Abstract
This prespecified subanalysis of the global, randomized controlled phase III KEYNOTE‐024 study of pembrolizumab vs chemotherapy in previously untreated metastatic non‐small‐cell lung cancer without EGFR/ALK alterations and a programmed death ligand 1 (PD‐L1) tumor proportion score of 50% or higher evaluated clinical outcomes among patients enrolled in Japan. Treatment consisted of pembrolizumab 200 mg every 3 weeks (35 cycles) or platinum‐based chemotherapy (four to six cycles). The primary end‐point was progression‐free survival; secondary end‐points included overall survival and safety. Of 305 patients randomized in KEYNOTE‐024 overall, 40 patients were enrolled in Japan (all received treatment: pembrolizumab, n = 21; chemotherapy, n = 19). Median progression‐free survival was 41.4 (95% confidence interval [CI], 4.2‐42.5) months with pembrolizumab and 4.1 (95% CI, 2.8‐8.3) months with chemotherapy (hazard ratio [HR], 0.27 [95% CI, 0.11‐0.65]; one‐sided, nominal P = .001). Median overall survival was not reached (NR) (95% CI, 22.9‒NR) and 21.5 (95% CI, 5.2‐35.0) months, respectively (HR, 0.39 [95% CI, 0.17‐0.91]; one‐sided, nominal P = .012). Treatment‐related adverse events occurred in 21/21 (100%) pembrolizumab‐treated and 18/19 (95%) chemotherapy‐treated patients; eight patients (38%) and nine patients (47%), respectively, had grade 3‐5 events. Immune‐mediated adverse events and infusion reactions occurred in 11 pembrolizumab‐treated patients (52%) and four chemotherapy‐treated patients (21%), respectively; four patients (19%) and one patient (5%), respectively, had grade 3‐5 events. Consistent with results from KEYNOTE‐024 overall, first‐line pembrolizumab improved progression‐free survival and overall survival vs chemotherapy with manageable safety among Japanese patients with metastatic non‐small‐cell lung cancer without EGFR/ALK alterations and a PD‐L1 tumor proportion score of 50% or higher. The trial is registered with Clinicaltrials.gov: NCT02142738.
Keywords: Japan, non‐small‐cell lung carcinoma, PD‐L1 protein, pembrolizumab, treatment outcome
This prespecified subanalysis of the global, randomized controlled phase III KEYNOTE‐024 study of pembrolizumab vs chemotherapy in previously untreated metastatic non‐small‐cell lung cancer without EGFR/ALK alterations and a PD‐L1 tumor proportion score of 50% or higher evaluated clinical outcomes among patients enrolled in Japan. Consistent with results from KEYNOTE‐024 overall, first‐line pembrolizumab improved progression‐free survival and overall survival vs chemotherapy with manageable safety among 40 Japanese patients in the study.

Abbreviations
- AE
adverse event
- BICR
blinded, independent, central radiologic review
- CI
confidence interval
- CR
complete response
- ECOG
Eastern Cooperative Oncology Group
- HR
hazard ratio
- IgG4
immunoglobulin G4
- i.v.
intravenous
- mAb
monoclonal antibody
- MRI
magnetic resonance imaging
- NSCLC
non‐small‐cell lung cancer
- NR
not reached
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed death 1
- PD‐L1
programmed death ligand 1
- PFS
progression‐free survival
- PR
partial response
- RECIST
response evaluation criteria in solid tumors
- TPS
tumor proportion score
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths worldwide 1 and represents approximately 20% of all cancer‐related deaths in Japan. 2 Platinum‐based chemotherapy has historically been the standard first‐line treatment for patients with advanced‐stage non‐small‐cell lung cancer (NSCLC), particularly those without targetable EGFR and ALK alterations 3 , 4 , 5 ; however, immunotherapy directed at the PD‐1 checkpoint pathway has more recently provided patients with a therapeutic option that can improve clinical outcomes over standard chemotherapy regimens. 6 The most recent updates to lung cancer clinical practice guidelines in Japan now recommend the anti‐PD‐1 immunotherapy pembrolizumab as first‐line treatment in patients with metastatic NSCLC without targetable gene alterations and a PD‐L1 TPS of 50% or higher. 7
Pembrolizumab is a humanized IgG4 mAb that blocks the interaction between PD‐1 and its ligands PD‐L1 and PD‐L2, thereby promoting cytotoxic T‐cell‐mediated antitumor responses. 8 The phase I KEYNOTE‐001 trial was the first study to show an association between PD‐L1 expression and response to pembrolizumab, showing a higher response rate among patients with advanced NSCLC and a PD‐L1 TPS of 50% or higher. 9 The global phase III KEYNOTE‐024 study subsequently found that patients with previously untreated metastatic NSCLC without EGFR mutations or ALK translocations and a PD‐L1 TPS of 50% or higher had significantly longer OS (HR, 0.60; 95% CI, 0.41‐0.89; P = .005) and favorable safety outcomes with pembrolizumab vs platinum‐based chemotherapy. 10 A recent updated analysis from KEYNOTE‐024 showed that pembrolizumab continued to prolong OS compared with chemotherapy with longer follow‐up (HR for OS, 0.63; 95% CI, 0.47‐0.86; nominal P = .002), despite an increase in the number of patients who crossed over from chemotherapy to pembrolizumab (82 patients vs 66 patients who crossed over to pembrolizumab on study in the previous analysis). 11
Previous analyses from registry data and clinical trials of anticancer therapies for NSCLC suggest that Asian patients might have better survival outcomes than non‐Asian patients. 12 , 13 Here, we report results from patients enrolled in the KEYNOTE‐024 study in Japan. 10
2. MATERIALS AND METHODS
2.1. Patients
Eligibility criteria for enrollment in the KEYNOTE‐024 study have been previously published 10 ; this subanalysis included patients enrolled in KEYNOTE‐024 from 23 sites in Japan. In short, adult patients aged 18 years or older were eligible if they had previously untreated stage IV NSCLC without activating EGFR mutations or ALK translocations, a PD‐L1 TPS of 50% or higher, measurable disease based on RECIST version 1.1, and an ECOG performance status of 0 or 1. For evaluation of PD‐L1 status, patients must have provided a tumor tissue sample obtained at the time of or after diagnosis of metastatic disease and before any adjuvant or neoadjuvant therapy. Patients were ineligible if they had untreated brain metastases, active autoimmune disease that required systemic treatment, had received systemic steroid therapy within 3 days before the first dose of study medication or were receiving any other immunosuppressive medication, or had interstitial lung disease or a history of pneumonitis that required steroid treatment.
All patients provided written informed consent before enrollment. The trial protocol and all amendments were approved by an institutional review board or independent ethics committee at each study site, and the trial was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
2.2. Study design
This was a prespecified subanalysis of the phase III, open‐label, randomized KEYNOTE‐024 study (ClinicalTrials.gov identifier, NCT02142738). As described previously, 10 patients were randomly assigned in a 1:1 ratio to receive either i.v. pembrolizumab 200 mg every 3 weeks for up to 35 cycles or the investigator’s choice of one of the following five platinum‐based chemotherapy regimens, selected before randomization, for four to six cycles: carboplatin or cisplatin plus pemetrexed, carboplatin or cisplatin plus gemcitabine, or carboplatin plus paclitaxel. Pemetrexed‐containing regimens were permitted only for patients with nonsquamous tumors, and pemetrexed maintenance therapy could continue after the combination chemotherapy regimen was completed. Randomization was stratified by ECOG performance status (0 vs 1) and tumor histology (squamous vs nonsquamous). Treatment continued for the prespecified number of cycles or until radiologic disease progression per RECIST version 1.1 by investigator review, unacceptable toxicity, concurrent illness precluding further treatment, or a decision was made by the patient or the investigator to withdraw treatment. Crossover from chemotherapy to pembrolizumab was permitted for patients with documented disease progression (per RECIST version 1.1 by BICR) who met safety criteria. Patients in either treatment arm who were considered to be deriving clinical benefit and were clinically stable (ie no signs and symptoms of clinically significant disease progression, no rapid disease progression or progressive tumor requiring urgent alternative treatment, and no decline in ECOG performance status) could continue to receive treatment after disease progression. Patients in the pembrolizumab arm who achieved a complete response could discontinue treatment if they had been treated for at least 6 months and had received at least two treatments beyond the initial date of complete response. 10 Patients who stopped pembrolizumab after a complete response or after completing 2 years (35 cycles) of pembrolizumab and subsequently had disease progression could receive a second course of pembrolizumab for up to 17 cycles if they had received no other anticancer therapy since the last pembrolizumab dose and continued to meet the required eligibility criteria.
2.3. End‐points
The end‐points in this preplanned subgroup analysis of patients enrolled in KEYNOTE‐024 in Japan were the same as for the overall study. The primary end‐point in the KEYNOTE‐024 study was PFS, defined as the time from randomization to the first of either documented disease progression (per RECIST version 1.1 by BICR) or death from any cause. Secondary end‐points were OS, defined as the time from randomization to death from any cause; ORR, defined as the proportion of patients with a confirmed complete or partial response (per RECIST version 1.1 by BICR); and safety. Duration of response was an exploratory end‐point, and was defined as the time from the first documentation of a complete or partial response to disease progression. 10
2.4. Assessments
A central laboratory assessed PD‐L1 expression in formalin‐fixed tumor samples obtained through core‐needle or excisional biopsy or from tissue resected at the time of or after diagnosis of metastatic disease from a site not previously irradiated using the PD‐L1 IHC 22C3 pharmDx assay (Agilent Technologies). 14 Computed tomography (preferred) or MRI was carried out every 9 weeks, with tumor response assessed per RECIST version 1.1 by BICR. After the end of treatment, patients were monitored for disease status every 3 months until disease progression, initiation of new anticancer therapy, withdrawal of consent, loss to follow‐up, or death; once imaging assessments were stopped (ie for progressive disease or for starting a new anticancer therapy) survival follow‐up was undertaken approximately every 2 months until death or withdrawal of consent. Safety was monitored throughout the study and for 30 days or more after treatment discontinuation (90 days for serious AEs and AEs of interest). All AEs were graded in severity per the NCI’s Common Terminology Criteria for Adverse Events version 4.0.
2.5. Statistical analysis
Statistical methods for this subanalysis of the KEYNOTE‐024 study were the same as those of the primary analysis, 10 except that only those patients enrolled in Japan were included. Efficacy analyses included all randomized patients, according to the treatment assigned (intention‐to‐treat population); safety analyses included all patients who received at least one dose of treatment, according to the treatment received. Both PFS and OS were estimated using the Kaplan‐Meier method. For the analysis of PFS, patients who were alive without disease progression and had not initiated new anticancer therapy or who were lost to follow‐up were censored at the time of last tumor assessment. For the analysis of OS, patients without documented death were censored at the time of last follow‐up. Between‐group differences in PFS and OS were assessed using a stratified log‐rank test. Hazard ratios and associated 95% CIs were assessed using a stratified Cox proportional hazards model with Efron’s method of handling ties. The stratified Miettinen and Nurminen method was used to assess treatment differences in ORR; patients with missing data were considered nonresponders. Stratification factors used for randomization were also applied to the analyses. One‐sided nominal P values are provided for this subanalysis. The data cut‐off date for this analysis was 15 February 2019.
3. RESULTS
3.1. Patients and treatment
Among 305 patients randomized in the overall KEYNOTE‐024 study population (pembrolizumab, n = 154; chemotherapy, n = 151), 10 40 patients were randomized at Japanese sites (pembrolizumab, n = 21; chemotherapy, n = 19; Figure 1) between November 2014 and October 2015, all of whom received treatment as assigned. In the chemotherapy arm, 10 patients crossed over to pembrolizumab on study (53%) and an additional three patients received anti‐PD‐1 treatment outside of cross‐over, for an effective cross‐over rate of 68% in the intention‐to‐treat population. Of patients initially allocated to pembrolizumab, eight patients (38%) received subsequent platinum‐based chemotherapy.
FIGURE 1.
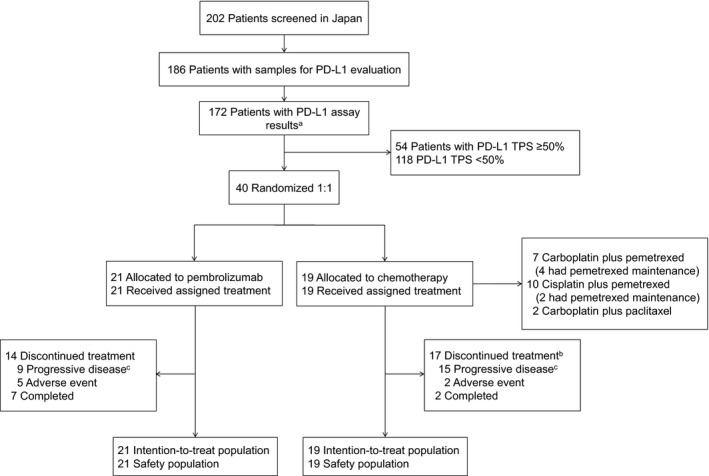
CONSORT flow diagram of Japanese patients enrolled in KEYNOTE‐024 to evaluate pembrolizumab vs chemotherapy in metastatic non‐small‐cell lung cancer. aThe remaining patients did not meet study eligibility criteria (n = 14). bIncludes 10 patients who crossed over to pembrolizumab treatment during the study. cIncludes clinical progression. PD‐L1, programmed death ligand 1; TPS, tumor proportion score
Patient demographic and baseline clinical characteristics were generally well balanced between the treatment arms (Table 1). Most patients had nonsquamous tumors (86% in the pembrolizumab arm and 95% in the chemotherapy arm) and were former or current smokers (95% and 100%, respectively). Median treatment exposure as of the data cut‐off date (15 February 2019) was 13.1 months (range, 0.03‐47.6 months) in the pembrolizumab arm and 3.5 months (range, 0.03‐11.8 months) in the chemotherapy arm, and median time from randomization to data cut‐off was 43.3 months (range, 40.7‐50.5 months).
TABLE 1.
Patient demographic and baseline clinical characteristics of Japanese patients enrolled in KEYNOTE‐024
| Characteristic | Pembrolizumab (n = 21) | Chemotherapy (n = 19) |
|---|---|---|
| n (%) a | n (%) a | |
| Age (y) | ||
| Median | 66 | 67 |
| Range | 40‐80 | 53‐77 |
| Male sex | 16 (76) | 18 (95) |
| ECOG performance status | ||
| 0 | 7 (33) | 8 (42) |
| 1 | 14 (67) | 11 (58) |
| Smoking status | ||
| Former/current | 20 (95) | 19 (100) |
| Never | 1 (5) | 0 |
| Histology | ||
| Squamous | 3 (14) | 1 (5) |
| Nonsquamous | 18 (86) | 18 (95) |
| Brain metastases | 1 (5) | 1 (5) |
| Prior neoadjuvant therapy | 0 | 0 |
| Prior adjuvant therapy | 0 | 0 |
Data are n (%), unless otherwise noted.
3.2. Efficacy outcomes
A total of 27 PFS events occurred among the 40 patients enrolled in Japan, with few events occurring in the pembrolizumab arm (n = 10). The median PFS was 41.4 (95% CI, 4.2‐42.5) with pembrolizumab and was 4.1 months (95% CI, 2.8‐8.3) with chemotherapy (HR for PFS, 0.27; 95% CI, 0.11‐0.65; one‐sided, nominal P = .001; Figure 2A). In addition, the estimated PFS rate at 1 year was higher in the pembrolizumab arm (64% [95% CI, 39%‐81%]) vs the chemotherapy arm (25% [95% CI, 9%‐46%]).
FIGURE 2.
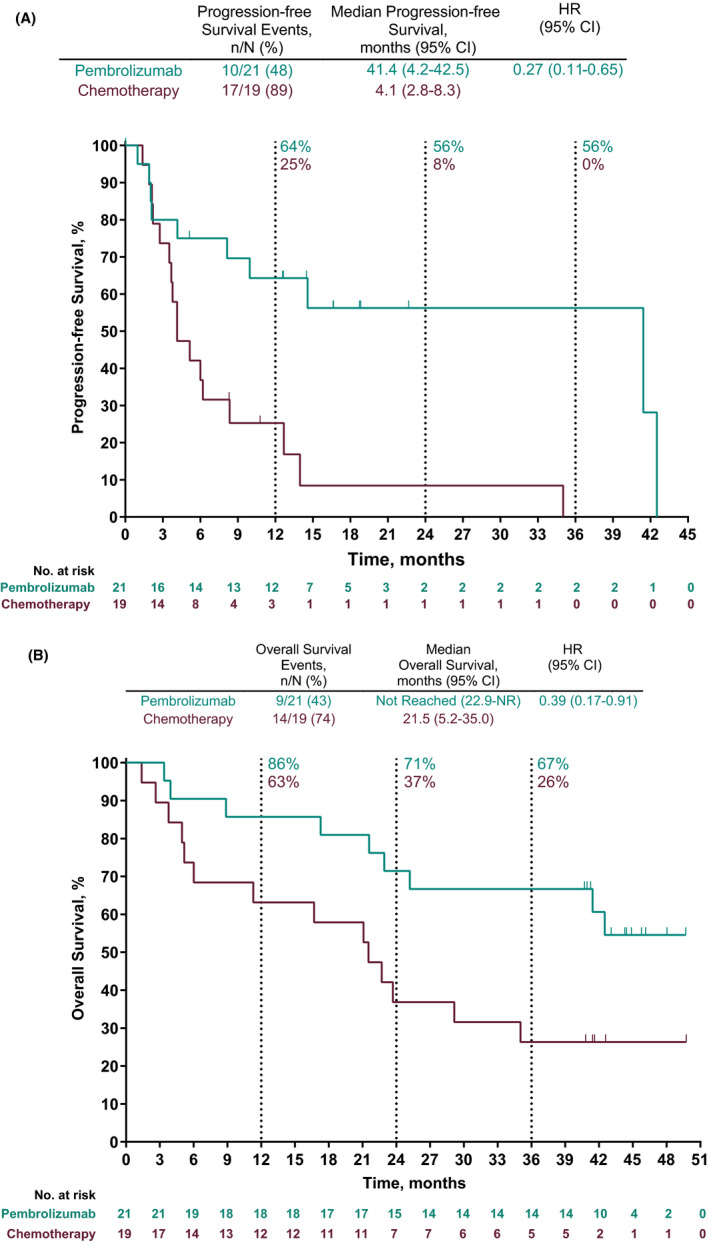
Kaplan‐Meier estimates of (A) progression‐free survival per RECIST version 1.1 per independent central review and (B) overall survival in patients with metastatic non‐small‐cell lung cancer treated with pembrolizumab or chemotherapy. CI, confidence interval; HR, hazard ratio; NR, not reached; RECIST, response evaluation criteria in solid tumors
At the time of data cut‐off, approximately half of the patients had died (pembrolizumab arm, n = 9; chemotherapy arm, n = 14). Overall survival was longer with pembrolizumab than with chemotherapy. Median OS was NR in the pembrolizumab arm (22.9‐NR) and was 21.5 months (95% CI, 5.2‐35.0) in the chemotherapy arm (HR for OS, 0.39; 95% CI, 0.17‐0.91; one‐sided, nominal P = .012; Figure 2B). The estimated OS rates in the pembrolizumab arm vs the chemotherapy arm were 86% (95% CI, 62%‐95%) vs 63% (95% CI, 38%‐80%) at 1 year, 71% (95% CI, 47%‐86%) vs 37% (95% CI, 17%‐58%) at 2 years, and 67% (95% CI, 43%‒83%) vs 26% (95% CI, 10%‐47%) at 3 years.
The ORR was 62% (95% CI, 38%‐82%) in the pembrolizumab arm and 26% (95% CI, 9%‐51%) in the chemotherapy arm (one‐sided, nominal P = .019). The median duration of response was NR (range, 6.2+ to 20.6+ months) in the pembrolizumab arm and 9.8 months (range, 4.8+ to 10.4 months) in the chemotherapy arm (plus signs in ranges indicate nondisease progression at the last assessment [censored] for the patient with the minimum/maximum response duration). Among patients in the pembrolizumab arm who had a complete (n = 1) or partial (n = 12) response, seven patients had completed 35 cycles (2 years) of treatment at the time of data cut‐off (Figure 3). Of these seven patients, three received a second course of pembrolizumab (one completed 17 cycles and two discontinued due to progressive disease; all three remained alive at data cut‐off).
FIGURE 3.
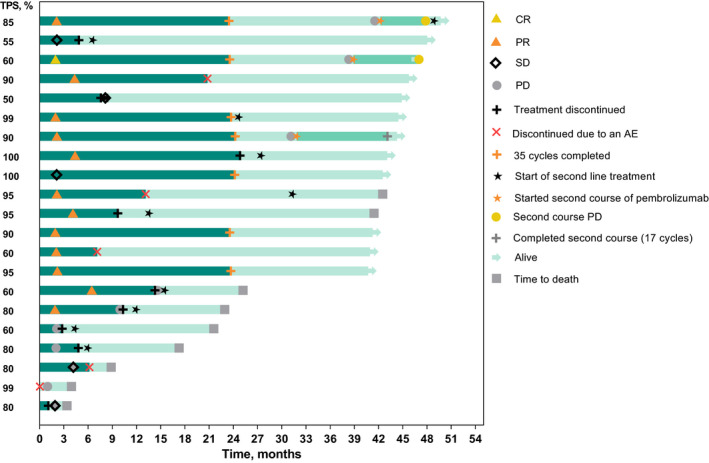
Duration of treatment and time to response among patients in the pembrolizumab arm of KEYNOTE‐024 with a complete response (CR) or partial response (PR) per RECIST version 1.1 by independent central review. Bar lengths indicate duration of treatment (first‐course, dark green; second‐course, medium green) and months of follow‐up (light green). Tumor response (ie, CR, PR, SD, PD) is expressed per RECIST version 1.1 by independent central review only. AE, adverse event; PD, progressive disease; PD‐L1, programmed death ligand 1; SD, stable disease; TPS, tumor proportion score
3.3. Safety
Treatment‐related AEs of any grade occurred in all 21 patients treated with pembrolizumab and 18 of the 19 patients (95%) treated with chemotherapy in this Japanese cohort (Table 2). The most common treatment‐related AEs in the pembrolizumab arm were pyrexia (n = 5), diarrhea (n = 4), and rash (n = 4), and the most common in the chemotherapy arm were decreased appetite (n = 12), nausea (n = 11), and anemia (n = 9). Treatment‐related AEs of grade 3‐5 occurred in eight patients (38%) in the pembrolizumab arm and nine patients (47%) in the chemotherapy arm. Four patients (19%) in the pembrolizumab arm and one patient (5%) in the chemotherapy arm discontinued treatment because of treatment‐related AEs. No patient in the pembrolizumab arm and one patient (5%) in the chemotherapy arm died due to a treatment‐related AE. Immune‐mediated AEs and infusion reactions of any grade, and regardless of relationship to treatment as assessed by the investigator, occurred in 11 patients (52%) in the pembrolizumab arm and in four patients (21%) in the chemotherapy arm (Table 2). The most common events in the pembrolizumab arm (occurring in at least 10% of patients) were infusion reactions (n = 4; 19%), and pneumonitis and hypothyroidism (each n = 3; 14%). Grade 3‐5 immune‐mediated AEs occurred in four patients (19%) in the pembrolizumab arm (grade 3 hepatitis, severe skin reaction, and uveitis, and grade 4 pneumonitis, all in one patient each) and one patient (5%) in the chemotherapy arm (grade 3 pneumonitis).
TABLE 2.
Summary of adverse events (AEs) among patients from Japan in the as‐treated population of KEYNOTE‐024 a
| Treatment‐related AEs b | Pembrolizumab (n = 21) | Chemotherapy (n = 19) |
|---|---|---|
| n (%) | n (%) | |
| Any grade | 21 (100) | 18 (95) |
| Grade 3‐5 | 8 (38) | 9 (47) |
| Led to discontinuation c | 4 (19) | 1 (5) |
| Led to death | 0 | 1 (5) |
| Treatment‐related AEs b occurring in ≥15% of patients in either arm | Any grade | Grade 3‐5 | Any grade | Grade 3‐5 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Pyrexia | 5 (24) | 0 (0) | 2 (11) | 0 (0) |
| Diarrhea | 4 (19) | 1 (5) | 3 (16) | 0 (0) |
| Rash | 4 (19) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 3 (14) | 0 (0) | 12 (63) | 2 (11) |
| Anemia | 2 (10) | 1 (5) | 9 (47) | 5 (26) |
| Malaise | 2 (10) | 0 (0) | 8 (42) | 0 (0) |
| Hypoalbuminemia | 2 (10) | 2 (10) | 4 (21) | 2 (11) |
| Hiccups | 1 (5) | 0 (0) | 6 (32) | 0 (0) |
| Constipation | 1 (5) | 0 (0) | 5 (26) | 0 (0) |
| Peripheral sensory neuropathy | 1 (5) | 0 (0) | 3 (16) | 0 (0) |
| Nausea | 0 (0) | 0 (0) | 11 (58) | 0 (0) |
| Platelet count decreased | 0 (0) | 0 (0) | 8 (42) | 3 (16) |
| White blood cell count decreased | 0 (0) | 0 (0) | 8 (42) | 1 (5) |
| Neutrophil count decreased | 0 (0) | 0 (0) | 4 (21) | 1 (5) |
| Immune‐mediated AEs and infusion reactions d | n (%) | n (%) | n (%) | n (%) |
|---|---|---|---|---|
| Any | 11 (52) | 4 (19) | 4 (21) | 1 (5) |
| Infusion reactions | 4 (19) | 0 (0) | 1 (5) | 0 (0) |
| Pneumonitis | 3 (14) | 1 (5) | 1 (5) | 1 (5) |
| Hypothyroidism | 3 (14) | 0 (0) | 2 (11) | 0 (0) |
| Colitis | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Hepatitis | 1 (5) | 1 (5) | 0 (0) | 0 (0) |
| Hyperthyroidism | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Severe skin reactions | 1 (5) | 1 (5) | 0 (0) | 0 (0) |
| Thyroiditis | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Uveitis | 1 (5) | 1 (5) | 0 (0) | 0 (0) |
| Adrenal insufficiency | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
The as‐treated population comprised all randomized patients who received at least one dose of study treatment, according to the treatment received.
Adverse events that were attributed to treatment by the investigator are listed.
Treatment‐related AEs that led to discontinuation were: pneumonitis (n = 2), fatigue (n = 1), and uveitis (n = 1) among four patients in the pembrolizumab group, and hypoxia (n = 1) and pulmonary alveolar hemorrhage (resulting in death) in one patient in the chemotherapy group.
Immune‐mediated AEs and infusion reactions are listed irrespective of attribution to study treatment by the investigator.
4. DISCUSSION
This prespecified subanalysis of KEYNOTE‐024 showed that pembrolizumab prolonged PFS over platinum‐based chemotherapy (HR for disease progression or death, 0.27; one‐sided, nominal P = .001) among patients enrolled in Japan with previously untreated metastatic NSCLC without targetable EGFR/ALK alterations and a PD‐L1 TPS of 50% or more. Of note, few PFS events had occurred in either treatment arm after a median study follow‐up of 43.3 months. In addition, pembrolizumab prolonged OS over chemotherapy (HR for death, 0.39; one‐sided, nominal P = .012). With few OS events occurring in the pembrolizumab arm, median OS was not reached. Treatment with pembrolizumab was associated with a higher ORR compared with chemotherapy (62% vs 26%). In addition, pembrolizumab had a manageable safety profile, and no new safety signals were identified in this subset of Japanese patients relative to previous studies evaluating pembrolizumab monotherapy in patients with advanced NSCLC. 9 , 10 , 15
The favorable efficacy observed with pembrolizumab in this subanalysis among patients in KEYNOTE‐024 enrolled in Japan is consistent with the significantly longer PFS (HR, 0.50; P < .001) and OS (HR, 0.60; P = .005) observed with pembrolizumab vs chemotherapy in the overall study population, 9 , 10 , 15 with somewhat lower HRs for PFS (0.27) and OS (0.39) in the current analysis. Although the reason for these lower HRs is uncertain, several factors might have contributed, including potential differences in baseline characteristics, patient care practices in Japan, or in treatment responses between Japanese and non‐Asian populations. 13 , 16 The smaller number of patients in this subgroup analysis and few PFS and OS events in either treatment arm might have also contributed. Notably, the positive results from the current analysis are consistent with the positive findings in the large, multicenter, randomized controlled phase III KEYNOTE‐042 study as well. 17 Similar to results from the overall KEYNOTE‐024 study noted above, KEYNOTE‐042 showed an OS benefit with pembrolizumab vs platinum‐based chemotherapy in patients with previously untreated locally advanced or metastatic NSCLC without EGFR or ALK alterations and a PD‐L1 TPS of 50% or higher (HR, 0.69; P = .0003), and additionally showed OS benefit in the overall population with PD‐L1 TPS of at least 1% (HR, 0.81; P = .0018). 17 Together, these findings provide support for the use of pembrolizumab monotherapy as first‐line treatment for PD‐L1‐positive (TPS ≥ 1%) advanced NSCLC, which has received regulatory approval in Japan. 18 Significant OS benefit has also been shown with pembrolizumab plus platinum‐based chemotherapy vs chemotherapy alone in patients with previously untreated metastatic NSCLC without EGFR or ALK alterations, irrespective of PD‐L1 expression, in the phase III placebo‐controlled studies KEYNOTE‐189 (nonsquamous; HR, 0.49; P < .001) and KEYNOTE‐407 (squamous; HR, 0.64; P < .001). 19 , 20
Importantly, the OS benefits with pembrolizumab monotherapy over chemotherapy among patients in this subanalysis and in the global KEYNOTE‐024 study were observed despite relatively high cross‐over rates (53% in the current analysis and 44% in the primary analysis of the global study). 10 Moreover, the effective cross‐over rate of 68% in the current analysis (after accounting for patients in the chemotherapy arm who received anti‐PD‐1/PD‐L1 therapy outside of on‐study cross‐over) was similar to the effective cross‐over rate in the most recent updated analysis from the KEYNOTE‐024 global study (65%), which continued to show an OS benefit with pembrolizumab (HR, 0.63; P = .002), further supporting the efficacy gains with pembrolizumab. 11
With a median treatment exposure of 13.1 months (range, 0.03‐47.6 months) in this subanalysis compared with 7 months (range, 0.03‐18.7 months) in the primary analysis of the KEYNOTE‐024 study, 10 there were no additional safety concerns identified, supporting the tolerability of pembrolizumab in Japanese patients with metastatic NSCLC. In addition, the immune‐mediated AEs and infusion reactions that occurred with pembrolizumab among the patients included in this subanalysis were consistent with those observed in previous clinical trials evaluating pembrolizumab monotherapy in advanced NSCLC,9,10,15 with few events of grade 3 or higher—a finding that is of particular clinical relevance, as monitoring for these events is necessary in practice.
Key limitations of this subanalysis are that no alpha was allocated, and because the data represent a subset of patients from the global KEYNOTE‐024 study, the sample size was smaller (40 patients of 305 who were randomized in the global study). Accordingly, with fewer patients, fewer events of PFS and OS occurred, as noted above. However, despite the limited power of this analysis, the HRs for these end‐points suggested substantial PFS and OS benefits with pembrolizumab over chemotherapy. Importantly, these results provide support for the efficacy and safety of pembrolizumab in Japanese patients with advanced NSCLC.
In conclusion, this subanalysis among patients enrolled in KEYNOTE‐024 in Japan demonstrated the efficacy and safety benefits of pembrolizumab vs platinum‐based chemotherapy, as also observed in the primary analysis. These findings provide further support for the use of pembrolizumab monotherapy as first‐line treatment in patients from Japan with metastatic NSCLC without activating EGFR mutations or ALK translocations and a PD‐L1 TPS of 50% or higher.
DISCLOSURE
Miyako Satouchi has received honoraria from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Taiho Pharmaceutical, Pfizer, Novartis, Eli Lilly, and Boehringer Ingelheim and grants from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Pfizer, Novartis, Boehringer Ingelheim, AbbVie, Takeda, and Eli Lilly. Toshiaki Takahashi has received research funds from AstraZeneca, Chugai Pharmaceutical, Eli Lilly Japan, Ono Pharmaceutical, MSD, Tokyo, and Pfizer Japan. Kazuhiko Nakagawa has received lecture fees, honoraria, or other fees from AstraZeneca, Astellas Pharma, MSD, Tokyo, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Eli Lilly Japan, Pfizer Japan, and Kyorin Pharmaceutical, research funds from MSD, Tokyo, A2 Healthcare, inVentiv Health Japan, Astellas Pharma, Daiichi Sankyo, Eisai, AbbVie, IQVIA Services Japan, ICON Japan, Chugai Pharmaceutical, Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Syneos Health, Pfizer Japan, Eli Lilly Japan, SymBio Pharmaceuticals, Bristol‐Myers Squibb, CMIC Shift Zero, Taiho Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and AstraZeneca and scholarship endowments or research grants from Takeda Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, Nippon Boehringer Ingelheim, Daiichi Sankyo, and Chugai Pharmaceutical. Keisuke Aoe has received lecture fees, honoraria, or other fees from Ono and Bristol‐Myers Squibb and research funds from Ono, Bristol‐Myers Squibb, MSD, AstraZeneca, Novartis, and Eli Lilly. Takayasu Kurata has received lecture fees, honoraria, or other fees from MSD, Ono, Bristol‐Myers Squibb, AstraZeneca, Chugai, Eli Lilly, and Boehringer Ingelheim and research funds from MSD, AstraZeneca, Takeda, Bristol‐Myers Squibb, and Novartis. Tatsuro Fukuhara has received research funds from MSD, Ono Pharma, Bristol‐Myers Squibb, and AstraZeneca. Shunichi Sugawara has received lecture fees from MSD. Shigeki Umemura has received research funds from MSD. Hideo Saka has received research funds from MSD, AstraZeneca, Ono, Parexel International, WJOG, Bristol‐Myers Squibb, Chugai, and Takeda. Isamu Okamoto has received lecture fees, honoraria, or other fees from MSD, Eli Lilly, Chugai, Ono, and AstraZeneca and research funds from MSD, Eli Lilly, Chugai, Ono, and AstraZeneca. Nobuyuki Yamamoto has received lecture fees, honoraria, or other fees from MSD, AstraZeneca, Ono Pharmaceutical, Eli Lilly, Boehringer Ingelheim, Novartis, and Pfizer and research funds from Bristol‐Myers Squibb, Amgen, MSD, Astellas, AstraZeneca, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Takeda Pharmaceutical, Chugai Pharmaceutical, Terumo, Toppan Printing, Eli Lilly, Boehringer Ingelheim, Novartis, and Pfizer. Kazuma Kishi has received research funds from MSD. Nobuyuki Katakami obtained speakers fees and research grants from AstraZeneca, Taiho, Boehringer Ingelheim Japan, MSD, and Chugai Pharma. Hidehito Horinouchi has received lecture fees, honoraria, or other fees from Eli Lilly, AstraZeneca, Kyowa Kirin, MSD, Ono, and Bristol‐Myers Squibb and research funds from Chugail, Daiichi Sankyo, AstraZeneca, MSD, Ono, Bristol‐Myers Squibb, and Genomic Health. Toyoaki Hida has received research funds from MSD, Bristol‐Myers Squibb, and Ono. Hiroaki Okamoto has received research funds from Taiho, Chugai, Astellas, Eli Lilly, Merck, and Bristol‐Myers Squibb. Shinji Atagi has received research funds from AstraZeneca, MSD, Eli Lilly, Chugai, Ono, Taiho, Boehringer Ingelheim, Pfizer, F. Hoffman‐La Roche, and Bristol‐Myers Squibb. Shi Rong Han and Kazuo Noguchi are employees of MSD, Tokyo, Japan. Victoria Ebiana is a former employee of Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA, and has received remuneration from MSD and Amgen. Katsuyuki Hotta has received lecture fees, honoraria, or other fees from MSD and AstraZeneca and research funds from AstraZeneca, Chugai, Eli Lilly, Bristol‐Myers Squibb, and Astellas. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). Medical writing assistance was provided by Madhura Mehta, PhD, and Christabel Wilson, MSc, of ICON (North Wales, PA, USA). This assistance was funded by MSD. A portion of the data reported in this manuscript were accepted for presentation at the 60th Annual Meeting of the Japanese Respiratory Society, 20‐22 September 2020.
Satouchi M, Nosaki K, Takahashi T, et al. First‐line pembrolizumab vs chemotherapy in metastatic non‐small‐cell lung cancer: KEYNOTE‐024 Japan subset. Cancer Sci. 2020;111:4480–4489. 10.1111/cas.14647
DATA AVAILABILITY STATEMENT
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. The Editorial Board of the Cancer Statistics in Japan , ed. Cancer Statistics in Japan ‐ 2017. Tokyo, Japan: Foundation for Promotion of Cancer Research (FPCR); 2018. [Google Scholar]
- 3. Japan Lung Cancer Society . Guidelines for lung cancer clinical practice 2018 edition: Non‐small cell lung cancer; 2018. https://www.haigan.gr.jp/guideline/2018/1/2/180102070100.html#j_7‐2_1. Accessed January 11, 2019
- 4. Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK, Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence‐Based Care . First‐line systemic chemotherapy in the treatment of advanced non‐small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5:260‐274. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non‐small cell lung cancer, version 5.2019; 2019. www.nccn.org. Accessed June 12, 2019
- 6. Khan M, Lin J, Liao G, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non‐small cell lung cancer: A meta‐analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non‐small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters S, Kerr KM, Stahel R. PD‐1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev. 2018;62:39‐49. [DOI] [PubMed] [Google Scholar]
- 9. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 10. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Rodríguez–Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE‐024: pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537‐546. [DOI] [PubMed] [Google Scholar]
- 12. Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and Caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8:451‐462. [DOI] [PubMed] [Google Scholar]
- 13. Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non‐small cell lung cancer: results of a meta‐analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030‐1038. [DOI] [PubMed] [Google Scholar]
- 14. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD‐L1 immunohistochemistry assay for pembrolizumab therapy in non‐small‐cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herbst RS, Baas P, Kim D‐W, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 16. Fukuoka M, Yano S, Giaccone G, et al. Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237‐2246. [DOI] [PubMed] [Google Scholar]
- 17. Mok TSK, Wu Y‐L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 18. Merck’s KEYTRUDA® (pembrolizumab) receives five new approvals in Japan, including in advanced non‐small cell lung cancer, as adjuvant therapy for melanoma, and in advanced microsatellite instability‐high tumors; 2019. https://www.merck.com/news/mercks‐keytruda‐pembrolizumab‐receives‐five‐new‐approvals‐in‐japan‐including‐in‐advanced‐non‐small‐cell‐lung‐cancer‐nsclc‐as‐adjuvant‐therapy‐for‐melanoma‐and‐in‐advanced‐microsa/. Accessed March 1, 2019
- 19. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 20. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379:2040‐2051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com


