Abstract
Anaplastic lymphoma kinase (ALK) inhibition is expected to be a promising therapeutic strategy for ALK‐positive malignancies. We aimed to examine the efficacy and safety of alectinib, a second‐generation ALK inhibitor, in patients with relapsed or refractory ALK‐positive anaplastic large cell lymphoma (ALCL). This open‐label, phase II trial included patients (aged 6 years or older) with relapsed or refractory ALK‐positive ALCL. Alectinib 300 mg was given orally twice a day (600 mg/d) for 16 cycles, and the duration of each cycle was 21 days. Patients who weighed less than 35 kg were given a reduced dose of alectinib of 150 mg twice a day (300 mg/d). Ten patients were enrolled, and the median age was 19.5 years (range, 6‐70 years). Objective responses were documented in eight of 10 patients (80%; 90% confidence interval, 56.2‐95.9), with six complete responses. The 1‐year progression‐free survival, event‐free survival, and overall survival rates were 58.3%, 70.0%, and 70.0%, respectively. The median duration of therapy was 340 days. No unexpected adverse events occurred. The most common grade 3 and higher adverse event was a decrease in neutrophil count in two patients. Alectinib showed favorable clinical activity and was well tolerated in patients with ALK‐positive ALCL who had progressed on standard chemotherapy. Based on the results of the current study, the Ministry of Health, Labour and Welfare of Japan approved alectinib for the treatment of recurrent or refractory ALK‐positive ALCL in February 2020.
Keywords: alectinib, ALK inhibitor, anaplastic large cell lymphoma, refractory, relapse
This phase II study aimed to assess the efficacy and safety of alectinib, an anaplastic lymphoma kinase (ALK) inhibitor, in patients with relapsed or refractory ALK‐positive anaplastic large cell lymphoma (ALCL). Alectinib showed favorable clinical activity, was well tolerated, and represents a good treatment option for pediatric or adult patients. Based on the results of this study, the Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare in Japan has approved alectinib for the treatment of recurrent or refractory ALK‐positive ALCL.

1. INTRODUCTION
Anaplastic large cell lymphoma (ALCL) is a rare peripheral T‐cell lymphoma that accounts for 10%‐15% of pediatric non‐Hodgkin lymphoma (NHL) cases and 1%‐2% of adult NHL cases. 1 Anaplastic large cell lymphoma is characterized by the presence of large CD30‐positive T cells. 2 According to anaplastic lymphoma kinase (ALK) expression, the WHO classifies ALCL into two subtypes: ALK‐positive or ALK‐negative. 3 Approximately 90% of pediatric and almost 50% of adult ALCL cases are ALK‐positive. 4 Most ALK‐positive ALCL cases show t(2;5)(p23;q35) chromosomal translocation, which produces ALK/nucleophosmin fusion proteins (p80), which represent the core pathophysiology of this disease. Anthracycline‐based combination chemotherapy is a standard frontline treatment for ALK‐positive ALCL, with a long‐term event‐free survival rate of 60%‐70%. 5 , 6 , 7 However, the standard treatment for relapsed or refractory ALK‐positive ALCL has not been established. Allogeneic hematopoietic stem cell transplantation (HSCT) has been reported as a curative treatment for relapsed or refractory ALK‐positive ALCL, with a long‐term event‐free survival rate of approximately 50%. 8 , 9 , 10 Recent studies have reported the efficacies of second‐line treatments for relapsed or refractory ALCL, including vinblastine monotherapy, brentuximab vedotin (BV), and ALK inhibitors. 11 , 12 Brentuximab vedotin is an anti‐CD30 Ab drug conjugated to the microtubule‐disrupting agent, monomethyl auristatin E, and has been associated with a high overall response rate. 12 , 13 Anaplastic lymphoma kinase inhibitors have dramatically improved the prognosis of non‐small‐lung cancer with ALK overexpression driven by ALK genetic alteration, and thus, ALK inhibitors have been proposed to be a promising treatment strategy for other kinds of cancers with ALK mutations. Anaplastic lymphoma kinase inhibitors inhibit the proliferation of ALK‐positive tumor cells and induce apoptosis in these cells. 14 In ALK‐positive ALCL, activated ALK acted as a driver mutation, thus the blockade of ALK could be an effective method for the control of this disease. For the treatment of ALCL, the Children’s Oncology Group (COG) reported the results of a clinical trial of the first‐generation ALK inhibitor crizotinib (ADVL0912 trial). 15 , 16 Nine patients with ALK‐positive ALCL were included, and the overall response rate was 89% (8/9) with 78% (7/9) complete remission (CR). These results indicated the high efficacy of this ALK inhibitor for ALK‐positive ALCL. Alectinib (Chugai Pharmaceutical Co.) is a second‐generation ALK inhibitor, given orally; its efficacy and safety have been reported in adult patients with non‐small‐cell lung cancer (NSCLC), and it is currently approved for ALK fusion gene‐positive NSCLC. 17 Furthermore, alectinib has shown superior efficacy to crizotinib based on a large randomized phase III study, compared to crizotinib, in untreated ALK‐positive NSCLC. 18 According to these reports, we hypothesized that alectinib should be effective for ALK‐positive ALCL, and undertook a phase II clinical trial to investigate the efficacy and safety of alectinib for relapsed or refractory ALK‐positive ALCL.
2. METHODS
2.1. Study design and patients
This single arm, open‐label phase II trial of patients with relapsed or refractory ALK‐positive ALCL was carried out to evaluate the efficacy and safety of alectinib, a second‐generation ALK inhibitor in Japan. Three institutes, the National Hospital Organization Kyushu Cancer Centre, National Hospital Organization Nagoya Medical Centre, and St. Marianna University School of Medicine Hospital, were involved in this trial. This study was registered in the UMIN‐CTR (unique trial no. UMIN000016991).
Patients with a minimum age limit of 6 years diagnosed with relapsed or refractory ALK‐positive ALCL were included in this study. Anaplastic lymphoma kinase‐positive ALCL was definitively diagnosed by histological examination with immunohistochemical staining. The major inclusion criteria were as follows: ECOG performance status of 0‐2, at least one measurable lesion, and preserved organ functions. Patients with central nervous system lesions were excluded. Full details of the inclusion and exclusion criteria were reported previously (Table S1). 19 Pathological diagnoses were reviewed centrally for all cases.
The trial was approved by the institutional review boards of each participating institution and carried out in accordance with Guidelines for Good Clinical Practice and the ethical principles written in the Declaration of Helsinki. Written informed consent is obtained from every patient prior to participation in the trial.
2.2. Procedures
Alectinib 300 mg was given orally twice a day (600 mg/d) continuously. One cycle consisted of 21 days. Patients who weighed less than 35 kg were given 150 mg twice a day (300 mg/d). This dose was determined based on the simulations to predict pediatric pharmacokinetics (PK). Treatment was continued for a maximum of 16 cycles if there was not progressive disease or unacceptable toxicity. From cycle 17 onwards, therapy was continued for patients who would benefit from further treatment according to the investigators’ assessments.
2.3. Outcomes
The primary efficacy end‐point was the objective response during the protocol treatment, which we defined as the proportion of patients who achieved complete remission (CR) or partial remission (PR). Evaluation was carried out before starting alectinib, at the completion of cycles 3, 7, 11, and 16, and then every 24 weeks from cycle 17 onwards. Evaluation was also carried out for all patients on completion of their treatment with alectinib. The best response was adopted for the evaluation of the response. The response was assessed by an independent central review board according to the Revised Response Criteria for Malignant Lymphoma (2007). 20 Positron emission tomography/computed tomography (CT) was used for the evaluation of CR, and CT was used for the evaluation of PR, stable disease, or progressive disease (PD). However, CT was also used for the evaluation of CR in cases with pretreatment PET negative. Secondary end‐points that were evaluated included pharmacokinetics, tolerability in patients aged 6 years and older and less than 15 years, CR rate, response duration, progression‐free survival (PFS), event‐free survival (EFS), overall survival (OS), and adverse events (AEs). All patients who received at least one dose of alectinib were evaluable for response and considered fully evaluable for toxicity. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.03. To evaluate the safety of pediatric patients, a review by data and safety monitoring board was held at the stage when the initial‐stage safety outcome is observed in subjects between the ages of 6 and 15 and at the stage when treatment cycle 1 in the three subjects is completed.
For patients aged 15 years and older, blood samples for PK analysis were collected at the following time points: cycle 1, day 1 (predose and at 0.5, 1, 2, 4, 6, 8, and 10 hours after the first dose), day 2 (at 24 hours after first dose), day 8 (before morning dose), day 15 (before morning dose), and day 21 (before morning dose and at 0.5, 1, 2, 4, 6, 8, and 10 hours post‐dose); cycle 3, day 21 (before morning dose); and cycle 7, day 21 (before morning dose). For patients younger than 15 years, blood samples were collected on cycle 1, day 1 (predose and at 1, 4, 6, and 10 hours after first dose), day 2 (at 24 hours after first dose), day 8 (before morning dose), day 15 (before morning dose), and day 21 (before morning dose and at 1, 4, 6, and 10 hours post‐dose); cycle 3, day 21 (before morning dose); and cycle 7, day 21 (before morning dose). Plasma alectinib concentrations were quantified using a liquid chromatography‐mass spectrometry method with a lower detection limit for quantification of 1.5 ng/mL. 21
For assessment of the molecular response caused by alectinib treatment, the expression of nucleophosmin (NPM)‐ALK in peripheral blood (PB) or bone marrow (BM) was evaluated using quantitative real‐time PCR. Normalized copy numbers (NCNs) were calculated as the number of copies of NPM‐ALK per 104 copies of ABL. We used previously reported protocols to evaluate NPM‐ALK expression. 22 , 23 , 24 NPM‐ALK expression in PB was measured before the beginning of alectinib therapy, at the end of the first, third, and 16th courses, and at the end or treatment. NPM‐ALK expression in BM was measured before the beginning of alectinib therapy. For patients with BM involvement, this was measured at the time of evaluation for CR.
2.4. Statistical analyses
A long‐term survival rate has been reported as 40%‐60% for recurrent or refractory ALCL in previous studies. 25 , 26 , 27 In addition, the objective response rate for relapsed or refractory ALCL patients treated with pralatrexate, a novel antifolic acid agent, in the PROPEL trial was 35% (6 of 17 patients). 28 Referencing these reports, the threshold response rate was set at 50%. Crizotinib showed a response rate of 89% (eight of nine patients) in the trial of pediatric recurrent or refractory ALCL patients. 15 Reported response rates to BV in recurrent or refractory ALCL patients was 86% (50 of 58 patients). 12 We expected that our drug would elicit response rates comparable to these drugs, and set the expected response rate as 85%. As a statistical power of 79% could be obtained with 10 patients with an alpha level of 0.05 (one‐tailed), the target sample size was found to be 10.
Response rate, CR rate, and their 90% confidence intervals (CIs) were calculated. To estimate PFS, EFS, and OS, the Kaplan‐Meier method was used. Their 90% CIs were calculated with Greenwood’s formula. Events for EFS included death, progression of disease, secondary cancer, and toxicity‐related discontinuation. The PK parameters were estimated using noncompartmental analysis. The incidence of AEs was calculated with respect to events and grade.
3. RESULTS
3.1. Patients
Eight male and two female patients (median age, 19.5 years; range, 6‐70 years) with ALK‐positive ALCL were enrolled between May 2015 and November 2017; all were eligible and were evaluable based on their responses (Tables 1 and S2). Histological diagnosis of ALK‐positive ALCL was centrally confirmed, and FISH analysis revealed NPM‐ALK fusion in all 10 patients. Four patients had primary induction failure, and six had relapsed ALCL. Six patients were diagnosed as having Ann‐Arbor clinical stage III or IV disease. Five patients had previously received BV. One patient had received prior radiation, and none had received prior HSCT. Eight patients were given 300 mg per dose, and two patients were given 150 mg per dose. The dose was determined according to the patient’s bodyweight defined in the protocol. The median duration of therapy was 340 days (range, 14‐925 days). The median follow‐up duration for the surviving patients was 508 days (range, 283‐925 days).
TABLE 1.
Characteristics of 10 patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma (ALCL)
| No. | Age (y) | Sex | BW (kg) | PS | Stage | Baseline B symptom | Extranodal disease | Disease status | Prior therapy | Prior radiation | Prior HSCT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Male | 65.20 | 0 | III | Yes | No | 1st relapse | ALCL99 | No | No |
| 2 | 20 | Male | 61.85 | 0 | II | No | No | Refractory | BV | No | No |
| 3 | 10 | Male | 30.50 | 0 | I | No | Skin | 1st relapse | ALCL99 | No | No |
| 4 | 19 | Female | 55.00 | 2 | III | Yes | No | 1st relapse | CHOP | No | No |
| 5 | 70 | Male | 57.40 | 1 | III | Yes | No | 2nd relapse | ESHAP | No | No |
| 6 | 6 | Male | 20.00 | 0 | I | No | No | 2nd relapse | ALCL99, VBL, BV | No | No |
| 7 | 12 | Female | 47.00 | 1 | III | Yes | No | 1st relapse | ALCL99 | No | No |
| 8 | 30 | Male | 59.20 | 2 | IV | Yes | No | Refractory | CHOP, ESHAP, BV | Yes | No |
| 9 | 38 | Male | 71.70 | 2 | III | Yes | Soft tissue | Refractory | CHOP, ESHAP, MA, BV | No | No |
| 10 | 29 | Male | 60.20 | 2 | II | Yes | Colon | Refractory | CHOP, EPOCH, CHASE, ESHAP, BV | No | No |
Abbreviations: ALCL99, standard chemotherapy for childhood ALCL 5 ; BV, brentuximab vedotin; BW, bodyweight; CHASE, cyclophosphamide, cytarabine, dexamethasone, etoposide; CHOP cyclophosphamide, hydroxydaunorubicin, vincristine, prednisolone; EPOCH, etoposide, prednisolone, vincristine, cyclophosphamide, hydroxydaunorubicin; ESHAP, etoposide, cisplatin, cytarabine, prednisolone; HSCT, hematopoietic stem cell transplantation; MA, methotrexate, cytarabine; PS, performance status; VBL, vinblastine.
3.2. Efficacy
The objective response rate was 80% (90% CI, 56.2‐95.9) with six patients achieving CR (60%) and two patients achieving PR (20%) (Table 2, Figure 1). The lower limit of the 90% CI of the overall response rate was above the threshold of 50%, and the primary end‐point met the predefined criteria. All six patients with CR had relapsed at the time of enrolment. Two patients received allogeneic HSCT in remission following alectinib treatment. At the point of data cut‐off, five patients continued to receive alectinib beyond 16 cycles (Figure 2). The 1‐year PFS, EFS, and OS rates were 58.3% (90% CI, 28.6‐79.3), 70.0% (90% CI, 39.6‐87.2), and 70.0% (90% CI, 39.6‐87.2), respectively (Figure 3). Two patients discontinued alectinib due to disease progression and three patients due to HSCT. Three patients died following disease progression. None of the patients discontinued alectinib due to toxicities.
TABLE 2.
Response to alectinib treatment in 10 patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma
| n (%) | 90% CI | |
|---|---|---|
| Objective response (CR + PR) | 8 (80) | 56.15‐95.91 |
| CR | 6 (60) | 34.33‐83.03 |
| PR | 2 (20) | |
| SD | 0 (0) | — |
| PD | 2 (20) | — |
Abbreviations: CI, confidence interval; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 1.
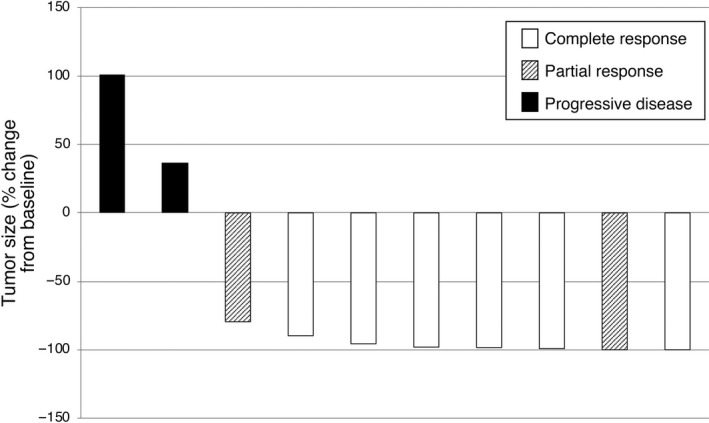
Waterfall plot of best percentage change of tumor size from baseline, based on investigator assessments, in 10 patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma treated with alectinib
FIGURE 2.
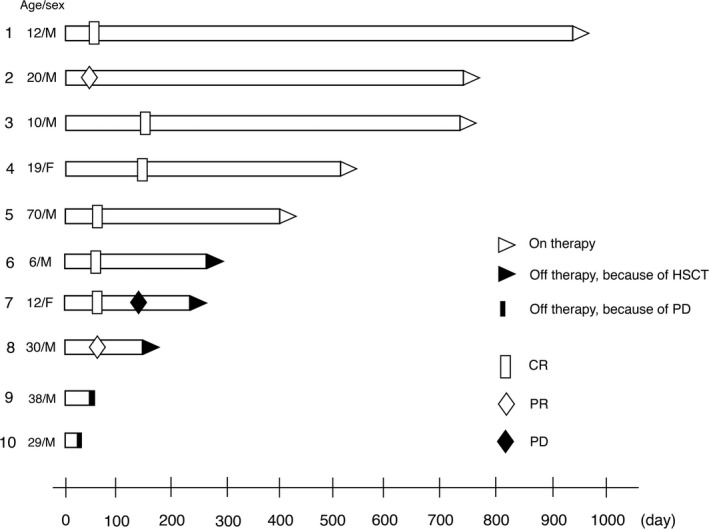
Response characteristics of 10 patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma treated with alectinib. Objective response was observed in eight patients. Two patients received hematopoietic stem cell transplantation (HSCT) in remission following alectinib treatment. Five patients continued to receive alectinib beyond 16 cycles. Three patients died following disease progression (patient nos. 8, 9, and 10). CR, complete remission; F, female; M, male; PD, progressive disease; R, partial remission
FIGURE 3.
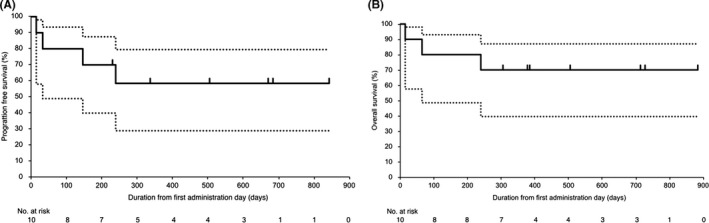
Progression‐free survival (A) and overall survival (B) of 10 patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma treated with alectinib. Solid and dotted lines show the survival estimate and its 90% confidence interval, respectively
3.3. Safety
Adverse events were observed in all 10 patients (Table S3); AEs reported in 10% or more of patients are shown in Table 3. Adverse events that occurred in 30% or more patients were oral mucositis, constipation, diarrhea, upper respiratory tract infection, maculopapular rash, raised alkaline phosphatase level, and headache. The most common grade 3 and higher AE was a decrease in neutrophil count in two patients. Other grade 3 and higher AEs were oral mucositis, duodenal stenosis, limb edema, pain, acute tonsillitis, cholangitis, bile duct stenosis, disseminated intravascular coagulation, febrile neutropenia, hypertriglyceridemia, tumor lysis syndrome, and anorexia in one patient each. Visual disturbance was not observed. One patient died following the progression of ALCL at 21 days after the first treatment with alectinib. No unexpected AE was experienced, and no patient required discontinuation or dose reduction because of AEs. The initial‐stage safety outcome for pediatric patients was evaluated according to the data of first three subjects between the ages of 6 and 15 years, and the safety of alectinib for pediatric patients was approved by the safety monitoring board.
TABLE 3.
Adverse events reported in 10% or more of patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma treated with alectinib (n = 10)
| All grades (%) | Grade ≥ 3 (%) | |
|---|---|---|
| Diarrhea | 4 (40) | 0 (0) |
| Upper respiratory infection | 4 (40) | 0 (0) |
| Rash | 4 (40) | 0 (0) |
| Increased blood ALP | 4 (40) | 0 (0) |
| Oral mucositis | 3 (30) | 1 (10) |
| Constipation | 3 (30) | 0 (0) |
| Headache | 3 (30) | 0 (0) |
| Nausea | 2 (20) | 0 (0) |
| Vomiting | 2 (20) | 0 (0) |
| Malaise | 2 (20) | 0 (0) |
| Edema in extremities | 2 (20) | 1 (10) |
| Fever | 2 (20) | 0 (0) |
| Pain | 2 (20) | 1 (10) |
| Bronchitis | 2 (20) | 0 (0) |
| Conjunctivitis | 2 (20) | 0 (0) |
| Allergic rhinitis | 2 (20) | 0 (0) |
| Coughing | 2 (20) | 0 (0) |
| Hyperuricemia | 2 (20) | 0 (0) |
| Pruritus | 2 (20) | 0 (0) |
| Dry skin | 2 (20) | 0 (0) |
| Increased AST | 2 (20) | 0 (0) |
| Decreased neutrophil count | 2 (20) | 2 (20) |
Abbreviations: ALP, alkaline phosphatase; AST, aspartate aminotransferase.
3.4. Pharmacokinetics of alectinib
We analyzed the pharmacokinetics of alectinib in all 10 patients. Patients were divided into three cohorts according to age and drug dose as follows: (A) younger than 15 years on 300 mg/d; (B) younger than 15 years on 600 mg/d; and (C) 15 years or older on 600 mg/d. The geometric mean steady‐state peak concentrations of alectinib in the cohorts A, B, and C were 479.5, 676.7, and 395.7 ng/mL, respectively, which were reached in 4, 5, and 4 hours as median values, respectively (Table 4).
TABLE 4.
Pharmacokinetics of alectinib according to three cohorts of patients with anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma
| Cohort | Cmax (ng/mL) | AUC0‐10 (ng h/mL) | |||||
|---|---|---|---|---|---|---|---|
| Identifier | Age (y) | Dose (mg/d) | N | Cycle 1 day 1 | Cycle 1 day 21 | Cycle 1 day 1 | Cycle 1 day 21 |
| A | <15 | 300 | 2 | 197.3 (11.3) | 479.5 (46.1) | 765.3 (48.3) | 3288.2 (47.9) |
| B | <15 | 600 | 2 | 84.2 (8.8) | 676.7 (44.4) | 538.7 (14.5) | 5597.6 (38.8) |
| C | ≥15 | 600 | 6 | 62.6 (74.0) | 395.7 (37.2) | 333.4 (87.5) | 2991.8 (38.2) |
Data are geometric mean (coefficient of variation [%]). AUC0–10, area under plasma concentration time curve from 0 h to 10 h; Cmax, maximum plasma concentration.
3.5. Analysis of NPM‐ALK expression
NPM‐ALK in PB and BM was measured in all 10 patients at the start of alectinib treatment by real‐time PCR, and quantifiable NPM‐ALK in PB or BM was observed in three patients; these cases were defined as minimum residual disease (MRD)‐positive. In the first patient, NPM‐ALK NCNs in PB and BM were 62 and 63, respectively. In the second patient, NPM‐ALK NCNs in PB were 27 and the BM sample was not available. Both patients had NPM‐ALK expression of less than 10 NCNs after the first course of alectinib. In the third patient, NPM‐ALK expression in PB was extremely high, at 1225 NCNs, and it reached 28 896 NCNs at the end of the first course of alectinib. Alectinib treatment was stopped because of PD for this patient.
4. DISCUSSION
Alectinib is a second‐generation ALK inhibitor, and its efficacy and safety has been previously reported in patients with NSCLC. 17 , 29 This is the first phase II clinical trial to evaluate the efficacy of alectinib for relapsed or refractory ALK‐positive ALCL, and we found that eight of 10 patients (80%) attained objective responses, with six patients (60%) attaining CR and two patients (20%) attaining PR. At the point of data cut‐off, four patients continued receiving alectinib in CR without severe AEs that would have required discontinuation or dose reduction. The median duration of alectinib treatment in those four patients was 733 days (range, 397‐925 days). Two patients received allogeneic HSCT, and were in good general condition. Based on the results of current study, the Ministry of Health, Labour and Welfare in Japan approved alectinib for the treatment of recurrent or refractory ALK‐positive ALCL in February 2020.
The efficacy of a first‐generation ALK inhibitor for ALK‐positive ALCL has been reported previously. 15 , 16 , 30 The COG undertook a phase I trial of crizotinib for refractory solid or central nervous system tumors and ALCL. The results of this study showed that crizotinib was well tolerated with a dose of 280 mg/m2 twice daily, and antitumor activity for malignancies harboring ALK translocations, particularly ALK‐positive ALCL, was suggested. 15 In the subsequent phase II trial (also carried out by COG), 26 patients with relapsed or refractory ALK‐positive ALCL received crizotinib. The overall response rates for patients with ALK‐positive ALCL were 83% for six patients with a dose of 165 mg/m2, and 90% for 20 patients with a dose of 280 mg/m2. Complete remission was observed in 83% of the patients with a dose of 165 mg/m2 and 80% of the patients with a dose of 280 mg/m2. 16 In another phase Ib trial of crizotinib (PROFILE 1013) for ALK‐positive tumors excluding NSCLC, there was an objective response rate of 53%, with eight CRs and one PR, for 18 patients with ALK‐positive lymphoma. 30 These clinical trials revealed the high efficacy of crizotinib for relapsed or refractory ALK‐positive ALCL; however, AEs were observed, including visual disturbance. Alectinib is a second‐generation ALK inhibitor that overcomes the gatekeeper mutation of crizotinib. 14 Furthermore, more favorable efficacy and safety in the case of alectinib compared to crizotinib has been reported in untreated ALK‐positive NSCLC. 29 The development of alectinib treatment regimens for patients with ALK‐positive ALCL is therefore indispensable.
Although some treatment options, such as vinblastine monotherapy, BV, and HSCT have been reported to be effective, there is no standard treatment recommended for relapsed or refractory ALK‐positive ALCL at the current time. Patients with disease that is refractory to conventional chemotherapies have particularly poor prognoses. Brugieres et al 11 studied 36 pediatric patients treated weekly with vinblastine for relapsed or refractory ALCL; this treatment was highly efficacious, with a CR rate of 83%. However, the 5‐year EFS rate was 30%, and the long‐term prognosis was often poor. In adults, a phase II trial of BV was carried out in patients with relapsed or refractory systemic ALCL. In that trial, 50 of 58 patients (86%) achieved an objective response, with 33 patients (57%) achieving CR. 12 Peripheral neuropathies occurred in 41% of the patients, which is common to vinblastine, but caution is needed regarding the long‐term safety of BV. It has also been reported that in patients who achieved CR with BV, the 5‐year OS and PFS were 79% and 57%, respectively; however, the percentage of patients without recurrence with no treatment after a BV treatment regime is low, at approximately 15%. 13 Allogeneic HSCT, a curative treatment for relapsed or refractory ALCL, has a long‐term survival rate of approximately 50% in pediatric patients. 8 , 9 , 10 However, allogeneic HSCT can cause toxicity long after treatment, affecting quality of life, and can lead to high rates of treatment‐related mortality. If alectinib can be safely taken over a long time period, it could be used to induce remission as a bridging therapy before implementing allogeneic HSCT. As a result, alectinib could reduce treatment‐related mortality in allogeneic HSCT due to its favorable efficacy and safety, and could be expected to improve overall treatment outcomes.
The PK parameter tended to be slightly higher in cohort B (younger than 15 years on 600 mg/d) compared with cohorts A (younger than 15 years on 300 mg/d) and C (15 years or older on 600 mg/d). Although limited by the small number of patients in each cohort, the efficacy and safety of each cohort were comparable. Importantly, these PKs were similar to those of ALK‐positive NSCLC patients. 31 The peak concentration of alectinib in the patient who died following disease progression after the first course of alectinib in this study was extremely low (16.97 mg/mL). The low mean steady‐state peak concentration of alectinib in cohort C could be attributed to this patient. This patient had a lymphoma mass in the duodenum, and the possibility of obstruction was suspected, making it likely that this was the major contributor to the patient’s death, rather than alectinib. Molecular monitoring of NPM‐ALK expression will be critical to predict prognosis accurately. 22 , 23 , 24 In the current study, three patients were MRD‐positive (detected by PCR) at the start of alectinib treatment. All MRD‐positive patients who attained an objective response became MRD‐negative immediately after the first course of alectinib. This result suggests that alectinib can lead to deep remission even for MRD‐positive patients.
The current study is limited by the fact that the number of patients was small due to the rarity of this disease, and the monitoring duration was relatively short. The long‐term safety and efficacy of alectinib for relapsed or refractory ALK‐positive ALCL cannot be clarified; therefore, further observation is needed. Nevertheless, it should be noted that five patients (50%) have achieved durable remission of more than approximately 400 days (397‐925 days) by treatment with alectinib alone.
In conclusion, alectinib showed favorable clinical activity and was well tolerated in patients with ALK‐positive ALCL who had progressed on standard chemotherapy. Moreover, alectinib is expected to innovate the treatment strategy of ALK‐positive ALCL with minimum toxicity, including first‐line treatment.
CONFLICT OF INTEREST
Hirokazu Nagai has received honoraria from Bayer, Bristol‐Myers, Celgene, Chugai, Eisa, Janssen, Kyowa Kirin, MSD, Mundi, Novartis, Ono, Sanofi, SymBio, Takeda, and Zenyaku Kogyo, and received research funding from Bayer, Bristol‐Myers, Celgene, Chugai, Eisa, HUYA, IQVIA, Janssen, Kyowa Kirin, Mundi, Ono, Otsuka, Solasia, SymBio, Takeda, and Zenyaku Kogyo. The other authors have no conflict of interest.
Supporting information
Table S1‐S3
ACKNOWLEDGMENTS
This study was supported by the Ministry of Health, Labour and Welfare Grants No. 14533440, and Japan Agency for Medical Research and Development Grants No.15ck0106162h0001, 16ck0106162h0002, and 17ck0106162h0003. We thank the patients and their families, and the participating study teams for making this study possible. This study was partly presented at the 60th American Society of Hematology Annual Meeting, San Diego, CA, 1‐4 December 2018.
Fukano R, Mori T, Sekimizu M, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma: An open‐label phase II trial. Cancer Sci. 2020;111:4540–4547. 10.1111/cas.14671
This clinical trial was carried out at three institutions: National Hospital Organization Kyushu Cancer Center, 3‐1‐1 Notame, Minami‐ku, Fukuoka, Fukuoka 855‐1395, Japan; St. Marianna University School of Medicine Hospital, 2‐16‐1 Sugao, Kawasaki, Kanagawa 216‐8511, Japan; and National Hospital Organization Nagoya Medical Center, 4‐1‐1 Sannomaru, Naka‐ku, Nagoya, Aichi 460‐0001, Japan
Reiji Fukano, Tetsuya Mori, and Hirokazu Nagai contributed equally to this work.
Funding informationMinistry of Health, Labour and Welfare of Japan, Grant/Award Number: 14533440; Japan Agency for Medical Research and Development, Grant/Award Numbers: 15ck0106162h0001, 16ck0106162h0002, and 17ck0106162h0003.
REFERENCES
- 1. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560‐572. [DOI] [PubMed] [Google Scholar]
- 2. Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki‐1 in reactive and neoplastic lymphoid tissue: evidence that Reed‐Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848‐858. [PubMed] [Google Scholar]
- 3. Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 4. Stein H, Foss H‐D, Dürkop H, et al. CD301 anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681‐3695. [PubMed] [Google Scholar]
- 5. Brugières L, Le Deley MC, Rosolen A, et al. Impact of the Methotrexate Administration Dose on the Need for Intrathecal Treatment in Children and Adolescents With Anaplastic Large‐Cell Lymphoma: Results of a Randomized Trial of the EICNHL Group. J Clin Oncol. 2009;27(6):897‐903. [DOI] [PubMed] [Google Scholar]
- 6. Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high‐risk anaplastic large‐cell lymphoma: results of the randomized ALCL99‐vinblastine trial. J Clin Oncol. 2010;28(25):3987‐3993. [DOI] [PubMed] [Google Scholar]
- 7. Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93(11):3913‐3921. [PubMed] [Google Scholar]
- 8. Fukano R, Mori T, Kobayashi R, et al. Haematopoietic stem cell transplantation for relapsed or refractory anaplastic large cell lymphoma: a study of children and adolescents in Japan. Br J Haematol. 2015;168(4):557‐563. [DOI] [PubMed] [Google Scholar]
- 9. Gross TG, Hale GA, He W, et al. Hematopoietic stem cell transplantation for refractory or recurrent non‐Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16(2):223‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strullu M, Thomas C, Le Deley MC, et al. Hematopoietic stem cell transplantation in relapsed ALK+ anaplastic large cell lymphoma in children and adolescents: a study on behalf of the SFCE and SFGM‐TC. Bone Marrow Transplant. 2015;50(6):795‐801. [DOI] [PubMed] [Google Scholar]
- 11. Brugieres L, Pacquement H, Le Deley MC, et al. Single‐drug vinblastine as salvage treatment for refractory or relapsed anaplastic large‐cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27(30):5056‐5061. [DOI] [PubMed] [Google Scholar]
- 12. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN‐35) in patients with relapsed or refractory systemic anaplastic large‐cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190‐2196. [DOI] [PubMed] [Google Scholar]
- 13. Pro B, Advani R, Brice P, et al. Five‐year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709‐2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679‐690. [DOI] [PubMed] [Google Scholar]
- 15. Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large‐cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children's Oncology Group Study. J Clin Oncol. 2017;35(28):3215‐3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK‐rearranged advanced non‐small‐cell lung cancer (AF‐001JP study): a single‐arm, open‐label, phase 1–2 study. Lancet Oncol. 2013;14(7):590‐598. [DOI] [PubMed] [Google Scholar]
- 18. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK ‐positive non‐small‐cell lung cancer (J‐ALEX): an open‐label, randomised phase 3 trial. Lancet. 2017;390(10089):29‐39. [DOI] [PubMed] [Google Scholar]
- 19. Nagai H, Fukano R, Sekimizu M, et al. Phase II trial of CH5424802 (alectinib hydrochloride) for recurrent or refractory ALK‐positive anaplastic large cell lymphoma: study protocol for a non‐randomized non‐controlled trial. Nagoya J Med Sci. 2017;79:407‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579‐586. [DOI] [PubMed] [Google Scholar]
- 21. Heinig K, Miya K, Kamei T, et al. Bioanalysis of alectinib and metabolite M4 in human plasma, cross‐validation and impact on PK assessment. Bioanalysis. 2016;8(14):1465‐1479. [DOI] [PubMed] [Google Scholar]
- 22. Mussolin L, Pillon M, d'Amore ES, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005;19(9):1643‐1647. [DOI] [PubMed] [Google Scholar]
- 23. Damm‐Welk C, Busch K, Burkhardt B, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM‐ALK‐positive anaplastic large‐cell lymphoma. Blood. 2007;110(2):670‐677. [DOI] [PubMed] [Google Scholar]
- 24. Iijima‐Yamashita Y, Mori T, Nakazawa A, et al. Prognostic impact of minimal disseminated disease and immune response to NPM‐ALK in Japanese children with ALK‐positive anaplastic large cell lymphoma. Int J Hematol. 2018;107(2):244‐250. [DOI] [PubMed] [Google Scholar]
- 25. Attarbaschi A, Dworzak M, Steiner M, et al. Outcome of children with primary resistant or relapsed non‐Hodgkin lymphoma and mature B‐cell leukemia after intensive first‐line treatment: a population‐based analysis of the Austrian Cooperative Study Group. Pediatr Blood Cancer. 2005;44(1):70‐76. [DOI] [PubMed] [Google Scholar]
- 26. Mori T, Takimoto T, Katano N, et al. Recurrent childhood anaplastic large cell lymphoma: a retrospective analysis of registered cases in Japan. Br J Haematol. 2006;132(5):594‐597. [DOI] [PubMed] [Google Scholar]
- 27. Woessmann W, Zimmermann M, Lenhard M, et al. Relapsed or refractory anaplastic large‐cell lymphoma in children and adolescents after Berlin‐Frankfurt‐Muenster (BFM)‐type first‐line therapy: a BFM‐group study. J Clin Oncol. 2011;29(22):3065‐3071. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor OA, Pro B, Pinter‐Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T‐cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK‐positive non–small‐cell lung cancer. N Engl J Med. 2017;377(9):829‐838. [DOI] [PubMed] [Google Scholar]
- 30. Gambacorti‐Passerini C, Orlov S, Zhang L, et al. Long‐term effects of crizotinib in ALK‐positive tumors (excluding NSCLC): a phase 1b open‐label study. Am J Hematol. 2018;93(5):607‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hida T, Nakagawa K, Seto T, et al. Pharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non‐small‐cell lung cancer with or without prior crizotinib therapy. Cancer Sci. 2016;107(11):1642‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


