbers of immune cells and molecules. Underlying the notion of ‘regulation’ is the existence of feedback loops, which in the AIR are found through loops in the directed graph that is the lower molecular interaction map layer. The imbalance in the performance of each subprocess in the lower layer is balanced by the coordination layers which provide signals across subprocesses. These coordination layers in acute inflammation resolution can be summarized as the molecular switches, such as lipid mediator class switch which are responsible for the production of either PIM or SPM from the same precursor (Fig. 3). The AIR provides a platform to initiate detailed investigation of the dynamics of regulatory subprocesses and coordination layer events in the context of different clinical disease phenotypes.
Fig. 3.
Acute inflammation and inflammation resolution follows the concept of Mesarovic's Interaction Balance Coordination Principle. Two subprocesses (neutrophil dynamics and macrophage dynamics) are controlled by their respective miRNA, cytokines and transcription factors (TF). These subprocesses communicate together in the regulation of phenotype. If there is any imbalance in the desired and actual outputs by these processes (shown by ‘+’ sign, higher level coordination layer (shown here by ‘SPM Production’, ‘Lipid Mediator Switching’) provide signals and make the balance between subprocesses to return to homeostasis. The AIR provides molecular level details of these coordination layers and offers an opportunity to harness these layers for therapeutic purposes.
3.2. Potential uses of the AIR
3.2.1. The AIR as a portal to connect public databases
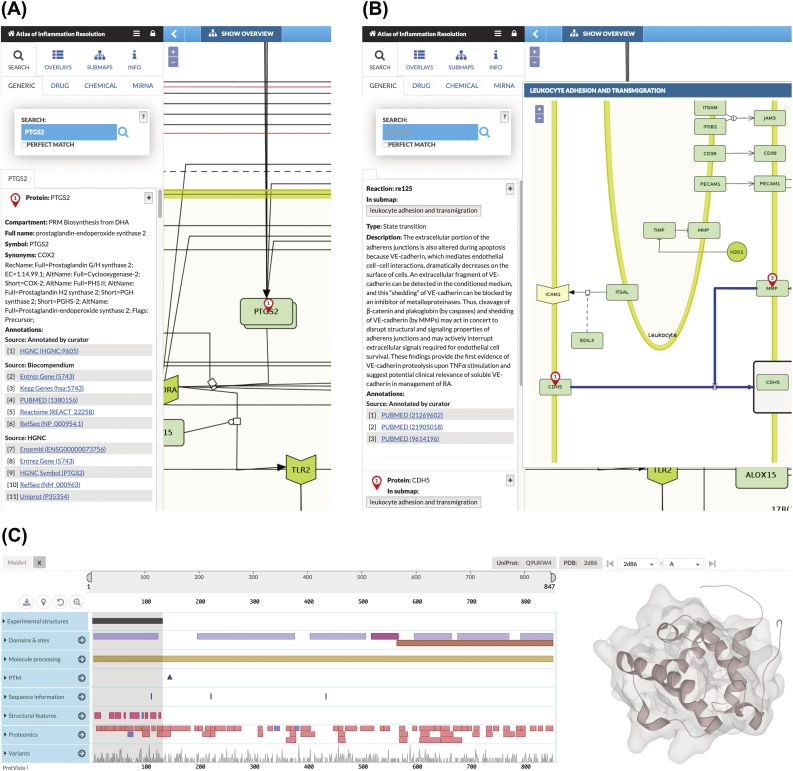
All biomolecules and chemicals present in the AIR are manually annotated with official names (official HGNC symbol for genes and proteins, ChEBI name for drug and chemicals) and reactions are manually annotated with PubMed IDs. The majority of the state-of-the-art databases containing additional information (such as detailed description of the molecules, full chemical names, regulatory molecules, downstream signaling cascade, disease association) can be directly searched by selecting nodes or reactions present in the AIR (Fig. 4A and B).
Fig. 4.
The AIR as a portal to connect public databases. (a) A node ‘PTGS2’ is selected. All the links to various state-of-the-art databases (HGNC, Entrez gene, KEGG gene, PubMed, Reactome, RefSeq, UniProt etc.) along with compartment (AIR submap) information, full name, synonyms are directly available on the left panel. (b) A reaction is selected and the literature from where the reaction is derived is provided as PubMed IDs. Wherever possible, we also summarize the reaction in the context of acute inflammation and inflammation resolution in the description section. (c) Snapshot of MolArt plugin integrated with MINERVA interface. The 3D structure of the CH domain of VAV-3 protein is shown as an example.
3.2.2. The AIR as a ready-to-use resource from structure analysis to dynamic models
All the submaps available on the AIR are prepared in standard SBML notation along with complete annotation using CellDesigner tools to ensure their reusability. These submaps can be directly downloaded from the AIR and used for in silico simulation, perturbation experiments or network analysis. The majority of the proteins that have a 3D structure already resolved and available in Protein Data Bank can be directly visualized using MolArt visualization plugin (Hoksza et al., 2018) integrated in MINERVA environment (Fig. 4C). This plugin helps users in exploring both sequence and structural features (including protein variation data from large scale studies) associated with proteins. In addition to the sequence and structure level features of the protein, one can connect 3D structures of protein complexes, drugs and chemicals bound to their receptor proteins which can be directly integrated into structure-based drug development pipelines. Users can develop their own Minerva plugins to analyze the content of the molecular interaction map (MIM).
3.2.3. Visualization of time-series omics data
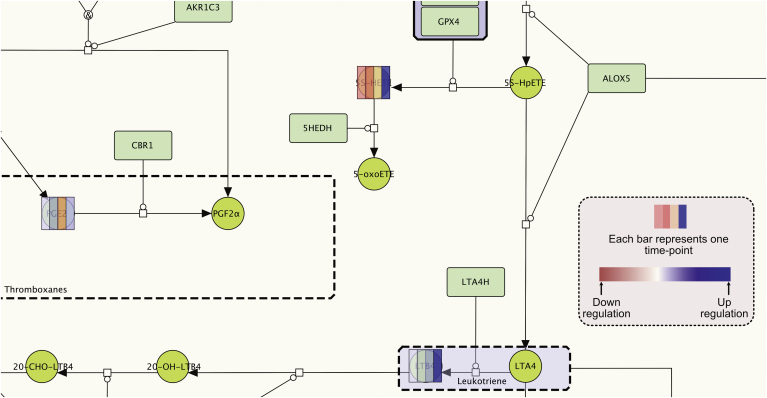
AIR hosted on the MINERVA platform provides the user with an interactive interface to map time-series omics data. Once uploaded on the AIR, these data can be visualized on all the interconnected submaps. Nodes are overlaid with multiple color bars depending on the number of associated time-points (Fig. 5). By providing a visual representation of change in the node expression profile over time, this feature helps users in designing new hypotheses on the role of connected nodes regulating a phenotype (see Fig. 6).
Fig. 5.
Mapping of time-series data onto the AIR. Nodes are overlaid with colored bars where each bar indicates the data at a given time point. In this example, log2 concentration fold change values of selected SPM were calculated at 4 time points (0 h, 12 h, 24 h and 48 h) and mapped onto the AIR when mice were challenged with higher titre E. coli (107 c.f.u.) compared to self-resolving E. coli infection (105 c.f.u.) (Chiang et al., 2012). The color gradient from red to blue indicates downregulation and upregulation. The color bars demonstrate that all the pro-inflammatory lipid mediators (PGE2, 5 S-HETE and LTB4) are mostly upregulated from time point 0 h–48 h in response to the stimulus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
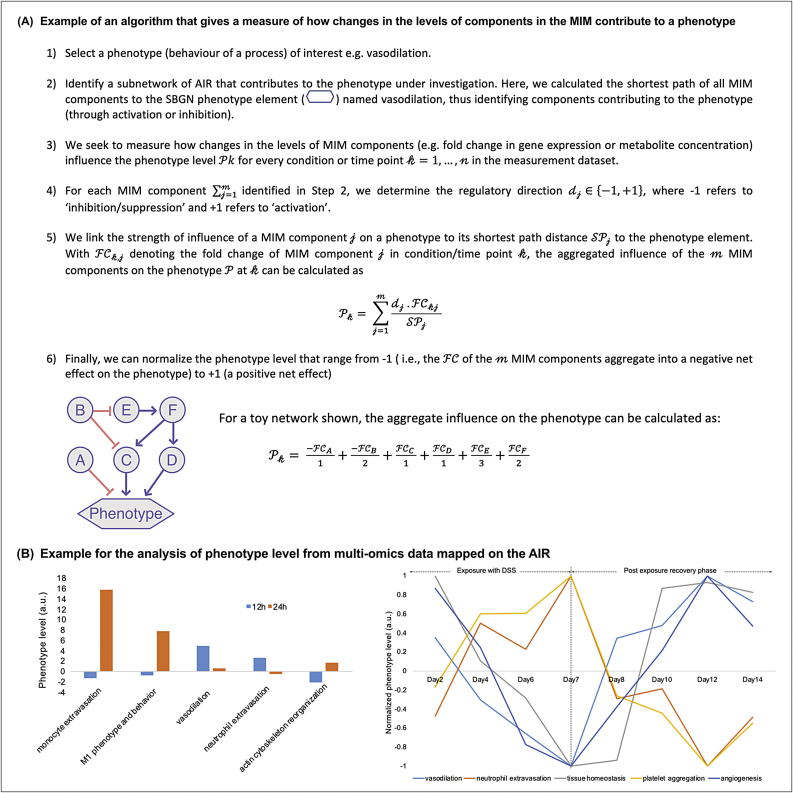
Fig. 6.
Examples using the AIR for bioinformatic analyses. (A) Algorithm to determine the aggregated influence of change in the MIM components on phenotype level. A toy network in the bottom highlights the aggregate influence on the phenotype due to the expression or concentration fold change in the network components A to F. (B) Examples for the analysis of phenotype level from multi-omics data mapped on the AIR. Left: An example where we measure the influence on the phenotype level (a.u.) from MIM components after mapping of miRNA log fold change data from the zymosan-induced peritonitis mouse model treated with/without resolvin D1 (RvD1) at time point 12 h and 24 h (Recchiuti et al., 2011). The bar in the plot indicates influence on the phenotype levels when RvD1 was co-administered. The graph indicates that vasodilation was quickly downregulated which is also supported by the low level of neutrophil extravasation. Other phenotypes (monocyte extravasation; M1 phenotype and behavior; acting cytoskeleton reorganization) were upregulated, suggesting that RvD1 brought the whole systems quickly towards the inflammation resolution phase in comparison to the exposure with zymosan alone. Right: In another example, we highlight the influence of MIM components on various processes associated with the acute inflammation onset and resolution after mapping of time-series transcriptomics profile from mouse colitis model (Czarnewski et al., 2019). The graph indicates normalized phenotype levels (‘vasodilation’, ‘neutrophil extravasation’, ‘tissue homeostasis’, ‘platelet aggregation’, and ‘angiogenesis’) from mouse model exposed with dextran sodium sulfate (DSS) for 7 days to induce acute colitis followed by 7 days of recovery phase. In this study transcriptomics profiling of colon samples were carried out for 9 different timepoints (Day 0, Day 2, Day 4, Day 6, Day 7, Day 8, Day 10, Day 12 and Day 14). Results suggest that the ‘neutrophil extravasation’ and ‘platelet aggregation’ increases until the DSS exposure (i.e. 7days, inflammation initiation phase) followed by sharp decline during the post exposure recovery phase. On the other hand, ‘vasodilation’ increases from day 7–12 (inflammation transition phase) and then a sharp decline in the phenotype was observed suggesting that the system is in inflammation resolution phase.
3.2.4. The identification of core regulatory processes
In the last decade, several methodologies have been developed to define and identify core regulatory networks, disease modules or context-specific subnetworks from large molecular interaction network (Khan et al., 2018; Park et al., 2019; Rush and Repsilber, 2018; Dreyer et al., 2018, Jaitly et al., 2020, Saelens et al., 2018, Singh et al., 2020). The AIR allows interfacing with such approaches in general through additional plugins. As an example, we provide the user with an interface to identify a core regulatory network from the AIR responsible for the overall dynamics associated with the acute inflammatory process or phenotype under investigation. The detailed methodology for the prediction of the core regulatory network is summarized in our previous publications (Khan et al., 2018, 2017). The methodology is based on the prioritization of feedback loops derived from user-provided multi-omics data, integration of prioritized motifs and finally the preparation of ready-to-use network files in standardized SBML format for in silico analysis.
3.2.5. The AIR helps in analysing the modulation of molecular processes and clinical phenotypes
The AIR contains information on relationships between proteins, small molecules, non-coding RNA, and their effects on biological processes. This information not only represents a comprehensive resource on acute inflammation but, furthermore, provides the basis to elucidate functions of elements and core networks in the regulation of inflammatory processes. Many scientific questions that exceed the capacity of in vitro and in vivo research may be answered by integrating state of the art in silico methods to the AIR, either depending on data input by the user or stand-alone.
Omics data can be considered as a snapshot of the molecular state, i.e. the current molecular activity, of a sample at the measured time. By mapping context-specific data to the AIR as shown in section 3.2.3, plugins integrated into the AIR are able to explore the influence of molecules on the major biological processes of clinical interest which are otherwise difficult, time-consuming and mostly invasive. Estimating changes in these processes from high-throughput -omics data can provide a fast assessment of how the sample was affected in the given context and scale-up molecular changes up to the whole tissue or even organism. Biological processes are included in the AIR as “phenotype” elements of gene ontology terms, e.g. as defined by the Gene Ontology Resource (http://geneontology.org) or Mammalian Phenotype Ontology (http://informatics.jax.org). To facilitate a rapid assessment of the phenotype level (e.g. increased acute inflammation; increased vasodilation; decreased neutrophil numbers; efferocytosis etc.) based on the level of regulating elements in the data sample at various time-points and/or in various experimental conditions, the AIR implements various logic-based rules. An example of an algorithm that gives a measure of how changes in the levels of components in the MIM contribute to a phenotype is provided in Fig. 7.
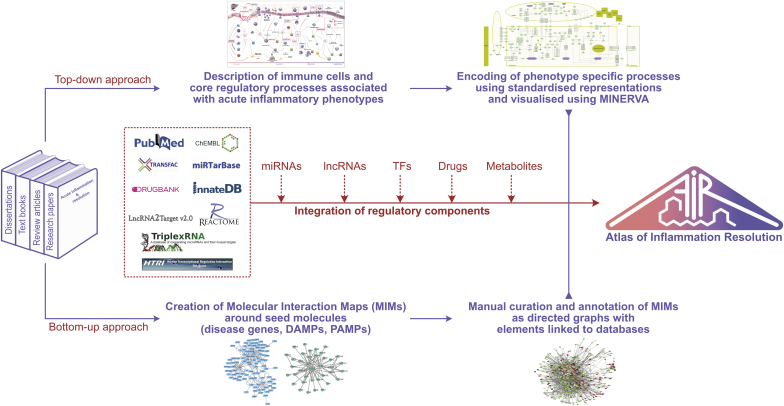
Fig. 7.
Workflow for the construction of the Atlas of Inflammation Resolution (AIR). The AIR is constructed both bottom-up and top-down. In case of the top-down approach, higher level processes, phenotypes and interplay between immune cells were identified in various stages of acute inflammation. These processes and phenotypes were extended in the form of information flow diagrams in standard SBML notations. In the bottom-up approach, first seed molecules were identified from damage-associated molecular patterns (DAMPs), Pathogen-associated molecular patterns (PAMPs) and key disease genes associated with selected clinical phenotypes of acute inflammation. Each seed molecule is then extended with the experimentally validated interacting partners. Models generated using bottom-up and top-down approaches were later merged and integrated with experimentally validated regulatory layers including transcription factors, miRNAs, lncRNAs, drugs and metabolites to prepare the AIR.
In case when no specific clinical and experimental data is available for the disease under investigation, the AIR can still be used to generate new hypotheses about the disease mechanisms using network topological and functional analyses. For this, elements can be ranked by various centrality measures either in the whole MIM or in defined subnetworks, to assess their importance in biological subprocesses. Because most interactions in the AIR are directed, Boolean rules are defined to create executable models of subnetworks to perform in silico perturbation experiments for the identification of molecular switches whose dysregulation may result in the disease phenotypes.
3.2.6. AIR as a platform to connect inflammation communities
The AIR is freely available to the community on the MINERVA platform hosted on ELIXIR, an intergovernmental organization that brings together life science resources from across Europe (https://air.elixir-luxembourg.org). The AIR also provides an interface where communities can directly raise questions, suggest the inclusion of molecules and processes and update information.
The AIR is designed with the aim to connect clinicians, biochemists, systems biologists, computer scientists and drug developers. Depending on the need of the end-user, the AIR provides various levels of representation and organization of data and models. Resources such as AIR need continuous improvement with the inclusion of missing links at the molecular level as soon as they are published. This can be realized only through community efforts. The AIR provides an interactive platform to connect the community; thus, we hope that the AIR will be sustained in future by the community.
4. Methods
4.1. Construction of AIR combining bottom-up and top-down approaches
Classical disease networks are designed using a bottom-up approach, where the phenotype is represented by interacting subsystems (functional modules), each containing evidence-based molecular interactions of clinical relevance (Fisher et al., 2014). With the availability of omics data, disease networks are frequently built top-down, where signatures (e.g. differentially expressed genes) are being first identified and subsequently connected to their interacting and regulatory partners. All the connected components are then analyzed to access overrepresented molecular processes and pathways, which together are supposed to coordinate for the emergence of disease phenotype (Butcher et al., 2004; Wang and Marincola, 2008). Because of the pros and cons associated with both approaches, the AIR is conceptualized as an integration of the models generated in bottom-up and top-down approaches (Fisher et al., 2014) (Fig. 7).
In case of the bottom-up approach, we first reviewed published literature to identify molecular processes, cell and tissue specific phenotypes associated with one of the four acute inflammatory phases i.e. inflammation initiation, transition, resolution and return to homeostasis (Fig. 1). For each of the processes/phenotypes, we manually screened literature and databases (Reactome (https://reactome.org), KEGG (https://www.genome.jp/kegg), InnateDB (https://innatedb.com)) to extract experimentally validated signaling and regulatory events. These are finally represented by a process diagram using the CellDesigner software (http://www.celldesigner.org) in standard systems biology graphics notation (SBGN) representation.
The top-down approach to the acute inflammation response started with the collection of key molecular signatures (seed molecules). These molecular signatures are then extended with known interacting and regulatory partners. As acute inflammation can be triggered by a variety of etiological agents, from which we considered three sets of seed molecules for the construction of AIR, these are 1) damage associated molecular patterns (DAMPs); 2) receptors recognizing pathogen associated molecular patterns (PAMPs); and 3) disease genes from selected acute inflammatory clinical phenotypes. While DAMPS and PAMPs- recognizing receptors were mainly identified through research articles, key molecules associated with acute inflammatory clinical indications were screened through disease-gene association databases. To this end, we mainly used DisGeNET (https://disgenet.org), eDGAR (http://edgar.biocomp.unibo.it), KEGG disease (https://www.genome.jp/kegg/disease) databases. As many of the disease-gene association databases are based on text mining algorithms, we manually cross checked the disease-gene association from associated research papers. For each of the seed molecules, we identified interacting molecular components from literature and databases to prepare a molecular interaction network. We also used the Bisogenet 3.0.0 app (Martin et al., 2010) available on Cytoscape 3.7.0 which connects large number of biological databases (e.g. DIP (https://dip.doe-mbi.ucla.edu), BioGRID (https://thebiogrid.org), HPRD (https://hprd.org), IntAct (https://www.ebi.ac.uk/intact/), MINT (https://mint.bio.uniroma2.it)) to first create biological networks around each of the seed molecule. We extracted only experimentally validated interactions.
Networks generated using bottom-up and top-down approaches were merged together to present a comprehensive molecular interaction network of acute inflammation on AIR. This approach not only enabled us to expand various acute inflammatory processes/phenotypes (identified in bottom-up approach) with underlying molecular level interactions but also helped in annotating several molecular interactions (top-up approach) with specific process/phenotype. In addition, many chemical mediators play central roles in the onset of inflammation and later in its resolution. Considering this, we have currently included biosynthesis pathways and downstream signaling cascades of PIM and SPM into the AIR.
Experimentally validated regulatory layers, which include miRNAs from miRbase (http://www.mirbase.org), miRTarBase (http://mirtarbase.mbc.nctu.edu.tw), TriplexRNA (https://triplexrna.org); transcription factors from TRNSFAC (http://genexplain.com/transfac), TRRUST (https://www.grnpedia.org/trrust) and HTRIdb (http://www.lbbc.ibb.unesp.br/htri); long noncoding RNAs from EVLncRNAs (http://biophy.dzu.edu.cn/EVLncRNAs), lncRNADisease (http://www.cuilab.cn/lncrnadisease) databases are also integrated with the AIR using an in-house script. The overall workflow for the construction of AIR is described in Fig. 7.
4.2. AIR as a directed graph
Providing direction (e.g. activation, inhibition) to the edges connecting various nodes in the network is a crucial step for network topological analyses and for initiating dynamic systems biological models for the prediction of biomarkers and therapeutic candidates. Only a directed graph will provide mechanistic insights through the study of network motifs and system dynamics. Giving direction to interactions is however a manual effort. Databases using text mining approaches are prone to include false positive information about regulatory directions. We therefore manually cross-checked associated publications before providing directions to the connected edges. More than 80% of the total interactions in the network are directed as of May 2020. Subsets of the AIR that are directed graphs can be used to construct mathematical models that describe acute inflammation and inflammation resolution as dynamical systems. While such systems biology approaches are already well established in cancer research (Khan et al., 2017), acute inflammation and inflammation resolution offer plenty new opportunities for more interdisciplinary approaches using mathematical modelling and computer simulations. The AIR provides a valuable starting point to identify core regulatory networks that can be subjected to dynamic mechanistic modelling. The entire molecular interaction map of the AIR is encoded using standardized representations, allowing the use of bioinformatics approaches to study it, using graph theoretic and statistical approaches. The Minerva platform allows the integration of plugins for this purpose.
4.3. Annotation of AIR content
We enriched the annotation of every gene/protein in the AIR with specific UniProt-, HGNC-, RefSeq-, Ensembl- and NCBI-ID as well as common aliases with the full name of the encoding protein. In case of small molecules, the ChEBI-ID is provided. Every interaction in AIR is hyperlinked to the respective literature and database. With all the manual curation and annotation, we make AIR a reliable resource for studying processes related to acute inflammation and its resolution.
4.4. AIR technical implementation
We used OpenLayer and Google Maps based techniques to bring the AIR to the web-browser for easy visualization implemented on the MINERVA platform developed by University of Luxembourg (Gawron et al., 2016). Considering various user groups (clinicians, research scientists, pharmaceutical companies), we divided the AIR into three layers (Fig. 3). The top layer consists of immune cell types, cellular processes and compartments. Middle or process layer is comprised of sub-modules and biomolecular species and finally the bottom layer provides information about molecular level interactions. To reduce the computational effort in processing network images and the requirements to the high-speed local internet, we tiled the CellDesigner representation of the AIR. Tiling is a technique to cut images into a matrix of smaller images of the same size and store it in a specific folder structure. With this, only the currently viewed parts of the AIR can be loaded and displayed. We wrote small Python scripts for tiling and layering to test if the CellDesigner export of the map fits to the postproduction process of the AIR. A local MINERVA instance was installed and tested with AIR for various security and reliability issues before its deployment on the Elixir server.
Funding
S.K.Gupta and O.Wolkenhauer acknowledge support from Bundesministerium für Bildung und Forschung (BMBF) grants [MelAutim (01ZX 1905B) and SASkit (012X 1903B)] and funding received from the European Union's Horizon 2020 research and Innovation programme under the Marie Skłodowska-Curie grant agreement No 765274. C.N.Serhan acknowledges support from USA NIH GM038765. V.Chiurchiù acknowledges support from FISM 2017/R/08 and GR-2016-02362380 grants. C.Godson and E.Brennan are supported by a JDRF Strategic Research Award, Science Foundation Ireland 15/IA/3152 and a US-Ireland R&D partnership award15/US/B3130. O.Werz acknowledges support by the Deutsche Forschungsgemeinschaft (SFB1127 ChemBioSys and SFB1278 Polytarget). J.G.Filep acknowledges support from the Canadian Institutes of Health Research (MOP-97742and MOP-102619). M.Perretti acknowledges the financial support of the Medical Research Council (grant MR/K013068/1) and Versus Arthritis UK (grant 21,274). B.D.Levy was supported in part by the USA NIH P01-GM095467 and R01-HL122531. The project was in part supported by Heel GmbH. The funders had no role in study design, data collection, curation of content and analysis.
Author contributions
C.N.Serhan, S.K.Gupta and O.Wolkenhauer conceived the manuscript and drafted the first versions. All authors contributed to the scientific content and helped writing the text. All authors approved of the submitted version. O.Wolkenhauer and S.K.Gupta supervised projects that included the curation of content to the MIM, or layouting submaps. S.K.Gupta, S.S.Gupta, P.Schopohl, M.Hoch, D.Brauer, F.M.Khan and D.Gjorgevikj designed various submaps. O.Wolkenhauer, S.K.Gupta, S.S.Gupta and C.N.Serhan equally contributed to the quality check of content. Furthermore, all authors contributed to the interpretation and quality control of information contained in the AIR.
Data and materials availability
The AIR is hosted on ELIXIR, an intergovernmental organization that brings together life science resources from across Europe and can be accessed through https://air.elixir-luxembourg.org. All the submaps included in the AIR can be directly downloaded in SBML notations. Tutorials to use the AIR are available on https://air.bio.informatik.uni-rostock.de.
Declaration of competing interest
The AIR is built from experimentally validated information from the literature, with no information related to products of pharma companies being referred to. All authors declare that there are no competing financial interests that could undermine the objectivity, integrity and value of a publication.
Acknowledgments
The AIR uses Minerva, developed by the Luxembourg Centre for Systems Biomedicine (LCSB). We are grateful for the Minerva support provided by Piotr Gawron and Marek Ostaszeweski. The LCSB is also the elixir node hosting the AIR. We acknowledge Tom Gebhardt and Martin Scharm for providing IT support for the implementation and installation of the AIR.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mam.2020.100894.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anthony P. Robbins' pathologic basis of disease. Journal of Clinical Pathology. W.B. Saunders, Philadelphia. 1990 doi: 10.1136/jcp.43.2.176-a. fifth ed. [DOI] [Google Scholar]
- Bara O., Day J., Djouadi S.M. Proceedings of the IEEE Conference on Decision and Control. 2013. Nonlinear state estimation for complex immune responses; pp. 3373–3378. [DOI] [Google Scholar]
- Butcher E.C., Berg E.L., Kunkel E.J. Systems biology in drug discovery. Nat. Biotechnol. 2004;22(10):1253–1259. doi: 10.1038/nbt1017. [DOI] [PubMed] [Google Scholar]
- Calzone L., Gelay A., Zinovyev A., Radvanyi F., Barillot E. A comprehensive modular map of molecular interactions in RB/E2F pathway. Mol. Syst. Biol. 2008;4:173. doi: 10.1038/msb.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N., Fredman G., Bäckhed F., Oh S.F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484(7395):524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V., Leuti A., Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnewski P., Parigi S.M., Sorini C., Diaz O.E., Das S., Gagliani N., Villablanca E.J. Conserved transcriptomic profile between mouse and human colitis allows unsupervised patient stratification. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-10769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhalle S., Bode S.F.N., Balling R., Ollert M., He F.Q. A roadmap towards personalized immunology. npj Syst. Biol. Appl. 2018;4 doi: 10.1038/s41540-017-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R., Sadlier D.M., Godson C. Pro-resolving lipid mediators: agents of anti-ageing? Semin. Immunol. 2018;40:36–48. doi: 10.1016/j.smim.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Dreyer F.S., Cantone M., Eberhardt M., Jaitly T., Walter L., Wittmann J., Gupta S.K., Khan F.M., Wolkenhauer O., Pützer B.M., Jäck H., Heinzerling L., Vera J. A web platform for the network analysis of high-throughput data in melanoma and its use to investigate mechanisms of resistance to anti-PD1 immunotherapy. Biochim Biophys Acta Mol Basis Dis . 2018;1864(6 Pt B):2315–2328. doi: 10.1016/j.bbadis.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Fisher C.P., Kierzek A.M., Plant N.J., Moore J.B. Systems biology approaches for studying the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2014;20(41):15070–15078. doi: 10.3748/wjg.v20.i41.15070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K.A., Ostaszewski M., Matsuoka Y., Ghosh S., Glaab E., Trefois C., Crespo I., Perumal T.M., Jurkowski W., Antony P.M.A., Diederich N., Buttini M., Kodama A., Satagopam V.P., Eifes S., Del Sol A., Schneider R., Kitano H., Balling R. Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol. Neurobiol. 2014;49(1):88–102. doi: 10.1007/s12035-013-8489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 2016;15(8):551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- Gawron P., Ostaszewski M., Satagopam V., Gebel S., Mazein A., Kuzma M., Zorzan S., McGee F., Otjacques B., Balling R., Schneider R. MINERVA—a platform for visualization and curation of molecular interaction networks. npj Syst. Biol. Appl. 2016;2 doi: 10.1038/npjsba.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Greten F.R., Karin M. 2010. Immunity, Inflammation, and Cancer. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoksza D., Gawron P., Ostaszewski M., Schneider R. MolArt: a molecular structure annotation and visualization tool. Bioinformatics. 2018;34(23):4127–4128. doi: 10.1093/bioinformatics/bty489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoksza D., Gawron P., Ostaszewski M., Smula E., Schneider R., Cowen L. MINERVA API and plugins: opening molecular network analysis and visualization to the community. Bioinformatics. 2019;35(21):4496–4498. doi: 10.1093/bioinformatics/btz286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucka M., Finney A., Sauro H.M., Bolouri H., Doyle J.C., Kitano H., Arkin A.P., Bornstein B.J., Bray D., Cornish-Bowden A., Cuellar A.A., Dronov S., Gilles E.D., Ginkel M., Gor V., Goryanin I.I., Hedley W.J., Hodgman T.C., Hofmeyr J.H., Hunter P.J., Juty N.S., Kasberger J.L., Kremling A., Kummer U., Le Novère N., Loew L.M., Lucio D., Mendes P., Minch E., Mjolsness E.D., Nakayama Y., Nelson M.R., Nielsen P.F., Sakurada T., Schaff J.C., Shapiro B.E., Shimizu T.S., Spence H.D., Stelling J., Takahashi K., Tomita M., Wagner J., Wang J. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19(4):524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Jaitly T., Gupta S.K., Wolkenhauer O., Vera J. Cancer Immunology. Springer; Cham.: 2020. Envisioning the Application of Systems Biology in Cancer Immunology; pp. 599–624. [DOI] [Google Scholar]
- Khan F.M., Marquardt S., Gupta S.K., Knoll S., Schmitz U., Spitschak A., Engelmann D., Vera J., Wolkenhauer O., Pützer B.M. Unraveling a tumor type-specific regulatory core underlying E2F1-mediated epithelial-mesenchymal transition to predict receptor protein signatures. Nat. Commun. 2017;8(1) doi: 10.1038/s41467-017-00268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.M., Sadeghi M., Gupta S.K., Wolkenhauer O. Methods in Molecular Biology. Vol. 1702. Humana Press; New York, NY: 2018. A network-based integrative workflow to unravel mechanisms underlying disease progression. [DOI] [PubMed] [Google Scholar]
- Kitano H., Funahashi A., Matsuoka Y., Oda K. Using process diagrams for the graphical representation of biological networks. Nat. Biotechnol. 2005;23(8):961–966. doi: 10.1038/nbt1111. [DOI] [PubMed] [Google Scholar]
- Kohn K.W. Molecular interaction map of the mammalian cell cycle control and DNA repair systems. Mol. Biol. Cell. 1999;10(8):2703–2734. doi: 10.1091/mbc.10.8.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.D., Serhan C.N. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Tabas I., Fredman G., Fisher E.A. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 2014;114(12):1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Ochagavia M.E., Rabasa L.C., Miranda J., Fernandez-de-Cossio J., Bringas R. BisoGenet: a new tool for gene network building, visualization and analysis. BMC Bioinf. 2010;11 doi: 10.1186/1471-2105-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y., Matsumae H., Katoh M., Eisfeld A.J., Neumann G., Hase T., Ghosh S., Shoemaker J.E., Lopes T.J., Watanabe T., Watanabe S., Fukuyama S., Kitano H., Kawaoka Y. A comprehensive map of the influenza A virus replication cycle. BMC Syst. Biol. 2013;7 doi: 10.1186/1752-0509-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mesarovic M.D., Sreenath S.N., Keene J.D. Search for organising principles: understanding in systems biology. Syst. Biol. 2004;1(1):19–27. doi: 10.1049/sb:20045010. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., Golenbock D., Gresnigt M.S., Heneka M.T., Hoffman H.M., Hotchkiss R., Joosten L.A.B., Kastner D.L., Korte M., Latz E., Libby P., Mandrup-Poulsen T., Mantovani A., Mills K.H.G., Nowak K.L., O’Neill L.A., Pickkers P., Van Der Poll T., Ridker P.M., Schalkwijk J., Schwartz D.A., Siegmund B., Steer C.J., Tilg H., Van Der Meer J.W.M., Van De Veerdonk F.L., Dinarello C.A. A guiding map for inflammation. Nat. Immunol. 2017;18(8):826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novershtern N., Itzhaki Z., Manor O., Friedman N., Kaminski N. A functional and regulatory map of asthma. Am. J. Respir. Cell Mol. Biol. 2008;38(3):324–336. doi: 10.1165/rcmb.2007-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gómez A., Perretti M., Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol. Med. 2013;5(5):661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Hwang D., Yeo Y.S., Kim H., Kang J. CONFIGURE: a pipeline for identifying context specific regulatory modules from gene expression data and its application to breast cancer. BMC Med. Genom. 2019;12(5) doi: 10.1186/s12920-019-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Cooper D., Dalli J., Norling L.V. Immune resolution mechanisms in inflammatory arthritis. Nat. Rev. Rheumatol. 2017;13(2):87–99. doi: 10.1038/nrrheum.2016.193. [DOI] [PubMed] [Google Scholar]
- Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C.N. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25(2):544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush S.T.A., Repsilber D. Capturing context-specific regulation in molecular interaction networks. BMC Bioinf. 2018;19(1) doi: 10.1186/s12859-018-2513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens W., Cannoodt R., Saeys Y. A comprehensive evaluation of module detection methods for gene expression data. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-03424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough J., Majno G. 1st. Vol. 82. Harvard University Press; Cambridge: 1977. pp. 66–67. (The Healing Hand: Man and Wound in the Ancient World, the American Historical Review). [DOI] [Google Scholar]
- Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. The FASEB Journal. 2017;31(4):1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Lipid mediators in Health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- Simon T.L. Transfusion. 2nd. Vol. 44. Lippincott Williams & Wilkins; Philadelphia, Pa.: 2004. BLOOD, PRINCIPLES AND PRACTICE OF HEMATOLOGY. [DOI] [Google Scholar]
- Singh N., Eberhardt M., Wolkenhauer O., Vera J., Gupta S.K. An integrative network-driven pipeline for systematic identification of lncRNA-associated regulatory network motifs in metastatic melanoma. BMC Bioinformatics. 2020;21(1) doi: 10.1186/s12859-020-03656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T.E., Serhan C.N. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 2003;82(2):82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- Varaiya P. Theory of hierarchical, multilevel systems. IEEE Trans. Automat. Contr. 1972;17(2):280–281. doi: 10.1109/tac.1972.1099964. [DOI] [Google Scholar]
- Viola J., Soehnlein O. Atherosclerosis - a matter of unresolved inflammation. Semin. Immunol. 2015;27(3):184–193. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Wang E., Marincola F.M. Bottom up: a modular view of immunology. Immunity. 2008;29(1):9–11. doi: 10.1016/j.immuni.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Zhu L., Dent J.E., Nardini C. A comprehensive molecular interaction map for rheumatoid arthritis. PloS One. 2010;5(4) doi: 10.1371/journal.pone.0010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Pan W., Qian L. Identification of the miRNA–mRNA regulatory network in multiple sclerosis. Neurol. Res. 2017;39(2):142–151. doi: 10.1080/01616412.2016.1250857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.