Abstract
Immune checkpoint inhibitors (ICIs) have dramatically changed the strategy used to treat patients with non‐small‐cell lung cancer (NSCLC); however, the vast majority of patients eventually develop progressive disease (PD) and acquire resistance to ICIs. Some patients experience oligoprogressive disease. Few retrospective studies have evaluated clinical efficacy in patients with oligometastatic progression who received local therapy after ICI treatment. We conducted a retrospective analysis of advanced NSCLC patients who received PD‐1 inhibitor monotherapy with nivolumab or pembrolizumab to evaluate the effects of ICIs on the patterns of progression and the efficacy of local therapy for oligoprogressive disease. Of the 307 patients treated with ICIs, 148 were evaluated in our study; 42 were treated with pembrolizumab, and 106 were treated with nivolumab. Thirty‐eight patients showed oligoprogression. Male sex, a lack of driver mutations, and smoking history were significantly correlated with the risk of oligoprogression. Primary lesions were most frequently detected at oligoprogression sites (15 patients), and 6 patients experienced abdominal lymph node (LN) oligoprogression. Four patients showed evidence of new abdominal LN oligometastases. There was no significant difference in overall survival (OS) between the local therapy group and the switch therapy group (reached vs. not reached, P = .456). We summarized clinical data on the response of oligoprogressive NSCLC to ICI therapy. The results may help to elucidate the causes of ICI resistance and indicate that the use of local therapy as the initial treatment in this setting is feasible treatment option.
Keywords: local therapy, nivolumab, oligometastasis, oligoprogression, pembrolizumab
This study showed the efficacy of local therapy for oligoprogressive disease after PD‐1 blockade in advanced non‐small‐cell lung cancer.

Abbreviations
- ALK
anaplastic lymphoma kinase
- AR
acquired resistance
- B2M
beta 2 microglobulin
- BRAF
B‐RAF proto‐oncogene, serine/threonine kinase
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- EORTC
European Organisation for Research and Treatment of Cancer Lung Cancer Group
- HE
hematoxylin‐eosin
- ICI
immune checkpoint inhibitor
- IHC
immunohistochemistry
- KRASv‐Ki‐ras2
Kirsten rat sarcoma viral oncogene homolog
- LAT
local ablative therapy
- LN
lymph node
- MET
mesenchymal‐to‐epithelial transition
- NSCLC
non‐small‐cell lung cancer
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed cell death 1
- PD‐L1
programmed cell death ligand 1
- PET
positron emission tomography
- PFS
progression‐free survival
- PS
performance status
- QOL
quality of life
- RESIST
Response Evaluation Criteria in Solid Tumors
- RFA
radiofrequency ablation
- SBRT
stereotactic body radiation therapy
- TTF
time to treatment failure
1. INTRODUCTION
A treatment strategy that blocks the pathway that involves PD‐1 and PD‐L1 proteins has shown marked efficacy in the treatment of patients with various kinds of solid tumors, especially NSCLC. 1 , 2 , 3 Among the anti‐PD‐1 protein antibodies, nivolumab resulted in significantly longer OS than docetaxel among patients with advanced NSCLC who showed disease progression after platinum‐based chemotherapy. 4 , 5 , 6 , 7 Pembrolizumab resulted in significantly longer PFS and OS than platinum‐based chemotherapy among patients with previously untreated advanced NSCLC and high PD‐L1 expression. 8 , 9 The anti‐PD‐L1 antibody atezolizumab has been shown to prolong OS in patients with previously treated advanced NSCLC. 10 The addition of pembrolizumab and atezolizumab to platinum‐based chemotherapy also improved PFS and OS among patients with advanced NSCLC who did not have driver gene alterations. 11 , 12 , 13 , 14 As described above, nivolumab, pembrolizumab, and atezolizumab have been approved as the standard treatment for advanced NSCLC. The TTF of these ICIs in patients with advanced NSCLC ranges from 12 to 25 mo, which is 2‐3 times greater than that of cytotoxic chemotherapy 4 , 5 , 15 , 16 and, in some cases, the tumor response has been reported to last for more than 5 y. 17 Moreover, ICI responders with NSCLC who experience prolonged PFS also experience prolonged OS. 18
In 1995, Hellman and Weichselbaum described the concept of the oligometastatic state, in which metastases are limited in number and location (which enables local radical treatment). 19 In some oligometastatic patients, it is possible that the LAT of all metastatic lesions may result in a substantial survival advantage and even a cure. After the concept of “oligometastasis” was first described in 2002, the first molecular targeting agent gefitinib was introduced into clinical practice with NSCLC patients, and multiple actionable oncogenic drivers and the corresponding targeted agents were identified. Most patients treated with these agents will experience some degree of tumor shrinkage, however almost all patients eventually experience tumor progression. Oligoprogressive disease describes the situation in which a patient develops disease progression in a limited number of sites after a targeted therapy has resulted in some degree of response. 20 Similar to that observed in an oligometastatic state, the LAT of lesions resulting in progression could contribute to an increase in disease control by targeted agents, and the results of several retrospective studies regarding the outcomes of patients who developed oligoprogressive disease have been reported. 21 , 22 , 23 , 24
Various local therapies have been used for the treatment of limited metastases, including surgery, SBRT, and RFA, and various factors, such as the site of metastasis and PS, determine which treatments are optimal. The most robust randomized data that are favorable toward the treatment of oligometastatic cancers are derived from patients with limited brain metastases. 25 Both surgery and SBRT are efficacious, but no large prospective trial has compared the 2 techniques in the treatment of oligometastases confined to the adrenals. 26 , 27 Radiotherapy‐induced cell death can be immunogenic via various mechanisms 28 , 29 , 30 , however the effects of radiotherapy on tumor immunogenicity are not well known, and this is an exciting area currently under study. There have also been few reports that have described the progression patterns in patients treated with ICIs and the clinical outcomes in the course of subsequent treatment, including local therapies.
In this study, we retrospectively reviewed the progression patterns resulting from treatment with ICIs according to different TTFs and evaluated the clinical outcomes in the course of treatment with subsequent local therapies.
2. MATERIALS AND METHODS
2.1. Patients and data analysis
At our institute, we retrospectively evaluated 307 patients treated with anti‐PD‐1 antibodies (nivolumab or pembrolizumab) between May 2013 and September 2018. Patients who were not evaluated according to the RECIST criteria or who had been previously treated with ICIs were excluded from the analysis. In total, 148 patients were included in this study, all of whom had progressed after treatment with ICIs. We analyzed detailed information regarding the progressive organ sites and lesions resulting from ICI progression. This retrospective study was approved by the Institutional Review Board of the Aichi Cancer Center (IRB number: 2018‐1‐205).
2.2. PD‐1 inhibitor treatment and response
Patients received at least 1 course of nivolumab (3 mg/kg every 2 wk) or pembrolizumab (2 mg/kg every 3 wk) as monotherapy. The ICI treatments were continued until patients developed PD or experienced unacceptable adverse events. In general, patients who underwent radiographic imaging 3 mo before the initiation of ICI treatment were evaluated for tumor response according to the RECIST, version 1.1. 31 In this study, oligoprogression and oligometastasis were defined as the presence of 1 progressive/metastatic organ site within 3 lesions after PD‐1 blockade, and oligometastasis was defined as the presence of 1 metastatic organ site within 3 lesions. Of the 3 to 6 lesions of oligoprogressive disease that have been reported, 32 , 33 , 34 we used the smallest number in this study. The imaging evaluations were performed mostly during the therapeutic period with CT scan, however in 9 of 10 cases (90.0%) of oligoprogression and oligometastasis the details of metastasis before or soon after LAT intervention were evaluated by PET‐CT.
2.3. Statistical analysis
Differences in the categorical variables were evaluated using chi‐square (χ2) test or Fisher exact test, as appropriate. PFS was measured from the start of ICI therapy (nivolumab or pembrolizumab) until the date of RECIST‐defined PD. OS was measured from the initiation of ICI treatment until death and was censored at the date of the last visit or telephone contact for patients whose death could not be confirmed. The survival probabilities were estimated using the Kaplan‐Meier method, in which differences in the variables were calculated using the log‐rank test. All reported P‐values are two‐sided and a P‐value < .05 was considered statistically significant unless stated otherwise. We analyzed the data using GraphPad Prism software version 7 for Windows (GraphPad Software Inc) or the JMP statistical software package for Windows version 9 (SAS Institute).
3. RESULTS
3.1. Patient clinicopathological characteristics
Baseline patient demographics and disease characteristics are listed in Table 1. The majority of patients (59%) had adenocarcinoma. Forty‐three patients (29%) had driver mutations, 23 patients harbored EGFR mutations, 7 patients had Kirsten rat sarcoma (KRAS) mutations, 4 patients had MET exon 14 skipping variants, 3 patients had ALK rearrangements, and 3 patients had v‐RAF murine sarcoma viral oncogene homolog B1 (BRAF) V600E mutations. The overall response rate was 22.3%. PFS and OS were 3.02 mo (95% CI: 2.67‐3.87) and 18.4 mo (95% CI: 15.2‐23.0), respectively (Figure 1A,B). Forty‐nine patients presented with the exacerbation of lesion(s) in a single organ at the time of PD, and among these 49 patients, 38 showed oligoprogression (Figure 2). Ten patients with oligoprogression underwent LAT, surgery, or radiation. After local therapy, 6 patients continued ICI therapy, and 2 patients received no systemic therapy. The percentages of male patients, patients with adenocarcinoma histology and patients who had never smoked were significantly greater in the non‐oligoprogression group than in the oligoprogression group. Patients who developed oligoprogression showed a higher response rate (52.6% vs. 11.8%, P < .0001) and a PFS of more than 6 mo (63.2% vs. 18.2%, P < .0001) compared with patients who did not develop oligoprogression. The oligoprogression group showed better PFS (median PFS 7.37 mo vs. 2.50 mo, P < .0001) and OS (median OS; not reached vs. 12.94 mo, P < .0001) than the non‐oligoprogression group (Figure 3).
TABLE 1.
Characteristics of patients who received ICI treatment (n = 148)
| n |
Oligo n = 38 |
Non‐oligo n = 110 | P‐value | |
|---|---|---|---|---|
| Age, median (range) | 65 (37‐87) | 68 (48‐87) | 64 (37‐82) | |
| Sex, female/male | 41/107 | 5/33 | 36/74 | .021 |
| ECOG performance status, 0/1/2 | 52/91/5 | 17/20/1 | 35/71/4 | .35 |
| Driver mutation | ||||
| Positive (%) | 43 a (29%) | 8(21%) | 35 (32%) | .300 |
| Histology | ||||
| Ad/Sq/other | 87/41/20 | 15/15/8 | 72/26/12 | .019 |
| Smoking history | ||||
| Yes/No | 118/30 | 35/3 | 83/27 | .034 |
| Stage | ||||
| Postoperative recurrence/III/IV | 16/29/103 | 6/6/26 | 10/23/77 | .458 |
| ICI treatment | ||||
| Nivolumab/Pembrolizumab | 106/42 | 25/13 | 81/29 | .406 |
| ICI line | ||||
| 1/2/3 or later | 20/76/52 | 9/20/9 | 11/56/43 | .054 |
| Response to PD‐1 blockade | ||||
| PR | 33 | 20 | 13 | |
| SD | 20 | 7 | 13 | |
| PD | 95 | 11 | 84 | |
| ORR | 22.3% | 52.6% | 11.8% | <.0001 |
| PFS | ||||
| Within 6 mo/over 6 mo | 104/44 | 14/24 | 90/20 | <.0001 |
Abbreviations: Ad, adenocarcinoma; ECOG, Eastern Cooperative Group; ICI, immune checkpoint inhibitor; Non‐oligo, non‐oligoprogression; Oligo, oligoprogression; ORR, objective response rate; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; Sq, squamous cell carcinoma.
Major driver gene alterations were not evaluated in 4 patients because of the presence of pure squamous cell carcinoma histology.
FIGURE 1.
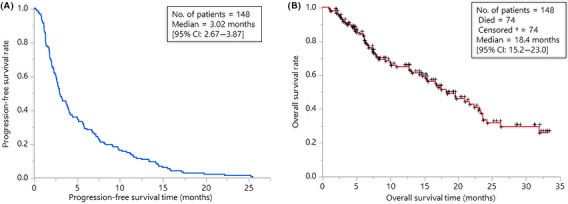
Kaplan‐Meier estimates of progression‐free survival (A) and overall survival (B) among the patients treated with ICIs in our cohort (n = 148) are shown
FIGURE 2.
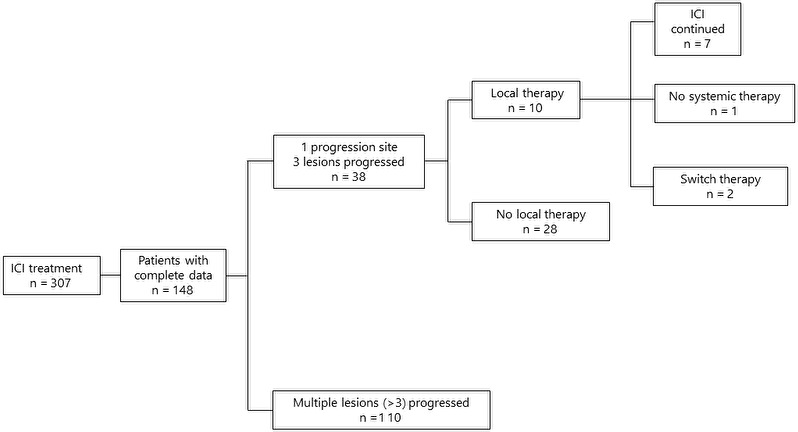
Analytical flowchart of this study
FIGURE 3.
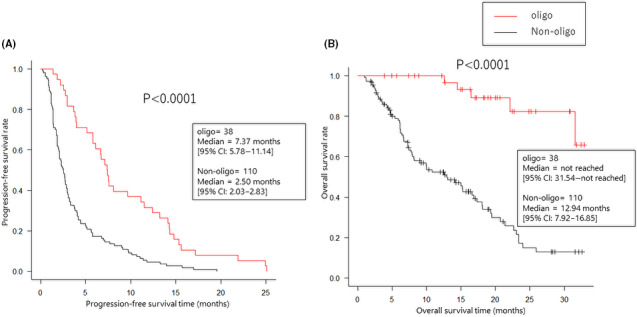
Kaplan‐Meier estimates of progression‐free survival and overall survival among the patients with and without oligoprogression or oligometastasis
3.2. Oligoprogression and oligometastasis sites in organs
The sites of oligoprogression and oligometastasis are shown in Figure 4. Primary lung lesions (15 patients) were the most common site for oligoprogression; 6 patients experienced abdominal LN oligoprogression, and among these 6 patients, 4 showed evidence of new abdominal LN oligometastasis without other PD sites.
FIGURE 4.
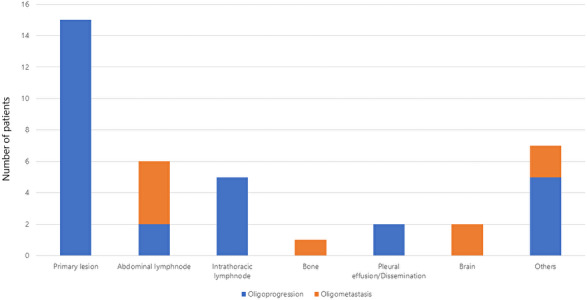
Details of oligoprogressive (blue) and oligometastatic (orange) sites
3.3. Local therapy for oligoprogression and oligometastasis
The characteristics of patients who received or did not receive local therapy for oligoprogression/oligometastasis are shown in Table 2. The details of the clinical course used are shown in Figures 5 and S1, and Tables 3 and S1. There was no difference in characteristics between these 2 groups. Among the 38 patients with oligoprogression, 10 received definitive local therapy at the oligoprogressive site (Figure 5). Six patients continued ICI treatment after progression, 2 patients receive another systemic therapy, and 2 patients had no further systemic treatment. Six of the 10 patients maintained a progression‐free status for at least 3 mo; among these 6 patients, 3 received local therapy at the abdominal LNs. The timing to next therapy after LAT was similar in each patient. There was no difference in OS between the groups that received and did not receive local therapy (Figure 6).
TABLE 2.
Characteristics of patients with oligoprogression or oligometastasis who received ICI treatment (n = 38)
|
Received LAT n = 10 |
Did not receive LAT n = 28 |
P‐value | |
|---|---|---|---|
| Age, median (range) | 63.5 (53‐78) | 70.0 (48‐87) | |
| Sex, female/male | 1/9 | 4/24 | 1.00 |
|
ECOG P performance status, 0/1/2 |
3/7/0 | 14/13/1 | .475 |
| Driver mutation | |||
| Positive (%) | 1 (10%) | 5 (18%) | 1.00 |
| Histology | |||
| Ad/Sq/other | 2/4/4 | 13/11/4 | .197 |
| Smoking history | |||
| Yes/No | 9/1 | 26/2 | 1.00 |
| Stage | |||
| Postoperative recurrence/III/IV | 3/1/6 | 3/5/20 | .521 |
| ICI treatment | |||
| Nivolumab/Pembrolizumab | 8/2 | 17/11 | .406 |
| ICI line | |||
| 1/2/3 or later | 1/7/2 | 8/13/7 | .499 |
Abbreviations: Ad, adenocarcinoma; ECOG, Eastern Cooperative Group; ICI, immune checkpoint inhibitor; LAT, local ablative therapy; Non‐oligo, non‐oligoprogression; Oligo, oligoprogression; PFS, progression‐free survival; Sq, squamous cell carcinoma.
Major driver gene alterations were not evaluated in 5 patients because of the presence of pure squamous cell carcinoma histology.
FIGURE 5.
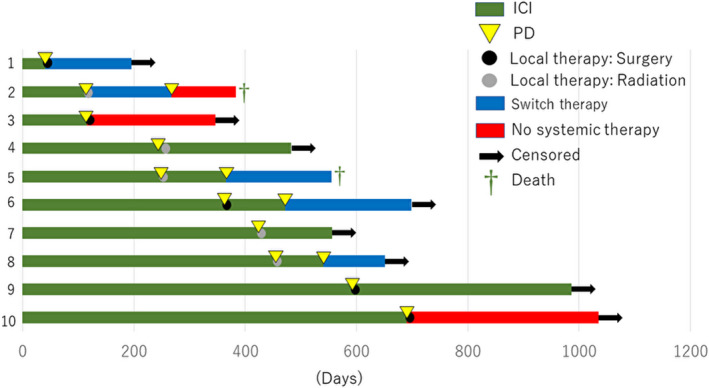
Development and management of acquired resistance to ICIs. Swimmer plot showing the time to resistance after the initial response to ICIs and local therapy among 10 patients. Each bar represents 1 patient
TABLE 3.
Clinical outcomes in patients who showed oligoprogressive disease during ICI therapy and who underwent local ablative therapy
| No. | Age |
Sex |
ICI | Histology | Major driver mutation | BI | Treatment lines | PFS (mo) | Progression sites | LAT | Days to next therapy after LAT | Subsequent therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | PB | NOS | None | 720 | 1 | 1.4 | Abdominal LN | Ope | 33 | CBDCA + nabPTX |
| 2 | 58 | M | NV | Sq | None | 760 | 2 | 3.7 | Bone | Rad | 28 | DTX |
| 3 | 61 | M | NV | Pleo | None | 800 | 2 | 3.9 | Abdominal LN | Ope | (no therapy) | No systemic therapy |
| 4 | 53 | M | PB | Sarco | None | 600 | 3 | 7.5 | Gallbladder | Rad | 14 | PB beyond PD |
| 5 | 72 | M | NV | Sq | None | 0 | 2 | 8.2 | Abdominal LN | Rad | 1 | NV beyond PD |
| 6 | 58 | M | NV | LCNEC | None | 760 | 2 | 12.6 | Adrenal gland | Ope | 16 | NV beyond PD |
| 7 | 74 | M | NV | Ad | None | 675 | 2 | 14.3 | Brain | Rad | 10 | NV beyond PD |
| 8 | 78 | M | NV | Sq | None | 1050 | 4 | 14.5 | Abdominal LN | Rad | 64 | NV beyond PD |
| 9 | 72 | M | NV | Sq | None | 820 | 2 | 15.8 | Abdominal LN | Ope | 34 | NV beyond PD |
| 10 | 66 | F | NV | Ad | EGFR Exon 20 insertion | 840 | 2 | 22.2 | Chest wall LN | Ope | (no therapy) | No systemic therapy |
Abbreviations: Ad, adenocarcinoma; BI, Brinkman index; CBDCA, carboplatin; DTX, docetaxel; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; LAT, local ablative therapy; LCNEC, large cell neuroendocrine carcinoma; LN, lymph node; nabPTX, nab‐paclitaxel; NOS, not otherwise specified; NV, nivolumab; Ope, operation; PB, pembrolizumab; PD, progressive disease; PFS, progression‐free survival; Pleo, pleomorphic carcinoma; Rad, radiation; Sarco, sarcomatoid carcinoma; Sq, squamous cell carcinoma.
FIGURE 6.
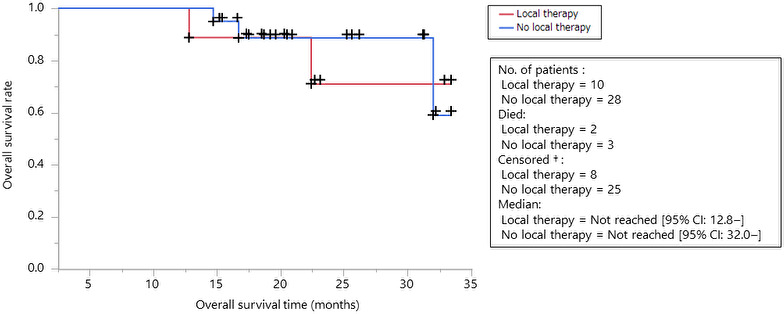
Kaplan‐Meier estimates of progression‐free survival among the patients with oligoprogression or oligometastasis according to local therapy intervention are shown (red: with local therapy, blue: without local therapy)
3.4. PD‐L1 expression in oligometastatic LNs
PD‐L1, PD‐1, and HE IHC are shown in Figure 7A,B. Four patients showed evidence of 3 new abdominal LN oligometastases and 1 new chest LN oligometastasis, which were surgically resected. No considerable change was observed in PD‐L1 IHC before and after treatment with ICIs. Tumor cells spread throughout the whole LN, and PD‐L1‐positive cells seemed to localize within the cortex more than the medulla. PD‐1‐positive T cells seemed to be relatively sparsely distributed in all patients.
FIGURE 7.
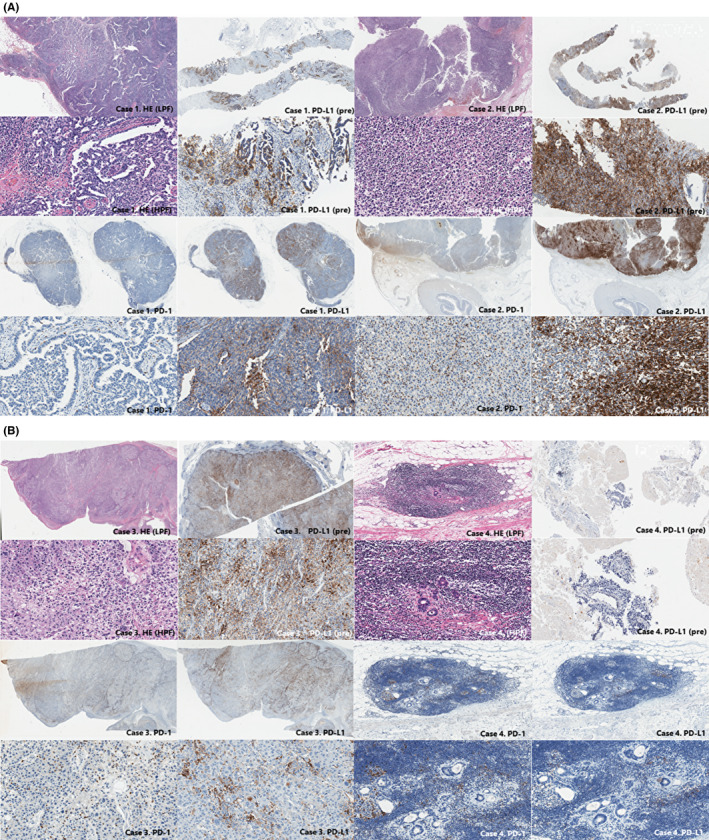
Histological sections of resected oligoprogressive lymph nodes with hematoxylin and eosin staining and findings on immunostaining for PD‐1 and PD‐L1 in (A) cases 1 and 2, and (B) cases 3 and 4. The patient number is the same as Table 3. Findings on immunostaining for PD‐L1 is also shown before treatment with ICIs (PD‐L1 IHC of cases 2 and 3 were performed with clone 22C3.). PD‐L1 TPS is high before and after treatment in cases 1, 2, and 3, negative in case 4. Infiltrating PD‐1 positive lymphocyte are sparsely distributed throughout the tumor
4. DISCUSSION
Most patients with advanced NSCLC, and who are treated with systemic therapy, relapse at a site of preexisting disease. 35 Even after ICI treatment, oligoprogression is observed in existing lesions, including the primary lesion. Regarding cytotoxic chemotherapy, some data suggest that the larger the tumor deposits, the more likely it is that resistant clones will remain and that local control of the lesion will be more beneficial. 36 Oligoprogressive disease is believed to be the result of the development of an isolated resistant subclone that usually occurs only at a few metastatic sites. 21 Theoretically, disease control is attained by the LAT of newly developed oligometastatic sites before the systemic dissemination of resistant clones occurs. In addition to the elimination of resistant subclones, LAT is also beneficial in terms of the prevention of local symptoms and complications from a growing tumor. For example, the LAT of abdominal LN metastases can prevent further neuropathic pain due to the invasion of the peritoneal plexus by metastatic cancer cells. 37 As a consequence, currently, the most common method used to treat oligoprogression in NSCLC patients with driver mutations is to continue targeted therapy to suppress the growth of the sensitive clone and to use LAT to eliminate the lesion containing the resistant clone. 38
Some patients treated by immunotherapy show a marked and durable response, similar to that observed in patients with driver mutations treated with suitable targeted agents. Gettinger S et al reported the results of a retrospective analysis regarding the clinical characteristics and outcomes of patients who acquired resistance to PD‐1 axis inhibitors. 17 Of the 26 patients who were evaluated, 15 received LAT, and the median survival time after AR was not reached in these 15 patients, who had a 2‐y survival rate of 92%. Eleven patients continued PD‐1 axis inhibitor therapy after local therapy. In our study, 6 of 10 patients in an oligoprogressive state after ICI therapy were treated with a course of therapy, and successful results were obtained; this finding supports the initial use of LAT except in patients in whom widespread dissemination of the resistant clone has occurred.
To obtain robust conclusions regarding the clinical benefit of LAT for patients who show oligoprogressive disease during ICI therapy, a formal statistical analysis with established endpoints (eg, OS or PFS) is required, however this is impossible in any retrospective setting, including our study, because of the extremely heterogeneous background of the evaluated patients. 21 Recently, the idea that local therapy can be used to treat oligoprogressive disease has received increasing attention from the European Organisation for Research and Treatment of Cancer Lung Cancer Group (EORTC). 39 It is also difficult to perform prospective clinical trials under these circumstances. There is no consensus concerning the maximum tolerated number of courses of LAT, the optimal timing, and regimen of subsequent systemic therapy, the necessity of the distinction between brain and nonbrain progression, and the independent protocol to be used to determine the positive/negative population for each driver mutation. Currently, LAT in the oligoprogressive state during ICI therapy could be considered in selected patients, and practitioners should pay attention to the following 3 points. First, careful patient selection, including intensive clinical tumor staging (including PET‐CT), is essential before treatment. Second, to shorten the interruption of ICI treatment, suitable treatment modalities should be selected. Finally, a careful and close follow‐up of treated patients is strictly required.
Another notable observation in our cohort was that most patients experienced oligoprogressive disease; 56% of patients had a primary site of progression, 21% had LN progression and oligometastatic disease, and 44% had LN metastases. Similar findings were also observed in 26 patients with NSCLC who developed AR to PD‐1 axis inhibitor therapy. Gettinger S et al reported that 11 (70%) patients experienced LN‐only progression after treatment with a PD‐1 axis inhibitor. 17 Previously, it was not reported that the primary site was the most frequent lesion site that AR to ICIs. The presence of liver and lung metastases was reported to be an independent predictor of nivolumab efficacy in patients with advanced NSCLC. 17 In this report, heterogeneity in PD‐L1 expression and heterogeneity in the genetic profiles of primary and metastatic sites may have led to AR to ICIs in metastatic lesions. The mechanisms underlying the development of AR to ICIs in NSCLC remain largely unknown. Several of the possible mechanisms of resistance include the upregulation of alternate immune checkpoints, 17 defects in interferon signaling pathways, 40 the enhanced expression of IL‐34, 41 the loss of B2M, which prevents the presentation of tumor antigens by major histocompatibility complex class I, 42 loss‐of‐function B2M, JAK1 and JAK2 mutations resulting in insensitivity to interferon gamma, 43 and somatic neoantigen loss that compromises immune recognition. 44 However, a definitive understanding of the mechanism involved in AR has remained elusive.
In our study of patients with AR, similar findings to those of a previous report were observed, 17 which included a tendency for the development of AR within LNs in the case of both oligometastasis and oligoprogression. A potential hypothesis explaining this finding can be offered. Several receptors, including LFA‐1 and S1PR1, enable naïve CD8 T cells to enter the LN to search for dendritic cells expressing tumor‐specific antigens. When naïve CD8 T cells differentiate into activated effector CD8 T cells, they undergo a dramatic shift in the expression of surface proteins that regulate cellular trafficking. Importantly, effector CD8 T cells lose the expression of both CD62L and CCR7, and prevents these cells from gaining access to LNs through high endothelial venules. 45 In other words, it may be inherently difficult for tumor‐specific CD8 T cells to effectively maintain anatomical localization to secondary LNs (especially sentinel LNs) due to this mechanism.
Among the patients with oligometastases, 4 with abdominal LN metastases had abdominal organ metastases (3 liver and 1 adrenal) before ICI treatment. These LN metastatic lesions showed high PD‐L1 expression; according to the proposed hypothesis that heterogeneity in PD‐L1 expression may be a possible contributor to resistance, it is difficult to explain this result. 46 PD‐1‐positive lymphocyte infiltration appears to have been decreased in an evaluated patient, which may support the explanation that effector CD8 T cells have difficulty infiltrating secondary LNs. In addition, it can be speculated that oligoprogressive and oligometastatic lesions in the central nervous system (CNS) and the pleural fluid may be related to the low penetration of the CNS by ICIs and the fact that this environment is difficult for ICIs to navigate, however this is merely speculation.
This study has limitations. The greatest limitations include the small sample size and the retrospective nature of the study, which prevents the comparison of our findings to nonregional or pretreatment results and causes a selection bias for LAT. A larger prospective study with strict enrollment criteria is urgently needed to overcome these limitations. Specifically, it is necessary to evaluate whether local treatment may improve QOL and prevent complications and to verify whether similar progressive patterns can be reproduced in another larger cohort. It is also important to strictly define the criteria used for the local treatment of these lesions based on definitive results.
In this study, we summarized the clinical data regarding oligoprogressive NSCLC during ICI therapy and showed that the use of LAT as the initial treatment in this setting is a feasible treatment option.
DISCLOSURE STATEMENT
Dr. Niimi has a financial relationship as a lecturer with Eli Lily Inc, Novartis Pharma Inc, AstraZeneca Inc, MSD Inc, Ono Pharmaceutical Industries, Kyorin Pharmaceutical Inc, Daiichi Sankyo Inc, Boehringer Ingelheim Inc, Kyowa Hakko Kirin Inc, Taiho Pharmaceutical Inc, GlaxoSmithKline, and Sanofi Inc, and has received grants from Eli Lily Inc and Novartis Pharma Inc. Dr. Hida has received grants from Pfizer, MSD, Kissei, AstraZeneca, Clovis Oncology, Novartis, Ono Pharmaceutical Co., Ignyta, Merck Serono, Daiichi Sankyo, Nippon Boehringer Ingelheim, Eisai, Kyowa Hakko Kirin, Taiho Pharmaceutical Co., Bristol‐Meyers Squibb, Chugai Pharmaceutical Co., Astellas, AbbVie, Takeda Pharmaceutical Co., and Dainippon Sumitomo Pharma. Other authors (than Dr. Niimi and Dr. Hida) have no conflict of interest.
Supporting information
Figure S1
Table S1
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of all patients included in this study. We would also like to express our deepest appreciation to Dr. Yasushi Yatabe for his sincere efforts and guidance in obtaining the pathological findings. The authors wish to thank Miss Nanae Ueda for performing immunohistochemistry of PD‐1 and PD‐L1.
Kagawa Y, Furuta H, Uemura T, et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non‐small cell lung cancer. Cancer Sci. 2020;111:4442–4452. 10.1111/cas.14605
Yusuke Kagawa, Hiromi Furuta, Katsuhiro Masago and Toyoaki Hida Authors contributed equally to this work.
REFERENCES
- 1. Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res. 2014;2:846‐856. [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non‐small‐cell lung cancer previously treated with platinum‐containing chemotherapy regimens. The TAX 320 Non‐Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354‐2362. [DOI] [PubMed] [Google Scholar]
- 7. Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non‐small‐cell lung cancer previously treated with platinum‐based chemotherapy. J Clin Oncol. 2000;18:2095‐2103. [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 10. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 12. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379:2040‐2051. [DOI] [PubMed] [Google Scholar]
- 13. Horn L, Mansfield AS, Szczesna A, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379:2220‐2229. [DOI] [PubMed] [Google Scholar]
- 14. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288‐2301. [DOI] [PubMed] [Google Scholar]
- 15. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): a phase 2, single‐arm trial. Lancet Oncol. 2015;16:257‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gettinger S, Horn L, Jackman D, et al. Five‐Year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: results from the CA209‐003 study. J Clin Oncol. 2018;36:1675‐1684. [DOI] [PubMed] [Google Scholar]
- 18. Shukuya T, Mori K, Amann JM, et al. Relationship between overall survival and response or progression‐free survival in advanced non‐small cell lung cancer patients treated with anti‐PD‐1/PD‐L1 antibodies. J Thorac Oncol. 2016;11:1927‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8‐10. [DOI] [PubMed] [Google Scholar]
- 20. Campo M, Al‐Halabi H, Khandekar M, Shaw AT, Sequist LV, Willers H. Integration of stereotactic body radiation therapy with tyrosine kinase inhibitors in stage IV oncogene‐driven lung cancer. Oncologist. 2016;21:964‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laurie SA, Banerji S, Blais N, et al. Canadian consensus: oligoprogressive, pseudoprogressive, and oligometastatic non‐small‐cell lung cancer. Curr Oncol. 2019;26:e81‐e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non‐small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197‐203. [DOI] [PubMed] [Google Scholar]
- 23. Petty WJ, Urbanic JJ, Ahmed T, et al. Long‐term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102:527‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene‐addicted non‐small‐cell lung cancer. J Thorac Oncol. 2012;7:1807‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bansal P, Osman D, Gan GN, Simon GR, Boumber Y. Recent advances in targetable therapeutics in metastatic non‐squamous NSCLC. Front Oncol. 2016;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non‐small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001;71:981‐985. [DOI] [PubMed] [Google Scholar]
- 27. Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non‐small cell lung cancer. Strahlenther Onkol. 2011;187:245‐251. [DOI] [PubMed] [Google Scholar]
- 28. Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsumura S, Wang B, Kawashima N, et al. Radiation‐induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation‐induced IFN‐gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132‐3139. [DOI] [PubMed] [Google Scholar]
- 31. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 32. Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non‐small‐cell lung cancer without progression after first‐line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non‐small‐cell lung cancer. Clin Lung Cancer. 2014;15:346‐355. [DOI] [PubMed] [Google Scholar]
- 34. Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non‐small‐cell lung cancer. J Clin Oncol. 2014;32:3824‐3830. [DOI] [PubMed] [Google Scholar]
- 35. Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first‐line systemic therapy for metastatic lung cancer? A patterns‐of‐failure analysis. Acta Oncol. 2009;48:578‐583. [DOI] [PubMed] [Google Scholar]
- 36. Strom HH, Bremnes RM, Sundstrom SH, Helbekkmo N, Flotten O, Aasebo U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non‐small‐cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109:1467‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karyagar S, Koc ZP, Karyagar SS, et al. Abdominal lymph node metastasis in patients with non‐small‐cell lung cancer as shown by PET/CT. Clin Nucl Med. 2013;38:691‐694. [DOI] [PubMed] [Google Scholar]
- 38. NCCN Clinical Practice Guidelines in Oncology . Advanced/Metastatic NSCLC (Stage IV) (NSCL‐C 5 of 10). https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=1536.
- 39. Levy A, Hendriks LEL, Berghmans T, et al. EORTC lung cancer group survey on the definition of NSCLC synchronous oligometastatic disease. Eur J Cancer. 2019;122:109‐114. [DOI] [PubMed] [Google Scholar]
- 40. Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447‐3453. [DOI] [PubMed] [Google Scholar]
- 41. Han N, Baghdadi M, Ishikawa K, et al. Enhanced IL‐34 expression in Nivolumab‐resistant metastatic melanoma. Inflamm Regen. 2018;38:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gettinger S, Choi J, Hastings K, et al. Impaired HLA Class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7:1420‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaretsky JM, Garcia‐Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med. 2016;375:819‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non‐small cell lung cancer. Cancer Discov. 2017;7:264‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nolz JC, Starbeck‐Miller GR, Harty JT. Naive, effector and memory CD8 T‐cell trafficking: parallels and distinctions. Immunotherapy. 2011;3:1223‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamiya M, Tamiya A, Inoue T, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non‐small cell lung cancer: A retrospective multicenter trial. PLoS One. 2018;13:e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


