ABSTRACT
Objectives: The purpose of this study was to compare the effects of deep dry needling (DN) with and without needle manipulation on pressure pain thresholds (PPTs) and electromyographic (EMG) amplitude of the lumbosacral multifidus (LM) in adults with low back pain (LBP).
Methods: Participants were randomized into two treatment groups: with needle manipulation (n = 21) and without needle manipulation (n = 21). All participants received a single session of the assigned DN intervention. PPTs and EMG amplitude of the LM muscle were collected three times: before DN, immediately after DN, and one week after DN.
Results: The needle manipulation group had a significantly greater increase in PPT immediately after the intervention and at the one-week follow-up as compared to the no needle manipulation group. The increase of PPT in the needle manipulation group was significant immediately after the intervention, and the increase remained significant at the one-week follow-up. However, there was no significant difference in EMG amplitude of the LM muscle between groups across the three time points.
Discussion: Deep DN with needle manipulation appeared to reduce mechanical pressure sensitivity more than DN without manipulation for patients with LBP. Although a single session of DN could reduce pressure pain sensitivity, it may not be sufficient to improve LM muscle function. Level of Evidence: 1b. Trial registration numbers: NCT03970486.
KEYWORDS: Acupuncture, mechanical pain sensitivity, electromyographic activity, lumbar spine, needle manipulation; orthopedics; manual therapy; physical therapy
Introduction
Literature has shown decreased lumbosacral multifidus (LM) muscle function in patients with low back pain (LBP) [1–3]. To address this deficit, specific segmental spinal stabilization exercises were developed to facilitate LM muscle function for treating LBP [4,5]. Several strategies commonly are used by orthopedic manual physical therapists (OMPTs) to increase LM muscle function, such as verbal cues [3,6,7], tactile feedback [3,8–10], and recently, deep dry needling (DN) [11,12]. Two studies have shown increased muscle thickness of the LM muscle after DN in both healthy and LBP populations as measured by ultrasound imaging [11,12]. However, because ultrasound imaging captures only a 2-D image, it cannot represent muscle contraction fully. Although a recent systematic review and meta-analysis suggested that DN could enhance force production in those with neck pain, it is unclear if the effects would be the same for individuals with LBP [13].
Two common deep DN techniques are used by OMPTs for treating patients with LBP: with and without needle manipulation. DN without manipulation is similar to Chinese acupuncture in that it leaves the needle in situ after it is inserted, but does not follow acupuncture principles, such as inserting needles on acupoints along the meridians and limiting needle insertion to a depth of approximately 5–10 mm [14–17]. In addition, DN with manipulation specifically targets the muscle belly and employs manipulation approaches such as pistoning or sparrow-pecking/coning the needle by pulling it in and out within the muscle or by redirecting it in small angles [11,12,18]. In contrast, the needle manipulation in Chinese acupuncture only involves rotating the needle in place and the needle is left for 10–30 minutes after needle manipulation [17]. The deep muscular DN technique was designed originally to elicit local twitch responses in the muscle, thus leading to chemical interaction (e.g. acetylcholine depletion) at the neuromuscular junction, and therefore facilitating muscle training [13,15]. However, recent evidence suggests that local twitch responses may not be necessary for successful outcomes [19,20]. Because side effects such as increased soreness and nausea are associated with needle manipulation [19,20], needling techniques without manipulation may be preferred if they demonstrate benefits similar to those of needling with manipulation.
Although DN with or without needle manipulation has shown to be a useful technique to reduce pain in patients with LBP [14,21–23], it is unclear which of these two DN techniques would have a greater effect on mechanical pain sensitivity and LM muscle function, which is considered to be an important component in LBP rehabilitation. Therefore, the primary purpose of the study was to compare the effects of two DN techniques on pressure pain sensitivity and electromyographic (EMG) amplitude of the LM in individuals with LBP. The secondary purpose was to compare self-reported low back pain intensity between the two techniques before and after DN.
Methods
Participants
This study was approved by the investigators’ affiliated Institutional Review Board and was registered with ClinicalTrials.gov (NCT03970486). Before data collection began, a power analysis was performed using G*Power 3.1.3 to estimate an adequate sample size [24]. Using a medium effect size of 0.25 and an alpha level of 0.05, a minimum of 36 participants, 18 in each group, was required to reach a power of 0.90. Considering a 10% attrition, 40 participants, 20 in each group, were planned for enrollment in the study. Participants were recruited from the physical therapy clinics at which two investigators were employed, or were employees/students at the investigators’ affiliated institutions.
The eligible participants were individuals who had existing LBP in the L4-S2 levels with tenderness to palpation at this area, and an average pain intensity score ≥ 2/10 on the Numeric Pain Rating Scale (NPRS) in the 24 hours prior to study enrollment. Exclusion criteria included: bleeding disorders, use of anti-coagulants, previous low back surgery, systemic joint disease (e.g. rheumatoid arthritis), cancer of the lower quadrant, neurological disorders, allergic reaction to adhesive tape, and inability to obtain the testing position (i.e. prone lying). Each participant was informed of the risks and procedures of the study, and then signed a written informed consent form.
Outcome measures
Pressure pain threshold (PPT)
A hand-held computerized pressure algometer (Medoc ltd., Ramat Yishai, Israel) was used to determine the PPT over the most tender point between L4-S2. The algometer consists of a 1-cm2 round tip that was pressed perpendicularly to the skin on the target location (Figure 2(a)). To provoke the participant’s pain or discomfort, pressure was increased at a rate of 40 kPa/sec until the first perception of pain, at which time the participant pressed the unit’s response button and the test was terminated [25,26].
Figure 2.
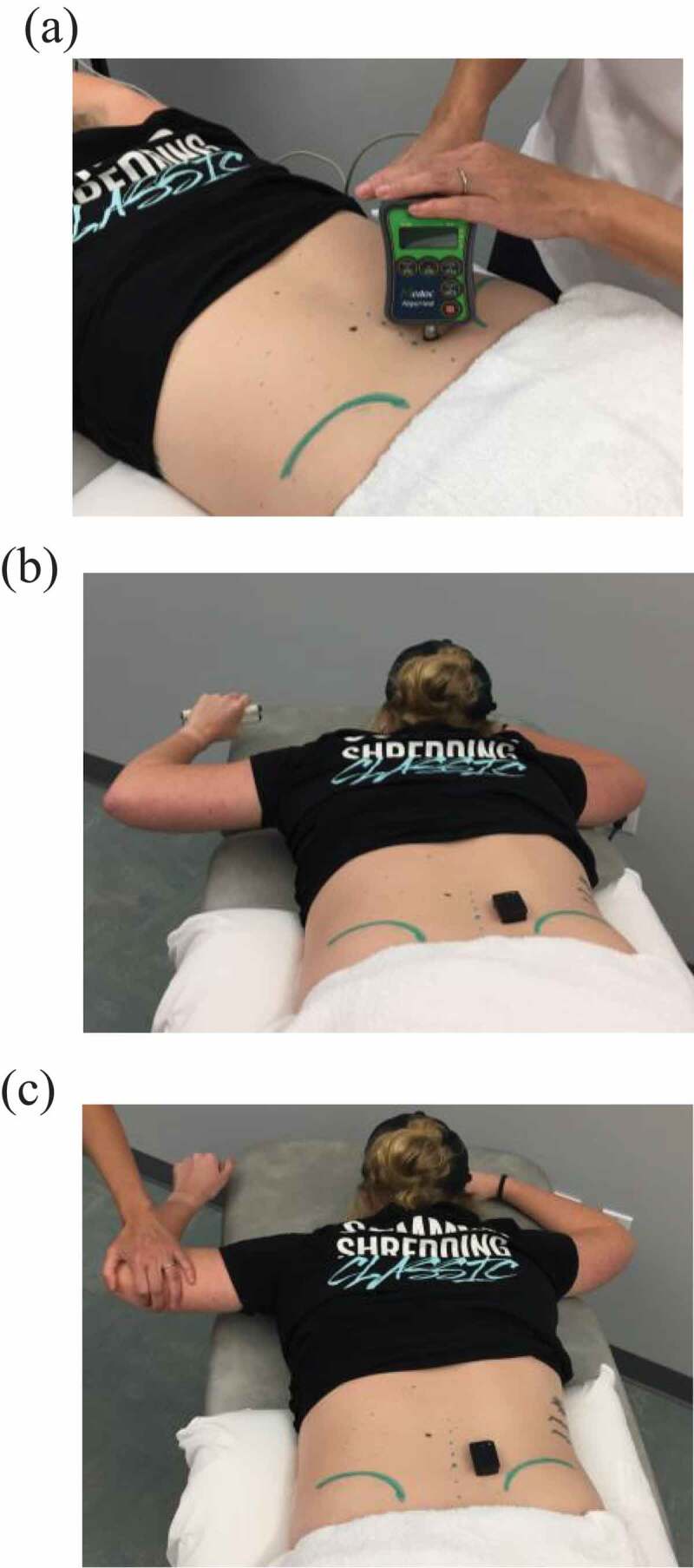
(a) Pressure pain threshold testing, (b) contralateral arm lift task while holding a hand weight, and (c) contralateral arm lift task for maximal voluntary isometric contraction
Electromyographic (EMG) amplitude
An EMG system with a wireless surface electrode (Delsys Trigno®, Delsys Inc., Natick, MA) was used to determine the amplitude of LM muscle excitation. EMG amplitude was collected at the location corresponding to the PPT testing site. The wireless EMG electrode contained a built-in pre-amplifier, a Butterworth filter and two sets of parallel silver contact bars with a fixed distance of 1 cm between the recording bars. The EMG system’s bandwidth was set at 20 to 450 Hz with a gain of 1,000, and the sampling rate was 2,000 Hz. The Common Mode Rejection Ratio (CMRR) was >80 dB for this EMG system. These EMG specifications were selected to record sufficient surface EMG signals from a target muscle or muscle group with minimal noise. The surface EMG rather than needle or fine-wire EMG was chosen because needle and fine-wire EMG has been shown to have poor-to-fair between-day reliability and could potentially affected the pressure pain threshold test when the needle or fine-wire was left in the muscle during the EMG testing [27].
Self-reported low back pain intensity
The NPRS was used to assess each participant’s self-reported LBP intensity. The NPRS is an 11-point Likert scale, ranging from 0 being no pain and 10 being worst pain imaginable. Both the minimal detectable change (MDC) and minimal clinical important difference (MCID) of the NPRS were reported to be two points for patients with LBP [28], and 2.4 points specifically for chronic LBP [29].
Procedure
On the first visit, eligible participants were asked about their demographic information and past medical history. Participants also were asked about their pain location, duration, and intensity at present, at worst, and at best in the past 24 hours using the NPRS. The Modified Oswestry Disability Index (ODI) was administered to all participants to determine their perceived disability and functional limitations due to LBP [30,31].
Next, PPTs and EMG amplitude were collected from the painful side of the participants. When the participants had bilateral LBP, these outcome measures were collected from the most painful side. If both sides were equally painful, a coin toss was performed to select the side. During the PPT and EMG testing, participants were asked to lie in a prone position on an examination table with their arms to their sides. A pillow was placed under the participant’s abdomen to reduce the lumbar lordotic curve and an inclinometer was placed on the lumbosacral junction to ensure that the lumbar curve was ≤10° [32].
To prepare for EMG recording, the participant’s skin over the LM muscles (mostly between L4-S2) was cleaned with alcohol and, if needed, excessive hair was shaved using a disposable razor. Adhesive tape was used to affix a wireless EMG electrode to the skin over the LM muscles. Following the recommendations of the Surface ElectroMyoGraphy for the Noninvasive Assessment of Muscles (SENIAM) project [33,34], the electrode for the LM was placed at the level of the PPT recording site, approximately 2–3 cm from the midline, and was aligned with a line from the posterior superior iliac spine (PSIS) to the interspace between the L1 and L2 spinous process. This electrode placement was chosen because an EMG electrode placed near the L5 segment has been shown to best capture LM EMG excitation [35].
Pressure-pain threshold testing
First, participants were given a response button to stop testing on their own, and were instructed to stop the test as soon as the pressure became uncomfortable or painful, and not to allow an uncomfortable or painful sensation to continue. Four research team members were responsible for administering the PPT testing depending on their availability. However, the same pair of research team members tested the same participant throughout the study (i.e. before, immediately after, and one week after DN). Four trials were tested on the most tender point as described earlier, but the first trial was counted as a practice trial. The average of the last three trials was used for data analysis [26,36].
EMG recording
During EMG recording, participants were in the same position as during the PPT testing, and were asked to abduct the contralateral shoulder to approximately 90° and flex the contralateral elbow to approximately 90° while holding a 1.5 or 2 lb. hand weight. A participant held a 1.5 lb. hand weight if his/her body weight was less than 175 lbs., and held a 2 lb. hand weight if his/her body weight was more than 175 lbs [11,37]. First, the EMG amplitude of the LM muscle was recorded during a contralateral arm lift, which has been shown to best elicit LM muscle activation [37]. A verbal instruction was given before the participant performed each contralateral arm lift: ‘Breathe normally. Without moving your pelvis or spine, raise your arm above the table’ [7]. Each participant was given one practice trial before EMG recording began, and then performed a 5-second contralateral arm lift five times while holding the hand weight (Figure 2(a)).
Next, two trials of maximal voluntary isometric contraction (MVIC) of the LM muscles were obtained by asking the participant to perform a contralateral arm lift without holding a hand weight while the investigator applied a steady downward force on the elbow opposite side of the tested LM muscle (Figure 2(b)). Two 5-second MVIC trials were recorded with a minimum of a 1-minute rest between the two trials. During both the contralateral arm lift and MVIC tasks, if a participant demonstrated difficulty in holding for 5 seconds, the participant was allowed to hold for a shorter time, but a minimum of 3 seconds was required. The highest EMG amplitude of the two MVIC trials was used to normalize EMG amplitude collected during a contralateral arm lift with a hand weight.
Prior to this study, a pilot study (n = 15 healthy asymptomatic adults) was conducted to establish the reliability of the EMG testing protocol used in this study. In the pilot study, intraclass correlation coefficients (ICCs) showed good-to-excellent within-session (0.811–0.906) and between-day (0.831–0.906) reliability.
Interventions
The two investigators (ZC, SWP) who performed the interventions were experienced physical therapists in treating patients with LBP and were recognized as certified manual therapists and Fellows of the American Academy of Orthopaedic Manual Physical Therapy. One investigator (ZC) had practiced DN for 10 years and the other investigator (SWP) had practiced DN for 5 years. Before data collection began, the two investigators met and standardized the two DN interventions so that the treating investigators were able to administer either one of the two DN techniques. In addition, the research team members (PA, SL, TH, LS) who collected the outcome measures (EMG amplitude, PPTs, and NPRS scores) were blinded to the type of DN technique.
An opaque envelope containing 40 cards, 20 marked ‘A’ for the needle manipulation intervention and 20 marked ‘B’ for no needle manipulation intervention, was used to implement the randomization. The treating therapists (ZC and SWP) were responsible for administering the randomization after baseline outcome measures were collected. Each participant was randomly assigned into one of the DN interventions by drawing a card from the envelope. If a participant did not return for the second visit or dropped out from the study, a card with this participant’s intervention assignment was returned to the envelope.
The insertion of the needle was the same for both techniques, following the previously reported protocol [11,12]. Two lengths of sterile, disposable, 0.30 mm x 60 mm solid filament needle (Seirin Corp., Shizuoka, Japan) and 0.30 mm x 100 mm solid filament needle (Shanghai Kangnian Medical Device Co., Ltd., Shanghai, China) were used in the study. The length of the needle for each participant was selected based on the size of the participant. Two needles were inserted on or near the most tender point. Two additional needles were inserted on the opposite side at the level of the most tender point regardless of unilateral or bilateral LBP. After piercing the skin, the needle was directed toward the spinous process in a slight inferior-medial angle (approximately 20–30°). This angle was chosen because the needle likely was stopped by the bony lamina of the vertebra to ensure a safe intervention and to standardize the depth of the needle insertion.
For the needle manipulation technique, after the needle was inserted, it was pulled slightly in and out within the muscle and redirected in small angles for 10 seconds after insertion [11,12]. The needle was then withdrawn immediately at the end of needle manipulation. For the DN without manipulation technique, the needles stayed (in situ) in the LM for approximately 10 minutes after the insertion [16]. Although each treatment session mostly lasts 20–30 minutes in Chinese acupuncture [38], a duration of 10 minutes was allotted for the no needling manipulation intervention, thus minimizing time discrepancy between the two interventions.
Reassessment
Immediately after and approximately one-week after the DN intervention, the three outcome measures (i.e. EMG amplitude, PPTs and NPRS scores) were collected. In addition, each participant was asked about any presence of common adverse symptoms, including increased soreness, nausea, and dizziness. If bleeding occurred, the participant was informed of the occurrence. All participants were asked to resume normal daily activity, but to avoid strenuous activity or exercises between the two visits.
Data analysis
Descriptive statistics were calculated for participant characteristics and normalized EMG amplitude of all participants. Independent t-tests were used to compare the ratio data of participant characteristics as well as the baseline (i.e. before DN) PPT, EMG, and NPRS data between groups. Chi-square statistics were used to analyze categorical data of participant characteristics. Root-mean square (RMS) values were extracted from each EMG trial using Delsys EMGWorks (Delsys Inc., Natick, MA) for quantifying LM muscle excitation. The middle three seconds of each 5-second EMG recording during the contralateral arm lift and MVIC tasks were used for statistical analysis. The larger EMG RMS value of the two MVIC tasks was used for normalization. Each EMG RMS value during a contralateral arm lift was normalized to the EMG RMS value of the MVIC using the following formula: (EMG RMS during contralateral arm lift/EMG RMS of MVIC) x 100.
IBM SPSS Version 25.0 (IBM Corp., Armonk, NY, USA) was used to analyze normalized EMG data (% of MVIC) of the LM muscle and the PPT data. Two separate 2 (group) x 3 (time) ANOVAs with repeated measures were performed to determine differences in PPT and normalized EMG data between groups over time, respectively. The α level was set at 0.05 for each ANOVA with repeated measures.
Results
Participants
Fifty-four participants were screened for eligibility and 41 participants were enrolled in the study from April 2018 – August 2019. These 41 participants were randomly assigned into either the needle manipulation or no needle manipulation group. Figure 1 illustrates participant enrollment, allocation, follow-up, and analysis. Two participants in the needling manipulation group did not return for a follow-up visit. The Little’s Missing Completely at Random analysis for the PPT data was significant (p = 0.039), indicating that the missing data for these two participants was not random; therefore, imputation for missing data replacement was performed to include 21 participants in the needle manipulation group and 20 participants in the no needle manipulation group for statistical analysis. Table 1 lists the characteristics of participants, including age, gender, body mass index, painful side(s), testing side, onset of pain, duration of pain, NPRS scores (current, worst, least and average), ODI scores, as well as three baseline outcome measurements. There were no significant differences in any of the participants’ characteristics and baseline outcome measurements between groups, although the no needle manipulation group had more female participants and had a longer duration of LBP, primarily due to two participants’ history of LBP for more than 20 years. The average NPRS score (NPRS: 3.7 ± 1.8) and the average ODI score (20.9 ± 11.6) indicate that the participants had mild disability due to a low-to-moderate level of LBP. However, the long duration of LBP (average 6 years) indicates that their LBP had become chronic. Table 2 lists means and standard deviations of PPTs, normalized LM muscle EMG amplitude and NPRS scores collected at 3 different time points.
Figure 1.
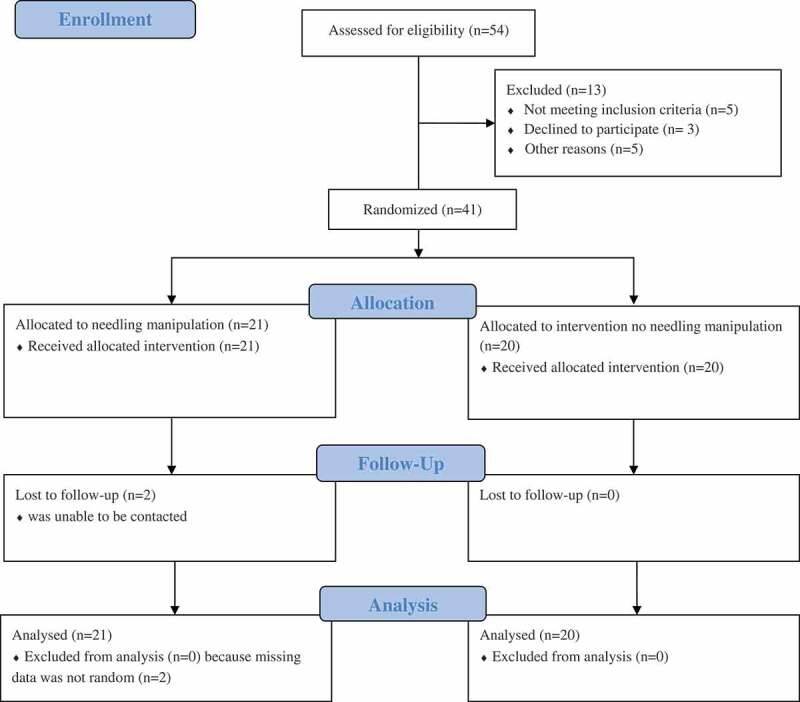
The consort diagram for participant enrollment, allocation, follow-up and analysis
Table 1.
Characteristics of all participants (analyzed, n = 41), the needle manipulation group (n = 21) and the no needle manipulation group (n = 20)
| Variables | All | Needle Manipulation | No Needle Manipulation |
p value Between Groups |
|---|---|---|---|---|
| Age (years) | 38.9 ± 15.5 | 37.7 ± 14.1 | 40.3 ± 17.2 | 0.601 |
| Gender (female) | 76% | 62% | 90% | 0.067 |
| BMI | 26.9 ± 5.7 | 26.2 ± 6.2 | 27.7 ± 5.2 | 0.431 |
| Painful side (bilateral/right/left) | 28/7/6 | 15/2/4 | 13/5/2 | 0.355 |
| Testing side (right/left) | 25/16 | 13/8 | 12/8 | 0.577 |
| Onset of pain (traumatic/gradual) | 9/32 | 6/15 | 3/17 | 0.454 |
| Duration of pain (weeks) | 324.3 ± 326.1 | 242.7 ± 250.3 | 410.0 ± 378.0 | 0.101 |
| NPRS (0–10) | ||||
| Current | 3.7 ± 2.0 | 3.5 ± 1.7 | 3.9 ± 2.2 | 0.577 |
| Worst | 5.4 ± 2.1 | 5.5 ± 1.7 | 5.3 ± 2.6 | 0.716 |
| Least | 2.1 ± 2.2 | 2.0 ± 2.2 | 2.2 ± 2.2 | 0.827 |
| Average | 3.7 ± 1.8 | 3.7 ± 1.6 | 3.8 ± 2.1 | 0.879 |
| ODI (%) | 20.9 ± 11.6 | 21.0 ± 12.3 | 20.8 ± 11.0 | 0.967 |
| Normalized EMG Amplitude (%MVIC) | 53.7 ± 30.7 | 55.8 ± 29.0 | 51.4 ± 33.0 | 0.650 |
| PPT (kPa) | 383.4 ± 185.5 | 377.3 ± 170.0 | 389.9 ± 204.9 | 0.830 |
BMI = Body mass index, NPRS = Numerical Pain Rating Scale, ODI = Oswestry Disability Index, EMG = Electrographic, MVIC = maximal isometric voluntary contraction, PPT = pressure pain threshold.
Table 2.
Pressure pain thresholds (PPTs), normalized electromyographic (EMG) amplitude of the lumbosacral multifidus muscle during contralateral arm lift, and Numerical Pain Rating Scale (NPRS) scores before, immediately after, and one week after a single session of dry needling intervention
| Variables | All (n = 41) | Needle Manipulation (n = 21) | No Needle Manipulation (n = 20) | p value (interaction) |
|---|---|---|---|---|
| PPT (kPa) | 0.023* | |||
| Pre needling | 383.4 ± 185.5 | 377.3 ± 170.0 | 389.9 ± 204.9 | |
| Immediately post | 432.3 ± 186.7 | 460.2 ± 199.6 | 403.1 ± 172.3 | |
| 1-week follow-up | 415.0 ± 177.2 | 455.7 ± 176.9 | 372.3 ± 171.4 | |
| EMG amplitude (%MVIC) | 0.772 | |||
| Pre needling | 53.7 ± 30.7 | 55.8 ± 29.0 | 51.4 ± 33.0 | |
| Immediately post | 69.3 ± 82.1 | 72.3 ± 79.6 | 66.1 ± 86.6 | |
| 1-week follow-up | 55.7 ± 35.1 | 53.2 ± 23.9 | 58.4 ± 44.4 | |
| NPRS (current) | 0.238 | |||
| Pre needling | 3.7 ± 2.0 | 3.5 ± 1.7 | 3.9 ± 2.2 | |
| Immediately post | 2.0 ± 1.7 | 2.5 ± 1.7 | 1.5 ± 1.7 | |
| 1-week follow-up | 2.4 ± 2.1 | 2.4 ± 2.0 | 2.5 ± 2.1 |
MVIC: maximal voluntary isometric contraction. *p < 0.05.
Pressure pain threshold
There was a significant interaction between groups across the 3 time points for the PPT data (p = 0.023). Post-hoc comparisons showed that the needle manipulation group had a significantly greater increase in PPT immediately after the intervention (p = 0.025), and at the one week follow-up (p = 0.018), as compared to the no needle manipulation group (see Figure 3). In addition, the increase of PPT in the needle manipulation group was significant immediately after the intervention (p = 0.001), and the increase remained significant at the one-week follow-up (p = 0.019), whereas there was no difference in PPTs in the no needle manipulation group between any two time points.
Figure 3.
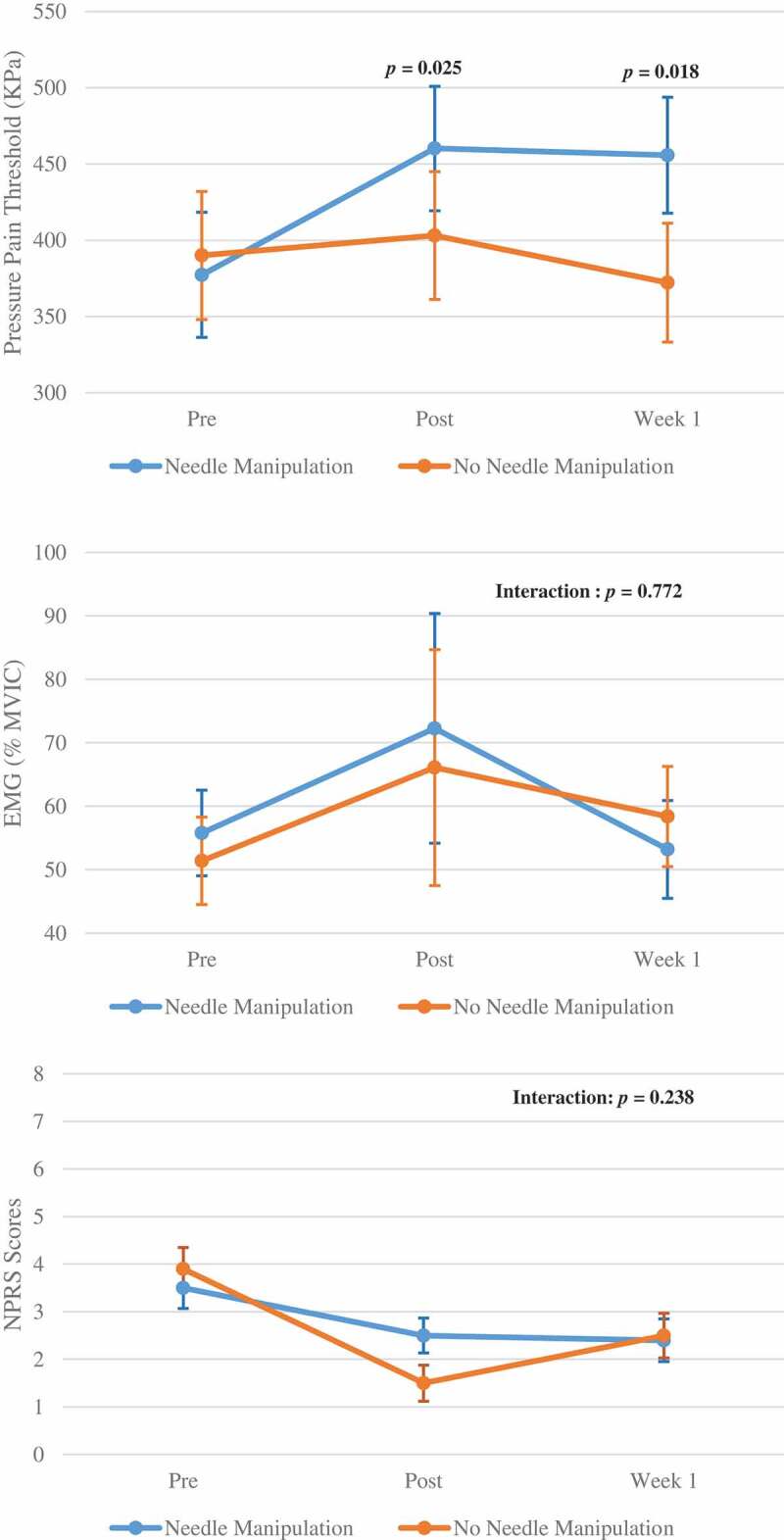
Pressure pain thresholds, normalized electromyographic (EMG) amplitude (% maximal voluntary isometric contraction) of the lumbosacral multifidus, and Numerical Pain Rating Scale (NPRS) scores between the two needling manipulations before, immediate after and one week after intervention
Electromyographic amplitude of the lumbosacral multifidus muscle
In contrast to the PPT results, there was no significant interaction between groups across the 3 time points (p = 0.772) for the normalized EMG data, and there was no significant main effect of time (p = 0.299) (see Figure 3).
Numerical pain rating scale score
The results of the NPRS scores showed that there was no significant interaction between groups across the 3 time points (p = 0.238), but there was a significant main effect of time (p < 0.001). Post-hoc comparisons showed that all participants had significant pain reduction immediately after DN (p = 0.001) and one week after DN (p = 0.019), although the NPRS scores remained decreased significantly at one week, but not as much as those immediately after DN (see Figure 3).
Adverse effects after needling
Both groups reported minor adverse effects. Among 21 participants in the needle manipulation group, 1 reported a slight dizziness immediately after DN, 4 had minor bleeding in one of the four needling sites, and 5 reported soreness immediately after DN and all 5 reported the soreness lasted a few hours to two days. Among 20 participants in the no needle manipulation group, only 1 had bleeding in one of the four needling sites, 2 reported soreness immediately after DN, and 2 reported soreness the following day.
Discussion
Although studies have shown positive effects of DN on PPT in patients with musculoskeletal pain [11,39,40], this study demonstrated that DN with needle manipulation resulted in a greater reduction in mechanical pressure sensitivity than the in-situ (no needle manipulation) approach. This finding is consistent with what has been found in previous studies in healthy adults, who had increased PPT from needling with rotation as compared to those who received needling without rotation [41,42]. Therefore, generalized pain modulation produced by needle insertion and by piercing the skin and subcutaneous tissues cannot explain the finding of this study [42,43]. Langvin et al. [43,44], demonstrated changes in connective tissue architecture (e.g. thickening or forming of spiral pattern) due to needle manipulation, and therefore hypothesized that the movement of the needle could deliver a mechanical signal, thus triggering the cellular changes in the connective tissue. However, the mechanism by which this immediate mechanical change produces analgesic effects is unclear. A recent in-vivo study of rats [45] found that the action of needle manipulation does not cause changes in the connective tissue, and suggested that neural tissues are responsible for needle manipulation effects.
Increased levels of adenosine in rats and humans also have been observed when needling with manipulation [46,47]. It was hypothesized that adenosine binds to adenosine A1 receptors on afferent nerves that transmit nociceptive signals to the spinal cord, and therefore reduce transmission of nociceptive input. In addition, adenosine A1 receptor activation can initiate inhibition of cyclic adenosine monophosphate (cAMP) which has been shown to increase in patients with chronic pain [47]. Recently, needle manipulation was shown to induce a local cutaneous release of nitric oxide, a vasodilator, which could affect regional blood flow and allow an influx of analgesic substances, leading to pain reduction [48]. Further, needling with a lift-thrusting technique, similar to the needle manipulation approach used in this study, significantly reduced subjective pain perception greater than needling with a rotation technique [49]. Because the lift-thrust technique induced a stronger feeling of tenderness and numbness perceived by the participants, the stronger sensation could result in subjective pain reduction.
The results of the study showed that the needle manipulation group had reduced mechanical pressure sensitivity immediately after needling with manipulation, and the effect maintained one week later. Using a similar DN approach (i.e. needling with manipulation), Koppenhaver et al. [11] also found increased PPT one week later, but not immediately after DN. However, a study of DN in patients with neck pain demonstrated increased PPT immediately and one week after DN [50]. Koppenhaver et al. attributed the delayed response of PPT to their participants’ delayed clinical improvement in disability. Interestingly, our PPT results were different from the self-reported NPRS scores. Objective PPT measurements have been shown to have weak correlation with pain perception [51,52]. Other factors, such as emotion and sympathetic responses, could affect the NPRS score, supporting the notion that PPT assesses only a specific point along the nociceptive pathway [52]. Post needling soreness and pain from needle manipulation also could affect NPRS scores, particularly those collected immediately after needling. Five participants from the needle manipulation group reported increased soreness after dry needling whereas only two participants from the no needle manipulation group had immediate post-needling soreness.
Although the needle manipulation significantly increased PPT, it did not increase muscle activity. Increased EMG amplitude has been observed in the upper trapezius muscle after DN therapy for patients with neck pain [53,54]. In addition, increased lumbar multifidus muscle thickness on ultrasonography was observed after DN in healthy adults [55] and patients with LBP [11]. The increased EMG amplitude and muscle thickness are believed to be the direct result of pain reduction. Similar to the EMG results of our study, several studies did not find increased EMG amplitude after DN [56,57]. The large variance in the EMG data collected immediately after needling (Figure 3) could have resulted in the lack of difference finding. It appears that DN could have an inhibitory effect in some participants and a facilitator effect in other participants. We cannot explain the underlying physiological mechanism for the opposite responses and only can speculate that other factors, such as sympathetic responses to DN, may have contributed to this result [58].
In addition, finding no significant change in EMG amplitude one week after DN could suggest that the degree of pain reduction or increased PPT was not sufficient to improve muscle function. This phenomenon was observed a few decades ago by Hides et al. [1], who demonstrated that recovery of the LM muscle does not occur concomitantly with pain reduction in patients with LBP. Considering that muscle atrophy from fatty infiltrates [59,60] would lead to decreased muscle contractility, particularly for the participants with chronic LBP, a single DN session without reinforced muscle training may not have been sufficient to observably increase muscle function in the short term.
One limitation of this study is the inability to control participants’ activity or medication intake between the two visits, although the participants were advised not to engage in non-routine activities during the study period. It was the authors’ intention that the use of randomization would minimize this factor. Another limitation was the use of surface EMG to study muscle contraction. However, the between-day reliability of the EMG testing protocol used in this study was shown to be good in the pilot study. Nevertheless, high body mass index and/or thick subcutaneous tissues could have affected surface EMG recordings [61]. In addition, surface EMG may not be ideal for recording deeper LM muscle contraction. Indwelling EMG, such as fine-wire or needle EMG, may be an optimal alternative for studying deeper LM contraction [62], but indwelling EMG could have caused discomfort during contralateral arm lift tasks, thus affecting the PPT and NPRS results. Lastly, the cross-talk effect from other paraspinal muscles cannot be completely eliminated [62,63]. However, the placement of electrodes used in this study has been shown to be optimal for recording LM muscle excitation [34,35,64].
Conclusion
The results of the study show that DN with needle manipulation appeared to reduce mechanical pressure sensitivity more than DN without needle manipulation for patients with LBP, although DN with needle manipulation had a greater occurrence of post-needling soreness and bleeding than DN without needle manipulation. DN with or without needle manipulation appears to equally reduce pain perception. Although a single session of DN could reduce pressure sensitivity or perception, it may not be sufficient to improve LM muscle function. Future studies should examine the effects of combining DN and muscle training for facilitating LM muscle contraction, and for functional improvements in activities of daily living. In addition, future studies may consider the use of indwelling EMG for studying LM muscle contraction.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Paige Allen and Stefan Lloyd for their assistance in data collection.
Biographies
Sharon Wang-Price, PT, PhD is a Professor in the School of Physical Therapy at Texas Woman’s University in Dallas, Texas. Dr. Wang-Price graduated from National Taiwan University in 1987 with a Bachelor of Science degree in Physical Therapy. She received her Master of Science degree in Sports Physical Therapy from University of Pittsburgh in 1991 and her Doctor of Philosophy degree in Physical Therapy from Texas Woman’s University – Houston in 1999. She became board certified in orthopedics through the American Board of Physical Therapy Specialties in 1999 and became a fellow of the American Academy of Orthopaedic Manual Physical Therapists in 2009 after she completed her fellowship in manual therapy from the North American Institute of Orthopaedic Manual Therapy. She maintains her clinical practice with Baylor Scott & White Institute for Rehabilitation.
Jason Zafereo, PT, PhD is an Associate Professor in the Department of Physical Therapy at the University of Texas (UT) Southwestern Medical Center in Dallas, Texas, and also is a faculty member in the UT Southwestern Orthopedic Physical Therapy Residency program. Dr. Zafereo graduated from Baylor University in 1999 with a Bachelor of Arts degree in Biology. He received his Master of Physical Therapy degree from UT Southwestern in 2001, and his Doctor of Philosophy degree in Physical Therapy from Texas Woman’s University – Dallas in 2015. Dr. Zafereo became board certified in orthopedics through the American Board of Physical Therapy Specialties in 2007 and became a fellow of the American Academy of Orthopaedic Manual Physical Therapists in 2007 after he completed his fellowship in manual therapy from the Manual Therapy Institute. He maintains a clinical practice in an interdisciplinary pain program at the Eugene McDermott Center for Pain Management at UT Southwestern Medical Center.
Zach Couch, PT, MPT is a clinical director for Texas Physical Therapy Specialist - Dallas and an adjunct faculty in the School of Physical Therapy at the Texas Woman’s University in Dallas, Texas. Mr. Couch received his Bachelor of Science degree in Physical Therapy and his Master of Physical Therapy degree in 2002 from the University of Evansville. Mr. Couch became board certified in orthopedics through the American Board of Physical Therapy Specialties in 2007 and became a fellow of the American Academy of Orthopaedic Manual Physical Therapists in 2011 after he completed his fellowship in manual therapy from the North American Institute of Orthopaedic Manual Therapy.
Kelli Brizzolara, PT, PhD PT, PhD is an Assistant Professor in the School of Physical Therapy at the Texas Woman’s University in Dallas, Texas. Dr. Brizzolara graduated from Texas A&M University in 2001 with a Bachelor of Science degree in Exercise Technology. She received her Master of Science degree in Physical Therapy from Texas Woman’s University - Houston in 2003, and her Doctor of Philosophy degree in Physical Therapy from Texas Woman’s University – Dallas in 2013. She became board certified in orthopedics through the American Board of Physical Therapy Specialties in 2006. She maintains her clinical practice with Texas Health Resources.
Taylor Heins, BS is a physical therapy student in the School of Physical Therapy at Texas Woman’s University in Dallas, TX. Taylor graduated from Emporia State University in Emporia, Kansas with a Bachelor of Science degree in Health & Human Performance in 2017. Taylor will graduate from Texas Woman’s University - Dallas with a Doctor of Physical Therapy degree in 2020. After graduation, she plans to return to her hometown of Emporia, Kansas to begin her clinical practice.
Lindsey Smith, BS is a physical therapy student in the School of Physical Therapy at Texas Woman’s University in Dallas, TX. Lindsey graduated from Cameron University, Oklahoma with a Bachelor of Science degree in Interdisciplinary Studies with a concentration in Biology and Sports and Exercise Science in 2017. Lindsey will graduate from Texas Woman’s University - Dallas with a Doctor of Physical Therapy degree in 2020. After graduation, Lindsey plans to move to Wichita Falls, Texas to begin her clinical practice.
Funding Statement
This work was supported by a Texas Woman’s University Small Grant.
Data Sharing
The data that support the findings of this study are available on request from the corresponding author, SWP. The data is not publicly available due to their containing information that could compromise the privacy of research participants.
Disclosure statement
No potential conflict of interest was reported by the authors.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed here.
References
- [1].Hides JA, Richardson CA, Jull GA.. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769. [DOI] [PubMed] [Google Scholar]
- [2].Hides JA, Stokes MJ, Saide M, et al. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19(2):165–172. [DOI] [PubMed] [Google Scholar]
- [3].Wallwork TL, Stanton WR, Freke M, et al. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther. 2009;14:496–500. [DOI] [PubMed] [Google Scholar]
- [4].Hicks GE, Fritz JM, Delitto A, et al. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86:1753–1762. [DOI] [PubMed] [Google Scholar]
- [5].O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22(24):2959–2967. [DOI] [PubMed] [Google Scholar]
- [6].Van K, Hides JA, Richardson CA. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J Orthop Sports Phys Ther. 2006;36(12):920–925. [DOI] [PubMed] [Google Scholar]
- [7].Wang-Price S, Zafereo J, Brizzolara K, et al. Effects of different verbal instructions on change of lumbar multifidus muscle thickness in asymptomatic adults and in patients with low back pain. J Man Manip Ther. 2017;25(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Henry SM, Westervelt KC. The use of real-time ultrasound feedback in teaching abdominal hollowing exercises to healthy subjects. J Orthop Sports Phys Ther. 2005;35(6):338–345. [DOI] [PubMed] [Google Scholar]
- [9].Vega Toro AS, Cools AM, de Oliveira AS. Instruction and feedback for conscious contraction of the abdominal muscles increases the scapular muscles activation during shoulder exercises. Man Ther. 2016;25:11–18. [DOI] [PubMed] [Google Scholar]
- [10].Wang-Price S, Zafereo J, Brizzolara K, et al. Effects of tactile feedback on lumbar multifidus muscle activity in asymptomatic healthy adults and patients with low back pain. J Bodyw Mov Ther. 2018;22(4):956–962. [DOI] [PubMed] [Google Scholar]
- [11].Koppenhaver SL, Walker MJ, Su J, et al. Changes in lumbar multifidus muscle function and nociceptive sensitivity in low back pain patient responders versus non-responders after dry needling treatment. Man Ther. 2015;20(6):769–776. [DOI] [PubMed] [Google Scholar]
- [12].Dar G, Hicks GE. The immediate effect of dry needling on multifidus muscles’ function in healthy individuals. J Back Musculoskelet Rehabil. 2016;29(2):273–278. [DOI] [PubMed] [Google Scholar]
- [13].Mansfield CJ, Vanetten L, Willy R, et al. The effects of needling therapies on muscle force production: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2019;49(3):154–170. [DOI] [PubMed] [Google Scholar]
- [14].Brinkhaus B, Witt CM, Jena S, et al. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med. 2006;166(4):450–457. [DOI] [PubMed] [Google Scholar]
- [15].Chou LW, Kao MJ, Lin JG. Probable mechanisms of needling therapies for myofascial pain control. Evid Based Complement Alternat Med. 2012;2012:705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dunning J, Butts R, Mourad F, et al. Dry needling: a literature review with implications for clinical practice guidelines. Phys Ther Rev. 2014;19(4):252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Myburgh C, Hartvigsen J, Aagaard P, et al. Skeletal muscle contractility, self-reported pain and tissue sensitivity in females with neck/shoulder pain and upper Trapezius myofascial trigger oints- a randomized intervention study. Chiropr Man Therap. 2012;20(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ga H, Choi JH, Park CH, et al. Dry needling of trigger points with and without paraspinal needling in myofascial pain syndromes in elderly patients. J Altern Complement Med. 2007;13(6):617–624. [DOI] [PubMed] [Google Scholar]
- [19].Koppenhaver SL, Walker MJ, Rettig C, et al. The association between dry needling-induced twitch response and change in pain and muscle function in patients with low back pain: a quasi-experimental study. Physiotherapy. 2017;103(2):131–137. [DOI] [PubMed] [Google Scholar]
- [20].Perreault T, Dunning J, Butts R. The local twitch response during trigger point dry needling: is it necessary for successful outcomes? J Bodyw Mov Ther. 2017;21(4):940–947. [DOI] [PubMed] [Google Scholar]
- [21].Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23(5):640–646. [DOI] [PubMed] [Google Scholar]
- [22].Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29(2):172–185. [DOI] [PubMed] [Google Scholar]
- [23].Tough EA, White AR, Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain. 2009;13(1):3–10. [DOI] [PubMed] [Google Scholar]
- [24].Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- [25].Johnston V, Jimmieson NL, Jull G, et al. Quantitative sensory measures distinguish office workers with varying levels of neck pain and disability. Pain. 2008;137(2):257–265. [DOI] [PubMed] [Google Scholar]
- [26].Persson AL, Brogårdh C, Sjölund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil Med. 2004;36(1):17–27. [DOI] [PubMed] [Google Scholar]
- [27].Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80(5):485–498. [PubMed] [Google Scholar]
- [28].Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30(11):1331–1334. [DOI] [PubMed] [Google Scholar]
- [29].Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010;19(9):1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82:8–24. [DOI] [PubMed] [Google Scholar]
- [31].Fritz JM, Irrgang JJ. A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys Ther. 2001;81:776–788. [DOI] [PubMed] [Google Scholar]
- [32].Kiesel KB, Uhl T, Underwood FB, et al. Rehabilitative ultrasound measurement of select trunk muscle activation during induced pain. Man Ther. 2008;13(2):132–138. [DOI] [PubMed] [Google Scholar]
- [33].Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles . The Netherlands: roessingh research and development [Internet]. [cited 2019. October 19]. Available from: http://www.seniam.org/
- [34].Djordjevic O, Konstantinovic L, Miljkovic N, et al. Relationship between electromyographic signal amplitude and thickness change of the trunk muscles in patients with and without low back pain. Clin J Pain. 2015;31(10):893–902. [DOI] [PubMed] [Google Scholar]
- [35].Sung W, Hicks GE, Ebaugh D, et al. Individuals with and without low back pain use different motor control strategies to achieve spinal stiffness during the prone instability test. J Orthop Sports Phys Ther. 2019;49(12):899–907. [DOI] [PubMed] [Google Scholar]
- [36].Chesterton LS, Sim J, Wright CC, et al. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23(9):760–766. [DOI] [PubMed] [Google Scholar]
- [37].Kiesel KB, Uhl TL, Underwood FB, et al. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther. 2007;12(2):161–166. [DOI] [PubMed] [Google Scholar]
- [38].Chen YJ, Chen CT, Liu JY, et al. What is the appropriate acupuncture treatment schedule for chronic pain? Review and analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2019;2019:5281039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Casanueva B, Rivas P, Rodero B, et al. Short-term improvement following dry needle stimulation of tender points in fibromyalgia. Rheumatol Int. 2014;34:861–866. [DOI] [PubMed] [Google Scholar]
- [40].Llamas-Ramos R, Pecos-Martín D, GallegoIzquierdo T, et al. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44:852–861. [DOI] [PubMed] [Google Scholar]
- [41].Choi YJ, Lee JE, Moon WK, et al. Does the effect of acupuncture depend on needling sensation and manipulation? Complement Ther Med. 2013;21(3):207–214. [DOI] [PubMed] [Google Scholar]
- [42].Zaslawski CJ, Cobbin D, Lidums E, et al. The impact of site specificity and needle manipulation on changes to pain pressure threshold following manual acupuncture: a controlled study. Complement Ther Med. 2003;11(1):11–21. [DOI] [PubMed] [Google Scholar]
- [43].Langevin HM, Churchill DL, Fox JR, et al. Biomechanical response to acupuncture needling in humans. J Appl Physiol. 2001;91(6):2471–2478. [DOI] [PubMed] [Google Scholar]
- [44].Langevin HM, Churchill DL, Wu J, et al. Evidence of connective tissue involvement in acupuncture. Faseb J. 2002;16(8):872–874. [DOI] [PubMed] [Google Scholar]
- [45].Chang S, Kwon OS, Bang SK, et al. Peripheral sensory nerve tissue but not connective tissue is involved in the action of acupuncture. Front Neurosci. 2019;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Goldman N, Chen M, Fujita T, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13(7):883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Takano T, Chen X, Luo F, et al. Traditional acupuncture triggers a local increase in adenosine in human subjects. J Pain. 2012;13(12):1215–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma SX, Lee PC, Anderson TL, et al. Response of local nitric oxide release to manual acupuncture and electrical heat in humans: effects of reinforcement methods. Evid Based Complement Alternat Med. 2017;2017:4694238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang T, Zhang W, Jia S, et al. A transcontinental pilot study for acupuncture lifting-thrusting and twisting-rotating manipulations. Evid Based Complement Alternat Med. 2012;2012:157989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mejuto-Vázquez MJ, Salom-Moreno J, Ortega-Santiago R, et al. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44(4):252–260. [DOI] [PubMed] [Google Scholar]
- [51].Sanches ML, Juliano Y, Novo NF, et al. Correlation between pressure pain threshold and pain intensity in patients with temporomandibular disorders who are compliant or non-compliant with conservative treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(4):459–468. [DOI] [PubMed] [Google Scholar]
- [52].Stuginski-Barbosa J, Silva RS, Cunha CO, et al. Pressure pain threshold and pain perception in temporomandibular disorder patients: is there any correlation? Rev Dor. São Paulo. 2015;16(1):22–26. [Google Scholar]
- [53].Silva AC, Biasotto-Gonzalez DA, Dos Santos DM, et al. Evaluation of the immediate effect of auricular acupuncture on pain and electromyographic activity of the upper trapezius muscle in patients with nonspecific neck pain: a randomized, single-blinded, sham-controlled, crossover study. Evid Based Complement Alternat Med. 2015;2015:523851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].de Souza LL, de Araujo FL, da Silva FA, et al. Unilateral and immediate stimulation of acupuncture points Xiaohai (SI8) and Jianwaishu (SI14) of the small intestine meridian increases electromyographic activity and strength in the ipsilateral and contralateral upper trapezius muscle. J Acupunct Meridian Stud. 2016;9(5):250–256. [DOI] [PubMed] [Google Scholar]
- [55].Puentedura EJ, Buckingham SJ, Morton D, et al. Immediate changes in resting and contracted thickness of transversus abdominis after dry needling of lumbar multifidus in healthy participants: a randomized controlled crossover trial. J Manipulative Physiol Ther. 2017;40(8):615–623. [DOI] [PubMed] [Google Scholar]
- [56].Calamita SAP, Biasotto-Gonzalez DA, De Melo NC, et al. Immediate effect of acupuncture on electromyographic activity of the upper trapezius muscle and pain in patients with nonspecific neck pain: a randomized, single-blinded, sham-controlled, crossover study. J Manipulative Physiol Ther. 2018;41(3):208–217. [DOI] [PubMed] [Google Scholar]
- [57].Silva de Camargo P, Lima CR, de Andrade E, et al. The effect of auricular and systemic acupuncture on the electromyographic activity of the trapezius muscle with trigger points-a pilot study. J Acupunct Meridian Stud. 2018;11(1):18–24. [DOI] [PubMed] [Google Scholar]
- [58].Calvo-Lobo C, Pacheco-da-Costa S, Martínez-Martínez J, et al. Dry needling on the infraspinatus latent and active myofascial trigger points in older adults with nonspecific shoulder pain: a randomized clinical trial. J Geriatr Phys Ther. 2018;41(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55(2):145–149. [DOI] [PubMed] [Google Scholar]
- [60].Kjaer P, Bendix T, Sorensen JS, et al. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cho SH, Kim SH, Park SY. Effect of the body mass index and sexual difference on the muscle activity during trunk exercise: a preliminary study. Exercise rehabil. 2018;14(5):778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stokes IA, Henry SM, Single RM. Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin Biomech. 2003;18(1):9–13. [DOI] [PubMed] [Google Scholar]
- [63].De Luca CJ, Merletti R. Surface myoelectric signal cross-talk among muscles of the leg. Electroencephalogr Clin Neurophysiol. 1988;69(6):568–575. [DOI] [PubMed] [Google Scholar]
- [64].Ekstrom RA, Osborn RW, Hauer PL. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J Orthop Sports Phys Ther. 2008;38(12):736–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles . The Netherlands: roessingh research and development [Internet]. [cited 2019. October 19]. Available from: http://www.seniam.org/


