Abstract
PD‐1/PD‐L1 immune checkpoint inhibitors are promising cancer immunotherapies however responses are still limited and the development of more effective combination immunotherapy is needed. We previously reported that STAT3 activation in cancer cells and immune cells was involved in immune‐resistant mechanisms. In this study, we evaluated the effect of highly absorptive forms of curcumin extracts and synthetic curcumin on anti‐tumor T cell responses. The curcumin po administration resulted in the significant augmentation of in vivo induction of tumor antigen‐specific T cells through restoration of dendritic cells (DCs) by inhibiting directly STAT3 in DCs and indirectly via reduced IL‐6 production from STAT3 activated cancer cells in 2 syngeneic MC38 and CT26 murine tumor models. Curcumin also showed direct DC enhancing activity and enhanced T cell induction for the immunized antigens in non‐tumor‐bearing mice and human hosts. Curcumin restored DC functions in xenogeneic nude mouse model implanted with high IL‐6‐producing human clear cell ovarian cancer cells. The combination of curcumin and PD‐1/PD‐L1 Abs demonstrated a synergistic anti‐tumor activity in MC38 murine tumor models. These results indicated that curcumin augments the induction of tumor antigen‐specific T cells by restoring the T cell stimulatory activity of DCs targeting activated STAT3 in both cancer cells and immune cells. Combination immunotherapy with curcumin and PD‐1/PD‐L1 Ab is an attractive strategy in the development of effective immunotherapy against various cancers.
Keywords: cancer immunotherapy, curcumin, dendritic cells, PD‐1/PD‐L1 blockade, STAT3
Highly absorptive form of curcumin can restore the T cell stimulatory activity of DCs and augment the induction of tumor antigen‐specific T cells by targeting activated STAT3 in both cancer cells and immune cells. Combination immunotherapy with curcumin and PD‐1/PD‐L1 Ab is an attractive strategy in the development of effective immunotherapy against various cancers.

Abbreviations
- BMDC
bone marrow‐derived DC
- CFA
complete Freund's adjuvant
- DC
dendritic cell
- dLN
draining lymph node
- HPMC
hydroxypropyl methyl cellulose
- MDSC
myeloid‐derived suppressor cell
- OVA
ovalbumin
- QOL
quality of life
- SD
standard deviation
- Syn‐CUR
amorphized synthetic curcumin
- TAM
tumor‐associated macrophage
- THC
Theracurmin
1. INTRODUCTION
Clinical approaches that utilize the immune system against cancers have been studied extensively, and various immunotherapies have been developed. In particular, immune checkpoint inhibitors targeting PD‐1/PD‐L1 and CTLA‐4 are promising therapeutic approaches to achieve long‐lasting responses in some patients with various advanced cancers. However, only a subset of patients responds to this treatment. Unresponsiveness to these immune checkpoint inhibitors may be mediated by multiple primary and adaptive immune‐resistant mechanisms that inhibit anti‐tumor T cell responses. 1 Thus, combined therapies that can reverse such immuno‐resistance in non‐responders are urgently needed.
STAT3, which is activated in various immunosuppressive immune cells and cancer cells, contributes to immunosuppression in the tumor microenvironment. 2 We have previously reported that the depletion of STAT3 in dendritic cells (DCs) resulted in the enhancement of their function and subsequent T cell induction, 3 suggesting that STAT3 in immune cells might be a promising therapeutic target. 4 STAT3 in cancer cells is also involved in the production of immunosuppressive cytokines such as IL‐6, IL‐8, and VEGF. 2 A high percentage of p‐STAT3‐positive tumor cells was a significant independent prognostic indicator for poor clinical outcome in cancer patients. 5 Thus, targeting STAT3 in both immune cells and cancer cells may restore the immunocompetence of cancer patients.
Curcumin (Curcuma longa), a major active component of turmeric, has been reported to have anti‐tumor effects by targeting various oncogenes including STAT3 in various cancers. 6 Its absorption and bioavailability by oral administration are limited in humans, however, 7 resulting in insufficient in vivo efficacy. To overcome this limitation, various highly absorptive forms of curcumin with improved bioavailability have been developed. Per oral (po) administration of a submicron crystal solid dispersion form of curcumin extracted from turmeric root (THC) resulted in high plasma concentration 8 and improved QOL scores in cancer patients 9 , 10 and those with inflammatory diseases such as osteoarthritis, 11 muscle damage, 12 and atherosclerotic hyperlipidemia 13 without severe adverse effect. Another form of curcumin for increasing absorptivity is amorphization. 14 We generated an amorphous formulation with high bioavailability; a mixture of synthetic curcumin and hydroxypropyl methyl cellulose (HPMC) (Syn‐CUR).
In this study, we demonstrated that highly absorptive curcumin extract (THC) and synthetic pure curcumin (Syn‐CUR) reversed cancer‐induced immunosuppression by inhibiting STAT3 in both cancer cells and DCs, and significantly enhanced the anti‐tumor effects of immune checkpoint inhibitors targeting PD‐1 and PD‐L1.
2. MATERIALS AND METHODS
2.1. Cells, culture supernatants, and reagents
Human epithelial ovarian cancer cell line JHOC‐5 was kindly provided by Dr. Juri Sugiyama and Dr. Hiroshi Nishio (Department of Obstetrics and Gynecology, Keio University, Tokyo, Japan). 15 Murine colon cancer cell line MC38 was kindly provided by the Surgery Branch, National Cancer Institute, National Institutes of Health (MD, USA). CT26 was purchased from ATCC. See Appendix S1 for more information on the cell lines. Highly absorptive forms of curcumin are a submicron crystal solid dispersion of curcumin (THC: Theracurmin) and amorphized synthetic curcumin (Syn‐CUR). Syn‐CUR is prepared by mixing and milling with HPMC for an amorphous formulation in which the ratio of HPMC to curcumin (Tokyo Chemical Industry) is 2:1. Both were dissolved in DMSO for in vitro study and diluted in water for in vivo study at various concentrations.
2.2. In vivo mice models
Mice were bred at the animal facilities of Keio University in accordance with guidelines for animal experimentation. JHOC‐5 cells (5 × 106/mouse), MC38 cells (5 × 105/mouse) and CT26 cells (5 × 105/mouse) were injected subcutaneously into the right flank of 6‐ to 8‐wk‐old female BALB/c‐nu/nu, C57BL/6J and BALB/c mice (CLEA). See Appendix S1 for more information of “Induction of tumor antigen‐specific T cells,” “T cell stimulatory activities of DCs,” “T cell suppression assay,” “Flow cytometric analysis” and “Real‐time RT‐PCR.” Tumor volume measurements were performed using calipers by measuring the largest diameter and perpendicular length and calculated in accordance with the following formula: ½ × (largest diameter) × (perpendicular diameter)2.
2.3. In vivo mice ovalbumin models
Purified chicken ovalbumin (OVA; albumin from chicken egg white) was purchased from Sigma, and CFA was purchased from Difco. Native OVA (25 µg/location) in PBS (25 µL/location) mixed with CFA (25 µL/location) was injected into the hind footpads (fp) and both flanks (total of 4 injection sites/mouse) of 6‐wk‐old female C57BL/6J mice.
2.4. Preparation of nuclear extracts and STAT3 and NFκB transcription activation assay
Nuclear extracts of MC38 and JHOC‐5 treated with curcumin for 6 h in vitro and tumor‐infiltrating CD11c+ and CD11b+ cells purified from MC38‐implanted C57BL/6 mice using CD11c and CD11b MACS beads (Miltenyi Biotec) were prepared by NE‐PER Nuclear and Cytoplasmic Extraction Reagents (Signosis). STAT3 and NFκB transcription activities in nuclear extracts were measured using a STAT3 and NFκB Filter Plate Assay (Thermo Scientific) in accordance with the manufacturer's instructions.
2.5. CMV‐specific T cell responses by IFN‐γ release
Human PBMCs were from HLA‐A*0201+ or HLA‐A*2402+ healthy volunteers with approval of Keio University School of Medicine Ethic Committee (Approval number 20130112) and acquisition of donors’ informed consents. The PBMCs (2 × 106 cells in AIM‐V including 10% AB serum) were pulsed with 2.5 µg/mL CMV pp65‐A2 (NLVPMVATV) or pp65‐A‐24 (QYDPVAALF) peptides in 24‐well plates (2 mL/well) and then IL‐2 at a concentration of 20 IU/mL and IL‐7 of 10 ng/mg was added for 6 d of culture at 37°C with or without THC or Syn‐CUR. After the stimulation, the responder T cells were collected and incubated with T2 (HLA‐A2.1+: from ATCC, HLA‐A24+: overexpression of A24 in T2) as stimulators at a responder‐to‐stimulator ratio of 1:1 (1‐2 × 105 cells) in 96‐well plates (100 µL/well), and triplicate wells were prepared. At 20 h after T cell stimulation with CMV or HIV (irrelevant peptide, 1 µg/mL), the quantity of IFN‐γ released into the culture supernatant was measured by ELISA.
2.6. Combination therapy with curcumin and immune checkpoint blockade
For combination with PD‐1/PD‐L1 blockade, mice received po administration with 5 mg/kg of curcumin diluted in water every day starting on day 3 after implantation with MC38 until killed (day 20‐25). Control mice received water. Anti‐PD‐1 Ab (BioXcell, clone: RMP1‐14) and anti‐PD‐L1 Ab (BioXcell, clone: 10F.9G2) were given ip on days 7, 10, and 13 at a dose of 200 µg/100 µL/mouse. Rat IgG2a (BioXcell, clone: 2A3) and rat IgG2b (BioXcell, clone: LTF‐2) were injected ip in the control group and the curcumin single treatment group on days 7, 10, and 13 at a dose of 200 µg/100 µL/mouse.
3. RESULTS
3.1. A highly absorptive form of extracted curcumin (THC) augments tumor antigen‐specific T cell induction through the restoration of DC function in tumor‐bearing mice
To evaluate the anti‐tumor immune responses of curcumin having STAT3 inhibiting activity, we first tested the effect of a highly absorptive form submicron crystal solid dispersion of the extracted curcumin from turmeric root (Theracurmin: THC) on the induction of gp70 tumor antigen‐specific T cells using syngeneic murine MC38 colon cancer cell line in C57BL/6 mouse models. Although no anti‐tumor effect by THC alone was observed (Figure 1A), THC augmented the induction of gp70‐specific T cells in tumor tissues (Figure 1B: left), draining lymph nodes (dLNs) (Figure 1B: middle), and spleens (Figure 1B: right). To elucidate the mechanism for the enhanced T cell induction by THC, we examined the phenotypes of DCs, MDSCs, TAMs and regulatory T cells in tumors by flow cytometric analysis (Figure S1A‐C). CD83 on CD11c+ I‐Ab+ DCs in dLNs was significantly increased (Figure S1A) and PD‐L1 expression on CD11c+ I‐Ab+ DCs in dLNs was decreased (Figure S1B) by THC treatment. Numbers of CD11b+F4/80+ TAMs and MDSCs including CD11b+F4/80‐Ly6G+ granulocytic‐MDSCs and CD11b+F4/80‐Ly6C+ monocytic‐MDSCs in tumors, were not altered by THC (Figure S1C).
FIGURE 1.
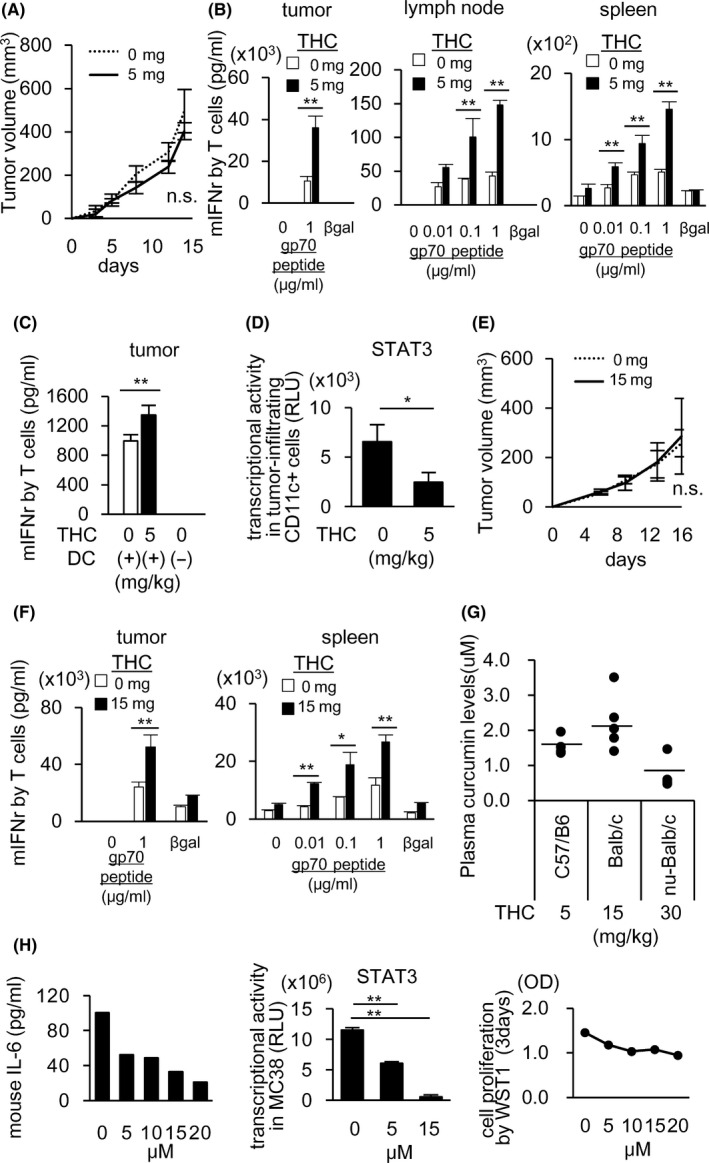
THC augments tumor antigen‐specific T cell induction possibly thorough the restoration of DC function to activate T cells in tumor‐bearing mice. MC38‐bearing C57/B6 mice (A‐D) or CT26‐bearing BALB/c mice (F, G) received po administration of THC every day starting on day 3 after tumor implantation. Control mice received water. Mean tumor size ± standard deviation (SD) (n = 5). Tumor growth of MC38 was not changed by THC (A). On day 15, CD8+ cells from tumor tissues or whole cells from dLNs and spleens were stimulated in vitro with gp70 peptides for 2 or 5 d. Lymphocytes isolated from the cultures were incubated with irradiated splenocytes from healthy C57BL/6 in the presence of gp70 or β‐gal peptides (irrelevant peptide, 1 µg/mL) for 24 h. The induction of gp70‐specific T cells was evaluated by IFN‐γ production from T cells (B). CD11c+ DC cells purified from tumor tissues were co‐cultured for 3 d with splenic T cells from syngeneic C57BL/6 mice in the presence of anti‐CD3 Ab. The T cell stimulatory activity of DCs was evaluated by IFN‐γ production from T cells (C). STAT3 transcription activities were evaluated using nuclear extracts of tumor‐infiltrating CD11c+ cells by a Filter Plate Assay (D). Plasma curcumin levels 2 h after THC administration were measured in C57BL/6 mice (5 mg/kg THC), BALB/c mice (15 mg/kg THC), and nude mice (30 mg/kg THC) by HPLC‐MS/MS (E). Tumor growth of CT26 was not changed by THC (F). The gp70‐specific T cell induction in the tumor tissues (G: left) and spleen (G: right) of CT26‐bearing BALB/c was evaluated by the same experiment as in (B). After treating MC38 with THC for 6 h, IL‐6 in the culture supernatants was measured by ELISA (H: left) and STAT3 transcriptional activity was evaluated using nuclear extracts of MC38 by a Filter Plate Assay (H: middle). Cell proliferation was measured after 72 h THC treatment by WST1 assay (H: right). Data in (A‐E) and (F‐H) in this figure are from 3 and 2 independent experiments, respectively. Error bars indicate SD. *P < .05, **P < .01 using a t test
We have previously reported that immunosuppressive cytokines produced by cancer cells caused impairment of DC functions through STAT3 activation in DCs. 2 , 3 , 4 Thus, we evaluated the effect of THC on DC function for stimulation of IFN‐γ production by T cells, and found that the T cell stimulatory activity of DCs in the MC38 tumors was restored in vivo by THC administration (Figure 1C). The gene expression and transcription activity of STAT3 in CD11c+ DCs in MC38 tumors was inhibited by THC as shown in Figures 1D and S2A, whereas both of them in CD11b+ cells were not changed (Figure S2A,B). Next, we evaluated the T cell suppressive activity of tumor‐infiltrating CD11b+ cells including TAMs and MDSCs in mice treated with THC, and no significant change was observed (Figure S2C), suggesting that immunosuppressive activity of tumor‐infiltrating TAMs and MDSCs were not altered by THC.
THC activities to enhance T cells were similarly observed in another murine colon cancer cell CT26/ BALB/c mouse model (Figure 1E,F) in which 15 mg/kg of THC was administered to obtain similar plasma curcumin concentrations to the 5 mg/kg administration in C57BL/6 mice (Figure 1G). The plasma concentration of curcumin was equivalent to that of pancreatic or biliary cancer patients whose QOL scores were improved during gemcitabine‐based chemotherapy by combined administration of THC. 9
As IL‐6 is one of the important factors involved in the suppression of DC function through activation of STAT3, 4 , 15 , 16 we evaluated IL‐6 production by tumor cells, and found that MC38, but not CT26, produced IL‐6. The production of IL‐6 from MC38 was inhibited by in vitro treatment with curcumin accompanied by reduced STAT3 activity (Figure 1H: left and middle) without affecting the cell proliferation (Figure 1H: right). We tested whether THC inhibits STAT3 in DCs treated with exogenous IL‐6 in vitro, and found that THC inhibited STAT3 expression induced by exogenous IL‐6 (Figure S3A). These results suggested that curcumin could restore DC function through not only directly inhibiting STAT3 in DCs induced by IL‐6 or other cytokines from non‐cancer cells, but also indirectly inhibiting production of IL‐6 in cancer cells. These results indicated that THC could augment the induction of tumor antigen‐specific T cells by inhibiting STAT3 in both cancer cells and DCs in tumor microenvironments.
3.2. THC augments induction of antigen‐specific T cells for immunized antigens through stimulating DCs in a non‐tumor‐bearing host
To evaluate the direct effect of THC on immune cells, C57BL/6 mice without tumors were immunized with OVA with or without daily po THC administration. At 10 d after immunization with the OVA protein, induction of OVA‐specific T cells was evaluated by in vitro stimulation with OVA‐peptides of lymphocytes from dLNs and spleens, and THC was found to augment OVA‐specific T cells in vivo in dLNs (Figure 2A). The T cell stimulatory activity of CD11c+ DCs in dLNs and spleens was also evaluated, and the T cell stimulatory activity of DCs in the dLNs (Figure 2B: left) and spleens (Figure 2B: right) was also enhanced by THC, indicating that THC augmented T cell induction partly by stimulating DCs even in non‐tumor‐bearing mice.
FIGURE 2.
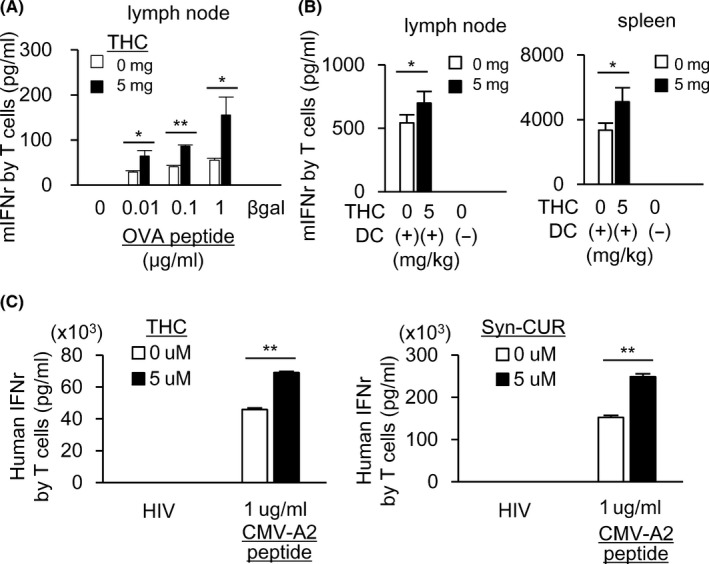
THC augments antigen‐specific T cell induction in healthy mice and human individuals. A, B, C57BL/6 mice were vaccinated with ovalbumin (OVA) in CFA on day 1 and received po administration of THC every day. On 10 d, OVA‐specific T cell induction in dLNs (A) and the T cell stimulatory activity of CD11c+ cells purified from dLNs and spleen (B) were evaluated by the same experiment as in Figure 1B,C. Five mice were included in each group. Human PBMCs from the peripheral blood of HLA*A02‐positive individuals (n = 2, or HLA*A24: n = 1 in Figure S4A) were stimulated in the presence of CMV‐A2 peptides for 6 d with or without THC or Syn‐CUR. After mixing with T2 cells pulsed with CMV or HIV (irrelevant peptide, 1 µg/mL) for 20 h, CMV‐specific T cell responses were measured by IFN‐γ ELISA (C). All data in this figure are from 2 independent experiments. Error bars indicate SD. *P < .05, **P < .01 using a t test
We then evaluated the effect of curcumin on in vitro induction of antigen‐specific T cells in human. PBMCs from HLA‐A2 positive healthy donors were stimulated in vitro with CMV HLA‐A2 binding epitope peptides for 6 d with or without curcumin (THC or synthetic curcumin, Syn‐CUR). Curcumin significantly enhanced the in vitro induction of CMV‐specific T cells (Figures 2C and S4A). These results indicated that curcumin could augment the induction of antigen‐specific T cells in non‐tumor‐bearing hosts partly by stimulating DC function.
3.3. Combined therapies of curcumin and anti‐PD‐1/PD‐L1 Abs synergistically augment tumor antigen‐specific T cell induction and the anti‐tumor effect
Although THC significantly augmented tumor antigen‐specific T cell induction, inhibition of tumor growth was not observed in the mouse models (Figure 1A, F). To clarify the reasons for this difference, we analyzed PD‐L1 expression in the tumor cells of THC‐treated mice, and a substantial amount of PD‐L1 expression on MC38 tumor cells and PD‐1 expression on tumor‐infiltrating CD8+ T cells was detected (Figure 3A), suggesting that PD‐L1 on cancer cells inhibited tumor‐specific T cells activated by THC. Thus, we performed a combination of PD‐1/PD‐L1 blockade with THC administration, and found that the combination of THC and anti‐PD‐1 Ab showed a significant synergistic anti‐tumor activity compared with treatment with THC or anti‐PD‐1 Ab alone (Figure 3B). The combined therapy also augmented gp70 tumor antigen‐specific T cells in tumors and dLNs (Figure 3C). Similar results were obtained in the combination treatment of anti‐PD‐L1 Ab and THC (Figure 3D). These results indicated that the combined therapies of THC and PD‐1/PD‐L1 blockade are attractive strategies for development of effective cancer immunotherapy.
FIGURE 3.
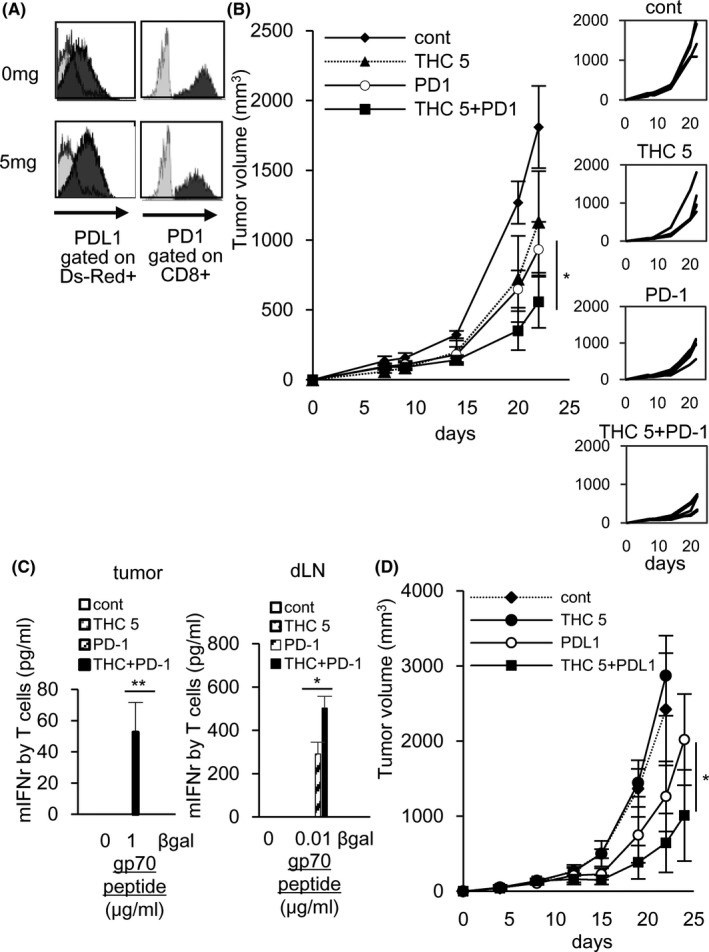
Combined therapies of THC and anti‐PD‐1/PD‐L1 Ab synergistically augment the gp70‐specific T cell induction and the anti‐tumor effect. The combined therapy of THC and anti‐PD‐1 Ab (A‐C) or anti‐PD‐L1 Ab (D) given ip on days 7, 10, and 13, was performed. On day 16 after implantation of DsRed‐labeled MC38 to C57BL/6 mice, tumor was collected from the mice. The expression of PD‐L1 on MC38 cells and the expression of PD‐1 on tumor‐infiltrating CD8+ T cells were evaluated by flow cytometry (A). On day 22, the combined therapy of THC and anti‐PD‐1 Ab synergistically inhibited tumor growth compared with each single treatment (B) and the combined therapy augmented the induction of gp70‐specific T cells in tumor tissues (C: left) and dLNs (C: right) by the same experiment as Figure 1B. The combined therapy of THC and anti‐PD‐L1 Ab synergistically inhibited tumor growth compared with each single treatment (D). Mean tumor size ± SD. Five mice were included in each group (4 mice were included in the control group). All results in this figure are from 2 independent experiments. Error bars indicate SD. *P < .05, **P < .01 using a t test
3.4. Amorphized synthetic curcumin (Syn‐CUR) showed enhanced anti‐tumor T cell induction and anti‐tumor effects similar to THC
Because THC contains curcumin from turmeric root extracts, we also evaluated the effects of the synthetic pure curcumin on anti‐tumor immune responses. We utilized highly absorptive amorphized synthetic curcumin (Syn‐CUR). Plasma curcumin concentrations in C57BL/6 mice after Syn‐CUR administration (5 mg/kg) were similar to those after administration of THC (5 mg/kg) (Figure 4A). The combination of Syn‐CUR and anti‐PD‐1 Ab showed synergistic anti‐tumor activity on day 20, accompanied by enhanced tumor‐specific T cells compared with treatment of Syn‐CUR or anti‐PD‐1 Ab alone (Figure 4B, C). The T cell stimulatory activity of DCs in the MC38 tumors was also restored by Syn‐CUR (Figure 4D) with STAT3 inhibition (Figure 4E). These results indicated that pure synthetic curcumin had enhancing activities of anti‐tumor T cells by acting on both cancer cells and DCs, and that the major active compound in THC appeared to be curcumin. Therefore, synthetic curcumin may also be an attractive candidate for combination immunotherapy with PD‐1/PD‐L1 Abs.
FIGURE 4.
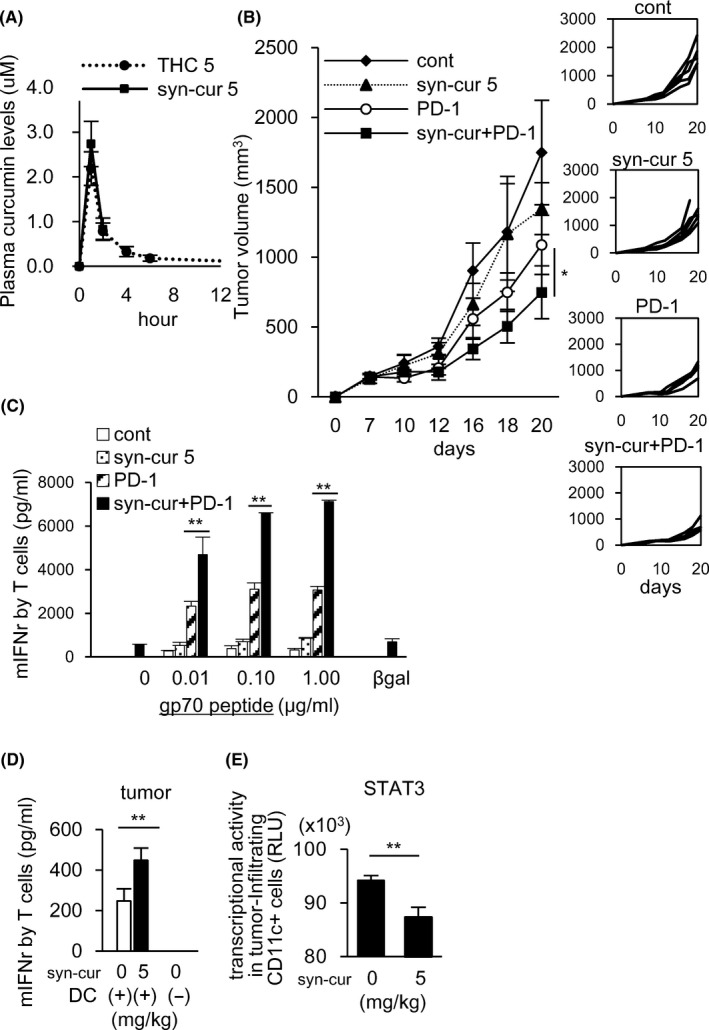
Amorphized synthetic curcumin (Syn‐CUR) showed the same anti‐tumor effect as THC. Plasma curcumin levels after Syn‐CUR administration were observed in MC38‐bearing C57BL/6 mice (A). Syn‐CUR treatment combined with anti‐PD‐1 Ab given ip on days 7, 10, and 13 was performed. On day 20, the combined therapy of Syn‐CUR and anti‐PD‐1 Ab synergistically inhibited tumor growth compared with each single treatment (B). Combined therapy augmented the induction of gp70‐specific T cells in the spleen by the same experiment as Figure 1B (C). Five mice were included in each group. The T cell stimulatory activity of DCs (CD11c+ cells) purified from tumor tissues was evaluated by the same experiment as Figure 1C (D). STAT3 transcription activities were evaluated using nuclear extracts of tumor‐infiltrating CD11c+ cells by a Filter Plate Assay (E). All results in this figure are from 2 independent experiments. Error bars indicate SD. *P < .05, **P < .01 using a t test
3.5. THC may augment immune response to human ovarian cancer though acting on STAT3
We then evaluated the augmenting activities of curcumin on immune responses to human cancer. We utilized human ovarian clear cell carcinoma JHOC‐5, which produces high amounts of IL‐6 and IL‐8. IL‐8 has an activity to promote nuclear translocation of STAT3. 17 THC significantly reduced the production of IL‐6 and IL‐8 by JHOC‐5 in vitro without affecting cell proliferation (Figure 5A) accompanied by inhibition of STAT3 transcription activities in the nucleus (Figure 5B). Next, we investigated the in vivo effects of THC using a xenogeneic nude mouse model implanted with human JHOC‐5 cancer cells. Systemic daily po administration of THC decreased human IL‐6 produced by JHOC‐5 in mouse serum (Figure 5D), although there was no significant change in tumor size (Figure 5C). The plasma curcumin concentrations 2 h after THC administration (30 mg/kg) were observed in the nude mouse model to be similar to those in the C57BL/6 and BALB/c mouse models (Figure 1G). At 25 d after tumor implantation, CD11c+ cells obtained from the spleens and tumors of nude mice were co‐cultured with splenic T cells from syngeneic BALB/c mice in the presence of anti‐CD3 Ab for 3 d to evaluate DC activity for T cell stimulation. THC administration was found to restore the T cell stimulatory activity of the murine DCs in JHOC‐5‐bearing mice, in which DC function was suppressed compared with that of non‐tumor‐bearing mice (Figure 5E). In this xenogeneic tumor model, human IL‐6 capable of binding to murine IL‐6R appears to suppress murine DCs. 18 These results indicated that THC may be useful for combination immunotherapy in patients with cancers that produce IL‐6, including clear cell ovarian cancer as described in the syngeneic mouse models in this study.
FIGURE 5.
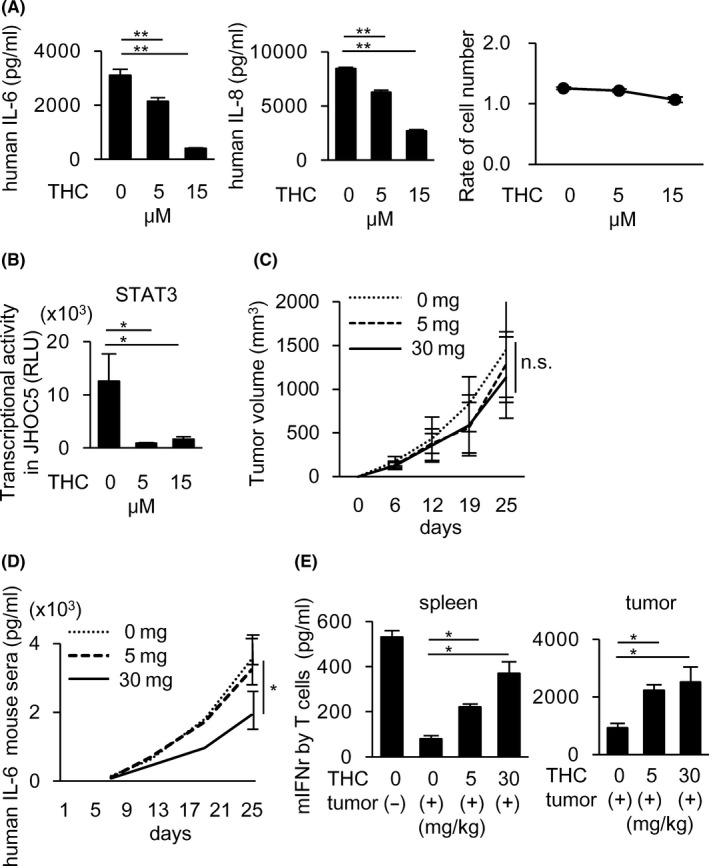
THC reduces IL‐6 and IL‐8 production from human ovarian cancers by inhibiting STAT3, leading to the restoration of suppressed DCs function to activate T cells in human ovarian cancer‐bearing nude mice. A, B, A human ovarian cancer cell line, JHOC‐5, was treated with THC for 6 h. IL‐6 and IL‐8 production in the culture supernatants were measured by ELISA (A: left and middle). The cell proliferation was measured after 72 h THC treatment by WST1 assay (A: right). STAT3 transcriptional activity was measured using nuclear extracts in the cultured JHOC‐5 with THC for 6 h by a Filter Plate Assay (B). C‐E, JHOC‐5‐bearing nude mice received po administration of THC every day from day 6 until killed. Four mice were included in each group. Tumor size was not changed by THC administration (C). Human IL‐6 detected in mice serum was decreased by THC administration (D). T cell stimulatory activity of DCs purified from the spleen (E: left panel) and tumor tissues (E: right panel) of JHOC‐5‐bearing nude mice was evaluated by IFN‐γ production from T cells. Data in (A, B) and (C‐E) of this figure are from 3 and 2 independent experiments, respectively. Error bars indicate SD. *P < .05, **P < .01 using a t test
Altogether, our study demonstrated that curcumin augmented the induction of tumor antigen‐specific T cells by inhibiting STAT3 signaling in both DCs and cancer cells, and that the combination of curcumin and PD‐1/PD‐L1Abs had a significant synergistic anti‐tumor activity and is an attractive strategy for effective combination immunotherapy, particularly for patients with IL‐6 producing cancers.
4. DISCUSSION
Constitutively activated STAT3 has been reported in various cancers, where it contributes to malignant phenotypes of cancer cells, including not only increased cell proliferation, apoptotic resistance, and enhanced invasive and metastatic abilities, but also the production of immune‐suppressive molecules. STAT3 is also activated in various immune cells including DCs, MDSCs/TAMs, and regulatory T cells in cancer microenvironments and is involved in the suppression of anti‐tumor immune responses. The present study demonstrated that curcumin could augment anti‐tumor T cell responses by inhibiting STAT3 activated in both cancer cells and dendritic cells and had a synergistic anti‐tumor effect with PD‐1/PD‐L1 Abs, although curcumin might target multiple molecules. As THC has been reported in previous clinical trials to produce no severe adverse effects, 9 curcumin is an attractive reagent for development of more effective combination cancer immunotherapy with immune checkpoint inhibitors.
In this study, we showed that curcumin enhanced anti‐tumor T cell responses through restoration of DC function via STAT3 inhibition. The effective dose (5 mg/kg) of THC for T cell responses (Figures 1B, F and 4C) only effected on DCs, but not on CD11b+ TAMs and MDSCs (Figure S2). This dose resulted in STAT3 inhibition, but not constant NFκB inhibition of DCs (Figure S5A). However, a higher dose (30 mg/kg) of THC suppressed both the induction of tumor‐specific T cells (Figure S5B: left) and DC function (Figure S5B: right). Plasma curcumin levels 2 h after intake of 30 mg/kg THC were increased than 6 times, compared with 5 mg/kg THC (Figure S5C). Therefore, the in vivo effect of THC on DCs and macrophages, as well as NFκB, appeared to be dose dependent. In fact, relatively high doses of curcumin such as 50 mg/kg have been shown previously to inhibit macrophage‐related inflammation, such as in atherosclerosis models, 19 and to induce apoptosis of MDSCs in a tumor model. 20 Curcumin is currently used as an anti‐inflammatory drug in ongoing clinical trials for various diseases including arthritis, diabetes, cardiovascular disease, Crohn's disease, and other inflammatory conditions, in which the curcumin dose ranges from 0.18 to 8 g/d. These observations indicated that the optimum dose of curcumin needs to be applied for augmentation of anti‐tumor T cell responses.
We showed that one of the mechanisms for restoration of DCs by curcumin was due to reduced IL‐6 production by murine and human cancer cells via their STAT3 inhibition. Various human cancer cells continuously produce various immunosuppressive cytokines including IL‐6, 21 and is related to poor prognosis of patients with some cancers. IL‐6 and IL‐8 play various immune‐suppressive roles in anti‐tumor immune responses, such as the inhibition of DCs, generation of MDSCs and TAMs, and recruitment of regulatory T cells. IL‐6 and IL‐8 are produced in large amounts by clear cell renal cell carcinoma and ovarian clear cell carcinoma. We previously reported that IL‐6 and IL‐8 produced by ovarian clear cell carcinoma induced immune suppression, such as the inhibition of DC function. 15 A meta‐analysis of randomized controlled trials suggested that curcumin significantly reduced circulating IL‐6 levels in various human diseases. 22 In this study, we showed in a xenogeneic tumor model that curcumin inhibited the production of IL‐6 and IL‐8 by ovarian clear cell carcinoma by inhibiting STAT3 activity, leading to the restoration of DC function. This mechanism may apply to a variety of human cancers.
Curcumin has been reported to reduce PD‐L1 expression in cancer cells. 23 , 24 Lim et al reported that curcumin reduced PD‐L1 expression by inhibiting CSN5, which is involved in the deubiquitination of PD‐L1. 23 Liao et al reported that curcumin reduced PD‐L1 expression through STAT3 inhibition. 24 However, in this study, a significant reduction of PD‐L1 expression on cancer cells was not observed. This difference may be due to the different tumor models used, and doses of curcumin, and needs further investigation in clinical trials.
Curcumin has been shown to have direct anti‐tumor effects on several types of cancers by inducing apoptosis through blocking various signaling molecules and pathways such as cyclin D1, c‐myc, JNK, and AMPK. 25 However, in our tumor models, curcumin had no effect on the cell proliferation of cancer cells in vitro and in vivo, possibly due to the difference in tumors and administered curcumin doses. For cancer cell types for which curcumin has a direct ant‐tumor effects, curcumin may have additional anti‐tumor effects via direct cytotoxicity and immunogenic cell death that lead to anti‐tumor T cell induction.
Altogether, the use of optimum doses of curcumin may be important for the development of more effective combination immunotherapies with PD‐1/PD‐L1 blockade, by acting on activated STAT3 in both cancer cells and immune cells. Further investigations in clinical trials for patients with various cancers will be warranted, particularly for IL‐6‐producing cancers with constitutive STAT3 activation.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figs S1‐S5
Appendix S1
ACKNOWLEDGMENTS
This work was supported by Grants‐in‐aid for Scientific Research from The Ministry of Health, Labor and Welfare (MHLW) of Japan (Research on New Drug Development, H22), Grants‐in‐aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (23240128, 26221005); The Project for Development of Innovative Research on Cancer Therapeutics (P‐DIRECT) (14069014) and the Project for Cancer Research And Therapeutic Evolution (P‐CREATE) from Japan Agency for Medical Research and Development (AMED); a grant from Tokyo Biochemical Research Foundation; and A Keio Gijuku Academic Development Funds. We thank Kenji Morii for technical advice and helpful discussions and Misako Sakamoto and Ryoko Suzuki for preparation of the manuscript.
Hayakawa T, Yaguchi T, Kawakami Y. Enhanced anti‐tumor effects of the PD‐1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020;111:4326–4335. 10.1111/cas.14675
Contributor Information
Tomonori Yaguchi, Email: beatless@rr.iij4u.or.jp, Email: yaguchi.tomonori.4m@kyoto-u.ac.jp.
Yutaka Kawakami, Email: yutakawa@keio.jp, Email: yutakawa@iuhw.ac.jp.
REFERENCES
- 1. Yaguchi T, Kawakami Y. Cancer‐induced heterogeneous immunosuppressive tumor microenvironments and their personalized modulation. Int Immunol. 2016;28(8):393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yaguchi T, Sumimoto H, Kudo‐Saito C, et al. The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 2011;93(3):294‐300. [DOI] [PubMed] [Google Scholar]
- 3. Iwata‐Kajihara T, Sumimoto H, Kawamura N, et al. Enhanced cancer immunotherapy using STAT3‐depleted dendritic cells with high Th1‐inducing ability and resistance to cancer cell‐derived inhibitory factors. J Immunol. 2011;187(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 4. Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF‐MAPK signaling pathway is essential for cancer‐immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin GS, Yang LJ, Wang XF, et al. STAT3 Tyr705 phosphorylation affects clinical outcome in patients with newly diagnosed supratentorial glioblastoma. Med Oncol. 2014;31(4):924. [DOI] [PubMed] [Google Scholar]
- 6. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. BioFactors. 2013;39(1):56‐68. [DOI] [PubMed] [Google Scholar]
- 7. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807‐818. [DOI] [PubMed] [Google Scholar]
- 8. Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34(5):660‐665. [DOI] [PubMed] [Google Scholar]
- 9. Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71(6):1521‐1530. [DOI] [PubMed] [Google Scholar]
- 10. Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol. 2014;20(28):9384‐9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakagawa Y, Mukai S, Yamada S, et al. Short‐term effects of highly‐bioavailable curcumin for treating knee osteoarthritis: a randomized, double‐blind, placebo‐controlled prospective study. J Orthop Sci. 2014;19(6):933‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanabe Y, Maeda S, Akazawa N, et al. Attenuation of indirect markers of eccentric exercise‐induced muscle damage by curcumin. Eur J Appl Physiol. 2015;115(9):1949‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funamoto M, Sunagawa Y, Katanasaka Y, et al. Highly absorptive curcumin reduces serum atherosclerotic low‐density lipoprotein levels in patients with mild COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2029‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuah AM, Jacob B, Jie Z, et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014;156:227‐233. [DOI] [PubMed] [Google Scholar]
- 15. Nishio H, Yaguchi T, Sugiyama J, et al. Immunosuppression through constitutively activated NF‐kappaB signalling in human ovarian cancer and its reversal by an NF‐kappaB inhibitor. Br J Cancer. 2014;110(12):2965‐2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SJ, Nakagawa T, Kitamura H, et al. IL‐6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844‐3854. [DOI] [PubMed] [Google Scholar]
- 17. Waugh DJ, Wilson C. The interleukin‐8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735‐6741. [DOI] [PubMed] [Google Scholar]
- 18. van Dam M, Mullberg J, Schooltink H, et al. Structure‐function analysis of interleukin‐6 utilizing human/murine chimeric molecules. Involvement of two separate domains in receptor binding. J Biol Chem. 1993;268(20):15285‐15290. [PubMed] [Google Scholar]
- 19. Momtazi‐Borojeni AA, Abdollahi E, Nikfar B, Chaichian S, Ekhlasi‐Hundrieser M. Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy. Heart Fail Rev. 2019;24(3):399‐409. [DOI] [PubMed] [Google Scholar]
- 20. Tu SP, Jin H, Shi JD, et al. Curcumin induces the differentiation of myeloid‐derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res. 2012;5(2):205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL‐6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685‐6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derosa G, Maffioli P, Simental‐Mendia LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin‐6 concentrations: a systematic review and meta‐analysis of randomized controlled trials. Pharmacol Res. 2016;111:394‐404. [DOI] [PubMed] [Google Scholar]
- 23. Lim SO, Li CW, Xia W, et al. Deubiquitination and Stabilization of PD‐L1 by CSN5. Cancer Cell. 2016;30(6):925‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti‐tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. 2018;92:32‐37. [DOI] [PubMed] [Google Scholar]
- 25. Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053‐1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs S1‐S5
Appendix S1


