Abstract
Prolonged hypersecretion of catecholamine induced by chronic stress may correlate with malignant progression of cancer. β2‐adrenergic receptor (β2‐AR) overexpressed in certain cancer cells may translate the signals from neuroendocrine system to malignant signals by interacting with oncoproteins, such as Her2. In the present study, we demonstrate that catecholamine stimulation activates the expression and proteolytic activity of ADAM10 by modulating the expression of miR‐199a‐5p and SIRT1 and also confirm that catecholamine induction triggers the activities of γ‐secretase, leading to shedding of Her2 extracellular domain (ECD) by ADAM10 and subsequent intramembranous cleavage of Her2 intracellular domain (ICD) by presenilin‐dependent γ‐secretase, nuclear translocation of Her2 ICD, and enhanced transcription of tumor metastasis‐associated gene COX‐2. Chronic stimulation of catecholamine strongly promotes the invasive activities of cancer cells in vitro and spontaneous tumor lung metastasis in mice. Furthermore, nuclear localization of Her2 was significantly correlated with overexpression of β2‐AR in human breast cancer tissues, indicating that catecholamine‐induced β2‐AR activation plays decisive roles in tumor metastasis. Our data also reveal that an unknown mechanism by which the regulated intramembrane proteolysis (RIP) initiated by β2‐AR‐mediated signaling controls a novel Her2‐mediated signaling transduction.
Keywords: a disintegrin and metalloprotease 10, Her2, metastasis, β2‐adrenergic receptor, γ‐secretase
In the present study, we demonstrated that catecholamine stimulation activates the expression and proteolytic activity of ADAM10 by modulating the expression of miR‐199a‐5p and SIRT1 and also confirm that catecholamine induction triggers the activities of γ‐secretase, leading to shedding of Her2 ECD by ADAM10 and subsequent intramembranous cleavage of Her2 ICD by presenilin‐dependent γ‐secretase, nuclear translocation of Her2 ICD, and enhanced transcription of tumor metastasis‐associated gene COX‐2. Chronic stimulation of catecholamine strongly promotes the invasive activities of cancer cells in vitro and spontaneous tumor lung metastasis in mice. Furthermore, the nuclear localization of Her2 was significantly correlated with overexpression of β2‐adrenergic receptor (β2‐AR) in human breast cancer tissues, indicating that catecholamine‐induced β2‐AR activation plays decisive roles in tumor metastasis.

Abbreviations
- ADAM10
a disintegrin and metalloprotease 10
- CTF
C‐terminal fragment
- DAPT
N‐[N‐(3,5‐difluorophenacetyl)‐l‐alanyl]‐S‐phenylglycine t‐butyl ester
- ECD
extracellular domain
- EGFR
epidermal growth factor receptor
- ICD
intracellular domain
- IgG
immunoglobulin G
- ISO
isoproterenol
- p95Her2
Her2 fragment with a molecular weight of 95 kDa
- RIP
regulated intramembrane proteolysis
- RTK
receptor tyrosine kinases
- RT‐PCR
reverse transcription‐PCR
- β2‐AR
β2‐adrenergic receptor
1. INTRODUCTION
β2‐Adrenergic receptor (β2‐AR)‐mediated signaling can amplify multiple receptor‐signaling pathways. 1 , 2 , 3 Crosstalk of the cell signaling pathways mediated by β2‐AR and receptor tyrosine kinases (RTK) may promote a stronger or more sustained biological response in a variety of target tissues. 4 , 5 , 6 , 7 Our previous study disclosed a positive feedback loop comprised of β2‐AR and Her2: the chronic stimulation of catecholamine induced β2‐AR‐mediated upregulation of Her2 expression, whereas Her2 overexpression promoted autocrine release of catecholamine, leading to the elevation of β2‐AR levels. 8 Several lines of evidence have indicated that the prolonged hypersecretion of catecholamine induced by chronic stress may correlate with higher occurrence of malignancies in various organs 9 , 10 , 11 , 12 , 13 ; β2‐AR overexpressed in certain cancer cells may translate the signals from the neuroendocrine system to malignant signals by interacting with oncoproteins such as Her2. 8 , 14 , 15 However, the molecular mechanisms underlying cross‐communication between β2‐AR and Her2‐mediated signaling pathways are largely unexplored.
Receptor localization plays a crucial role in gathering paracrine signals from adjacent cells. 16 , 17 Unlike EGFR, which constantly shuttles and recycles through the cell, Her2 as a member of EGFR family is a highly internalization‐resistant receptor and primarily resides on the plasma membrane of epithelial cells, although its nuclear localization has been documented. 18 , 19 , 20 Nuclear translocation of Her2 was proposed to be related to endocytic internalization in a full‐length form and mediated by a conventional nuclear importing system associated with the nuclear pore complex. 21 However, there has been contradictory evidence regarding the nuclear localization of Her2. 16 , 17 It was also reported that soluble Her2 CTF synthesized by alternative initiation of translation was located in the nucleus. 22 In accordance with this study, we observed that the Her2 intracellular domain (ICD), but not full‐length Her2, was exclusively distributed in the nucleus. 23 Correspondingly, a nuclear localization signal was identified within the sequences of Her2 ICD. It is known that Her2 extracellular domain (ECD) can be separated proteolytically from full‐length Her2 and detected in cultural medium of human breast cancer cell line SKBR3, as well as in sera from patients with breast cancer. 24 The occurrence of Her2 ECD is a marker for production of the N‐terminally truncated and membrane‐associated Her2 fragment with a molecular weight of 95 kDa (p95Her2), which possesses constitutive ligand‐independent activity and enhanced transforming efficiency. 25 , 26 The elevation of soluble Her2 ECD levels in sera from patients has been correlated with recurrence, nodal metastasis, worse prognosis, and poor response to hormone therapy, chemotherapy, and targeted therapy in clinical studies. 24 , 27 However, the molecular mechanisms whereby Her2 is cleaved are poorly understood.
2. MATERIALS AND METHODS
2.1. Cell culture and treatment
MCF‐7, BT474, MDA‐453, and SKOV3 cells were obtained from the American Type Culture Collection. MCF‐7/Her2 cells stably overexpressing Her2 were established in our laboratory as described previously. 28 For treatment with β‐AR agonists and antagonist, the cells were incubated overnight in serum‐free medium and then treated with 10 μmol/L epinephrine, 10 μmol/L norepinephrine, 5 or 10 μmol/L isoproterenol (ISO), 0.5 or 2 μmol/L ICI‐118551, 1 or 5 μmol/L DAPT or 1 μmol/L Atenolol (ATEN) for the indicated time points. For treatment with γ‐secretase antagonist, the cells were treated with 0.5 or 2 μmol/L L685458.
2.2. Western blot
The whole‐cell lysates were separated by SDS‐PAGE and transferred to polyvinylidene difluoride membranes (Millipore). The membranes were probed with the primary antibodies against ADAM10 (Abcam), the C‐terminus and N‐terminus of Her2 (Cell Signaling), phosphorylated Her2 (Thermo) and phosphorylated Akt, GFP, presenilin 1, presenilin 2, nicastrin, PEN2, COX‐2, Na/K‐ATPase, histone H3, glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), β‐tubulin (Cell Signaling) and SIRT1 (Santa Cruz). Bands were visualized using the Enhanced Chemiluminescence System (Amersham Pharmacia Biotech). All experiments were performed in duplicate.
2.3. In vitro γ‐secretase assay
In vitro γ‐secretase activities were measured as described previously. 29 Cells were resuspended in lysis buffer and lysed by passing through a 30‐gage needle attached to a 1‐mL syringe. Membrane pellets were incubated at 37°C for 2 h in 50 μL of assay reaction buffer (pH 6.5) containing 12 μmol/L specific fluorogenic substrate (Calbiochem). Fluorescence was measured using a SpectraMax M5 spectrometer (Molecular Devices). The experiments were performed in duplicate.
2.4. Preparation of nuclear extracts and oligonucleotide pull‐down assays
The 5′‐biotinylated double‐stranded oligonucleotides (5′‐ATAAACTTCAAATTTCAGTA‐3′) corresponding to the positions −1770 to −1750 of the COX‐2 promoter were synthesized by Invitrogen Biotechnology. The same sequences but not biotinylated were used as the competitors. Biotinylated oligonucleotides containing the mutated Her2 binding sequences (5′‐ATAAACTGACCCGGGAAGTA‐3′), in which conserved nucleotides were replaced, and sequences lacking the Her2 binding motif (5′‐ATAAACTTCAAATTTCAGTA‐3′) were also synthesized. SKOV3 cells were treated with 5 μmol/L ISO for 0 or 9 h under serum‐free conditions after overnight starvation. Nuclear extracts were prepared using a Nuclear‐Cytosol Extraction Kit (Applygen Technologies); 200 μg of the nuclear extracts were incubated at 4°C for 4 h with each pair of oligonucleotides previously coupled to Dynabeads M‐280 (Invitrogen). The protein/DNA complexes were separated with a Dynal magnet. Binding of Her2 was detected by western blotting with the antibodies against N‐terminus or C‐terminus of Her2. The experiments were performed in duplicate.
2.5. In vivo tumor model
All animal experiments were carried out in accordance with the approval of the Animal Research Committee of Xuzhou Medical University. Five‐ to 6‐wk‐old athymic female BALB/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. A total of 0.1 mL of the cell suspension (108 cells/mL) was injected sc into the right upper flanks of the mice. After tumor cell injection for 4 d, the mice were treated daily ip with PBS or ISO (10 mg/kg). Each group contained 8 mice. At 60 d following tumor implantation, mice were sacrificed. Primary tumors were dissected, weighed, and fixed in formalin. The lungs of the mice were autopsied, fixed, and photographed.
To evaluate the role of γ‐secretase in adrenergic signaling‐triggered tumor metastasis, SKOV3 cells (8 × 105/mouse) were injected intravenously via the tail vein, into NCG mice that were purchased from GemPharmatech Co., Ltd. Then the mice were treated daily with ISO (10 mg/kg, ip), celecoxib (COX‐2 inhibitor, 5 mg/kg, ip), or LY411,575 (γ‐secretase inhibitor, 1 mg/kg, po). Each group contained 5 mice. At 2 wk later, the mice were sacrificed and H&E staining was performed on the dissected lung tissue.
2.6. Clinical samples
All clinical tissue samples were obtained from the Affiliated Hospital of Xuzhou Medical University with the informed consent of patients and with approval for experiments from the Hospital.
2.7. Statistical analysis
Data were expressed as mean ± SD. For comparisons among the groups in the experiments, an ANOVA test was performed. For evaluation of consistency between β2‐AR expression and Her2 nuclear localization in the tumor tissues, Kappa coefficients were calculated. A P‐value < .05 was considered statistically significant.
3. RESULTS
3.1. Catecholamine stimulation induces Her2 CTF production and phosphorylation
When we treated the human breast cancer cell line BT474 with β‐AR agonist isoproterenol (ISO), an extra ~80 kDa molecular mass accumulated in a time‐dependent manner, as detected by western blot with the antibody against the C‐terminus of Her2 (Figure 1A). A similar result was also obtained in the human ovarian cancer cell line SKOV3 (Figure 1B), suggesting that the 80 kDa fragment may represent Her2 CTF and appearance of the 80 kDa Her2 fragment (p80Her2) was associated with ISO stimulation. To determine whether the generation of p80Her2 was mediated by activation of β‐AR, we used β‐AR activators including ISO (5 μmol/L) and naturally occurring catecholamines, epinephrine (10 μmol/L) and norepinephrine (10 μmol/L), to treat the human breast cancer cell line MCF‐7. As shown in Figure 1C, levels of both full‐length Her2 and p80Her2 were markedly increased. Pretreatment with the specific inhibitor of β2‐AR ICI 118551 (1 μmol/L) strikingly impaired the effect of ISO on the formation of p80Her2, whereas the specific inhibitor of β1‐AR ATEN (1 μmol/L) had only a marginal effect (Figure 1D), indicating that activation of β2‐AR is a prerequisite for the generation of p80Her2.
FIGURE 1.
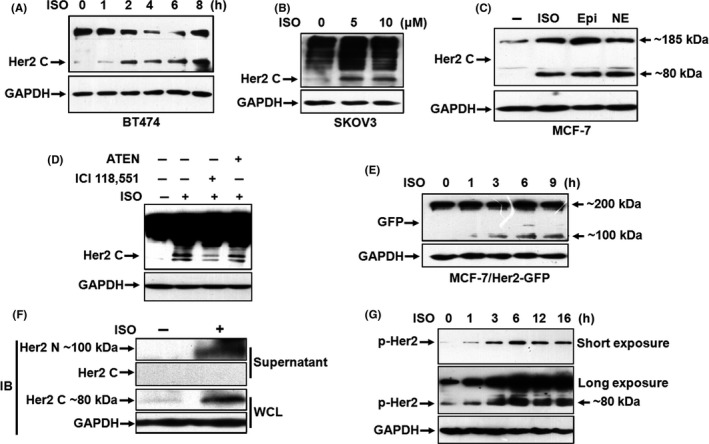
Catecholamine stimulation induces cleavage and phosphorylation of Her2. A, BT474 cells were starved overnight and then treated with 5 μmol/L isoproterenol (ISO) for 0, 1, 2, 4, 6 or 8 h. Expression of Her2 was analyzed by western blot with the antibodies against the C‐terminus of Her2. Equal loading was verified by detection of GAPDH. B, SKOV3 cells were treated with 0, 5 or 10 μmol/L ISO for 6 h. C, MCF‐7 cells were treated with 5 μmol/L ISO, 10 μmol/L epinephrine (Epi) or 10 μmol/L norepinephrine (NE). The arrows on the right indicate molecular weight of the bands. D, MCF‐7 cells were pretreated with 1 μmol/L ICI‐118551 or 1 μmol/L ATEN for 1 h and then incubated with 5 μmol/L ISO. E, MCF‐7/Her2‐GFP cells were treated with 5 μmol/L ISO for 0, 1, 3, 6 or 9 h. Western blot was performed by using the antibody against GFP. The arrows on the right indicate molecular weight of the bands. F, The supernatant and whole‐cell lysates of SKOV3 cells treated with 5 μmol/L ISO were collected and the fragments of Her2 detected by the antibodies against the N‐terminus and C‐terminus of Her2. G, SKOV3 cells were treated with ISO for 0, 1, 2, 6, 12 or 16 h. Phosphorylation of Her2 was detected by western blot
p95Her2 was assumed to contain the transmembrane and cytoplasmic domains. 24 To clarify whether p80Her2 had derived from Her2 CTF, we constructed MCF‐7 cells expressing Her2‐GFP fusion protein (MCF‐7/Her2‐GFP). Western blot analysis with the antibody against GFP showed that a new product, whose size perfectly fitted the molecular weight of p80Her2‐GFP fusion protein (~100 kDa), appeared after exposure of the cells to ISO (Figure 1E), testifying that the p80Her2 fragment was a product of Her2 CTF. We examined soluble Her2 ECD in the cultural supernatant of SKOV3 cells by using an antibody against the N‐terminus of Her2. Concomitant with markedly increased p80Her2 in the whole‐cell lysates, an c. 100 kDa protein was clearly detected in the cultural supernatant of the cells (Figure 1F), implying that catecholamine stimulation induced cleavage of Her2. Moreover, the phosphorylation of both full‐length Her2 and p80Her2 was prominently enhanced in a time‐dependent manner, indicating a rapid posttranslational modification of Her2 following ISO stimulation (Figure 1G).
3.2. Catecholamine modulates the cleavage of Her2 ECD by promoting ADAM10 expression through downregulation of miR‐199a‐5p and upregulation of SIRT1
Ectodomain cleavage of the transmembrane proteins is generally mediated by membrane‐associated metalloproteases under the regulation of multiple signaling pathways such as the activation of PKC. 30 , 31 Earlier studies have shown that Her2 ECD shedding could be suppressed by the broad‐spectrum metalloprotease inhibitors TNF Protease Inhibitor (TAPI), batimastat, and the tissue inhibitor of metalloproteases‐1. 24 A previous study utilizing siRNAs selectively inhibiting ADAM10 expression suggested that ADAM10 may be one of the proteases responsible for Her2 cleavage. 32 However, shedding of Her2 was inefficient in contrast with the majority of shedding events. In addition, how sheddase is controlled under physiological conditions is unclear. To test whether ADAM10 was engaged in catecholamine‐induced Her2 ECD cleavage, we examined the expression of ADAM10 in MCF‐7 cells stably transfected with an Her2 expression plasmid (MCF‐7/Her2). We found that ISO stimulation induced a significant upregulation of ADAM10 expression, which was obviously coherent with the accumulation of p80Her2 (Figure 2A). A recent study indicated that the NAD‐dependent deacetylase SIRT1 regulates the transcription of the gene encoding ADAM10 by direct interaction with the ADAM10 promoter. 33 Data in Figure 2B show that epinephrine stimulation dramatically promoted the expression of SIRT1 in a time‐dependent manner in MDA453 and SKOV3 cells. SIRT1 was recently identified as a direct target of miR‐199a‐5p. 34 Interestingly, expression of miR‐199a‐5p was strikingly repressed in SKOV3 and MDA453 cells treated with epinephrine, as determined by real‐time RT‐PCR analysis (Figure 2C). An experimental study demonstrated that β2‐AR can activate an antiapoptotic signal through Gi‐dependent coupling to phosphatidylinositol 3′‐kinase/Akt pathway and that activated Akt is sufficient for inducing downregulation of miR‐199a‐5p in cardiac myocytes. 35 We noticed that phosphorylation of Akt was significantly enhanced by epinephrine stimulation, accompanied by reduction in miR‐199a‐5p levels in MDA453 and SKOV3 cells (Figure 2B,C). Furthermore, knocking down ADAM10 expression by the siRNA, whose specificity and efficacy were verified in breast cancer cells by the previous study, 32 greatly inhibited the epinephrine‐induced shedding of Her2 (Figure 2D), implying that catecholamine modulates cleavage of Her2 ECD by promoting ADAM10 expression through downregulation of miR‐199a‐5p and thus upregulation of SIRT1. It is known that stimulation of β2‐AR with the agonists leads to shedding of heparin‐binding EGF‐like growth factor by ADAM17, which is also the major sheddase for Her3 and Her4, 36 , 37 and subsequent activation of EGFR in an autocrine/paracrine manner. 4 , 5 The findings in this study suggested that ADAM10 activities induced by catecholamine stimulation mediate cleavage of Her2 tyrosine kinase.
FIGURE 2.
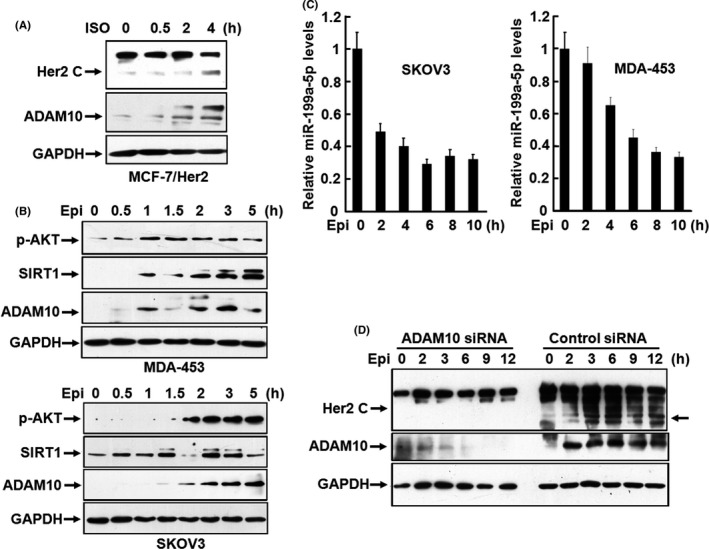
Catecholamine induces the cleavage of Her2 ECD by promoting ADAM10 expression. A, MCF‐7/Her2 cells were treated with 5 μmol/L isoproterenol (ISO) for 0, 0.5, 2 or 4 h. Expression of Her2 and ADAM10 was analyzed by western blot with the antibodies against the C‐terminus of Her2 and ADAM10. B, MDA‐453 and SKOV3 cells were treated with 10 μmol/L of epinephrine (Epi) for 0, 0.5, 1, 1.5, 2, 3, 5 or 7 h. Phosphorylated Akt, SIRT1, and ADAM10 were analyzed by western blot. C, SKOV3 and MDA453 cells were treated with 10 μmol/L of epinephrine for 0, 2, 4, 6, 8 or 10 h. The expression of miR‐199a‐5p was analyzed by real‐time PCR. D, SKOV3 cells were transfected with the specific siRNA targeting ADAM10 or control siRNA. After transfection for 48 h, the cells were treated with 10 μmol/L of epinephrine for 0, 2, 3, 6, 9 or 12 h and the expression of Her2 and ADAM10 was detected by western blot with the antibodies against the C‐terminus of Her2 and ADAM10. The arrow on the right indicates molecular weight of the bands
3.3. γ‐Secretase activity induced by catecholamine stimulation is responsible for the generation of p80Her2 ICD
Generation of CTFs from a transmembrane receptor involves cleavage within the transmembrane domain by γ‐secretase‐catalyzed proteolytic processing, whereas the activity of γ‐secretase is proposed to be regulated by the ADAM‐mediated ECD cleavage of transmembrane receptors. 38 Soluble Her4 ICD is produced by the sequential activities of ADAM‐17 and γ‐secretase after binding of its ligand heregulin or activation of protein kinase C (PKC) by 12‐O‐tetradecanoylphorbol‐13‐acetate. 39 However, proteolytic cleavage of Her2 by γ‐secretase has not been reported. As mentioned previously, p80Her2, the product of Her2 cleavage triggered by catecholamine stimulation, is somehow smaller compared with p95Her2. It was recently found that activation of β2‐AR provoked γ‐secretase activity in HEK293 cells. 40 We speculated that membrane‐associated Her2 ICD may undergo a secondary cleavage after shedding of Her2 ECD, possibly by γ‐secretase. Expression of γ‐secretase components, presenilin 1 (PS1), PS2, nicastrin, and PEN‐2 was found in MCF‐7, MCF‐7/Her2 and SKOV3 cells (Figure 3A). When we treated MCF‐7 and SKOV3 cells with ISO, γ‐secretase activities, determined by fluorogenic substrate assay, were prominently elevated in both cell lines (Figure 3B,C). From comparison of the known γ‐secretase substrates CD44, Notch, and E‐cadherin for homology of the amino acid sequences of their transmembrane domains Her2 did not share high homology with these sequences. However, these transmembrane domains, including that of Her2, frequently harbor several valine residues, some of which have been identified as potential γ‐secretase cleavage sites (Figure 3D). To investigate the role of γ‐secretase in Her2 intramembranous processing, we utilized the selective γ‐secretase inhibitor L685458 and a dipeptidic γ‐secretase specific inhibitor N‐[N‐(3,5‐difluorophenacetyl)‐l‐alanyl]‐S‐phenylglycine t‐butyl ester (DAPT). Pretreatment with L685458 or DAPT significantly blocked the production of p80Her2 in a concentration‐dependent manner (Figure 3E). The polytopic membrane proteins PS1 and PS2 are suggested to be the catalytic components of an active γ‐secretase complex. We employed specific siRNAs targeting PS1 and PS2 to transfect SKOV3 cells. Knock‐down of either PS1 or PS2 alone, or both, extraordinarily prevented the formation of p80Her2 (Figures 3F and S1), demonstrating that γ‐secretase activity induced by catecholamine stimulation is responsible for the generation of p80Her2 ICD.
FIGURE 3.
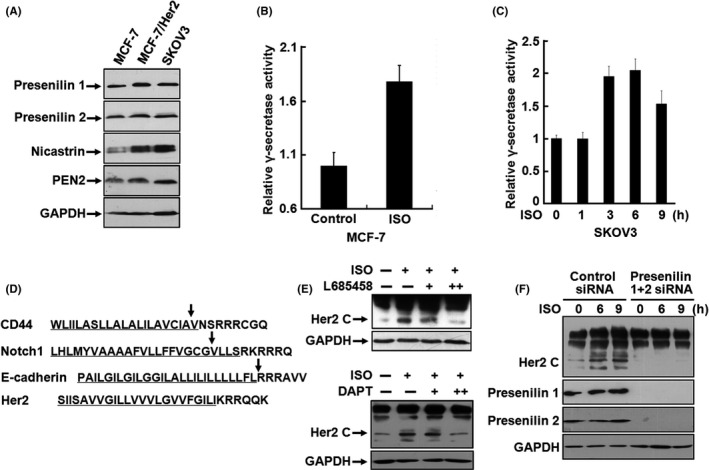
γ‐Secretase activity induced by catecholamine stimulation is responsible for the generation of p80Her2 intracellular domain (ICD). A, Expression of presenilin 1, presenilin 2, nicastrin, and PEN‐2 in MCF‐7, MCF‐7/Her2 and SKOV3 cells was analyzed by western blot. B, Cell lysates from MCF‐7 cells treated with 5 μmol/L ISO were prepared and γ‐secretase activities measured. C, SKOV3 cells were treated with 5 μmol/L of ISO for 0, 1, 3, 6 or 9 h. The activities of γ‐secretase were measured. D, Comparison of the known γ‐secretase substrates CD44, Notch and E‐cadherin with Her2 for homology of the amino acid sequences of the transmembrane domain (underlined sequences). The arrows indicated the identified cleavage sites. E, SKOV3 cells were pretreated with 0.5 and 2 μmol/L L685458 or 1 and 5 μmol/L DAPT and then incubated with 5 μmol/L ISO. Expression of Her2 was analyzed by western blot. F, SKOV3 cells were transfected with siRNA targeting presenilin 1 and presenilin 2. After transfection for 48 h, the cells were treated with 5 μmol/L isoproterenol (ISO) for 0, 6 or 9 h and the expression of Her2, presenilin 1, and presenilin 2 was detected by western blot
3.4. Catecholamine stimulation mediates nuclear translocation of Her2 ICD efficiently
Nuclear translocation of Her2 as a full‐length molecule was investigated in certain cell lines. 18 In contrast with other Her family members, the nuclear entry of Her2 was much less efficient. The regulatory mechanisms of nuclear translocation of full‐length Her2 receptor under physiological conditions remain elusive. To illustrate the subcellular distribution of Her2, we traced intracellular trafficking of GFP‐tagged Her2 after treatment of MCF‐7/Her2‐GFP cells with ISO. In unstimulated cells, ectopic overexpressed Her2‐GFP was defined at the cytoplasmic membrane (Figure 4A). No evidence for Her2‐GFP in the nucleus was found. However, in the presence of ISO, nuclear Her2 was readily visualized (Figure 4A). By immunofluorescence using the monoclonal antibodies against both C‐ and N‐terminus of Her2, we observed that Her2 molecules were predominantly located at the cytoplasmic membrane and no substantial nuclear Her2 could be detected with either antibody in unstimulated SKBR3 breast cancer cells (Figure 4B,C). The data are consistent with several previous observations that Her2 did not localize to the nucleus of several breast cancer cell lines spontaneously. 16 However, after treatment with ISO for 60‐90 min, Her2 hugely migrated into the nuclei (Figure 4B). Notably, nuclear Her2 could be easily detected by an antibody against the C‐terminus of Her2 (Figure 4B) but not by an antibody against the N‐terminus of Her2 (Figure 4C). Consistent with these data, p80Her2 was detected in the nuclei of ISO‐simulated SKOV3, MCF‐7/Her2‐GFP, and SKBR3 cells by cellular fractionation and western blotting (Figure 4D), but not in unstimulated cells, suggesting that Her2 ICD was liberated by γ‐secretase cleavage and entered the nuclei. Moreover, the features for uniform distribution of nuclear Her2 were distinguishingly different from those in the previous observation that Her2 in that the nuclear form appeared as discrete punctate spots under conventional culture conditions. Altogether, these results indicated that catecholamine stimulation mediates nuclear translocation of Her2 ICD efficiently.
FIGURE 4.
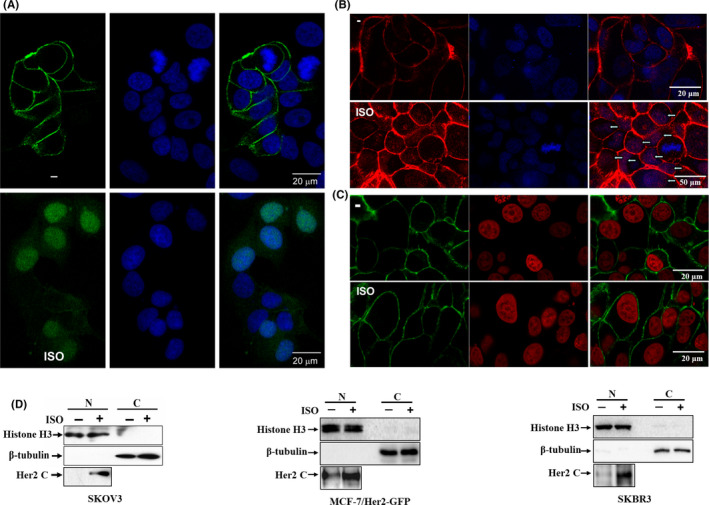
Catecholamine stimulation mediates nuclear translocation of Her2 intracellular domain (ICD). A, MCF‐7/Her2‐GFP cells were treated with 5 μmol/L of isoproterenol (ISO). The subcellular localization of Her2‐GFP fusion protein was observed under a confocal microscope. The nuclei were stained with 1 μg/mL DAPI. Bar = 20 μm B, SKBR3 cells were labeled with the antibody against the C‐terminus of Her2. Binding was detected with Alexa fluor 549 (Red)‐labeled secondary antibody. The arrows indicate the nuclei staining. Nuclei were also stained with DAPI. Bar = 20 μm (upper panel) or 50 μm (lower panel). C, SKBR3 cells were incubated with the antibody against N‐terminus of Her2 and binding was detected with Alexa fluor 488 (green)‐labeled secondary antibody. Nuclei were stained with propidium iodide (PI). Bar = 20 μm. D, SKOV3, MCF‐7/Her2 and SKOV3 cells were treated with 5 μmol/L of ISO for 0 or 9 h after overnight starvation. The nuclear extracts were prepared using a Nuclear‐Cytosol Extraction Kit. The expression of Her2 was analyzed by the antibody against the C‐terminus of Her2. Detection of histone H3 and β‐tubulin was used as the indicators of nuclear and cytoplasmic proteins
3.5. Her2 ICD physically binds to the promoter of COX2 gene and drives transactivation of COX2 gene
Sequential cleavage of transmembrane receptors can rapidly transform membrane‐associated proteins into soluble effectors, which enter the nucleus and regulate the transcription of their target genes. 31 To determine the functional significance of p80Her2 in the nucleus, we isolated the nuclear proteins from SKOV3 cells and performed oligonucleotide pull‐down assay. A previous study identified the Her2‐associated sequence (HAS), which was located at 1750 nucleotides upstream from the transcriptional initiation site in COX‐2, a known target gene of Her2. 18 We utilized the sequence as an oligonucleotide probe. By oligonucleotide pull‐down assay we could reproducibly detect the association of p80Her2 with oligonucleotide probes containing the HAS sequence in ISO‐simulated cells. No specific band was detected in the range from ~130 to 250 kDa (data not shown). Identical western blots with the mutated HAS sequence or nonspecific oligonucleotides exhibited no specific signal (Figure 5A). In addition, binding of p80Her2 with the HAS sequence was strongly impaired or completely abolished in a dose‐dependent manner by specific competitors. Furthermore, the expression of COX‐2 at both mRNA and protein levels was markedly upregulated by ISO stimulation (Figure 5B,C). These data provide further evidence to confirm that under catecholamine stimulation Her2 ICD migrates into the nucleus, physically binds to the promoter of the COX2 gene and drives transactivation of the COX2 gene.
FIGURE 5.
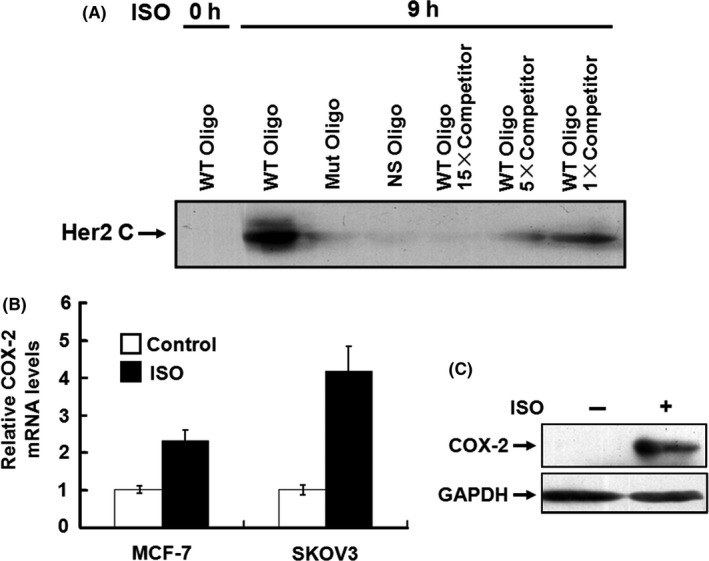
Her2 intracellular domain (ICD) physically binds to the promoter of COX2 gene and drives transactivation of COX2 gene. A, 200 μg of the nuclear extracts was incubated with the 5′‐biotinylated double‐stranded oligonucleotides (5′‐ATAAACTTCAAATTTCAGTA‐3′) corresponding to the positions −1770 to −1750 of the COX‐2 promoter previously coupled to Dynabeads M‐280. The protein/DNA complexes were separated with a Dynal magnet, denatured in SDS sample buffer and subjected to SDS‐PAGE. Binding of Her2 was detected by western blot with the antibodies against N‐terminus or C‐terminus of Her2. The same double‐stranded sequences that are not biotinylated were used as the competitors. The biotinylated oligonucleotides containing the mutated Her2 binding sequences (5′‐ATAAACTGACCCGGGAAGTA‐3′) and the sequences lacking the Her2 binding motif (5′‐ATAAACTTCAAATTTCAGTA‐3′) were used as the controls. B, C, MCF‐7 and SKOV3 cells were treated with 5 μmol/L of isoproterenol (ISO). The expression of COX‐2 at the mRNA and protein levels was detected by real‐time RT‐PCR (B) and western blot (C)
3.6. Catecholamine stimulation strongly promotes the invasive activities of cancer cells in vitro and spontaneous tumor lung metastasis in mice
In an effort to determine the effects of catecholamine stimulation on the biological behavior of tumor cells and relevance of Her2 nuclear localization in tumor development and metastasis, we investigated whether catecholamine stimulation conferred proliferation and invasion potential to Her2‐overexpressing tumor cells in a human ovarian cancer xenograft model. Treatment with ISO daily did not accelerate tumor growth in mice when compared with the control group (Figure S2). However, ISO stimulation significantly promoted the invasive capacity of SKOV3 cells in in vitro invasion assay using a Matrigel invasion chamber and caused spontaneous lung metastasis in nude mice (Figures 6A,B and S3). Metastatic colonization was observed by thorough examination and microscopic inspection of tissue sections (Figures 6B and S3). Surprisingly, nuclear staining of Her2 was mainly observed in metastatic tumor tissues by immunohistochemical labeling with an antibody against the C‐terminus of Her2 (Figure 6C), whereas nuclear Her2 was rarely seen in primary tumors (Figure S4). Moreover, using an antibody against the N‐terminus of Her2, nuclear Her2 could not be detected, but only membrane‐anchored Her2 was signaled (Figure 6C).
FIGURE 6.
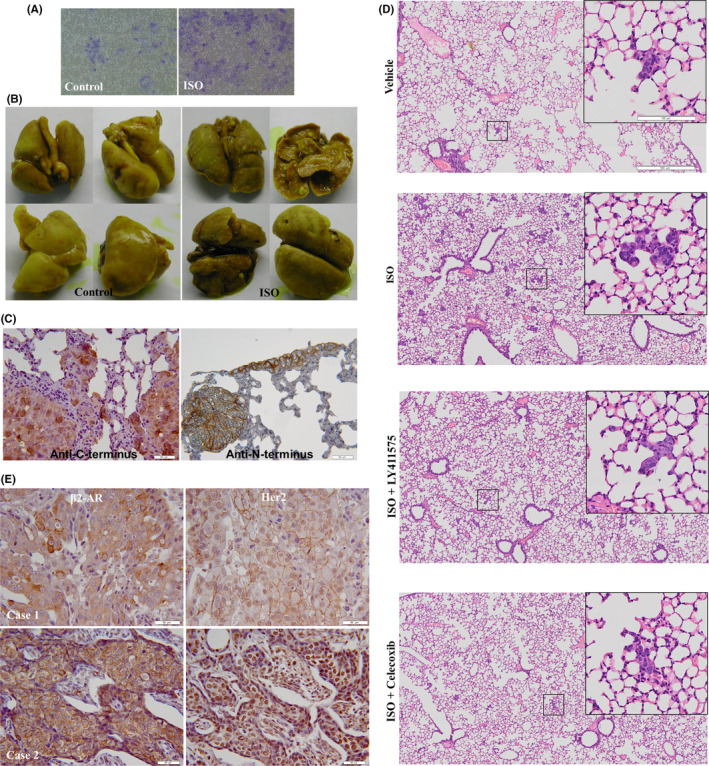
Catecholamine stimulation promotes the invasive and metastatic potentials of tumor cells. A, SKOV3 cells were chronically stimulated by 5 μmol/L isoproterenol (ISO) and invasive activities of the cells assessed by a cell invasion assay kit. The invasive cells were fixed and stained. B, A total of 0.1 mL of the cell suspension (108 cells/mL) was injected sc in the right upper flank of 5‐ to 6‐wk‐old athymic female BALB/c nude mice. After tumor cell injection for 4 d, the mice were treated daily ip with PBS or ISO (10 mg/kg). Eight mice were used in each group. At 60 d following tumor implantation, mice were sacrificed. The lungs of the mice were autopsied, fixed, and photographed. C, Lung metastatic tumors were dissected. Paraffin‐embedded tissue sections were stained with rabbit monoclonal antibodies against N‐terminus and C‐terminus of Her2. D, SKOV3 cells (8 × 105/mouse) were injected intravenously into NCG mice via the tail vein, and then the mice were treated daily with ISO (10 mg/kg, ip), celecoxib (COX‐2 inhibitor, 5 mg/kg, ip) or LY411,575 (γ‐secretase inhibitor, 1 mg/kg, po). Each group contained 5 mice. At 2 wk later, the mice were sacrificed and H&E staining was performed in the dissected lung tissue. E, Expression of β2‐AR and Her2 in breast cancer tissues was analyzed by immunohistochemistry with the antibodies against β2‐AR and C‐terminus of Her2. Bar = 50 μm
To explore the role of γ‐secretase and COX‐2 in adrenergic signaling‐triggered tumor metastasis, SKOV3 cells were injected intravenously into NCG mice via the tail vein. Then mice were treated with ISO, celecoxib, or LY411,575. Figure 6D shows that ISO treatment promoted lung metastasis of the cancer cells. Blockade of γ‐secretase or COX‐2 inhibited the enhancement of tumor metastasis triggered by ISO stimulation, suggesting the critical role of γ‐secretase and COX‐2 in adrenergic signaling‐mediated tumor metastasis (Figure 6D).
To gain further insight into correlation of Her2 nuclear localization with β2‐AR expression, we examined the expression of both Her2 and β2‐AR in 55 Her2‐positive human breast cancer tissues. In tissues that expressed relatively low levels of β2‐AR, Her2 molecules were distributed predominantly at the cytoplasmic membrane (Figure 6E, case 1). Nevertheless, in tissues expressing high levels of β2‐AR, nuclear Her2 was strongly positive (Figure 6E, case 2). In 62.6% of tissues with strong immunoreactivity for anti‐β2‐AR antibody (20/32), nuclear Her2 was positive. Only 13% of tissues that expressed low levels of β2‐AR (3/23) displayed positive staining for nuclear Her2 (Table S1). The difference between the 2 groups was highly significant (P < .0002). However, the kappa coefficient for β2‐AR expression and Her2 nuclear localization was moderate (0.46), suggesting that other molecular mechanisms may be involved in nuclear translocation of Her2. Simultaneous staining of β2‐AR and nuclear Her2 was also observed in human ovarian cancer tissues (Figure S5). These data demonstrated that nuclear localization of Her2 is intimately associated with overexpression of β2‐AR.
4. DISCUSSION
The present study demonstrated that catecholamine‐induced β2‐AR activation triggers shedding of Her2 ECD by ADAM10 and subsequent intramembranous cleavage of Her2 ICD by presenilin‐dependent γ‐secretase, resulting in nuclear translocation of p80Her2 and enhanced transcription of target genes (Figure 7). RIP has been highlighted as a novel mode of proteolysis‐dependent signal transduction. It is known that RIP is well controlled process and induced by specific agonists and intracellular signaling pathways. 41 , 42 The ADAMs (a disintegrin and metalloproteinases) as key sheddases for transmembrane receptors are now emerging as signaling scissors in diverse signal transduction pathways. The expression of ADAMs is not constitutive, but highly inducible and sensitive to stimuli from the microenvironment. Many of the ADAM substrates involved in signaling events are dysregulated in cancers or during tumor progression. 31 , 43 Our data confirmed that catecholamine stimulation activates the expression and proteolytic activity of ADAM10 by modulating the expression of miR‐199a‐5p and SIRT1 and that catecholamine induction triggers the activities of γ‐secretase, demonstrating that catecholamine‐induced β2‐AR activation plays decisive roles in cleavage and nuclear translocation of Her2 under physiological and pathological conditions.
FIGURE 7.
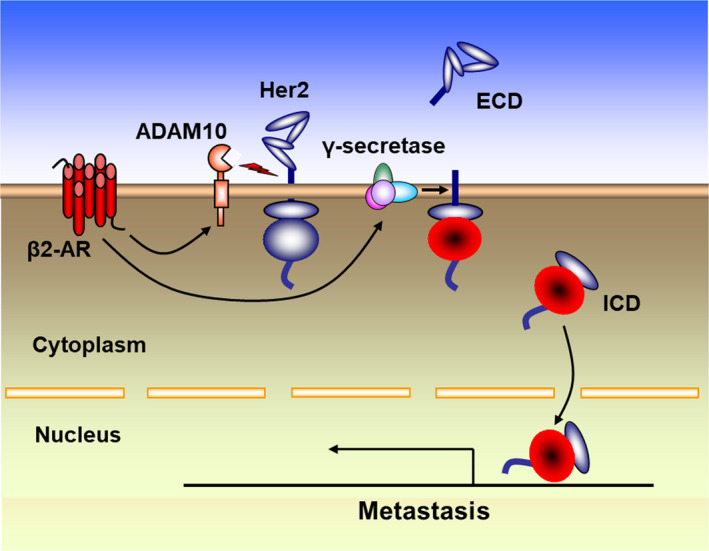
Molecular mechanism of cleavage and nuclear translocation of Her2. Catecholamine‐induced β2‐AR activation triggers shedding of Her2 extracellular domain (ECD) by ADAM10 and subsequent intramembranous cleavage of Her2 intracellular domain (ICD) by presenilin‐dependent γ‐secretase, resulting in nuclear translocation of p80Her2 and enhanced transcription of target genes
Interestingly, a recent phase II clinical trial showed that β‐blockade with propranolol reduced biomarkers of metastasis in breast cancer. 44 Our study showed that catecholamine stimulation strongly promoted the invasive activities of cancer cells in vitro and spontaneous tumor lung metastasis in mice. We noticed that the nuclear localization of Her2 was conspicuous in metastatic lung tissues. Furthermore, overexpression of β2‐AR significantly correlated with Her2 nuclear localization in human breast cancer tissues, implying the clinical significance of the crosstalk between β2‐AR and Her2. It is conceivable that ADAM10 and γ‐secretase shedding of Her2, subsequent activation of the PI3K‐Akt pathway, nuclear translocation of Her2 ICD, and ultimate transactivation of tumor metastasis‐associated genes may account for the metastatic progression in breast cancer. Nuclear Her2 and β2‐AR levels may be important markers for the progression and metastasis of Her2‐overexpressing cancers. Understanding the signaling characteristics of crosstalk between β2‐AR and oncoproteins, such as Her2, may shed a new light on complex signaling networks of human malignancy and improve the current paradigm of personalized cancer therapy.
DISCLOSURE
The authors declare that there are no potential conflicts of interest.
Supporting information
Fig S1‐5
Table S1
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Nos. 81972719, 81773258 and 81773086), Jiangsu Natural Science Foundation (No. BK20171161), Key University Science Research Project of Jiangsu Province (No. 17KJA320009), Key Research & Development Plan of Jiangsu Province (BE2018634), Key Young Talents in Medicine of Jiangsu Province (No. QNRC2016803), and Jiangsu Province Innovation and Entrepreneurship Talents Project.
Liu D, Zha L, Liu Y, et al. β2‐AR activation promotes cleavage and nuclear translocation of Her2 and metastatic potential of cancer cells. Cancer Sci. 2020;111:4417–4428. 10.1111/cas.14676
Contributor Information
Junnian Zheng, Email: jnzheng@xzhmu.edu.cn.
Ming Shi, Email: sm200@sohu.com.
References
- 1. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939‐944. [DOI] [PubMed] [Google Scholar]
- 2. Sood AK, Armaiz‐Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui B, Luo Y, Tian P, et al. Stress‐induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem‐like cells. J Clin Invest. 2019;129:1030‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noma T, Lemaire A, Naga Prasad SV, et al. Beta‐arrestin‐mediated beta1‐adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445‐2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engelhardt S. Alternative signaling: cardiomyocyte beta1‐adrenergic receptors signal through EGFRs. J Clin Invest. 2007;117:2396‐2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luttrell LM, Ferguson SS, Daaka Y, et al. Beta‐arrestin‐dependent formation of beta2 adrenergic receptor‐Src protein kinase complexes. Science. 1999;283:655‐661. [DOI] [PubMed] [Google Scholar]
- 7. Nilsson MB, Sun H, Diao L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: implications for combinations with beta‐blockers. Sci Transl Med. 2017;9(415):eaao4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi M, Liu D, Duan H, et al. The beta2‐adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351‐362. [DOI] [PubMed] [Google Scholar]
- 9. Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio‐behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094‐4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Renz BW, Takahashi R, Tanaka T, et al. beta2 adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zahalka AH, Arnal‐Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio‐metabolic switch in prostate cancer. Science. 2017;358:321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- 14. Shi M, Yang Z, Hu M, et al. Catecholamine‐Induced beta2‐adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol. 2013;190:5600‐5608. [DOI] [PubMed] [Google Scholar]
- 15. Liu D, Yang Z, Wang T, et al. beta2‐AR signaling controls trastuzumab resistance‐dependent pathway. Oncogene. 2016;35:47‐58. [DOI] [PubMed] [Google Scholar]
- 16. Ni CY, Murphy MP, Golde TE, Carpenter G. gamma‐Secretase cleavage and nuclear localization of ErbB‐4 receptor tyrosine kinase. Science. 2001;294:2179‐2181. [DOI] [PubMed] [Google Scholar]
- 17. Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c‐erbB‐3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang S‐C, Lien H‐C, Xia W, et al. Binding at and transactivation of the COX‐2 promoter by nuclear tyrosine kinase receptor ErbB‐2. Cancer Cell. 2004;6:251‐261. [DOI] [PubMed] [Google Scholar]
- 19. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463‐475. [DOI] [PubMed] [Google Scholar]
- 20. Cordo Russo RI, Béguelin W, Díaz Flaqué MC, et al. Targeting ErbB‐2 nuclear localization and function inhibits breast cancer growth and overcomes trastuzumab resistance. Oncogene. 2015;34:3413‐3428. [DOI] [PubMed] [Google Scholar]
- 21. Giri DK, Ali‐Seyed M, Li L‐Y, et al. Endosomal transport of ErbB‐2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005‐11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anido J, Scaltriti M, Bech Serra JJ, et al. Biosynthesis of tumorigenic HER2 C‐terminal fragments by alternative initiation of translation. EMBO J. 2006;25:3234‐3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Qian LU, Zhang Z, et al. Mutational analysis of ErbB2 intracellular localization. Histochem Cell Biol. 2007;128:473‐483. [DOI] [PubMed] [Google Scholar]
- 24. Arribas J, Baselga J, Pedersen K, Parra‐Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71:1515‐1519. [DOI] [PubMed] [Google Scholar]
- 25. Chumsri S, Sperinde J, Liu H, et al. High p95HER2/HER2 ratio associated with poor outcome in trastuzumab‐treated HER2‐positive metastatic breast cancer NCCTG N0337 and NCCTG 98–32‐52 (Alliance). Clin Cancer Res. 2018;24:3053‐3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rius Ruiz I, Vicario R, Morancho B, et al. p95HER2‐T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018;10:eaat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrier A, Gligorov J, Lefevre G, Boissan M. The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab Invest. 2018;98:696‐707. [DOI] [PubMed] [Google Scholar]
- 28. Yuan G, Qian LU, Shi M, et al. HER2‐dependent MMP‐7 expression is mediated by activated STAT3. Cell Signal. 2008;20:1284‐1291. [DOI] [PubMed] [Google Scholar]
- 29. Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma‐secretase from post‐mortem human brain. J Biol Chem. 2003;278:24277‐24284. [DOI] [PubMed] [Google Scholar]
- 30. Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32‐43. [DOI] [PubMed] [Google Scholar]
- 31. Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929‐941. [DOI] [PubMed] [Google Scholar]
- 32. Liu PCC, Liu X, Li Y, et al. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther. 2006;5:657‐664. [DOI] [PubMed] [Google Scholar]
- 33. Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta‐amyloid production by activating the alpha‐secretase gene ADAM10. Cell. 2010;142:320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Rane S, He M, Sayed D, et al. Downregulation of miR‐199a derepresses hypoxia‐inducible factor‐1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta‐adrenergic signaling pathways mediated through their reciprocal effects on miR‐199a‐5p. Cell Signal. 2010;22:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou B‐B, Peyton M, He B, et al. Targeting ADAM‐mediated ligand cleavage to inhibit HER3 and EGFR pathways in non‐small cell lung cancer. Cancer Cell. 2006;10:39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Citri A, Yarden Y. EGF‐ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505‐516. [DOI] [PubMed] [Google Scholar]
- 38. Fortini ME. Gamma‐secretase‐mediated proteolysis in cell‐surface‐receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673‐684. [DOI] [PubMed] [Google Scholar]
- 39. Lee HJ, Jung KM, Huang YZ, et al. Presenilin‐dependent gamma‐secretase‐like intramembrane cleavage of ErbB4. J Biol Chem. 2002;277:6318‐6323. [DOI] [PubMed] [Google Scholar]
- 40. Ni Y, Zhao X, Bao G, et al. Activation of beta2‐adrenergic receptor stimulates gamma‐secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12:1390‐1396. [DOI] [PubMed] [Google Scholar]
- 41. Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391‐398. [DOI] [PubMed] [Google Scholar]
- 42. Escamilla‐Ayala A, Wouters R, Sannerud R, Annaert W. Contribution of the Presenilins in the cell biology, structure and function of gamma‐secretase. Semin Cell Dev Biol. 2020;105:12‐26. [DOI] [PubMed] [Google Scholar]
- 43. Saha N, Robev D, Himanen JP, Nikolov DB. ADAM proteases: emerging role and targeting of the non‐catalytic domains. Cancer Lett. 2019;467:50‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hiller JG, Cole SW, Crone EM, et al. Preoperative beta‐blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26:1803‐1811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐5
Table S1


