Abstract
Adult T‐cell leukemia/lymphoma (ATL) is a mature T‐cell neoplasm and is classified into four subtypes (acute, lymphoma, chronic, and smoldering) according to the Shimoyama classification, established in 1991 through several nationwide surveys based on the clinical diversity of patients diagnosed in 1983‐1987 in Japan. Thereafter, no such studies have been conducted. Recently, we conducted a nationwide hospital survey using the method of the 1980s studies, collected baseline data on 996 ATL patients diagnosed in 2010‐2011 from 126 hospitals, and reported their unique epidemiological characteristics. Here, we report the follow‐up results of registered ATL patients with the goal of evaluating current prognoses and treatment modalities as of 2016‐2017. Of 770 evaluable patients, 391 (50.8%) had acute‐type, 192 (24.9%) had lymphoma‐type, 106 (13.8%) had chronic‐type, and 81 (10.5%) had smoldering‐type ATL. The initial therapy regimens used for acute/lymphoma‐type ATL were vincristine, cyclophosphamide, doxorubicin and prednisone, followed by doxorubicin, ranimustine, and prednisone and then by vindesine, etoposide, carboplatin, and prednisone (VCAP‐AMP‐VECP)‐like in 38.5/41.7% and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)‐like in 14.6/13.7% of patients. Allogeneic hematopoietic stem cell transplantation was used to treat 15.9/10.4% of acute/lymphoma‐type ATL patients. The 4‐year survival rates (the median survival time, days) for acute‐, lymphoma‐, unfavorable chronic‐, favorable chronic‐, and smoldering‐type ATL were 16.8% (252), 19.6% (305), 26.6% (572), 62.1% (1937), and 59.8% (1851), respectively. The 4‐year survival rates for acute‐ and lymphoma‐type ATL improved compared with those reported in 1991, but those for chronic‐ and smoldering‐type ATL were not. Further efforts are warranted to develop more efficient therapeutic strategies to improve the prognosis of ATL in Japan.
Keywords: ATL, clinical subtypes, HTLV‐1, Japanese nationwide survey, prognosis
The survival curve shows that the prognoses of patients with acute and lymphoma‐type ATL in Japan have improved modestly, but those of patients with chronic and smoldering‐type ATL have not improved.

Abbreviations
- allo‐HSCT
allogeneic hematopoietic stem cell transplantation
- ATL
adult T‐cell leukemia/lymphoma
- AZT
zidovudine
- BUN
blood urea nitrogen
- CCR4
CC chemokine receptor 4
- CHOP, cyclophosphamide
doxorubicin, vincristine, and prednisone
- HTLV‐1
human T‐cell leukemia virus type‐I
- IFN
interferon‐α
- IQR
interquartile range
- IRF4
interferon regulatory factor 4
- LDH
lactate dehydrogenase
- MST
median survival time
- OS
overall survival
- PD‐L1
programmed death‐ligand 1
- PS
performance status
- VCAP‐AMP‐VECP
vincristine, cyclophosphamide, doxorubicin and prednisone, followed by doxorubicin, ranimustine, and prednisone and then by vindesine, etoposide, carboplatin, and prednisone
1. INTRODUCTION
Adult T‐cell leukemia/lymphoma (ATL) is a mature, peripheral T‐cell malignancy 1 caused by human T‐cell leukemia virus type I (HTLV‐1) infection. 2 , 3 Regions of high HTLV‐1 endemicity and ATL prevalence are limited to Japan, sub‐Saharan Africa, South America, and central Australia. 4 Worldwide, at least 3000 new cases of ATL are diagnosed each year. 5 In Japan, there are one million HTLV‐1 carriers, 6 4000 new HTLV‐1 infections annually, 7 and 1000 deaths from ATL annually. 8
The diagnostic criteria and subtypes of ATL were first established in Japan in 1991 by the Lymphoma Study Group of Japan Clinical Oncology Group (JCOG) 9 based on the prognoses of patients enrolled in several nationwide hospital‐based surveys from 1983‐1987. 10 , 11 These criteria are now known as the “Shimoyama classification.”
It has been over 25 years since the establishment of the criteria for ATL, 9 and various treatment options are now available. These include “watchful waiting” for indolent ATL (smoldering‐ and favorable chronic‐types) 12 and a variety of intensive chemotherapies followed by allogeneic hematopoietic stem cell transplantation (allo‐HSCT) for aggressive ATL (acute‐, lymphoma‐, and unfavorable chronic‐types). 13 , 14 More recently, several promising new agents, including an anti‐CCR4 antibody (mogamulizumab) 15 and an immunomodulatory agent (lenalidomide) 16 have been approved for aggressive ATL. Therefore, it was expected that the prognoses of patients with ATL in Japan would improve compared with those reported in the 1980s‐1990s.
However, the majority of previous reports from Japan have suggested that ATL patient outcomes have not improved as expected. 17 , 18 , 19 As an example, a study of 1594 ATL patients diagnosed in 2000‐2009 19 reported that the 4‐year survival of smoldering‐type ATL was lower than in the 1991 report, 9 despite an improvement of survival rates for acute/lymphoma‐type ATL. However, most of the study procedures in the previously published reports differed from those of the 1991 report, 9 particularly in collecting data from participating hospitals. Therefore, to evaluate more precisely whether the prognostic features of current patients with ATL have changed compared with those in the 1991 report, 9 it is necessary to analyze data on recent ATL patients using similar procedures to those of the 1991 report.
We have already conducted a nationwide survey study of newly diagnosed ATL patients in 2010‐2011 in all of Japan 20 using similar procedures to those of the 1991 report, 9 , 10 , 11 accumulated data on 996 patients from 126 hospitals, and reported a significant shift toward older age at diagnosis and an increasing proportion of lymphoma‐type ATL in older patients compared with the 1991 report. However, we have not yet evaluated the outcomes of the registered patients. We therefore conducted this follow‐up survey study in a nationwide setting, using a similar method to that of the 1991 report. 10 , 11
The primary purpose of this study was to evaluate the prognosis of ATL patients by clinical subtypes and by treatment modality across Japan from the date of diagnosis in 2010‐2011 until the latest follow‐up or death by 2016‐2017. Then, we evaluated whether the prognoses of recently diagnosed ATL patients have improved compared with those described in the 1991 Shimoyama report in Japan. 9
2. MATERIALS AND METHODS
2.1. Study design and patients
This was a retrospective, observational, multicenter, hospital‐based study of ATL patients newly diagnosed in 2010‐2011 at 126 participating hematological and dermatological departments across Japan. The patients had already been registered in the baseline database developed through our nationwide, multicenter, hospital‐based study. 20 In this follow‐up study, we additionally collected data on treatments and outcomes for all registered patients as of 2016‐2017. The study protocol was approved by the ethics committee and institutional review board (IRB) of the National Cancer Center of Japan (approval no. 2014‐235). The ethical committee waived the need for written informed consent because of the retrospective nature of the study and anonymous data collection.
The study procedures followed as much as possible those of the 1991 report, 9 in which the diagnostic criteria for ATL were first described. 9 First, we inquired whether the chief physician of each hospital would be able to participate in this follow‐up study by sending a questionnaire to all 126 participating hospitals between April and June of 2016. The first questionnaires were returned by 113 hospitals (89.6%); of those, 98 (85.7%) hospitals agreed to participate in this follow‐up study after the approval of the study protocol by the regional IRB of each hospital. We then sent the follow‐up survey to each of the 98 hospitals. The survey consisted of the following elements: (a) patient baseline information recorded in the database of the first survey 20 ; (b) final outcome status, including causes of death; (c) chemotherapy status and response status to initial regimens; (d) status of radiotherapy, anti‐CCR4 antibody therapy, and allo‐HSCT; (e) information on ATL subtype and on acute transformation when any therapeutic options started in indolent subtypes. A reminder letter was mailed if the survey was not returned. All data were collected by a professional clinical research support office (Ata‐Life Inc, Tokyo, Japan) by the end of April 2017 and the data cleaning process was completed by the end of September 2018.
This follow‐up study focused on the treatment and outcome of ATL by clinical subtype, clinical condition at diagnosis, and treatment modality, but not on prognostic parameters, through multivariate analysis. We used the centrally reviewed subtypes of ATL in our previous baseline data. 20 The diagnostic agreement for subtype classification between participating institutions and central review was 0.72.
2.2. Statistical analysis
Continuous variables were summarized as medians [range or interquartile range (IQR)] and compared using Wilcoxon's rank‐sum test. Continuous variables were also stratified into groups as necessary. Differences in the frequencies of categorical variables were compared using the chi‐square test or Fisher's exact test. Follow‐up duration was calculated from the diagnosis date to the date of death or the last follow‐up at participating hospitals. A cumulative 4‐year overall survival (OS) rate and a median survival time (MST) were estimated using the Kaplan‐Meier method, and curve comparison was performed using both the log‐rank test and the generalized Wilcoxon test. MST was defined as the time at which the cumulative survival probability was 50%. Graphical and statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software) and JMP Pro 13 (SAS Institute). Two‐sided P‐values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Characteristics of patients
Figure 1A shows inclusion/exclusion of patients according to the STROBE guidelines. 21 Among 996 patients registered in the baseline study, 20 119 were excluded due to lack of participation information from hospitals. The remaining 877 patients (88.1%) from 98 hospitals were initially included. However, four hospitals that had previously registered 13 patients did not return outcome information, and thus we included 864 patients in the outcome dataset. Of 864 patients, 94 were excluded during the central review process. Finally, 770 patients were included in the prognosis analysis. Of 770 patients, 391 (50.8%) had acute‐type, 192 (24.9%) had lymphoma‐type, 106 (13.8%) had chronic‐type, and 81 (10.5%) had smoldering‐type ATL based on previous central review. 20 The demographic and clinical characteristics at diagnosis of the 770 patients are summarized by subtype in Table 1.
Figure 1.

A, Study flow chart for patient inclusion/exclusion. B, Overall survival (OS) and median survival time (MST) by adult T‐cell leukemia/lymphoma (ATL) subtype
Table 1.
Clinical characteristic of patients with adult T‐cell leukemia/lymphoma (ATL) at diagnosis by subtypes
| Characteristic | Summary unit | Total No. evaluated | ATL subtype according to Shimoyama's classification | |||
|---|---|---|---|---|---|---|
| Acute (n = 391) | Lymphoma (n = 192) | Chronic (n = 106) | Smoldering (n = 81) | |||
| Male Sex | n (%) | 770 | 211 (54.0) | 113 (58.9) | 43 (40.6) | 44 (54.3) |
| Age at diagnosis | Median (range) y | 770 | 68 (34‐94) | 70 (37‐91) | 65 (36‐85) | 68 (40‐89) |
| WBC count | Median (range) x109/L | 770 | 12.3 (2.3‐257) | 6.2 (1.1‐34.9) | 14.0 (7.6‐234.9) | 6.4 (1.5‐11.4) |
| Abnormal lymphocyte | % of WBC | 767 | 19 (0‐99.5) | 0‐1 | 37 (0‐95) | 6 (0‐34) |
| Serum albumin | Median (range) g/dL | 757 | 3.5 (0‐5) | 3.7 (1.6‐4.8) | 4 (0‐5) | 4.2 (0‐4.9) |
| BUN | Median (range) mg/dL | 767 | 16.4 (4.8‐170) | 15.1 (4.7‐57.5) | 13.5 (0‐26.7) | 15.5 (7‐45.2) |
| Creatinine | Median (range) mg/dL | 769 | 0.8 (0.3‐9.4) | 0.77 (0.29‐10.4) | 0.7 (0.4‐1.6) | 0.8 (0.3‐2.6) |
| CRP | Median (range) mg/dL | 765 | 0.9 (0‐34.9) | 0.9 (0‐29) | 0.2 (0‐17.6) | 0.1 (0‐5.1) |
| sIL‐2R | Median (range) U/mL | 720 | 23 900 (316‐41.7 × 106) | 10 661 (290‐4 × 105) | 6581 (200‐66.6 × 103) | 1076 (8.7‐52.8 × 103) |
| LDH | Median (range) IU/L | 769 | 583 (132‐13 990) | 398 (150‐16 900) | 257 (145‐467) | 216 (125‐341) |
| LDH > ULN | % | 770 | 87.7 | 87.0 | 56.6 | 38.3 |
| Hypercalcemia present (>11) | % | 744 | 30.3 | 14.4 | 0 | 0 |
| Ann Arbor stage, I‐II | % | 722 | 0.5 | 24.0 | 0 | 0 |
| ECOG PS (0‐1) | % | 750 | 53.5 | 65.1 | 88.4 | 89.7 |
| B symptoms present | % | 731 | 31.1 | 26.1 | 10.9 | 4.1 |
Abbreviations: BUN, blood urea nitrogen; CRP, C‐reactive protein; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status; sIL‐2R, soluble interleukin‐2 receptor; ULN, upper limit of normal; WBC, white blood cells.
3.2. Treatment regimens
Among 391 patients with acute‐type ATL, 343 (87.7%) received chemotherapies (Table 2). Of these patients, 161 (46.9%) received VCAP‐AMP‐VECP‐like regimens, 22 132 (38.5%) received CHOP‐like regimens, and the remainder received other regimens (Table S1). The choice of chemotherapy regimen for acute‐type ATL patients differed significantly by age and B‐symptom status, but not by Eastern Cooperative Oncology Group performance status (PS) nor by hypercalcemia status. For patients aged < 70 years, VCAP‐AMP‐VECP‐like regimens were the most common (61.6%) followed by CHOP‐like regimens, whereas for those aged ≥ 70 years, CHOP‐like regimens were the most common (49.7%). Allo‐HSCT was administered in 62 (15.9%) acute‐type ATL patients; the majority were those aged < 70 years (n = 61), those with good PS, and those without hypercalcemia at diagnosis. The chemotherapy regimens were decided by the attending hematologists or dermatologists in each hospital, and information on the reasons for the choice of chemotherapy regimen was not collected in this study.
Table 2.
Diversity in treatment options and chemotherapy response for acute or lymphoma type of adult T‐cell leukemia/lymphoma (ATL)
| Subtype and condition | Systematic chemotherapy received, n | First systematic chemotherapy regimens n (% of row) | P | Chemotherapy responses n (% of row) | P | Allo‐HSCT received n (% of row) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCAP‐AMP‐VECP regimen or the like | CHOP regimen or the like | Others a | CR | PR | SD (NC) | PD | Unkown | ||||||
| Acute type | |||||||||||||
| Chemotherapy received, all | 343 | 161 (46.9) | 132 (38.5) | 50 (14.6) | 49 (14.3) | 169 (49.3) | 39 (11.4) | 67 (19.5) | 19 (5.5) | 62 | |||
| Age at diagnosis | |||||||||||||
| <70 y | 198 | 122 (61.6) | 60 (30.3) | 16 (8.1) | <.0001 | 40 (20.5) | 90 (46.2) | 24 (12.3) | 32 (16.4) | 9 (4.6) | .0024 | 61 (30.8) | <.0001 |
| ≥70 y | 145 | 39 (26.9) | 72 (49.7) | 34 (23.4) | 9 (6.3) | 79 (55.6) | 15 (10.6) | 35 (24.7) | 4 (2.8) | 1 (0.7) | |||
| ECOG PS | |||||||||||||
| PS 0,1 | 188 | 96 (51.0) | 67 (35.6) | 25 (13.3) | .27 | 36 (19.4) | 90 (48.4) | 17 (9.1) | 39 (20.4) | 5 (2.7) | .05 | 50 (26.6) | <.0001 |
| PS 2‐4 | 149 | 63 (42.3) | 61 (40.9) | 25 (16.8) | 13 (9.0) | 75 (51.7) | 22 (15.2) | 28 (19.3) | 7 (4.8) | 12 (8.1) | |||
| Unknown | 6 | ||||||||||||
| Hypercalcemia | |||||||||||||
| Absent | 234 | 116 (49.6) | 89 (38.0) | 29 (12.4) | .19 | 38 (16.5) | 109 (47.4) | 29 (12.6) | 45 (19.6) | 9 (3.9) | .49 | 49 (20.9) | .14 |
| Present | 100 | 41 (41.0) | 40 (40.0) | 19 (19.0) | 10 (10.2) | 55 (56.1) | 10 (10.2) | 20 (20.4) | 3 (3.1) | 12 (10.4) | |||
| Unknown | 9 | ||||||||||||
| B symptoms | |||||||||||||
| Absent | 225 | 113 (50.2) | 74 (32.9) | 38 (16.9) | .009 | 32 (14.2) | 113 (50.2) | 26 (11.6) | 40 (17.8) | 14 (6.2) | .4 | 43 (19.4) | .65 |
| Present | 103 | 40 (38.8) | 52 (50.5) | 11 (10.7) | 14 (13.6) | 50 (48.5) | 13 (12.6) | 23 (22.3) | 3 (2.9) | 17 (16.7) | |||
| Unknown | 15 | ||||||||||||
| Lymphoma type | |||||||||||||
| Chemotherapy received, all | 175 | 78 (44.6) | 73 (41.7) | 24 (13.7) | 39 (22.3) | 79 (45.1) | 26 (14.9) | 26 (14.9) | 5 (2.8) | 20 | |||
| Age at diagnosis | |||||||||||||
| <70 y | 85 | 49 (57.6) | 32 (37.7) | 4 (4.7) | .0002 | 27 (31.7) | 35 (41.2) | 5 (5.9) | 15 (17.7) | 3 (33.5) | .001 | 20 (23.5) | <.0001 |
| ≥70 y | 90 | 29 (32.2) | 41 (45.6) | 20 (22.2) | 12 (13.5) | 44 (49.4) | 21 (23.6) | 11 (12.4) | 1 (1.1) | 0 | |||
| ECOG PS | |||||||||||||
| PS 0,1 | 112 | 51 (45.5) | 47 (42.0) | 14 (12.5) | 31 (27.7) | 50 (44.6) | 15 (13.4) | 13 (11.6) | 3 (2.7) | .12 | 17 (15.2) | .12 | |
| PS 2‐4 | 57 | 23 (40.4) | 24 (42.1) | 10 (17.5) | .63 | 7 (12.5) | 26 (46.4) | 9 (16.1) | 13 (23.1) | 1 (1.8) | 3 (5.3) | ||
| Unknown | 6 | ||||||||||||
| Hypercalcemia | |||||||||||||
| Absent | 146 | 68 (46.6) | 57 (39.0) | 21 (14.4) | .47 | 32 (22.1) | 67 (46.2) | 21 (14.5) | 23 (15.9) | 2 (1.4) | .27 | 15 (10.3) | .65 |
| Present | 25 | 9 (36.0) | 13 (52.0) | 3 (12.0) | 6 (24.0) | 29 (36.0) | 5 (20.0) | 3 (12.0) | 2 (8.0) | 4 (16.0) | |||
| Unknown | 4 | ||||||||||||
| B symptoms | |||||||||||||
| Absent | 127 | 59 (46.4) | 50 (39.4) | 18 (14.2) | .27 | 27(21.3) | 62 (48.8) | 21 (16.5) | 13 (10.2) | 4 (3.1) | .04 | 15 (10.3) | .2 |
| Present | 41 | 14 (34.1) | 22 (53.7) | 5 (12.2) | 9 (22.0) | 16 (39.0) | 3 (7.3) | 12 (29.3) | 1 (2.4) | 4 (9.8) | |||
| Unknown | 7 | ||||||||||||
Abbreviations: allo‐HSCT, allogeneic hematopoietic stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; ECOG, Eastern Cooperative Oncology Group; NC, no change; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease, VCAP‐AMP‐VECP, vincristine, cyclophosphamide, doxorubicin and prednisone, followed by doxorubicin, ranimustine, and prednisoneand then by vindesine, etoposide, carboplatin, and prednisone.
Other miscellaneous regimens are summarized in Table S1.
Among 192 patients with lymphoma‐type ATL, 175 (91.1%) received chemotherapies, 11 (5.7%) did not receive any chemotherapy, and the remainder had unknown status (3.1%). Of those who received any chemotherapies (Table 2), 78 (44.6%) received VCAP‐AMP‐VECP‐like regimens, 73 (41.7%) received CHOP‐like regimens, and the remainder received other regimens (Table S1). The chemotherapy regimens for lymphoma‐type ATL patients differed significantly by age (Table 2), but not by PS, hypercalcemia status, or B symptom status. Allo‐HSCT was administered in 20 (10.4%) lymphoma‐type ATL patients (only in those aged < 70 years); the majority were patients with good PS and without hypercalcemia.
Among 106 patients with chronic‐type ATL, 71 (67.0%) had unfavorable chronic‐type ATL, 33 (31.1%) had favorable chronic‐type ATL (Table 3), and the information on the poor prognostic factors was not available for the remaining patients. Among patients with unfavorable chronic‐type ATL, 54 (76.0%) received chemotherapies with a median interval from diagnosis to initiation of chemotherapy of 21 days (IQR, 7‐118 days) (Table 3). The chemotherapy regimens received were VCAP‐AMP‐VECP‐like regimens in 22 patients (40.8%), CHOP‐like regimens in 12 patients (22.2%), and other regimens in 20 patients (37.0%) (Table S1). Thirteen (18.3%) patients with unfavorable chronic‐type ATL received allo‐HSCT following chemotherapy, 41 (57.7%) received chemotherap y alone, 16 (22.5%) received no chemotherapy, and one had insufficient data. Among patients with favorable chronic‐type ATL, 20 (60.6%) received chemotherapy (Table 3) due to progression to unfavorable chronic‐type ATL (n = 9) or acute transformation (n = 11). The median interval from diagnosis to initiation of chemotherapy was 566 days, significantly longer compared with patients with unfavorable chronic‐type ATL (P = 0.002). The chemotherapy regimens used for favorable chronic‐type ATL were VCAP‐AMP‐VECP‐like regimens in 12 patients (60%) and CHOP‐like regimens in five patients (25%) (Table 3). Patients aged < 70 years received VCAP‐AMP‐VECP‐like regimens more often than those aged ≥ 70 years (P = .049). Eight (24.2%) patients with favorable chronic‐type ATL received allo‐HSCT following chemotherapy, 11 (33.3%) were treated with chemotherapy alone, one received chemotherapy but had no available data on allo‐HSCT, 12 (36.4%) received no chemotherapy, and one had no available data on chemotherapy.
Table 3.
Diversity in treatment options for chronic or smoldering subtypes of adult T‐cell leukemia/lymphoma (ATL)
| Subtype and condition | Systematic chemotherapy received, n | Days from diagnosis to chemotherapy, median (ranges) | P | First systematic chemotherapy regimens, n (% of row) | P*Fischer | Chemotherapy responses, n (% of row) | P*Fischer | allo‐HSCT received, n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCAP‐AMP‐VEC P or the like | CHOP or the like | Others a | CR | PR | SD (NC) | PD | Unknown | |||||||
| Unfaborable chronic type | ||||||||||||||
| N. received | 54 | 21 (0‐1862) | 22 (40.8) | 12 (22.2) | 20 (37.0) | ‐ | 8 (14.8) | 27 (50.0) | 6 (11.1) | 13 (24.1) | ‐ | 13 | ||
| Age at diagnosis | ||||||||||||||
| <70 y | 33 | 19 (0‐1862) | 0.9 | 19 (57.6) | 6 (18.2) | 8 (24.2) | .45 | 4 (12.2) | 18 (54.5) | 4 (12.2) | 7 (21.2) | .77 | 13 | |
| ≥70 y | 21 | 22 (1‐757) | 3 (14.3) | 6 (28.6) | 12 (57.1) | 5 (19.1) | 9 (42.9) | 2 (9.5) | 6 (28.6) | 0 | ||||
| ECOG PS at diagnosis | ||||||||||||||
| PS 0,1 | 47 | 19 (0‐757) | 0.7 | 21 (44.7) | 11 (23.4) | 15 (31.9) | .07 | 7 (14.9) | 24 (51.1) | 5 (10.6) | 11 (23.4 | .91 | 13 | |
| PS 2‐4 | 5 | 18 (2‐89) | 0 | 1 (20.0) | 4 (80.0) | 1 (20.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 0 | ||||
| Unknown | 2 | |||||||||||||
| Skin lesion | ||||||||||||||
| Absent | 31 | 15.5 (0‐1862) | 0.99 | 13 (41.9) | 6 (19.4) | 12 (38.7) | .78 | 4 (12.9) | 15 (48.4) | 3 (9.7) | 9 (29.0) | .82 | 5 | |
| Present | 22 | 26.5 (1‐477) | 9 (40.9) | 6 (27.3) | 7 (31.8) | 3 (13.6) | 12 (54.6) | 3 (13.6) | 4 (18.2) | 7 | ||||
| Unknown | 1 | |||||||||||||
| Favorable chronic type | ||||||||||||||
| N. received | 20 | 566 (2‐1460) | 12 (60.0) | 5 (25.0) | 3 (15.0) | ‐ | 3 (15.0) | 9 (45.0) | 6 (30.0) | 1 (5.0) | 1 | ‐ | 8 | |
| Age at diagnosis | ||||||||||||||
| <70 y | 17 | 566 (2‐1460) | 0.52 | 12 (70.6) | 3 (17.7) | 2 (11.7) | .049 | 3 (17.7) | 8 (47.1) | 4 (23.5) | 1 (5.9) | 1 | .67 | 8 |
| ≥70 y | 3 | 301 (6‐596) | 0 | 2 (66.7) | 1 (33.3) | 0 | 1 (33.3) | 2 (66.7) | 0 | 0 | ||||
| ECOG PS at diagnosis | ||||||||||||||
| PS 0,1 | 20 | 566 (2‐1460) | ‐ | 12 (60.0) | 5 (25.0) | 3 (15.0) | ‐ | 3 (15.0) | 9 (45.0) | 6 (30.0) | 1 (5.0) | 1 | ‐ | 8 |
| PS 2‐4 | 0 | 0 | 0 | 0 | 0 | |||||||||
| Skin lesion | ||||||||||||||
| Absent | 12 | 499 (2‐1460) | 0.14 | 8 (66.7) | 3 (25.0) | 1 (8.3) | .81 | 3 (25.0) | 7 (58.3) | 1 (8.3) | 1 (8.3) | .024 | 2 | |
| Present | 8 | 726 (86‐1098) | 4 (50.0) | 2 (25.0) | 2 (25.0) | 0 | 2 (25.0) | 5 (62.5) | 0 | 1 | 6 | |||
| Smoldering type | ||||||||||||||
| N received | 30 | 232 (23‐1344) | 9 (30.0) | 8 (26.7) | 13 (43.3) | ‐ | 3 (10.0) | 12 (40.0) | 6 (20.0) | 6 (20.0) | 3 | ‐ | 6 | |
| Age at diagnosis | ||||||||||||||
| <70 y | 22 | 228 (23‐1344) | 0.77 | 9 (40.9) | 7 (31.8) | 6 (27.3) | .01 | 3 (13.6) | 11 (50.0) | 2 (9.1) | 4 (18.2) | 2 | .06 | 6 |
| ≥70 y | 8 | 236 (63‐1186) | 0 | 1 (12.5) | 7 (87.5) | 0 | 1 (12.5) | 4 (50.0) | 2 (25.0) | 0 | ||||
| ECOG PS at diagnosis | ||||||||||||||
| PS 0,1 | 28 | 236 (23‐1344) | ‐ | 9 (32.1) | 8 (28.6) | 11 (39.3) | ‐ | 3 (10.7) | 12 (42.9) | 6 (21.4) | 4 (14.3) | 3 | ‐ | 6 |
| PS 2‐4 | 1 | 101 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | ||||
| Unknown | 1 | |||||||||||||
| Skin lesion | ||||||||||||||
| Absent | 8 | 417 (64‐1212) | 0.39 | 1 (12.5) | 1 (12.5) | 6 (75.0) | .14 | 1 (12.5) | 2 (25.0) | 2 (25.0) | 2 (25.0) | .8 | 1 | |
| Present | 21 | 228 (23‐1344) | 8 (38.1) | 7 (33.3) | 6 (28.6) | 2 (9.52) | 10 (47.6) | 4 (19.1) | 3 (14.3) | 2 | 5 | |||
| Unknown | 1 | |||||||||||||
Abbreviations: allo‐HSCT, allogeneic hematopoietic stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; ECOG, Eastern Cooperative Oncology Group; NC, no change; ND, not done; P*Fischer, Fisher's exact test; P, P‐value; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease, VCAP‐AMP‐VECP, vincristine, cyclophosphamide, doxorubicin and prednisone, followed by doxorubicin, ranimustine, and prednisoneand then by vindesine, etoposide, carboplatin, and prednisone.
Other miscellaneous regimens are summarized in Table S1.
Among 81 patients with smoldering‐type ATL, 43 (53.8%) had skin lesions and received a variety of topical treatments (data not shown). During follow‐up, 30 of 81 patients (37%) received chemotherapies due to acute crisis (n = 26) and worsened skin lesions (n = 4) (Table 3). The chemotherapy regimens used for smoldering‐type ATL were VCAP‐AMP‐VECP‐like regimens in nine patients (30.0%) and CHOP‐like regimens in eight patients (26.7%) (Table 3). Patients aged < 70 years were more commonly treated with VCAP‐AMP‐VECP‐like or CHOP‐like regimens, whereas those aged ≥ 70 years were more commonly treated with other regimens (Table S1) (P = .01). Among patients treated with any chemotherapy, six (7.4%) received allo‐HSCT following chemotherapy; all were < 70 years old at diagnosis with good PS, and five were positive for skin lesions (Table 3). Twenty‐four patients (29.6%) were treated with chemotherapy alone, and the remaining 51 (63.0%) received no chemotherapy.
3.3. Chemotherapy responses
Responses to initial chemotherapy for patients with acute/lymphoma‐type ATL are summarized in Table 2. Of 343 patients with acute‐type ATL receiving any chemotherapy, 49 (14.3%) achieved complete remission (CR) and 169 (49.3%) achieved partial remission (PR). Of 175 lymphoma‐type ATL patients treated with any chemotherapy, 39 (22.3%) and 79 (45.1%) achieved CR and PR, respectively. The CR/PR rates for acute/lymphoma‐type ATL were significantly lower in patients aged ≥ 70 years compared with those aged < 70 years. Moreover, CR/PR rates were significantly lower in patients with advanced PS for acute‐type ATL and in those with B symptoms for lymphoma‐type ATL. Patients treated with VCAP‐AMP‐VECP‐like regimens had significantly better response than those treated with CHOP‐like regimens in acute‐type ATL, and there was no significant difference in response between those treated with VCAP‐AMP‐VECP‐like regimens and those treated with CHOP‐like regimens in lymphoma‐type ATL (Table S4).
Responses to initial chemotherapy among patients with chronic/smoldering‐type ATL are summarized in Table 3. Of 54 patients with unfavorable chronic‐type ATL treated with any chemotherapy, 8 (14.8%) achieved CR and 27 (50.0%) achieved PR. The CR/PR rates for patients with unfavorable chronic‐type ATL did not differ significantly by age, PS, or skin lesion status. Of 20 patients with favorable chronic‐type ATL treated with any chemotherapy, 3 (15.0%) achieved CR and 9 (45.0%) achieved PR. The CR/PR rates did not differ significantly by age but were significantly lower in patients with skin lesions than in those without (P = .024). Of 30 patients with smoldering‐type ATL treated with any chemotherapy, 15 (50%) achieved CR/PR, but six (20%) progressed. The CR/PR rates tended to be lower in patients aged ≥ 70 years than in those aged < 70 years (P = .06) but did not differ significantly by skin lesion positivity.
In summary, the CR and CR/PR rates were lowest in patients with smoldering‐type ATL, intermediate in patients with acute‐ and favorable/unfavorable chronic‐type ATL, and highest in patients with lymphoma‐type ATL. However, the sample sizes were small, and the chemotherapy regimens and periods from diagnosis to initiation of chemotherapy were more diverse in patients with indolent ATL compared with aggressive ATL.
3.4. Prognoses
At the time of last observation, 243 (31.6%) of 770 patients were alive and 527 (68.4%) had died. The median follow‐up time for living patients was 1464 days (IQR, 176‐883.8 days). The 4‐year OS for all patients was 24.8% (95% confidence interval, 21.6‐28.2%) with a MST of 355 days (IQR, 151‐1438 days). The number of deaths and follow‐up duration by subtype are summarized in Table 4. The 4‐year OS and MST were lowest in acute‐type ATL, followed by lymphoma‐, chronic‐, and smoldering‐type ATL (Figure 1B and Table 4). The 4‐year OS and MST were significantly lower for unfavorable than for favorable chronic‐type ATL (Table 4, Figure 3A). Only 36 patients with acute/lymphoma‐type ATL received the new agent, mogamulizumab, 15 after its approval in Japan. The 3‐year OS and MST in patients treated with mogamulizumab were 9% and 4.8 months, respectively (figure not shown).
Table 4.
Prognosis variation of adult T‐cell leukemia/lymphoma (ATL) by subtype
| Items related to prognosis | Acute (n = 391) | Lymphoma (n = 192) | Chronic (n = 106) | Among chronic subtype | Smoldering (n = 81) | Among smoldering subtype | ||
|---|---|---|---|---|---|---|---|---|
| Unfavorable (n = 71) | Favorable (n = 33) | Skin lesion positive (n = 43) | Skin lesion negative (n = 37) | |||||
| Follow‐up duration for all, median (IQR), d | 210 (101‐468) | 252 (131‐619) | 631 (217‐1467) | 425 (183‐1467) | 1438 (612‐1607) | 968 (386‐1693) | 662 (394‐1701) | 1494 (370‐1692) |
| Follow‐up duration only for alive patients, median (IQR), d | 1354 (110‐1784) | 1440 (106‐1688) | 1491 (535‐1761) | 1543 (267‐1878) | 1481 (1060‐1690) | 1561 (419‐1744) | 1379 (293‐1731) | 1594 (1202‐1757) |
| No. of deaths (proportion of each total) | 297 (76.0%) | 139 (72.4%) | 61 (57.5%) | 47 (66.2%) | 13 (39.4%) | 30 (37.0%) | 18 (41.9%) | 11 (29.7%) |
| MST (95%CI), d | 252 (214‐301) | 305 (244‐364) | 778 (599‐1264) | 572 (339‐690) | 1937 (1137‐1937) | 1851 (1275‐NR) | 1739 (594‐NR) | NR |
| 4‐year survival rate (95%CI), % | 16.8 (13.2‐21.2) | 19.6 (14.2‐26.4) | 37.4 (28.2‐47.8) | 26.6 (17.1‐38.9) | 62.1 (43.4‐77.7) | 59.8 (47.6‐70.9) | 54.3 (37.6‐70.0) | 68.5 (50.6‐82.1) |
Abbreviations: CI, confidence interval; IQR, interquartile range; MST, median survival time; NR, not reached.
Figure 3.
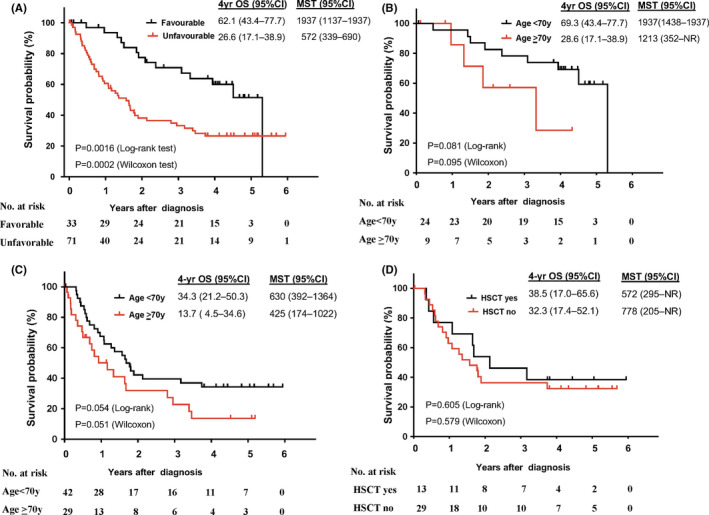
Survival analysis of chronic adult T‐cell leukemia/lymphoma (ATL) subtype. A, Overall survival (OS) in chronic ATL according to favorable and unfavorable subtype. B, OS in favorable chronic ATL by age. C, OS in unfavorable chronic ATL by age. D, OS in unfavorable chronic ATL by allogeneic hematopoietic stem cell transplantation (allo‐HSCT) in patients aged < 70 y. Abbreviations: CI, confidence interval; MST, median survival time; NR, not reached
The 4‐year OS and MST were higher for patients aged < 70 years than for those aged ≥ 70 years with acute‐ (Figure 2A), lymphoma‐ (Figure 2C), favorable chronic‐ (Figure 3B), and unfavorable chronic‐type (Figure 3C), but not with smoldering‐type ATL (data not shown). In patients aged < 70 years, the 4‐year OS of acute‐type ATL was significantly higher for those who received allo‐HSCT (38.9%) compared with those who did not (17.0%) (Figure 2B). This was not the case for lymphoma‐type ATL, although the OS tended to be higher in patients who received allo‐HSCT (39.1%) than in those who did not (26.3%) (Figure 2D). The survival of patients aged < 70 without allo‐HSCT was significantly higher than that of those aged ≥ 70, both in acute‐type ATL (P = .001 by log‐rank test) and in lymphoma‐type ATL (P = .0029 by log‐rank test). For patients with unfavorable chronic‐type ATL aged < 70 years, the 4‐year OS did not differ significantly in those who received and in those who did not receive allo‐HSCT (Figure 3D). Among patients with smoldering‐type ATL, there was no significant difference in 4‐year OS between those with skin involvement and those without, although the survival tended to be worse in those with skin involvement (Figure S1).
Figure 2.

Survival analysis of acute and lymphoma adult T‐cell leukemia/lymphoma (ATL) subtypes. A, Overall survival (OS) in acute ATL by age stratum at diagnosis. B, OS in acute ATL by allogeneic hematopoietic stem cell transplantation (allo‐HSCT) status among patients aged < 70 y. C, OS in lymphoma ATL by age stratum at diagnosis. D, OS in lymphoma ATL by allo‐HSCT status among patients aged < 70 y. Abbreviations: CI, confidence interval; MST, median survival time; NR, not reached
Finally, we summarized the clinical outcomes of patients by ATL subtype based on the 4‐year OS (Figure 4). The proportion (percentage of total) is shown for each column; by age status, by allo‐HSCT status, and by outcome status in acute‐ and lymphoma‐types; by poor prognostic factor status, by chemotherapy status, and by outcome status in chronic‐type; by skin lesion status, by chemotherapy status, and by outcome status in smoldering‐type. The 4‐year OS is also shown for patients aged < 70 treated with or without allo‐HSCT and for those aged ≥ 70 in acute‐ and lymphoma‐types. Among all patients with acute‐ and lymphoma‐type ATL, allo‐HSCT was applied for 18% and 11%, with a 4‐year OS of 7% and 4%, respectively (Figure 4A,B), and allo‐HSCT was not applied for 82% and 89%, with a 4‐year OS of 10% and 16%, respectively (Figure 4A,B). Among all patients with chronic‐type ATL, the 4‐year OS of those who received chemotherapy and those who did not was 10% and 8%, respectively, in unfavorable chronic‐type ATL, and 10% and 9%, respectively, in favorable chronic‐type ATL (Figure 4C). Among all patients with smoldering‐type ATL, the 4‐year OS of those who received chemotherapy and those who did not was 12% and 18%, respectively, in those with skin lesions, and 5% and 26%, respectively, in those without skin lesions (Figure 4D).
Figure 4.

Summary of outcomes of adult T‐cell leukemia/lymphoma (ATL) patients based on 4‐year overall survival (OS) in Japan by subtype: acute‐type (A), lymphoma‐type (B), chronic‐type (C), and smoldering‐type (D). The percentage in each box represents the estimated proportion of patients. Results based on the outcomes of patients who received any chemotherapy are shown for acute‐ and lymphoma‐type ATL. aDied despite allogeneic hematopoietic stem cell transplantation (allo‐HSCT) (only patients aged < 70 y). bDied despite chemotherapy without allo‐HSCT. cReceived chemotherapy during observation period. dDied despite chemotherapy. eAlive without chemotherapy in patients with unfavorable chronic‐type ATL. Abbreviations: Rate at 4‐y, survival rate at 4‐y follow‐up; UK, unknown
3.5. Causes of death
Among 527 patients who died, 414 (78.6%) died from ATL, 53 (10.0%) from infection, 11 (2.1%) from malignant diseases other than ATL, and the rest from other causes (Table S2). For patients with acute‐, lymphoma‐, and chronic‐type ATL, the second most common cause of death was infection. However, for patients with smoldering‐type ATL, the second most common cause of death was non‐ATL malignancy (accounting for 20% of deaths).
4. DISCUSSION
This report provides a comprehensive clinical picture of ATL patients in 2010‐2011 in Japan. Our main findings are that (a) the 4‐year OS for acute/lymphoma‐type ATL has improved compared with that in the 1991 report 9 despite an increasing number of older patients, 20 but (b) the 4‐year OS of smoldering/chronic‐type ATL has apparently not improved. In Table 5, we summarize the outcomes of ATL patients in Japan by subtype and by diagnosis year based on the 1991 report, 9 the 2015 report, 19 and the present report. The difference in the study design between the 2015 report and the present study is also summarized in Table S3. Although it is not possible to directly compare these studies with one another, our study provides important information on the current status of patients with ATL in clinical practice in Japan.
Table 5.
Summary of the prognosis of adult T‐cell leukemia/lymphoma (ATL) in literature and the present study
| Shimoyama report | Katsuya report | The present study | |
|---|---|---|---|
| Year of published (reference No.) | 1991 14 | 2015 23 | 2019 |
| Total | |||
| Year of diagnosis | 1983‐1987 | 2000‐2009 | 2010‐2011 |
| Year of the last follow‐up | 1990 | NA | 2016 |
| No. evaluated | 818 | 1594 | 770 |
| Age at diagnosis, median or mean (ranges), y | 57.1 (24‐92) | 61‐63 (NA) | 68 (34‐94) |
| No. dead (% of evaluated) | 565 (69.1) | 1128 (70.8) | 527 (68.4) |
| Acute | |||
| No. evaluated | 465 | 895 | 391 |
| MST, mo | 6.2 | 8.3 | 8.3 |
| 4‐y survival rate (%) | 5.0 | 11.4 | 16.8 |
| Lymphoma | |||
| No. evaluated | 156 | 355 | 192 |
| MST, mo | 10.2 | 10.6 | 10.0 |
| 4‐y survival rate (%) | 5.7 | 16.2 | 19.6 |
| Chronic, all | |||
| No. evaluated | 152 | 187 | 106 |
| MST, mo | 24.3 | 31.5 | 25.5 |
| 4‐y survival rate (%) | 26.9 | 35.6 | 37.4 |
| Unfavorable chronic | |||
| No. evaluated | NA | 15 | 71 |
| MST, mo | NA | NR | 18.8 |
| 4‐y survival rate (%) | NA | 29.0 | 26.6 |
| Favorable chronic | |||
| No. evaluated | NA | 172 | 33 |
| MST, mo | NA | NR | 63.5 |
| 4‐y survival rate (%) | NA | 60.0 | 62.1 |
| Smoldering | |||
| No. evaluated | 45 | 157 | 81 |
| MST, mo | NR | 55.0 | 60.7 |
| 4‐y survival rate (%) | 62.8 | 51.9 | 59.8 |
Abbreviations: MST, median survival time; NA, not available; NR, not reached.
Regarding the chemotherapy regimens used to treat patients with acute/lymphoma‐type ATL, VCAP‐AMP‐VECP‐like regimens (60%) were most common (Table 2). This is primarily because VCAP‐AMP‐VECP is considered a standard therapy for aggressive ATL in patients aged < 70 years in current Japan based on the results of a phase III trial. 22 The most common regimens differed from those in the 1991 report 9 , 23 and the 2015 report 19 ; in both of these previous reports, CHOP‐like regimens were most common. Differences in chemotherapy regimens might be related to improvements over time in the 4‐year OS of acute/lymphoma‐type ATL in Japan. However, among older patients with acute/lymphoma‐type ATL, the proportion who received VCAP‐AMP‐VECP‐like regimens was low, and both the CR/PR rate and OS were lower than in younger patients (Table 2). For such elderly patients, other chemotherapies as well as mogamulizumab, 15 lenalidomide, 16 and other promising agents 24 , 25 , 26 should be evaluated.
The application of allo‐HSCT may also have contributed to the improved 4‐year OS of acute/lymphoma‐type ATL compared with the 1991 report, as recommended for consideration in a recent consensus report. 27 However, we found that allo‐HSCT was applied in only 18% and 13% of acute‐ and lymphoma‐type ATL patients, respectively (Figure 4). About 20% of patients aged < 70 years with acute/lymphoma‐type ATL were still alive 4 years after diagnosis without allo‐HSCT (Figure 4A,B). By contrast with acute‐type ATL patients, improved survival for patients receiving allo‐HSCT was not significant in lymphoma/unfavorable chronic‐type ATL (Figures 2B, 2D, 3D, and Table 5). These findings suggest the possibility of further treatment stratification in these subtypes, although the sample size was small and the follow‐up period was not long enough in this study.
Prognoses have not improved for indolent ATL in this study. One reason might be the absence of strategies to prevent progression to aggressive ATL with patients under “watchful waiting” until disease progression. Further efforts to identify early markers for progression are needed for patients with indolent ATL. Genomic abnormalities associated with poor prognosis of indolent ATL, such as interferon regulatory factor 4 (IRF4) mutations and programmed death‐ligand 1 (PD‐L1) amplification, 28 are expected to be explored as new predictive biomarkers for early intervention. Recently, a combination of interferon alpha and azidothymidine (IFN/AZT) has shown promising effectiveness for indolent ATL in Western countries, although the evidence level was not sufficient. 29 In Japan, a phase III study of watchful waiting versus IFN/AZT for symptomatic indolent ATL is underway (JCOG1111C). 18 Deaths from comorbid diseases might be another reason for the disappointing outcome (Table S2), as deaths from infection and malignancies other than ATL were frequent in patients with chronic/smoldering‐type ATL in this study.
The limitations of this study were that (a) data were obtained retrospectively from participating institutions, (b) cytological/histological characteristics were not considered in the central review process, and (c) the effects of mogamulizumab on prognosis were not fully evaluated because the number of patients who received mogamulizumab was limited.
In conclusion, the prognoses of patients with acute/lymphoma‐type ATL in Japan have improved modestly over the past three decades, but those of patients with chronic/smoldering‐type ATL have not improved. Further efforts are warranted to develop better and safe therapeutic and preventive strategies to improve the prognoses of patients with ATL.
DISCLOSURE
Kisato Nosaka reports personal fees from Celgene KK; Kenji Ishitsuka reports grants from Kyowa Hakko Kirin Co., Ltd. and personal fees from Celgene KK and Kyowa Hakko Kirin Co., Ltd.; Kenichi Ishizawa reports grants from Novartis, Abbie, Bayer, and SymBio, and personal fees from Takeda, Celgene KK, Novartis, Ono Pharmaceutical, Chugai Pharma, and Eizai; Takashi Ishida reports grants from Kyowa Hakko Kirin Co., Ltd., Bayer AG, and Celgene KK and personal fees from Kyowa Hakko Kirin Co., Ltd., Celgene KK, and Mundipharma KK; Atae Utsunomiya reports personal fees from Kyowa Hakko Kirin Co., Ltd and Celgene KK; Kensei Tobinai reports grants from Chugai Pharma, Kyowa Hakko Kirin Co., Ltd., Ono Pharmaceutical, Celgene KK, Janssen, Eisai, Mundi Pharma, Takeda, and Abbvie and personal fees from Eisai, Takeda, Mundipharma, HUYA Bioscience International, Kyowa Hakko Kirin Co., Ltd., Celgene KK, Chugai Pharma, Ono Pharmaceutical, Yakult, Daiichi Sankyo, Bristol‐Myers Squibb, Meiji Seika Kaisha, Solasia Pharma, Verastem, and Zenyaku Kogyo; Toshiki Watanabe reports grants from Solasia Pharma; Kunihiro Tsukasaki reports scholarship from Chugai Pharma. All the remaining authors have declared no conflicts of interest.
Supporting information
FigS1
TableS1
TableS2
TableS3
TableS4
ACKNOWLEDGMENTS
We thank Dr Masanori Shimoyama for the expert opinions on this study. We thank all investigators in hospitals participating in this study. This work was partially supported by Grants‐in‐Aid from the Ministry of Health, Labour and Welfare of Japan (grant numbers: H23‐GanRinsho‐Ippan‐020 and H26‐GanSeisaku‐Ippan‐006) to MI, UK, TW, and KT and by the Japan Agency for Medical Research and Development (grant numbers: 17ck0106338h0001 and 18ck0106338s0502) to YI, MI, KN, YK, KI, AU, and KT. We also thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Appendix 1.
Collaborative Investigators in participating hospitals
Akifumi Takaori, Masakatsu Hishizawa (Kyoto University); Akira Kitanaka (Miyazaki University); Akiyoshi Takami (Aichi Medical University); Asahi Ito, Takashi Ishida (Nagoya City University); Atae Utsunomiya, Kentaro Yonekura (Imamura General Hospital); Atsuko Mugitani (Fuchu Hospital); Chiaki Kato (Meitetsu Hospital); Daisuke Ogawa (Nagasaki Prefecture Shimabara Hospital); Daisuke Tsuruta (Osaka City University); Eiichi Ohtsuka, Yoshio Saburi (Oita Prefectural Hospital); Eizaburo Sueoka (Saga University); Fujii Kazuyasu, Makoto Yoshimitsu (Kagoshima University); Fujio Matsubara (Shin‐Kokura Hospital); Fumi Miyagawa (Nara Medical University); Fumihiko Nakamura, Makoto Sugaya (The university of Tokyo); Hajime Kobayashi (Obihiro Kosei Hospital); Hideho Heizan (Hamanomachi Hospital); Hiroe Fuse (Matsudo City General Hospital); Hirohiko Shibayama (Osaka University); Hiroki Yamaguchi (Nippon Medical School); Hiromasa Harada (Yao Tokusyukai General Hospital); Hiroshi Ishikawa, Shinichiro Yoshida (Nagasaki Medical Center); Hiroshi Iwasaki (Sapporo‐Kosei General Hospital); Hiroshi Kawano (Koga General Hospital); Hiroshi Kazama (Tokyo Women's Medical University); Hiroshi Yamasaki (Kumamoto City Hospital); Hiroyuki Kuroda, Michiko Yamada (Steel Memorial Muroran Hospital); Hitoshi Suzushima (Kumamoto Shinto General Hospital); Ilseung Choi, Naokuni Uike (Kyushu Cancer Center); Kaname Miyashita (Saiseikai Fukuoka General Hospital); Katsuyasu Saigo (Kobe‐Kyodo Hospital); Kazuiku Ohshiro (Nanbu Medical Center/Nanbu Child Medical Center); Kazuki Tatsuno, Yoshiki Tokura, Takaaki Ono (Hamamatsu University School of Medicine); Keiji Sugimoto (Juntendo University Urayasu Hospital); Ken Ohmachi (Tokai University); Kenichiro Etoh (Kumamoto General Hospital); Kenji Ishitsuka, Monji Koga (Fukuoka University); Kensuke Narukawa (National Cancer Center Hospital East); Ki‐Ryang Koh (Osaka General Hospital of West Japan Railway Company); Kimiharu Uozumi (Kagoshima Medical Center); Kisato Nosaka (Kumamoto University); Koichi Nagai (Niigata Prefectural Central Hospital); Koji Adachi, Toru Motokura (Tottori University); Koji Izutsu (Toranomon Hospital); Koji Kato (Kyushu University); Koji Nagafuji (Kurume University); Masaaki Yuge (Ichinomiya Municipal Hospital); Masaharu Miyahara (Karatsu Red Cross Hospital); Masakazu Higuchi (Kyushu Hospital); Masaki Hayashi (Nakagami Hospital); Masaki Iino (Yamanashi Prefectural Central Hospital); Masanori Makita (Okayama Medical Center); Masao Hagihara (Eiju General Hospital); Masaru Shibano (Sakai City Medical Center); Masato Ito (Daido Hospital); Masato Saito (Nikko Memorial Hospital); Michiaki Koike (Juntendo University Shizuoka Hospital); Michihiro Hidaka (Kumamoto Medical Center); Mitsutoshi Kurosawa (Hokkaido Cancer Center); Motoharu Fukazawa (Funabashi Central Hospital); Motohiro Shindo (Asahikawa Medical University); Motoi Takenaka, Yoshitaka Imaizumi (Nagasaki University); Naoki Kobayashi (Sapporo Hokuyu Hospital); Nobuharu Kosugi (Numazu City Hospital); Nobuhiko Nakamura (Gifu University); Nobuhiko Tominaga (Suita Municipal Hospital); Noriko Fukuhara (Tohoku University); Rika Sakai (Kanagawa Cancer Center); Ryohei Nawata (Shimonoseki Medical Center); Satoshi Iyama (Sapporo Medical University); Satoshi Yamasaki (Kyushu Medical Center); Sawako Nakachi, Takeaki Tomoyose (University of the Ryukyus); Shigeki Ito (Iwate Medical University); Shigeru Chiba (University of Tsukuba); Shinya Rai (Kinki University); Takahiro Okada (Shimane University); Takahiro Shimano (Rinku General Medical Center); Takashi Inozume (University of Yamanashi); Takayoshi Ito (JA Toride Medical Center); Takayuki Ikezoe (Kochi University); Takeshi Fujimoto (Nagasaki National Hospital); Tatsuro Jo (Japanese Red Cross Nagasaki Genbaku Hospital); Tatsuya Kaji (Okayama University); Tohru Murayama (Hyogo Cancer Center); Tomohiro Myojo (Edogawa Hospital); Toru Takahashi (Tenshi Hospital); Toru Takahashi (Yamaguchi Prefectural Grand Medical Center); Toshiaki Yujiri (Yamaguchi University); Toshiyuki Nakayama (Tsurumi Hospital); Yoko Adachi (Kobe Central Hospital); Yoshiyasu Kato (Kagoshima Prefectural Satsunan Hospital); Yukio Kobayashi (National Cancer Center Hospital); Yukiyoshi Moriuchi (Sasebo City General Hospital); Yuko Ogata (Nishi‐Beppu National Hospital); Yuta Katayama (Hiroshima Red Cross Hospital & Atomic Bomb Survivors Hospital).
Imaizumi Y, Iwanaga M, Nosaka K, et al; for collaborative Investigators . Prognosis of patients with adult T‐cell leukemia/lymphoma in Japan: A nationwide hospital‐based study. Cancer Sci 2020;111:4567–4580. 10.1111/cas.14658
Yoshitaka Imaizumi, Masako Iwanaga and Kunihiro Tsukasaki are contributed equally to this work.
Contributor Information
Yoshitaka Imaizumi, Email: y-imaizm@nagasaki-u.ac.jp.
Masako Iwanaga, Email: masakoiwng@nagasaki-u.ac.jp.
Kunihiro Tsukasaki, Email: tsukasak@saitama-med.ac.jp.
for collaborative Investigators:
Akifumi Takaori, Masakatsu Hishizawa, Akira Kitanaka, Akiyoshi Takami, Asahi Ito, Kentaro Yonekura, Atsuko Mugitani, Chiaki Kato, Daisuke Ogawa, Daisuke Tsuruta, Eiichi Ohtsuka, Yoshio Saburi, Eizaburo Sueoka, Fujii Kazuyasu, Makoto Yoshimitsu, Fujio Matsubara, Fumi Miyagawa, Fumihiko Nakamura, Makoto Sugaya, Hajime Kobayashi, Hideho Heizan, Hiroe Fuse, Hirohiko Shibayama, Hiroki Yamaguchi, Hiroshi Ishikawa, Shinichiro Yoshida, Hiroshi Iwasaki, Hiroshi Kawano, Hiroshi Kazama, Hiroshi Yamasaki, Hiroyuki Kuroda, Michiko Yamada, Hitoshi Suzushima, Ilseung Choi, Naokuni Uike, Kaname Miyashita, Katsuyasu Saigo, Kazuiku Ohshiro, Kazuki Tatsuno, Takaaki Ono, Keiji Sugimoto, Ken Ohmachi, Kenichiro Etoh, Monji Koga, Kensuke Narukawa, Ki‐Ryang Koh, Kimiharu Uozumi, Koichi Nagai, Koji Adachi, Toru Motokura, Koji Izutsu, Koji Kato, Koji Nagafuji, Masaaki Yuge, Masaharu Miyahara, Masakazu Higuchi, Masaki Hayashi, Masaki Iino, Masanori Makita, Masao Hagihara, Masaru Shibano, Masato Ito, Masato Saito, Michiaki Koike, Michihiro Hidaka, Mitsutoshi Kurosawa, Motoharu Fukazawa, Motohiro Shindo, Motoi Takenaka, Naoki Kobayashi, Nobuharu Kosugi, Nobuhiko Nakamura, Nobuhiko Tominaga, Noriko Fukuhara, Rika Sakai, Ryohei Nawata, Satoshi Iyama, Satoshi Yamasaki, Sawako Nakachi, Takeaki Tomoyose, Shigeru Chiba, Shinya Rai, Takahiro Okada, Takahiro Shimano, Takashi Inozume, Takayoshi Ito, Takayuki Ikezoe, Takeshi Fujimoto, Tatsuro Jo, Tatsuya Kaji, Tohru Murayama, Tomohiro Myojo, Toru Takahashi, Toru Takahashi, Toshiaki Yujiri, Toshiyuki Nakayama, Yoko Adachi, Yoshiyasu Kato, Yukio Kobayashi, Yukiyoshi Moriuchi, Yuko Ogata, and Yuta Katayama
REFERENCES
- 1. Uchiyama T, Yodoi J, Sagawa K, et al. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. [PubMed] [Google Scholar]
- 2. Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415‐7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T‐cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV‐1 Infection. Front Microbiol. 2012;3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609‐e616. [DOI] [PubMed] [Google Scholar]
- 6. Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV‐1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84:327‐335. [DOI] [PubMed] [Google Scholar]
- 7. Satake M, Iwanaga M, Sagara Y, et al. Incidence of human T‐lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: a nationwide retrospective cohort analysis. Lancet Infectious Dis. 2016;16:1246‐1254. [DOI] [PubMed] [Google Scholar]
- 8. Iwanaga M, Watanabe T, Yamaguchi K. Adult T‐cell leukemia: a review of epidemiological evidence. Front Microbiol. 2012;10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimoyama M, The Lymphoma Study Group . Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79: 428‐437. [DOI] [PubMed] [Google Scholar]
- 10. Tajima K, the T‐ and B‐cell Malignancy Study Group and co‐authors . The 4th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. Int J Cancer. 1990;45:237‐243. [DOI] [PubMed] [Google Scholar]
- 11. Lymphoma Study Group (1984–1987) . Major prognostic factors of patients with adult T‐cell leukemia‐lymphoma: a cooperative study. Leuk Res. 1991;15:81‐90. [DOI] [PubMed] [Google Scholar]
- 12. Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia‐lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:15‐20. [DOI] [PubMed] [Google Scholar]
- 14. Utsunomiya A, Choi I, Chihara D, et al. Recent advances in the treatment of adult T‐cellleukemia‐lymphomas. Cancer Sci. 2015;106:344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishida T, Jo T, Takemoto S, et al. Dose‐intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: a randomized phase II study. Br J Haematol. 2015;169:672‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogura M, Imaizumi Y, Uike N, et al. Lenalidomide in relapsed adult T‐cell leukaemia‐lymphoma or peripheral T‐cell lymphoma (ATLL‐001): a phase 1, multicentre, dose‐escalation study. Lancet Haematol. 2016;3:e107‐e118. [DOI] [PubMed] [Google Scholar]
- 17. Fujiwara H, Fuji S, Wake A, et al. Dismal outcome of allogeneic hematopoietic stem cell transplantation for relapsed adult T‐cell leukemia/lymphoma, a Japanese nation‐wide study. Bone Marrow Transplant. 2017;52:484‐488. [DOI] [PubMed] [Google Scholar]
- 18. Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol. 2014;15:e517‐e526. [DOI] [PubMed] [Google Scholar]
- 19. Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570‐2577. [DOI] [PubMed] [Google Scholar]
- 20. Nosaka K, Iwanaga M, Imaizumi Y, et al. Epidemiological and clinical features of adult T‐cell leukemia‐lymphoma in Japan, 2010–2011: a nationwide survey. Cancer Sci. 2017;108:2478‐2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Medicine. 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458‐5464. [DOI] [PubMed] [Google Scholar]
- 23. Shimoyama M, Ota K, Kikuchi M, et al. Major prognostic factors of adult patients with advanced T‐cell lymphoma/leukemia. J Clin Oncol. 1988;6:1088‐1097. [DOI] [PubMed] [Google Scholar]
- 24. Yamagishi M, Nakano K, Miyake A, et al. Polycomb‐mediated loss of miR‐31 activates NIK‐dependent NF‐κB pathway in adult T cell leukemia and other cancers. Cancer Cell. 2012;21:121‐135. [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa H, Bissonnette RP, Gikkings M, et al. Induction of apoptosis by HBI‐8000 in adult T‐cell leukemia/lymphoma is associated with activation of Bim and NLRP3. Cancer Sci. 2016;25:575‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Q, Wang S, Chen J, et al. Histone deacetylases (HDACs) guided novel therapies for T‐cell lymphomas. Int J Med Sci. 2019;16:424‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook LB, Fuji S, Hermine O, et al. Revised adult T‐cell leukemia‐lymphoma international consensus meeting report. J Clin Oncol. 2019;37:677‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kataoka K, Iwanaga M, Yasunaga J‐I, et al. Prognostic relevance of integrated genetic profiling in adult T‐cell leukemia/lymphoma. Blood. 2018;131:215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta‐analysis on the use of zidovudine and interferon‐alfa in adult T‐cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177‐4183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigS1
TableS1
TableS2
TableS3
TableS4


